Clinical Development and Evaluation of a Multi-Component Dissolving Microneedle Patch for Skin Pigmentation Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Volunteers
2.3. Preparation of DMNs
2.4. Characterizations of DMNs
2.5. The PTIO Antioxidant Activity of DMNs
2.6. DPPH• Scavenging Efficiency of DMNs
2.7. Safety on B16-F10 Cells
2.8. Effects of Cellular Tyrosinase Activity
2.9. Melanin Measurement
2.10. Zebrafish Experiment
2.11. Clinical Research in DMNs
2.12. Statistical Analysis
3. Results and Discussion
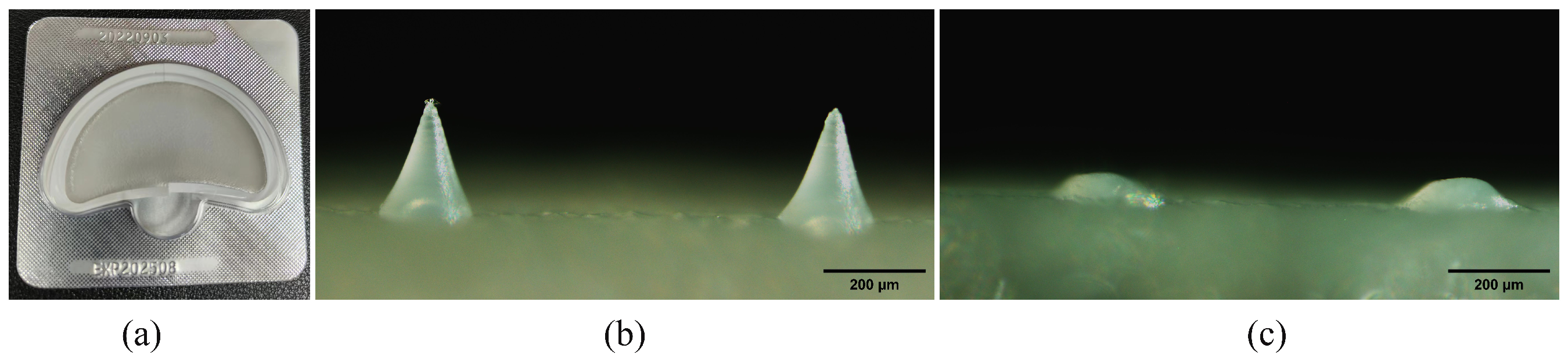
3.1. Preparation and Characterization of DMNs
3.2. Antioxidant Results of DMNs
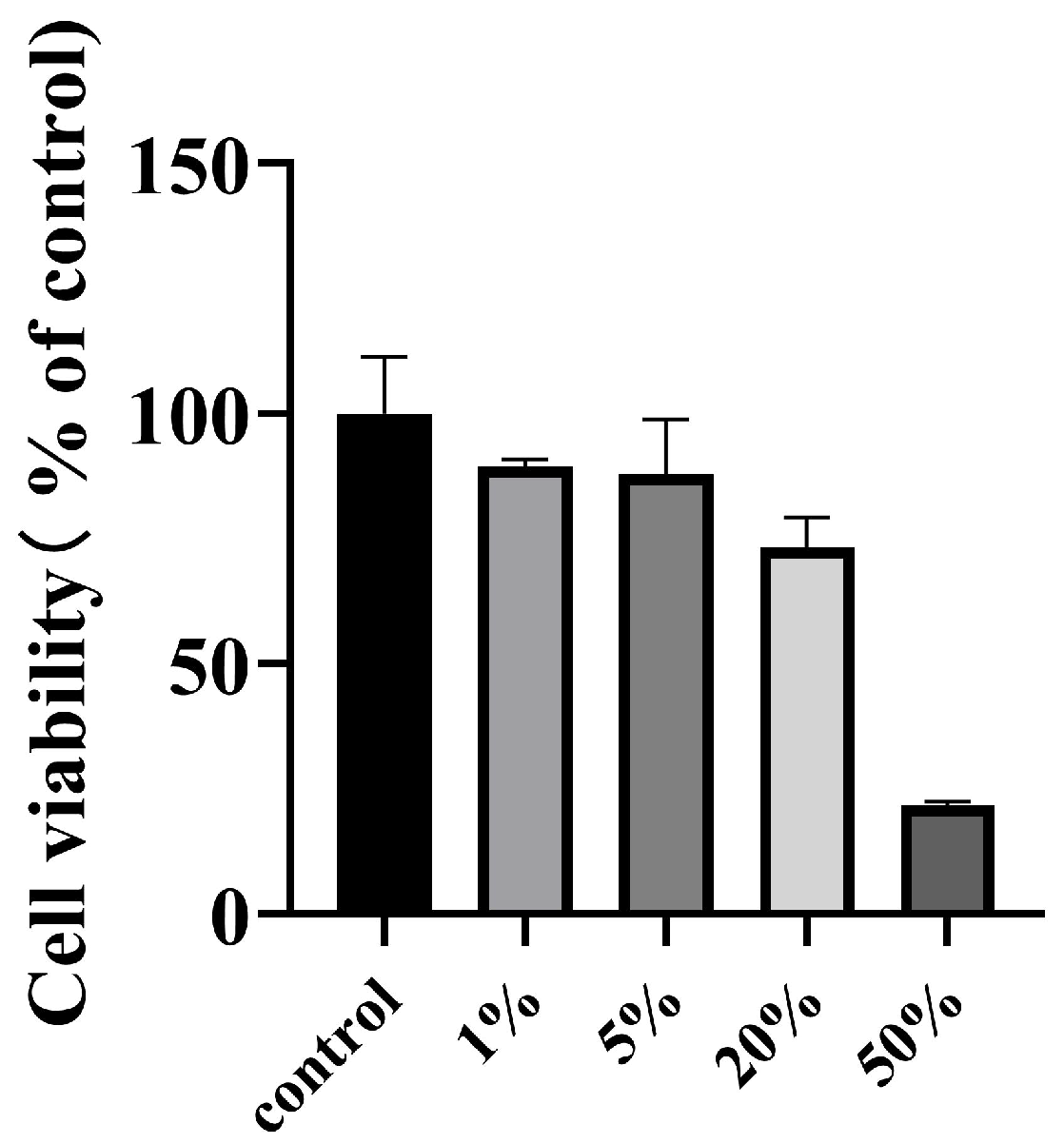
3.3. Cytotoxicity Results of DMNs
3.4. Effect of DMNs on The Activity of Tyrosinase in B16-F10 Cells
3.5. Effect of DMNs on Melanin Production in B16-F10 Cells
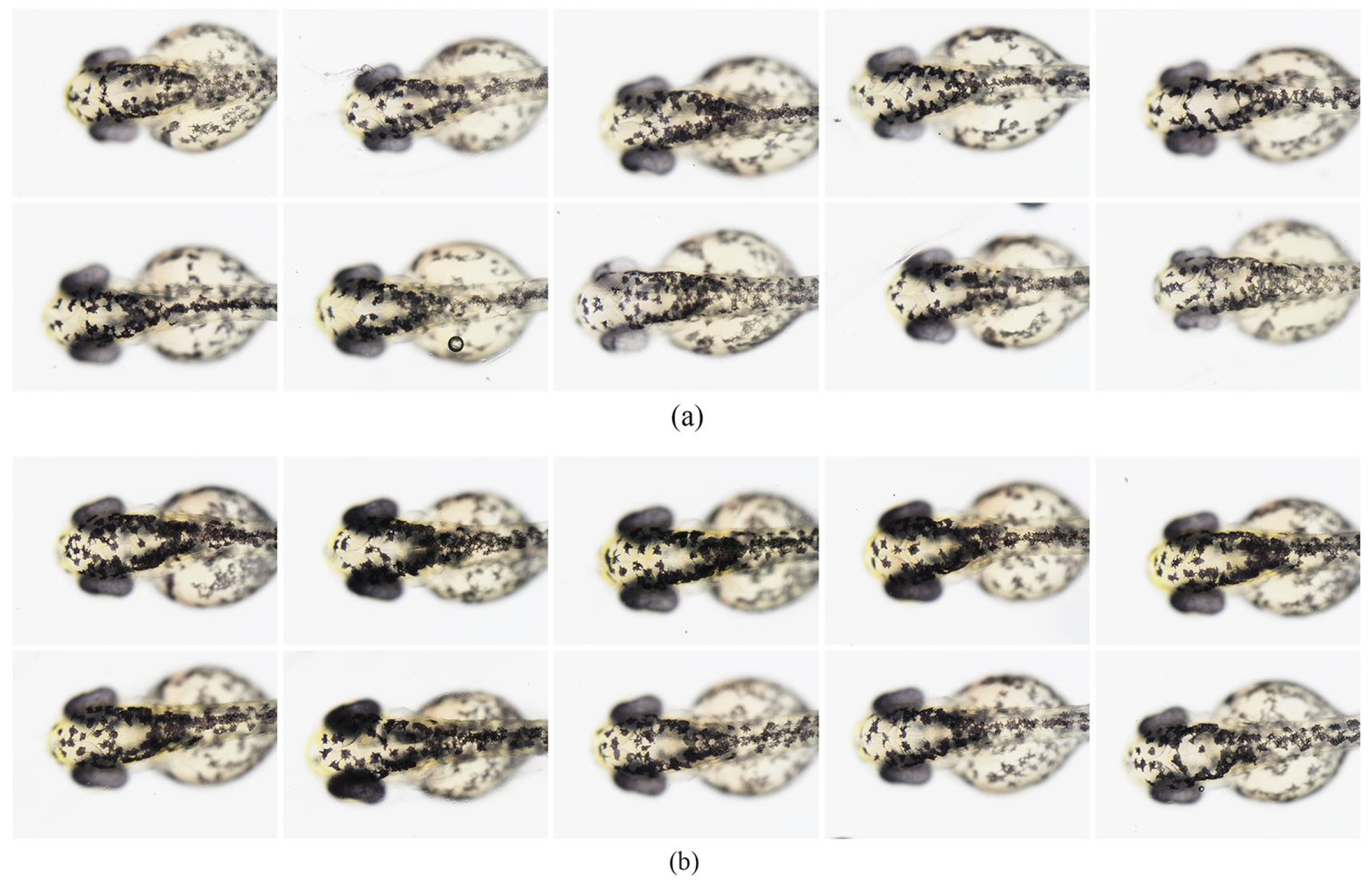
3.6. Effect of DMNs on Melanin Pigmentation of Zebrafish Embryos
3.7. The Clinical Trial of DMNs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Khalili, M.; Amiri, R.; Iranmanesh, B.; Zartab, H.; Aflatoonian, M. Safety and efficacy of mesotherapy in the treatment of melasma: A review article. J. Cosmet. Dermatol. 2022, 21, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Lee, J.W.; Papaccio, F.; Bellei, B.; Picardo, M. Alterations of the pigmentation system in the aging process. Pigment. Cell Melanoma Res. 2021, 34, 800–813. [Google Scholar] [CrossRef] [PubMed]
- Phansuk, K.; Vachiramon, V.; Jurairattanaporn, N.; Chanprapaph, K.; Rattananukrom, T. Dermal pathology in melasma: An update review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 11–19. [Google Scholar] [CrossRef]
- Choi, W.; Yin, L.; Smuda, C.; Batzer, J.; Hearing, V.J.; Kolbe, L. Molecular and histological characterization of age spots. Exp. Dermatol. 2017, 26, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Burestedt, E.; Narvaez, A.; Ruzgas, T.; Gorton, L.; Emnéus, J.; Domínguez, E.; Marko-Varga, G. Rate-limiting steps of tyrosinase-modified electrodes for the detection of catechol. Anal. Chem. 1996, 68, 1605–1611. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F. Hydroquinone: Myths and reality. Clin. Exp. Dermatol. 