Interpenetration Phenomena via Anion Template Effects in Fe(II) and Co(II) Coordination Networks with a Bis-(1,2,4-triazole) Ligand
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of 1,1′-(trans-2-butene-1,4-diyl)bis-1,2,4-triazole (tbbt)
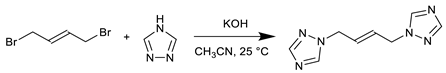
2.1.2. Synthesis of [Fe(tbbt)3](BF4)2 (1) (Catena-bis(µ-1,1′-(trans-2-butene-1,4-diyl)bis-1,2,4-triazole-κ2N4,N4’)iron bis(tetrafluoridoborate))
2.1.3. Synthesis of [Co(tbbt)3](BF4)2 (2) (Catena-bis(µ-1,1′-(trans-2-butene-1,4-diyl)bis-1,2,4-triazole-κ2N4,N4’)cobalt bis(tetrafluoridoborate))
2.1.4. Synthesis of [Fe(tbbt)3](ClO4)2 (3) (Catena-bis(µ-1,1′-(trans-2-butene-1,4-diyl)bis-1,2,4-triazole-κ2N4,N4′)iron bis(perchlorate))
2.1.5. Synthesis of [Co(tbbt)3](ClO4)2 (4) (Catena-bis(µ-1,1′-(trans-2-butene-1,4-diyl)bis-1,2,4-triazole-κ2N4,N4’)cobalt bis(perchlorate))
2.1.6. Synthesis of [Fe(NCS)2(tbbt)2] (5) (Catena-bis(thiocyanato-κN)-bis(µ-1,1’-(trans-2-butene-1,4-diyl)bis-1,2,4-triazole-κ2N4,N4’)iron)
2.1.7. Synthesis of [Co(NCS)2(tbbt)2] (6) (Catena-bis(thiocyanato-κN)-bis(µ-1,1′-(trans-2-butene-1,4-diyl)bis-1,2,4-triazole-κ2N4,N4’)cobalt)
2.1.8. Synthesis of [Fe(H2O)2(tbbt)2]Br2·2H2O (7) (Catena-diaqua-bis(µ-1,1′-(trans-2-butene-1,4-diyl)bis-1,2,4-triazole-κ2N4,N4’)iron Dibromide Dihydrate)
3. Results and Discussion
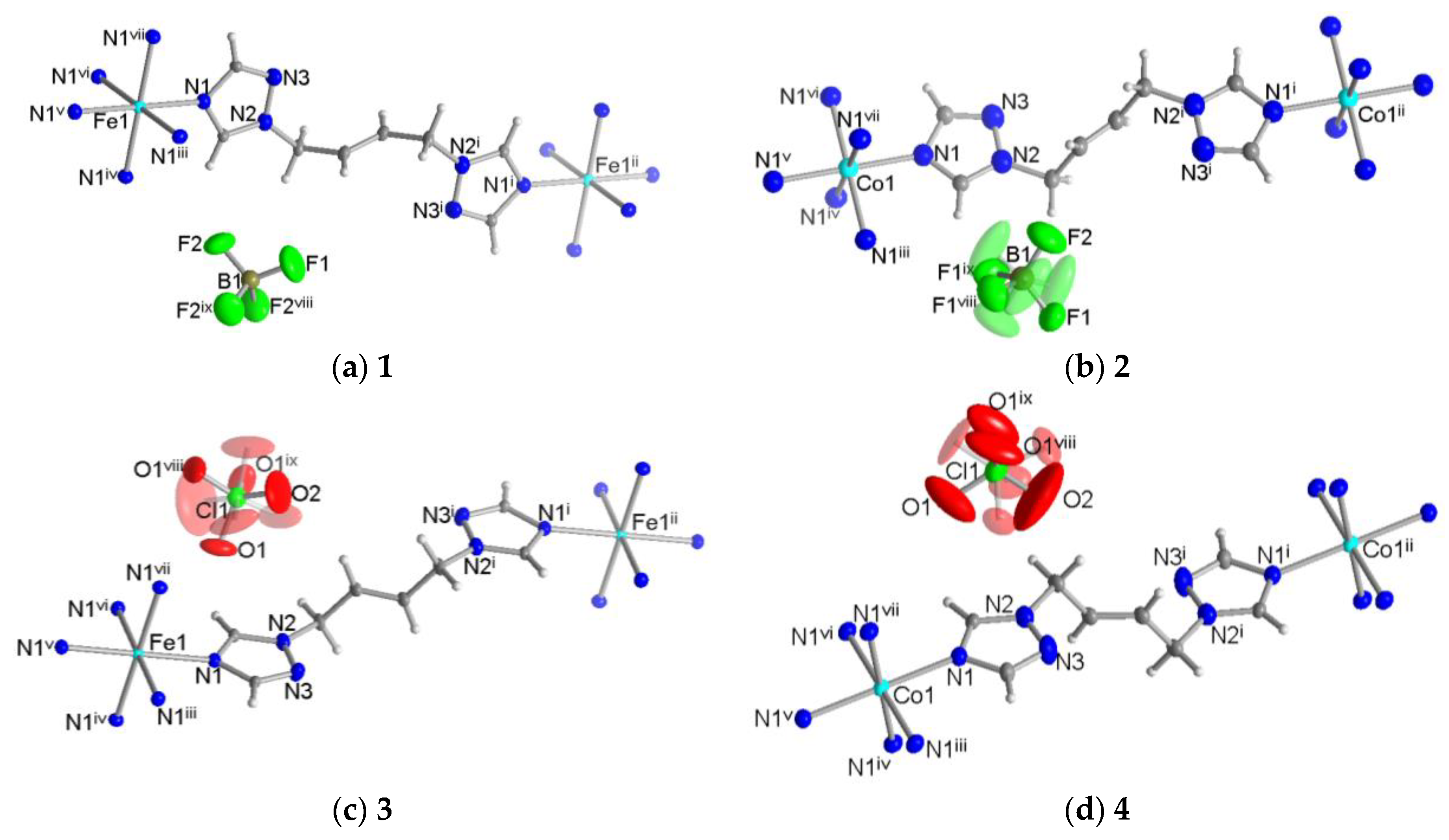
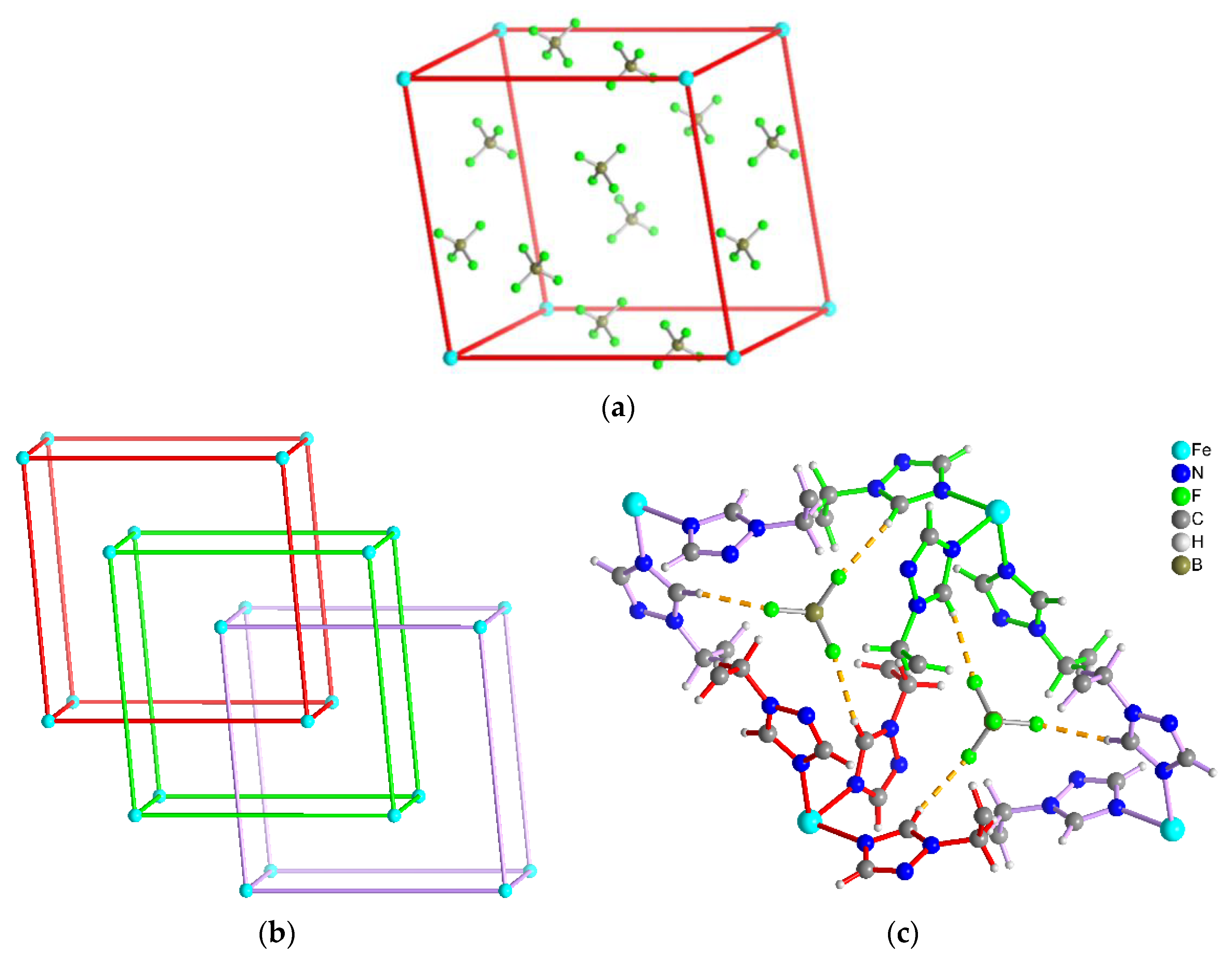
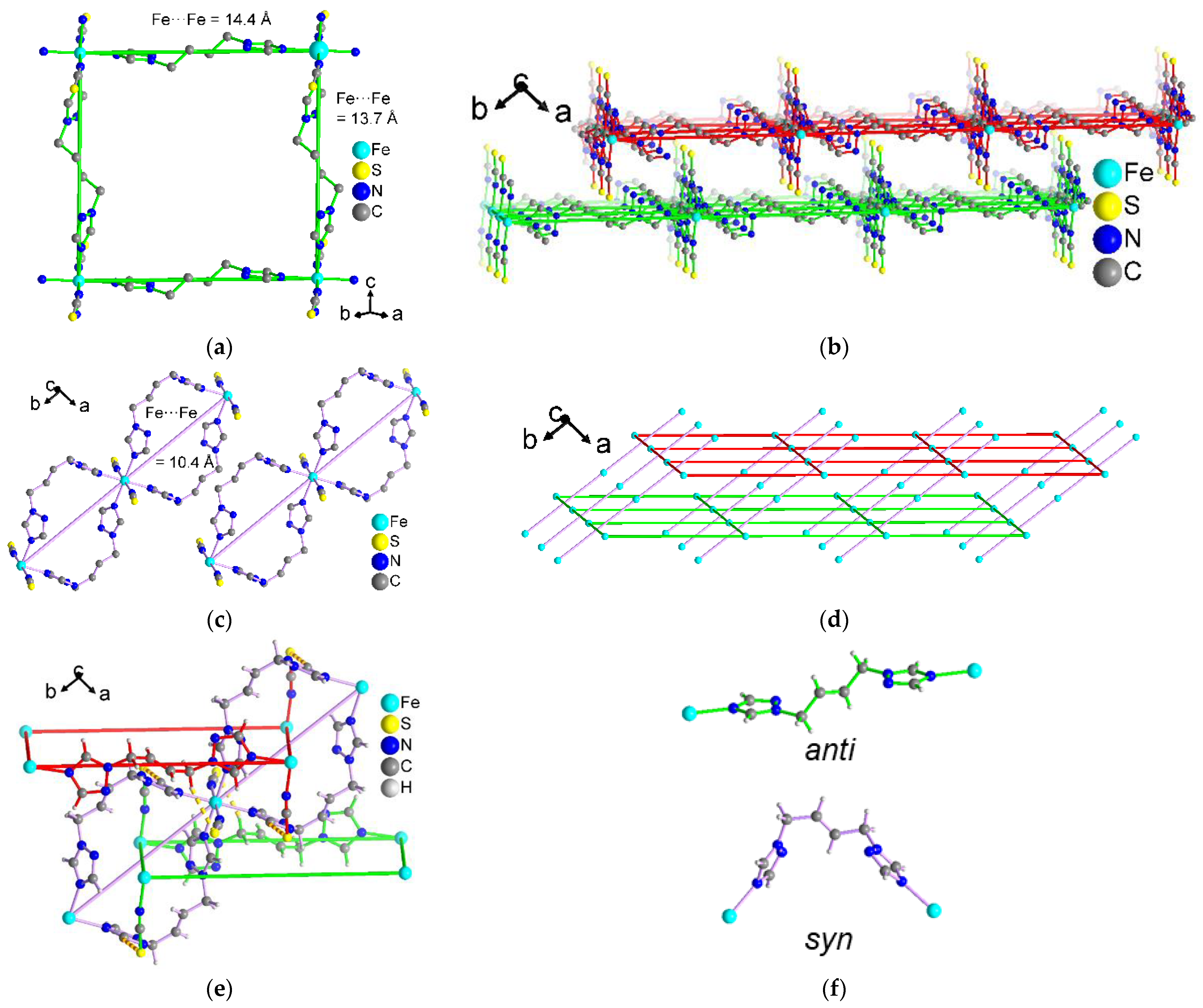
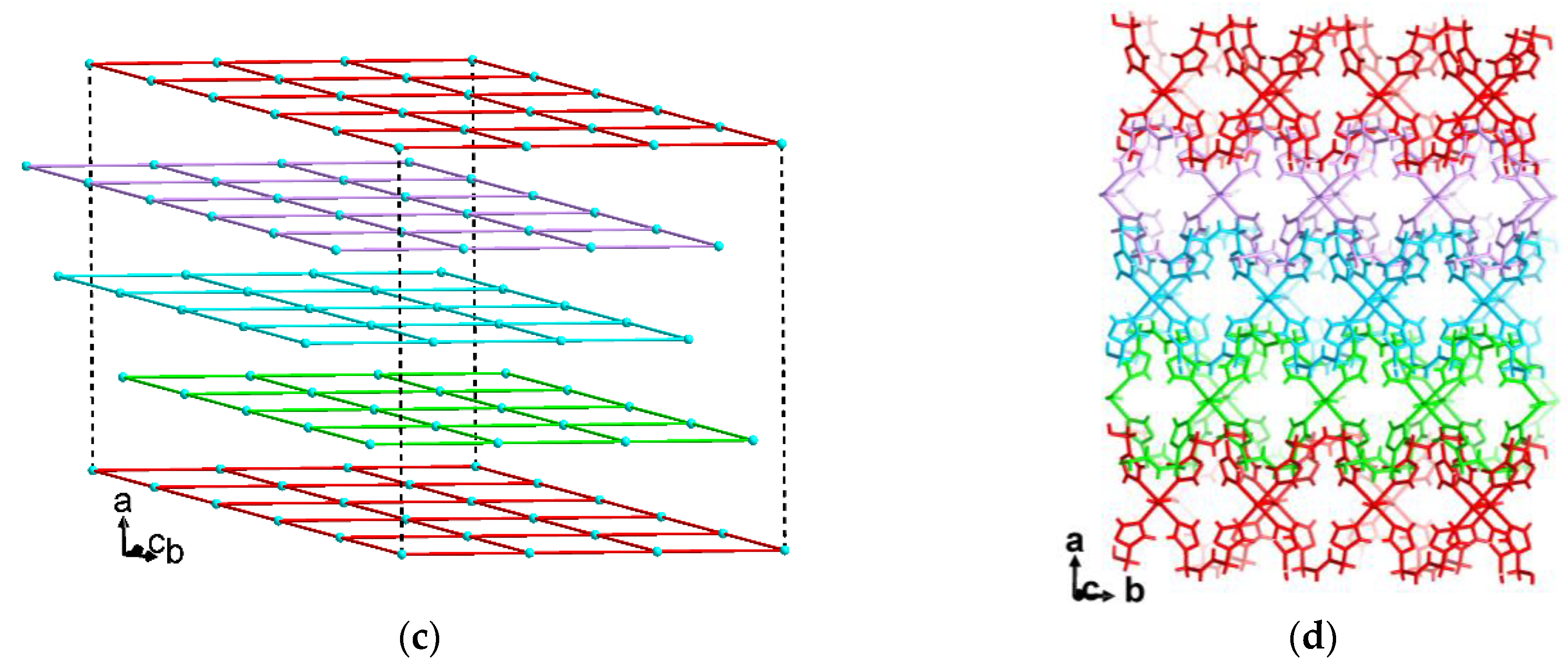
3.1. Crystal Structures of [M(tbbt)3](BF4)2 and [M(tbbt)3](ClO4)2 (M = Fe 1, 3; M = Co 2, 4)
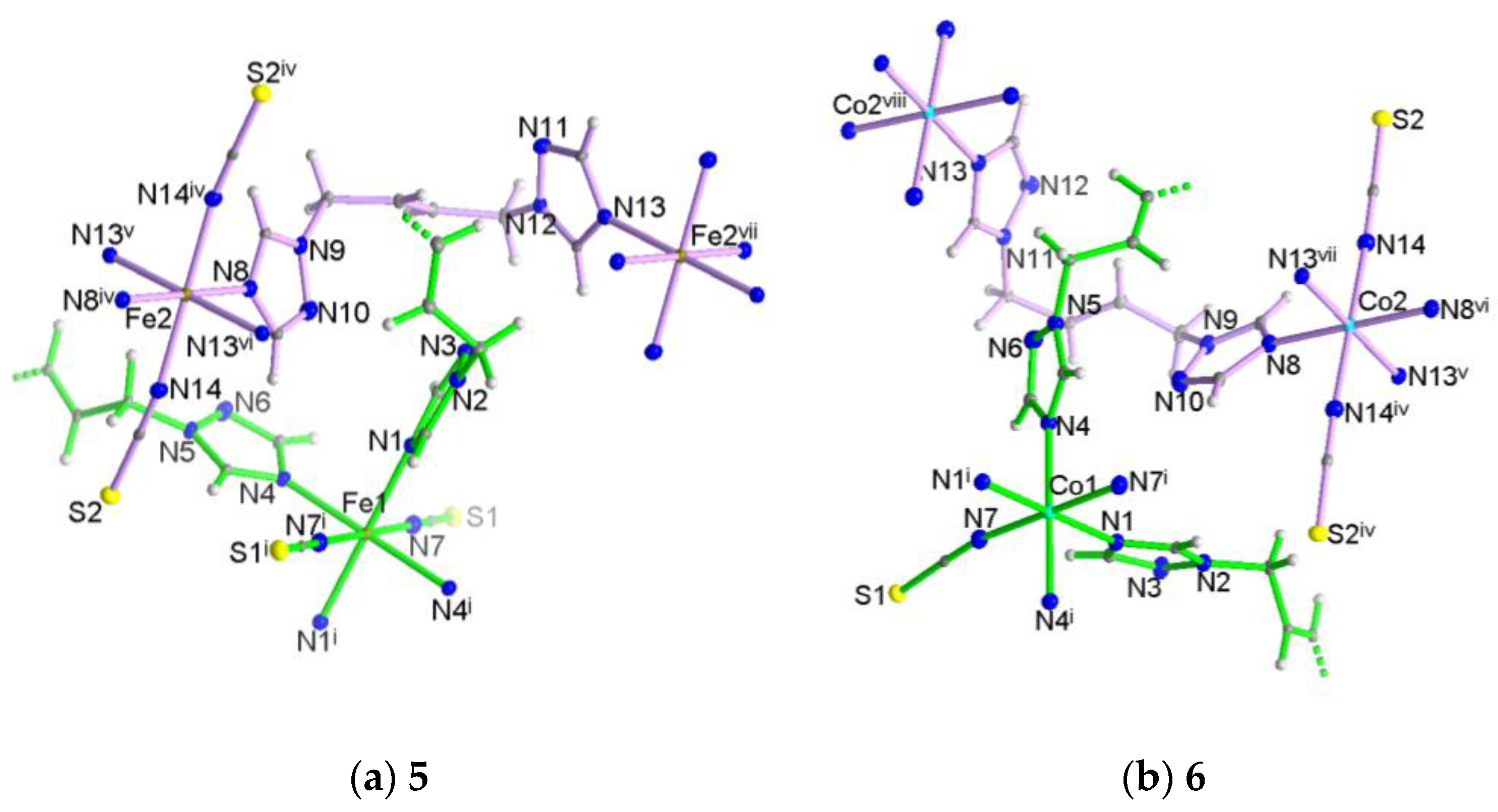
3.2. Crystal Structures of [M(NCS)2(tbbt)2] (M = Fe 5; M = Co 6)
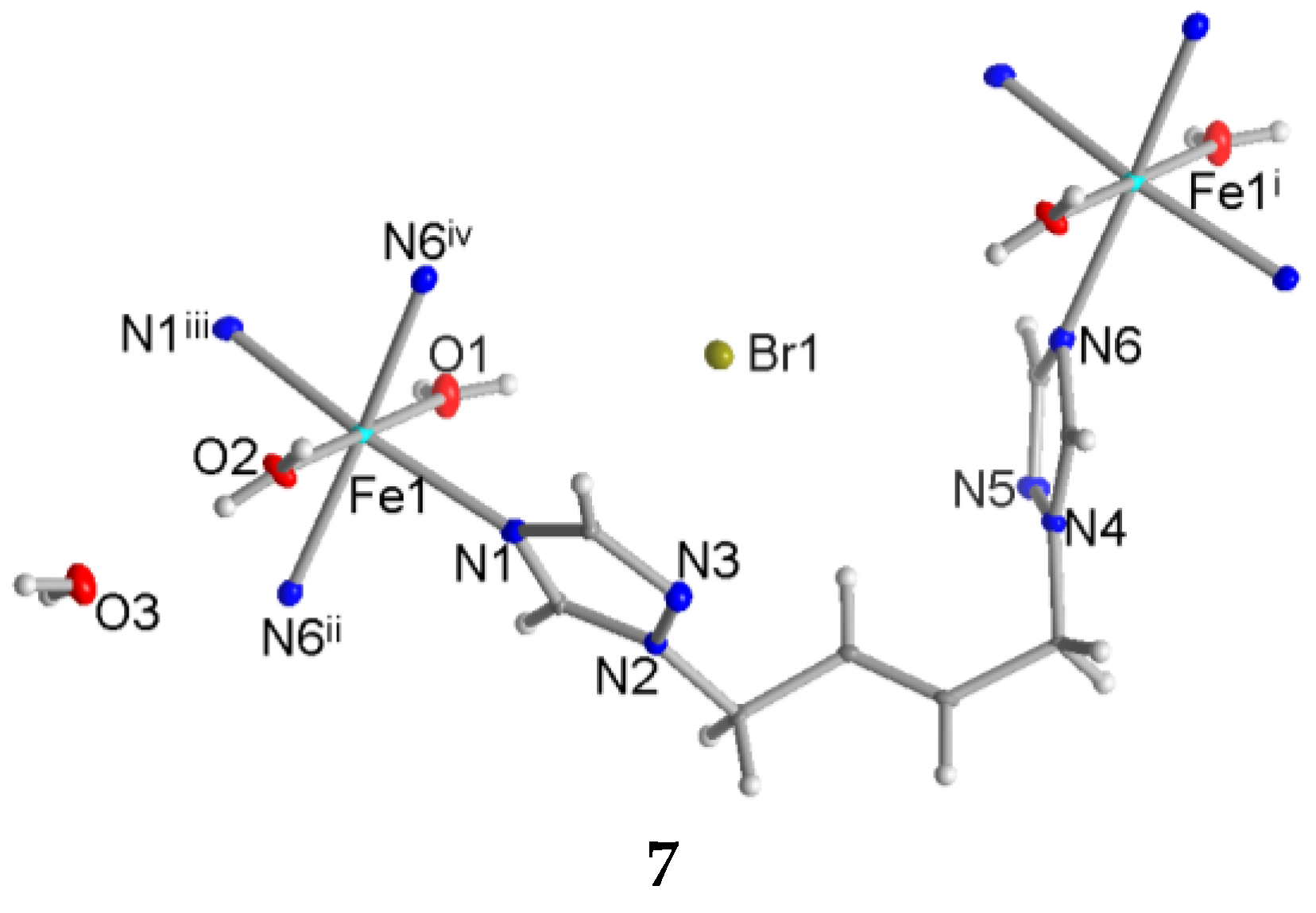
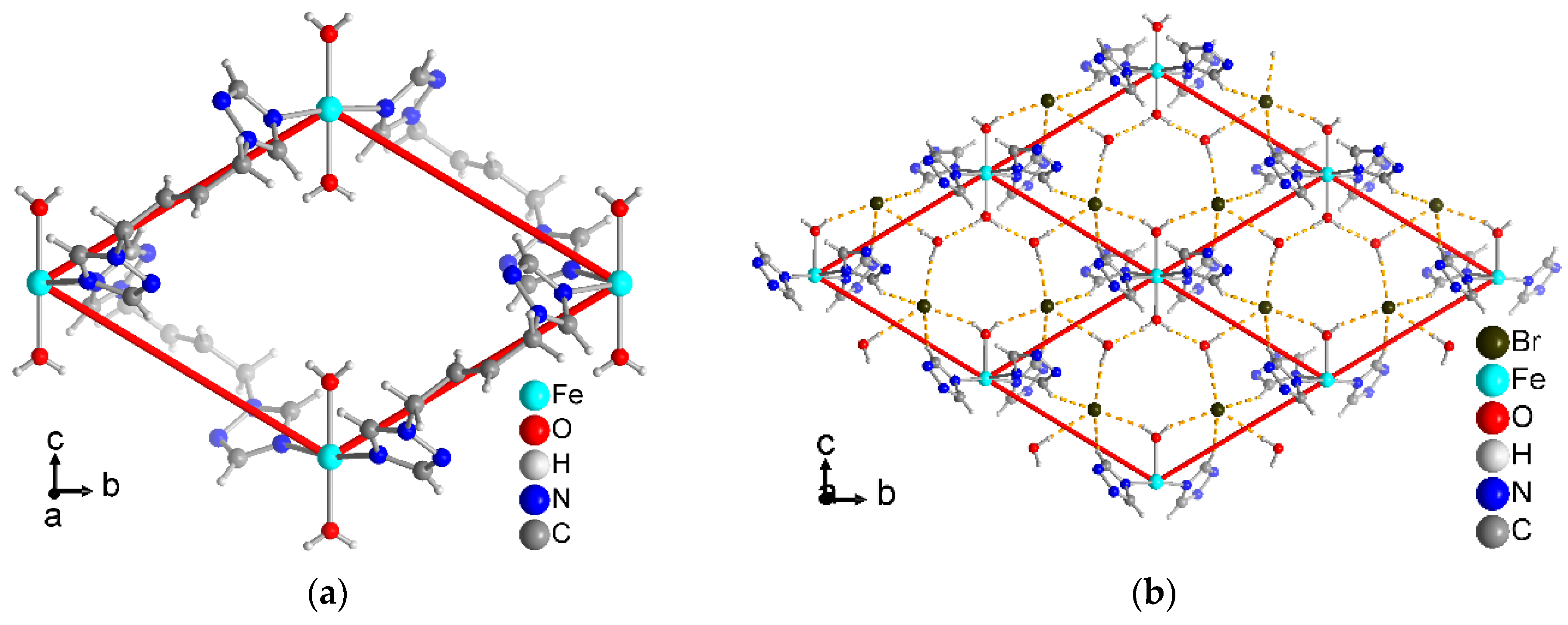
3.3. Crystal Structure of [Fe(H2O)2(tbbt)2]Br2·2H2O (7)
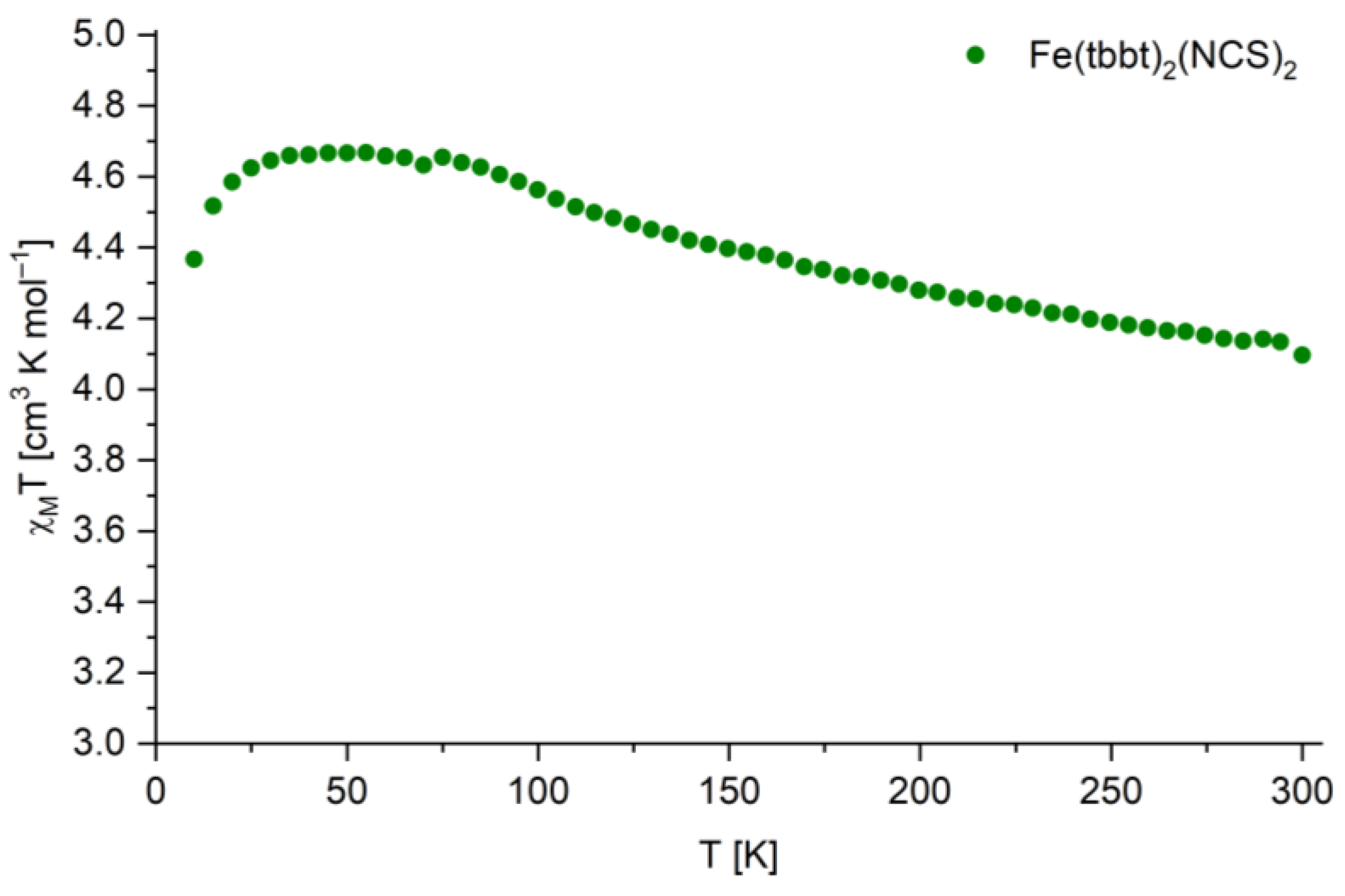
3.4. Spin-Crossover Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; Royal Society of Chemistry: Cambridge, UK, 2009; ISBN 978-0-85404-837-3. [Google Scholar]
- Batten, S.R.; Champness, N.R. Coordination polymers and metal-organic frameworks: Materials by design. Phil. Trans. R. Soc. A 2017, 375, 20160032. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Du, M.; Bu, X.-H.; Huang, Z.; Chen, S.-T.; Guo, Y.-M.; Diaz, C.; Ribas, J. From metallacyclophanes to 1-D coordination polymers: Role of anions in self-assembly processes of copper(II) and 2,5-bis(3-pyridyl)-1,3,4-oxadiazole. Inorg. Chem. 2003, 42, 552–559. [Google Scholar] [CrossRef]
- Mu, Y.; Han, G.; Ji, S.; Hou, H.; Fan, Y. Coordination polymers based on a flexible bis(triazole) ligand and aromatic polycarboxylate anions: Syntheses, topological structures and photoluminescent properties. CrystEngComm 2011, 13, 5943–5950. [Google Scholar] [CrossRef]
- Ma, J.-F.; Liu, J.-F.; Xing, Y.; Jia, H.-Q.; Lin, Y.-H. Networks with hexagonal circuits in co-ordination polymers of metal ions (ZnII, CdII) with 1,1′-(1,4-butanediyl)bis(imidazole). J. Chem. Soc. Dalton Trans. 2000, 2403–2407. [Google Scholar] [CrossRef]
- Hong, M.; Zhao, Y.; Su, W.; Cao, R.; Fujita, M.; Zhou, Z.; Chan, A.S.C. A Silver(I) Coordination Polymer Chain Containing Nanosized Tubes with Anionic and Solvent Molecule Guests. Angew. Chem. Int. Ed. 2000, 39, 2468–2470. [Google Scholar] [CrossRef]
- Lankshear, M.D.; Beer, P.D. Strategic anion templation. Coord. Chem. Rev. 2006, 250, 3142–3160. [Google Scholar] [CrossRef]
- Busch, D.H. Structural Definition of Chemical Templates and the Prediction of New and Unusual Materials. In The Pedersen Memorial Issue; Izatt, R.M., Bradshaw, J.S., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 389–395. ISBN 978-94-010-5118-7. [Google Scholar]
- Anderson, S.; Anderson, H.L.; Sanders, J.K.M. Expanding roles for templates in synthesis. Acc. Chem. Res. 1993, 26, 469–475. [Google Scholar] [CrossRef]
- Gimeno, N.; Vilar, R. Anions as templates in coordination and supramolecular chemistry. Coord. Chem. Rev. 2006, 250, 3161–3189. [Google Scholar] [CrossRef]
- Vilar, R. Anion-templated synthesis. Angew. Chem. Int. Ed. 2003, 42, 1460–1477. [Google Scholar] [CrossRef]
- Carpenter, J.P.; McTernan, C.T.; Ronson, T.K.; Nitschke, J.R. Anion Pairs Template a Trigonal Prism with Disilver Vertices. J. Am. Chem. Soc. 2019, 141, 11409–11413. [Google Scholar] [CrossRef] [PubMed]
- Horita, Y.; Ishimi, M.; Negishi, Y. Anion-templated silver nanoclusters: Precise synthesis and geometric structure. Sci. Technol. Adv. Mater. 2023, 24, 2203832. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, L.-P.; Guo, X.-Q.; Cai, L.-X.; Sun, Q.-F. Adaptive self-assembly and induced-fit transformations of anion-binding metal-organic macrocycles. Nat. Commun. 2017, 8, 15898. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, S.; Natarajan, R. Anion-Driven Programmable Chiral Self-Sorting in Metal-Organic Cages and Structural Transformations between Heterochiral and Homochiral Cages. Chemistry 2023, 29, e202203085. [Google Scholar] [CrossRef]
- Ni, J.; Wei, K.-J.; Liu, Y.; Huang, X.-C.; Li, D. Silver Coordination Polymers Based on Neutral Trinitrile Ligand: Topology and the Role of Anion. Cryst. Growth Des. 2010, 10, 3964–3976. [Google Scholar] [CrossRef]
- Reger, D.L.; Semeniuc, R.F.; Rassolov, V.; Smith, M.D. Supramolecular structural variations with changes in anion and solvent in silver(I) complexes of a semirigid, bitopic tris(pyrazolyl)methane ligand. Inorg. Chem. 2004, 43, 537–554. [Google Scholar] [CrossRef]
- Reger, D.L.; Watson, R.P.; Gardinier, J.R.; Smith, M.D. Impact of variations in design of flexible bitopic bis(pyrazolyl)methane ligands and counterions on the structures of silver(I) complexes: Dominance of cyclic dimeric architecture. Inorg. Chem. 2004, 43, 6609–6619. [Google Scholar] [CrossRef]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and Optical Switching of Iron(II) Complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Hauser, A. Intersystem Crossing in Iron(II) Coordination Compounds: A Model Process Between Classical and Quantum Mechanical Behaviour. Comments Inorg. Chem. 1995, 17, 17–40. [Google Scholar] [CrossRef]
- Gütlich, P. Spin crossover in iron(II)-complexes. In Metal Complexes. Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1981; Volume 44, pp. 83–195. [Google Scholar] [CrossRef]
- Białońska, A.; Bronisz, R.; Rudolf, M.F.; Weselski, M. HS⇄LS transition in iron(II) two-dimensional coordination networks containing tris(tetrazol-1-ylmethyl)methane as triconnected building block. Inorg. Chem. 2012, 51, 237–245. [Google Scholar] [CrossRef]
- Halcrow, M.A. Structure: Function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef]
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [PubMed]