2021, 46, 636–640. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.; Wang, W.; Zhang, J.; Yin, J.; Le, T.; Xue, J.; Engelhardt, U.H.; Jiang, H. Kojic acid showed consistent inhibitory activity on tyrosinase from mushroom and in cultured B16F10 cells compared with arbutins. Antioxidants 2022, 11, 502. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.; Xin, X.; Mo, L.; Zou, Y.; Zhao, G.; Yu, Y.; Chen, K. Antityrosinase mechanism and antimelanogenic effect of arbutin esters synthesis catalyzed by whole-cell biocatalyst. J. Agric. Food Chem. 2021, 69, 4243–4252. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoon, J.Y.; Yang, S.Y.; Choi, S.K.; Kwon, S.J.; Cho, I.S.; Jeong, M.H.; Ho Kim, Y.; Choi, G.S. Tyrosinase inhibitory components from Aloe vera and their antiviral activity. J. Enzym. Inhib. Med. Chem. 2017, 32, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chang, C.T.; Gowrisankar, Y.V.; Chen, X.Z.; Lin, H.C.; Yen, H.R.; Yang, H.L. Zerumbone exhibits antiphotoaging and dermatoprotective properties in ultraviolet A-irradiated human skin fibroblast cells via the activation of Nrf2/ARE defensive pathway. Oxidative Med. Cell. Longev. 2019, 2019, 4098674. [Google Scholar] [CrossRef] [Green Version]
- Panich, U.; Tangsupa-a nan, V.; Onkoksoong, T.; Kongtaphan, K.; Kasetsinsombat, K.; Akarasereenont, P.; Wongkajornsilp, A. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch. Pharmacal Res. 2011, 34, 811–820. [Google Scholar] [CrossRef]
- Dilokthornsakul, W.; Dhippayom, T.; Dilokthornsakul, P. The clinical effect of glutathione on skin color and other related skin conditions: A systematic review. J. Cosmet. Dermatol. 2019, 18, 728–737. [Google Scholar] [CrossRef]
- Zduńska-Pęciak, K.; Kołodziejczak, A.; Rotsztejn, H. Two superior antioxidants: Ferulic acid and ascorbic acid in reducing signs of photoaging—A split-face comparative study. Dermatol. Ther. 2022, 35, e15254. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef]