- Arcís-Castillo, Z.; Muñoz-Lara, F.J.; Muñoz, M.C.; Aravena, D.; Gaspar, A.B.; Sánchez-Royo, J.F.; Ruiz, E.; Ohba, M.; Matsuda, R.; Kitagawa, S.; et al. Reversible chemisorption of sulfur dioxide in a spin crossover porous coordination polymer. Inorg. Chem. 2013, 52, 12777–12783. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Meng, Y.; Dong, Y.-J.; Song, X.-W.; Huang, G.-Z.; Zhang, C.-L.; Ni, Z.-P.; Navařík, J.; Malina, O.; Zbořil, R.; et al. Light- and temperature-assisted spin state annealing: Accessing the hidden multistability. Chem. Sci. 2020, 11, 3281–3289. [Google Scholar] [CrossRef]
- Kusz, J.; Gütlich, P.; Spiering, H. Structural Investigations of Tetrazole Complexes of Iron(II). Top. Curr. Chem. 2004, 234, 129–153. [Google Scholar] [CrossRef]
- Weihermüller, J.; Schlamp, S.; Dittrich, B.; Weber, B. Kinetic Trapping Effects in Amphiphilic Iron(II) Spin Crossover Compounds. Inorg. Chem. 2019, 58, 1278–1289. [Google Scholar] [CrossRef]
- Bréfuel, N.; Watanabe, H.; Toupet, L.; Come, J.; Matsumoto, N.; Collet, E.; Tanaka, K.; Tuchagues, J.-P. Concerted Spin Crossover and Symmetry Breaking Yield Three Thermally and One Light-Induced Crystallographic Phases of a Molecular Material. Angew. Chem. 2009, 121, 9468–9471. [Google Scholar] [CrossRef]
- Létard, J.-F.; Guionneau, P.; Goux-Capes, L. Towards Spin Crossover Applications. Top. Curr. Chem. 2004, 235, 221–249. [Google Scholar] [CrossRef]
- Vela, S.; Paulsen, H. Cooperativity in Spin Crossover Systems. An Atomistic Perspective on the Devil’s Staircase. Inorg. Chem. 2018, 57, 9478–9488. [Google Scholar] [CrossRef]
- Li, W.; Sun, L.; Liu, C.; Rotaru, A.; Robeyns, K.; Singleton, M.L.; Garcia, Y. Supramolecular FeII4L4 cage for fast ammonia sensing. J. Mater. Chem. C 2022, 10, 9216–9221. [Google Scholar] [CrossRef]
- Muller, R.N.; Vander Elst, L.; Laurent, S. Spin transition molecular materials: Intelligent contrast agents for magnetic resonance imaging. J. Am. Chem. Soc. 2003, 125, 8405–8407. [Google Scholar] [CrossRef] [PubMed]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005, 2062–2079. [Google Scholar] [CrossRef] [PubMed]
- Lemercier, G.; Bréfuel, N.; Shova, S.; Wolny, J.A.; Dahan, F.; Verelst, M.; Paulsen, H.; Trautwein, A.X.; Tuchagues, J.-P. A range of spin-crossover temperature T1/2>300 K results from out-of-sphere anion exchange in a series of ferrous materials based on the 4-(4-imidazolylmethyl)-2-(2-imidazolylmethyl)imidazole (trim) ligand, Fe(trim)2X2 (X=F, Cl, Br, I): Comparison of experimental results with those derived from density functional theory calculations. Chem. Eur. J. 2006, 12, 7421–7432. [Google Scholar] [CrossRef]
- Klug, C.M.; McDaniel, A.M.; Fiedler, S.R.; Schulte, K.A.; Newell, B.S.; Shores, M.P. Anion dependence in the spin-crossover properties of a Fe(II) podand complex. Dalton Trans. 2012, 41, 12577–12585. [Google Scholar] [CrossRef]
- Dîrtu, M.M.; Rotaru, A.; Gillard, D.; Linares, J.; Codjovi, E.; Tinant, B.; Garcia, Y. Prediction of the spin transition temperature in Fe(II) one-dimensional coordination polymers: An anion based database. Inorg. Chem. 2009, 48, 7838–7852. [Google Scholar] [CrossRef]
- Kiehl, J.; Hochdörffer, T.; Carrella, L.M.; Schünemann, V.; Nygaard, M.H.; Overgaard, J.; Rentschler, E. Pronounced Magnetic Bistability in Highly Cooperative Mononuclear Fe(Lnpdtz)2(NCX)2 Complexes. Inorg. Chem. 2022, 61, 3141–3151. [Google Scholar] [CrossRef]
- Gütlich, P. Spin Crossover—Quo Vadis? Eur. J. Inorg. Chem. 2013, 2013, 581–591. [Google Scholar] [CrossRef]
- Bhar, K.; Khan, S.; Costa, J.S.; Ribas, J.; Roubeau, O.; Mitra, P.; Ghosh, B.K. Crystallographic evidence for reversible symmetry breaking in a spin-crossover d7 cobalt(II) coordination polymer. Angew. Chem. Int. Ed. 2012, 51, 2142–2145. [Google Scholar] [CrossRef]
- Murray, K.S. Advances in Polynuclear Iron(II), Iron(III) and Cobalt(II) Spin-Crossover Compounds. Eur. J. Inorg. Chem. 2008, 20, 3101–3121. [Google Scholar] [CrossRef]
- Beckmann, U.; Brooker, S. Cobalt(II) complexes of pyridazine or triazole containing ligands: Spin-state control. Coord. Chem. Rev. 2003, 245, 17–29. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Fallon, G.D.; Gatehouse, B.M.K.C.; Murray, K.S. Spin-state differences and spin crossover in five-coordinate Lewis base adducts of cobalt(II) Schiff base complexes. Structure of the high-spin (N,N’-o-phenylenebis(salicylaldiminato))cobalt(II)-2-methylimidazole adduct. Inorg. Chem. 1984, 23, 580–588. [Google Scholar] [CrossRef]
- Goodwin, H.A. Spin Crossover in Transition Metal Compounds II. Top. Curr. Chem. 2004, 234, 23–47. [Google Scholar] [CrossRef]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Yi, L.; Yang, X.; Lu, T.; Cheng, P. Self-Assembly of Right-Handed Helical Infinite Chain, One- and Two-Dimensional Coordination Polymers Tuned via Anions. Cryst. Growth Des. 2005, 5, 1215–1219. [Google Scholar] [CrossRef]
- Garcia, Y. Selected polyazole based coordination polymers displaying functional properties. Adv. Inorg. Chem. 2020, 76, 121–153. [Google Scholar] [CrossRef]
- Absmeier, A.; Bartel, M.; Carbonera, C.; Jameson, G.N.L.; Weinberger, P.; Caneschi, A.; Mereiter, K.; Létard, J.-F.; Linert, W. Both spacer length and parity influence the thermal and light-induced properties of iron(II) alpha,omega-bis(tetrazole-1-yl)alkane coordination polymers. Chem. Eur. J. 2006, 12, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Boland, Y.; Hertsens, P.; Marchand-Brynaert, J.; Garcia, Y. New Ditopic and Tripodal 1,2,4-Triazole- and Tetrazole-Based Ligands for Coordination Chemistry. Synthesis 2006, 9, 1504–1512. [Google Scholar] [CrossRef]
- Grunert, C.M.; Schweifer, J.; Weinberger, P.; Linert, W.; Mereiter, K.; Hilscher, G.; Müller, M.; Wiesinger, G.; van Koningsbruggen, P.J. Structure and physical properties of [μ-tris(1,4-bis(tetrazol-1-yl)butane-N4,N4′)iron(II)] bis(hexafluorophosphate), a new Fe(II) spin-crossover compound with a three-dimensional threefold interlocked crystal lattice. Inorg. Chem. 2004, 43, 155–165. [Google Scholar] [CrossRef]
- Quesada, M.; Kooijman, H.; Gamez, P.; Sánchez Costa, J.; van Koningsbruggen, P.J.; Weinberger, P.; Reissner, M.; Spek, A.L.; Haasnoot, J.G.; Reedijk, J. [Fe(μ-btzmp)2(btzmp)2(ClO4)2]: A doubly-bridged 1D spin-transition bistetrazole-based polymer showing thermal hysteresis behaviour. Dalton Trans. 2007, 46, 5434–5440. [Google Scholar] [CrossRef]
- Rentschler, E.; von Malotki, C. Spin transition in three-dimensional bridged coordination polymers of iron(II)–urea-triazoles. Inorg. Chim. Acta 2008, 361, 3646–3653. [Google Scholar] [CrossRef]