- Yu, J.S.; Kim, A.K. Effect of combination of taurine and azelaic acid on antimelanogenesis in murine melanoma cells. J. Biomed. Sci. 2010, 17, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Keragala, C.B.; Medcalf, R.L. Plasminogen: An enigmatic zymogen. Blood 2021, 137, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Choi, S.; Yang, S.; Choi, H.; Kang, H.; Park, K.C. Effect of tranexamic acid on melasma: A clinical trial with histological evaluation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Kanechorn Na Ayuthaya, P.; Niumphradit, N.; Manosroi, A.; Nakakes, A. Topical 5% tranexamic acid for the treatment of melasma in Asians: A double-blind randomized controlled clinical trial. J. Cosmet. Laser Ther. 2012, 14, 150–154. [Google Scholar] [CrossRef]
- Litaiem, N.; Daadaa, N.; Karray, M.; Chamli, A.; Zeglaoui, F. Hypopigmentation as a side effect of melasma treatment with tranexamic acid intradermal microinjections. Dermatol. Ther. 2020, 33, e13503. [Google Scholar] [CrossRef]
- Simmler, C.; Pauli, G.F.; Chen, S.N. Phytochemistry and biological properties of glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Liu, X.; Zheng, Y.; Zhang, Z.; Li, Y.; Che, B.; Liu, G.; Zhang, L.; Dong, C.; Aisa, H.A.; et al. The mechanisms of melanogenesis inhibition by glabridin: Molecular docking, PKA/MITF and MAPK/MITF pathways. Food Sci. Hum. Wellness 2023, 12, 212–222. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2016, 168, 111–117. [Google Scholar] [CrossRef]
- Chen, S.J.; Hseu, Y.C.; Gowrisankar, Y.V.; Chung, Y.T.; Zhang, Y.Z.; Way, T.D.; Yang, H.L. The anti-melanogenic effects of 3-O-ethyl ascorbic acid via Nrf2-mediated α-MSH inhibition in UVA-irradiated keratinocytes and autophagy induction in melanocytes. Free Radic. Biol. Med. 2021, 173, 151–169. [Google Scholar] [CrossRef]
- Le, Z.; Yu, J.; Quek, Y.J.; Bai, B.; Li, X.; Shou, Y.; Myint, B.; Xu, C.; Tay, A. Design principles of microneedles for drug delivery and sampling applications. Mater. Today 2022, 63, 137–169. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Oh, Y.; Kim, Y.; Shin, Y.; Baek, S.K.; Park, J.H. Progress in microneedle array patch (MAP) for vaccine delivery. Hum. Vaccines Immunother. 2021, 17, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, B.; Xing, M.; Meng, F.; Zhang, S.; Yang, G.; Cheng, A.; Yan, C.; Xu, B.; Gao, Y. Thermal stability of exenatide encapsulated in stratified dissolving microneedles during storage. Int. J. Pharm. 2023, 636, 122863. [Google Scholar] [CrossRef] [PubMed]