- van Koningsbruggen, P.J.; Garcia, Y.; Kahn, O.; Fournès, L.; Kooijman, H.; Spek, A.L.; Haasnoot, J.G.; Moscovici, J.; Provost, K.; Michalowicz, A.; et al. Synthesis, crystal structure, EXAFS, and magnetic properties of catena μ-tris(1,2-bis(tetrazol-1-yl)propane-N1,N1′)iron(II) bis(perchlorate). First crystal structure of an iron(II) spin-crossover chain compound. Inorg. Chem. 2000, 39, 1891–1900. [Google Scholar] [CrossRef]
- Attaryan, O.S.; Asratyan, G.V.; Liazyan, G.A.; Panosyan, G.A.; Kinoyan, F.S.; Darbinyan, E.G. Phase-transfer catalyzed alkylation of pyrazoles and 1,2,4-triazole with cis- and trans-1,4-dichloro-2-butenes. Chem. Heterocycl. Compd. 1989, 25, 414–420. [Google Scholar] [CrossRef]
- Zhou, Q.K.; Li, N.Y. A three-dimensional cadmium coordination polymer based on 1,4-bis(1,2,4-triazol-1-yl)but-2-ene and benzene-1,3,5-tricarboxylic acid. Acta Crystallogr. C Struct. Chem. 2017, 73, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-G.; Xu, Y.-F.; Zhou, X.-H.; Zuo, J.-L.; You, X.-Z. Assembly of Iron(II)-Triazole Polymers from 1D Chains to 3D Interpenetrated Frameworks: Syntheses, Structures, and Magnetic Properties. Cryst. Growth Des. 2008, 8, 1306–1312. [Google Scholar] [CrossRef]
- Dong, W.; Ouyang, Y.; Zhu, L.-N.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P.; Cheng, P. Structure and magnetic properties of a dtm-bridged two-dimensional supramolecular complex {[Fe(dtm)2(H2O)2](ClO4)2 · 2H2O} n (dtm = 4,4′-ditriazolemethane). Transit. Met. Chem. 2006, 31, 801–804. [Google Scholar] [CrossRef]
- Qi, J.; Tong, Y.-Z.; Tang, G.-T.; Wang, Q.-L.; Li, L.-C.; Ma, Y.; Yang, G.-M.; Liao, D.-Z. Structural diversities and magnetic properties of a series of Fe(II) coordination polymers based on bis(triazole) ligands. Polyhedron 2015, 90, 123–130. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.-Y.; Li, B.-L.; Wang, J.; Gao, S. Syntheses, structures and magnetic properties of five iron(II) coordination polymers with flexible bis(imidazole) and bis(triazole) ligands. Polyhedron 2012, 31, 77–81. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.-Y.; Zhao, X.-Q.; Zhang, J.-J.; Shi, W.; Cheng, P. Assembly of two ferrous coordination polymers with triazole derivative: Syntheses, structures and magnetic properties. Inorg. Chem. Commun. 2010, 13, 699–702. [Google Scholar] [CrossRef]
- Tian, A.; Ying, J.; Peng, J.; Sha, J.; Zhu, D.; Pang, H.; Zhang, P.; Chen, Y.; Zhu, M. Assembly of a new Wells–Dawson polyoxometalate-templated compound based on Fe-btb complex. Inorg. Chem. Commun. 2008, 11, 1132–1135. [Google Scholar] [CrossRef]
- Xing, X.-Y.; Mei, X.-L.; Li, L.-C. Iron(II) coordination polymers based on 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene: Structures and magnetic properties. J. Mol. Struct. 2011, 992, 89–95. [Google Scholar] [CrossRef]
- Calvo Galve, N.; Coronado, E.; Giménez-Marqués, M.; Mínguez Espallargas, G. A mixed-ligand approach for spin-crossover modulation in a linear Fe(II) coordination polymer. Inorg. Chem. 2014, 53, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro. Rigaku Oxford Diffraction; release 1.171.40.103a; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Diamond (Version 4.5), Crystal and Molecular Structure Visualization, Crystal Impact; Dr. H. Putz & Dr. K. Brandenburg GbR: Bonn, Germany, 2018. [Google Scholar]
- Ketkaew, R.; Tantirungrotechai, Y.; Harding, P.; Chastanet, G.; Guionneau, P.; Marchivie, M.; Harding, D.J. OctaDist: A tool for calculating distortion parameters in spin crossover and coordination complexes. Dalton Trans. 2021, 50, 1086–1096. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Xiang, B. Syntheses, crystal structures and luminescent properties of two new Zn(II) coordination polymers based on a dicarboxylate and different imidazole-containing ligands. J. Coord. Chem. 2016, 69, 1551–1558. [Google Scholar] [CrossRef]
- Shang, Q.; Zeng, T.; Gao, K.; Liu, N.; Cheng, Q.; Liao, G.; Pan, Z.; Zhou, H. A novel nitrogen heterocyclic ligand-based MOF: Synthesis, characterization and photocatalytic properties. New J. Chem. 2019, 43, 16595–16603. [Google Scholar] [CrossRef]
- Garcia, Y.; Bravic, G.; Gieck, C.; Chasseau, D.; Tremel, W.; Gütlich, P. Crystal structure, magnetic properties, and 57Fe Mössbauer spectroscopy of the two-dimensional coordination polymers M(1,2-bis(1,2,4-triazol-4-yl)ethane)2(NCS)2 (MII = Fe, Co). Inorg. Chem. 2005, 44, 9723–9730. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Yudina, N.G.; Ikorskii, V.N.; Varnek, V.A.; Oglezneva, I.M.; Larionov, S.V. Spin-crossover and thermochromism in complexes of iron(II) iodide and thiocyanate with 4-amino-1,2,4-triazole. Polyhedron 1995, 14, 1333–1337. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B.; Bienz, S.; Bigler, L.; Fox, T. Spektroskopische Methoden in der Organischen Chemie; 8 Auflage; Georg Thieme Verlag: Stuttgart, Germany, 2012; ISBN 9783135761084. [Google Scholar]
- Guionneau, P.; Marchivie, M.; Bravic, G.; Létard, J.-F.; Chasseau, D. Structural Aspects of Spin Crossover. Example of the [FeIILn(NCS)2] Complexes. In Spin Crossover in Transition Metal Compounds II; Gütlich, P., Goodwin, H.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 97–128. ISBN 978-3-540-40396-8. [Google Scholar]
- Ordejón, B.; de Graaf, C.; Sousa, C. Light-induced excited-state spin trapping in tetrazole-based spin crossover systems. J. Am. Chem. Soc. 2008, 130, 13961–13968. [Google Scholar] [CrossRef] [PubMed]
- Baburin, I.A.; Blatov, V.A.; Carlucci, L.; Ciani, G.; Proserpio, D.M. Interpenetrating metal-organic and inorganic 3D networks: A computer-aided systematic investigation. Part II [1]. Analysis of the Inorganic Crystal Structure Database (ICSD). J. Solid State Chem. 2005, 178, 2452–2474. [Google Scholar] [CrossRef]
- Baburin, I.A.; Blatov, V.A.; Carlucci, L.; Ciani, G.; Proserpio, D.M. Interpenetrated three-dimensional hydrogen-bonded networks from metal–organic molecular and one- or two-dimensional polymeric motifs. CrystEngComm 2008, 10, 1822–1838. [Google Scholar] [CrossRef]
- Blatov, V.A.; Carlucci, L.; Ciani, G.; Proserpio, D.M. Interpenetrating metal–organic and inorganic 3D networks: A computer-aided systematic investigation. Part I. Analysis of the Cambridge structural database. CrystEngComm 2004, 6, 377–395. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M. Polycatenation, polythreading and polyknotting in coordination network chemistry. Coord. Chem. Rev. 2003, 246, 247–289. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Proserpio, D.M.; Mitina, T.G.; Blatov, V.A. Entangled two-dimensional coordination networks: A general survey. Chem. Rev. 2014, 114, 7557–7580. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Proserpio, D.M. How 2-periodic coordination networks are interweaved: Entanglement isomerism and polymorphism. CrystEngComm 2017, 19, 1993–2006. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.-Y.; Lee, H. Structural and anionic effects of microcrystalline Zn-CPs on 4-nitrophenol sensing performances. RSC Adv. 2022, 12, 12957–12966. [Google Scholar] [CrossRef]