- Mönkäre, J.; Nejadnik, M.R.; Baccouche, K.; Romeijn, S.; Jiskoot, W.; Bouwstra, J.A. IgG-loaded hyaluronan-based dissolving microneedles for intradermal protein delivery. J. Control. Release 2015, 218, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Chen, M.C.; Wan, S.W. Sodium hyaluronate/chitosan composite microneedles as a single-dose intradermal immunization system. Biomacromolecules 2018, 19, 2278–2285. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Saha, I.; Rai, V.K. Hyaluronic acid based microneedle array: Recent applications in drug delivery and cosmetology. Carbohydr. Polym. 2021, 267, 118168. [Google Scholar] [CrossRef] [PubMed]
- Enggi, C.K.; Sulistiawati, S.; Stephanie, S.; Tangdilintin, F.; Achmad, A.A.; Putri, R.A.; Burhanuddin, H.; Arjuna, A.; Manggau, M.A.; Permana, A.D. Development of probiotic loaded multilayer microcapsules incorporated into dissolving microneedles for potential improvement treatment of vulvovaginal candidiasis: A proof of concept study. J. Colloid Interface Sci. 2023. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Chen, B.; Xie, H.; Chen, D. Antioxidant and cytoprotective effects of kukoamines A and B: Comparison and positional isomeric effect. Molecules 2018, 23, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.E.; Chang, B.Y.; Ham, S.O.; Kim, Y.C.; Kim, S.Y. Neobavaisoflavone inhibits melanogenesis through the regulation of Akt/GSK-3β and MEK/ERK pathways in B16F10 cells and a reconstructed human 3D skin model. Molecules 2020, 25, 2683. [Google Scholar] [CrossRef]
- Ullah, S.; Kang, D.; Lee, S.; Ikram, M.; Park, C.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Synthesis of cinnamic amide derivatives and their anti-melanogenic effect in α-MSH-stimulated B16F10 melanoma cells. Eur. J. Med. Chem. 2019, 161, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Wolnicka-Glubisz, A.; Nogal, K.; Żądło, A.; Płonka, P.M. Curcumin does not switch melanin synthesis towards pheomelanin in B16F10 cells. Arch. Dermatol. Res. 2015, 307, 89–98. [Google Scholar] [CrossRef]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An emerging transdermal drug delivery system. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef]