- Mingos, P.M.D.; Rohl, A.L. Size and shape characteristics of inorganic molecular ions and their relevance to crystallization problems. Inorg. Chem. 1991, 30, 3769–3771. [Google Scholar] [CrossRef]
- Campos-Fernández, C.S.; Schottel, B.L.; Chifotides, H.T.; Bera, J.K.; Bacsa, J.; Koomen, J.M.; Russell, D.H.; Dunbar, K.R. Anion template effect on the self-assembly and interconversion of metallacyclophanes. J. Am. Chem. Soc. 2005, 127, 12909–12923. [Google Scholar] [CrossRef]
- Coronado, E.; Giménez-Marqués, M.; Mínguez Espallargas, G. Combination of magnetic susceptibility and electron paramagnetic resonance to monitor the 1D to 2D solid state transformation in flexible metal-organic frameworks of Co(II) and Zn(II) with 1,4-bis(triazol-1-ylmethyl)benzene. Inorg. Chem. 2012, 51, 4403–4410. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, P.; Xie, Q.; Ma, Q.-Q.; Meng, Y.-H.; Wang, Z.-W.; Zhang, S.; Zhao, X.-J.; Chen, J.; Wang, Z.-L. Cadmium(II)-Triazole Framework as a Luminescent Probe for Ca2+ and Cyano Complexes. Chemistry 2016, 22, 10459–10474. [Google Scholar] [CrossRef] [PubMed]
- Martell, A.E.; Hancock, R.D. Metal Complexes in Aqueous Solutions; Fackler, J.P., Ed.; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Martin, R.B. Encyclopedia of Inorganic Chemistry, 2nd ed.; King, R.B., Ed.; Wiley: Chichester, England, 2005; Volume III, D-H, pp. 1762–1766. ISBN 978-0-470-86078-6. [Google Scholar]
- Atkins, P.W.; Overton, T.; Rourke, J.; Weller, M.; Armstrong, F. Shriver & Atkins’ Inorganic Chemistry, 5th ed.; Oxford University Press: Oxford, England, 2010; pp. 138–140. ISBN 978-0-19-923617-6. [Google Scholar]
- Paradis, N.; Chastanet, G.; Létard, J.-F. When Stable and Metastable HS States Meet in Spin-Crossover Compounds. Eur. J. Inorg. Chem. 2012, 2012, 3618–3624. [Google Scholar] [CrossRef]
- Paradis, N.; Chastanet, G.; Palamarciuc, T.; Rosa, P.; Varret, F.; Boukheddaden, K.; Létard, J.-F. Detailed Investigation of the Interplay Between the Thermal Decay of the Low Temperature Metastable HS State and the Thermal Hysteresis of Spin-Crossover Solids. J. Phys. Chem. C 2015, 119, 20039–20050. [Google Scholar] [CrossRef]
- Avila, Y.; Pérez, O.; Sánchez, L.; Vázquez, M.C.; Mojica, R.; González, M.; Ávila, M.; Rodríguez-Hernández, J.; Reguera, E. Spin crossover in Hofmann-like coordination polymers. Effect of the axial ligand substituent and its position. New J. Chem. 2023, 47, 10781–10795. [Google Scholar] [CrossRef]
- Avila, Y.; Mojica, R.; Vázquez, M.C.; Sánchez, L.; González, M.; Rodríguez-Hernández, J.; Reguera, E. Spin-crossover in the Fe(4X-pyridine)2 [Fe(CN)5NO] series with X = Cl, Br, and I. Role of the distortion for the iron atom coordination environment. New J. Chem. 2022, 47, 238–249. [Google Scholar] [CrossRef]
- Pittala, N.; Thétiot, F.; Triki, S.; Boukheddaden, K.; Chastanet, G.; Marchivie, M. Cooperative 1D Triazole-Based Spin Crossover Fe II Material With Exceptional Mechanical Resilience. Chem. Mater. 2017, 29, 490–494. [Google Scholar] [CrossRef]