- Xing, M.; Liu, H.; Meng, F.; Ma, Y.; Zhang, S.; Gao, Y. Design and Evaluation of Complex Polypeptide-Loaded Dissolving Microneedles for Improving Facial Wrinkles in Different Areas. Polymers 2022, 14, 4475. [Google Scholar] [CrossRef]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Chai, W.; Wang, T.; Zhang, Y. Effects of composition ratio on the properties of poly (vinyl alcohol)/poly (acrylic acid) blend membrane: A molecular dynamics simulation study. Mater. Des. 2016, 89, 848–855. [Google Scholar] [CrossRef]
- Li, X. Comparative Study of 1, 1-Diphenyl-2-picryl-hydrazyl Radical (DPPH•) Scavenging Capacity of the Antioxidant Xanthones Family. ChemistrySelect 2018, 3, 13081–13086. [Google Scholar] [CrossRef]
- Li, X. 2-Phenyl-4, 4, 5, 5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) radical scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef]
- Trouba, K.J.; Hamadeh, H.K.; Amin, R.P.; Germolec, D.R. Oxidative stress and its role in skin disease. Antioxidants Redox Signal. 2002, 4, 665–673. [Google Scholar] [CrossRef]
- Onkoksoong, T.; Jeayeng, S.; Poungvarin, N.; Limsaengurai, S.; Thamsermsang, O.; Tripatara, P.; Akarasereenont, P.; Panich, U. Thai herbal antipyretic 22 formula (APF22) inhibits UVA-mediated melanogenesis through activation of Nrf2-regulated antioxidant defense. Phytother. Res. 2018, 32, 1546–1554. [Google Scholar] [CrossRef]
- Nahhas, A.F.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol. Photoimmunol. Photomed. 2019, 35, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babbush, K.M.; Babbush, R.A.; Khachemoune, A. The therapeutic use of antioxidants for melasma. J. Drugs Dermatol. 2020, 19, 788–792. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Cheng, K.C.; Lin, Y.C.; Chen, C.Y.; Chou, H.Y.; Ma, D.L.; Leung, C.H.; Wen, Z.H.; D Wang, H.M. Synergistic effects of linderanolide B combined with arbutin, PTU or kojic acid on tyrosinase inhibition. Curr. Pharm. Biotechnol. 2015, 16, 1120–1126. [Google Scholar] [CrossRef]
- Lajis, A.F.B. A zebrafish embryo as an animal model for the treatment of hyperpigmentation in cosmetic dermatology medicine. Med. Lith. 2018, 54, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturm, R.A. A golden age of human pigmentation genetics. Trends Genet. 2006, 22, 464–468. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Praetorius, C.; Sturm, R.A.; Steingrimsson, E. Sun-induced freckling: Ephelides and solar lentigines. Pigment. Cell Melanoma Res. 2014, 27, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, D.; Sun, J.; Luo, X.; Li, H.; Sun, X.; Zheng, F. Analysis of antioxidant effect of two tripeptides isolated from fermented grains (Jiupei) and the antioxidative interaction with 4-methylguaiacol, 4-ethylguaiacol, and vanillin. Food Sci. Nutr. 2019, 7, 2391–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, W.; Wu, Z.; Zheng, R.; Yin, N.; Han, F.; Zhao, Z.; Dai, M.; Han, D.; Wang, W.; Niu, L. Inhibition mechanism of melanin formation based on antioxidant scavenging of reactive oxygen species. Analyst 2022, 147, 2703–2711. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Xing, M.; Zhang, S.; Gao, Y. Clinical Development and Evaluation of a Multi-Component Dissolving Microneedle Patch for Skin Pigmentation Disorders. Polymers 2023, 15, 3296. https://doi.org/10.3390/polym15153296
Yan C, Xing M, Zhang S, Gao Y. Clinical Development and Evaluation of a Multi-Component Dissolving Microneedle Patch for Skin Pigmentation Disorders. Polymers. 2023; 15(15):3296. https://doi.org/10.3390/polym15153296
Chicago/Turabian StyleYan, Chenxin, Mengzhen Xing, Suohui Zhang, and Yunhua Gao. 2023. "Clinical Development and Evaluation of a Multi-Component Dissolving Microneedle Patch for Skin Pigmentation Disorders" Polymers 15, no. 15: 3296. https://doi.org/10.3390/polym15153296
APA StyleYan, C., Xing, M., Zhang, S., & Gao, Y. (2023). Clinical Development and Evaluation of a Multi-Component Dissolving Microneedle Patch for Skin Pigmentation Disorders. Polymers, 15(15), 3296. https://doi.org/10.3390/polym15153296