- Pittala, N.; Thétiot, F.; Charles, C.; Triki, S.; Boukheddaden, K.; Chastanet, G.; Marchivie, M. An unprecedented trinuclear FeII triazole-based complex exhibiting a concerted and complete sharp spin transition above room temperature. Chem. Commun. 2017, 53, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.B.; Molnár, G.; Rotaru, A.; Shepherd, H.J. Pressure effect investigations on spin-crossover coordination compounds. C. R. Chim. 2018, 21, 1095–1120. [Google Scholar] [CrossRef]
- Tailleur, E.; Marchivie, M.; Itié, J.-P.; Rosa, P.; Daro, N.; Guionneau, P. Pressure-Induced Spin-Crossover Features at Variable Temperature Revealed by In Situ Synchrotron Powder X-ray Diffraction. Chemistry 2018, 24, 14495–14499. [Google Scholar] [CrossRef] [PubMed]
- Buron-Le Cointe, M.; Hébert, J.; Baldé, C.; Moisan, N.; Toupet, L.; Guionneau, P.; Létard, J.F.; Freysz, E.; Cailleau, H.; Collet, E. Intermolecular control of thermoswitching and photoswitching phenomena in two spin-crossover polymorphs. Phys. Rev. B 2012, 85, 064114. [Google Scholar] [CrossRef]
- McCusker, J.K.; Rheingold, A.L.; Hendrickson, D.N. Variable-Temperature Studies of Laser-Initiated 5T2 → 1A1 Intersystem Crossing in Spin-Crossover Complexes: Empirical Correlations between Activation Parameters and Ligand Structure in a Series of Polypyridyl Ferrous Complexes. Inorg. Chem. 1996, 35, 2100–2112. [Google Scholar] [CrossRef]
- Marchivie, M.; Guionneau, P.; Létard, J.F.; Chasseau, D. Photo-induced spin-transition: The role of the iron(II) environment distortion. Acta Crystallogr. B 2005, 61, 25–28. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Liu, C.-T.; Sheu, C.-F.; Ho, W.-L.; Lee, G.-H.; Wang, C.-C.; Wang, Y. New iron(II) spin crossover coordination polymers Fe(μ-atrz)3X2·2H2O (X = ClO4−, BF4−) and Fe(μ-atrz)(μ-pyz)(NCS)2·4H2O with an interesting solvent effect. Inorg. Chem. 2012, 51, 4663–4671. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordan, D.N.; Straßburg, P.G.; Woschko, D.; Carrella, L.M.; Cuignet, L.P.; Eickmeier, K.; Dronskowski, R.; Garcia, Y.; Rentschler, E.; Janiak, C. Interpenetration Phenomena via Anion Template Effects in Fe(II) and Co(II) Coordination Networks with a Bis-(1,2,4-triazole) Ligand. Polymers 2023, 15, 3286. https://doi.org/10.3390/polym15153286
Jordan DN, Straßburg PG, Woschko D, Carrella LM, Cuignet LP, Eickmeier K, Dronskowski R, Garcia Y, Rentschler E, Janiak C. Interpenetration Phenomena via Anion Template Effects in Fe(II) and Co(II) Coordination Networks with a Bis-(1,2,4-triazole) Ligand. Polymers. 2023; 15(15):3286. https://doi.org/10.3390/polym15153286
Chicago/Turabian StyleJordan, Dustin N., Patrick G. Straßburg, Dennis Woschko, Luca M. Carrella, Laure P. Cuignet, Katharina Eickmeier, Richard Dronskowski, Yann Garcia, Eva Rentschler, and Christoph Janiak. 2023. "Interpenetration Phenomena via Anion Template Effects in Fe(II) and Co(II) Coordination Networks with a Bis-(1,2,4-triazole) Ligand" Polymers 15, no. 15: 3286. https://doi.org/10.3390/polym15153286
APA StyleJordan, D. N., Straßburg, P. G., Woschko, D., Carrella, L. M., Cuignet, L. P., Eickmeier, K., Dronskowski, R., Garcia, Y., Rentschler, E., & Janiak, C. (2023). Interpenetration Phenomena via Anion Template Effects in Fe(II) and Co(II) Coordination Networks with a Bis-(1,2,4-triazole) Ligand. Polymers, 15(15), 3286. https://doi.org/10.3390/polym15153286









