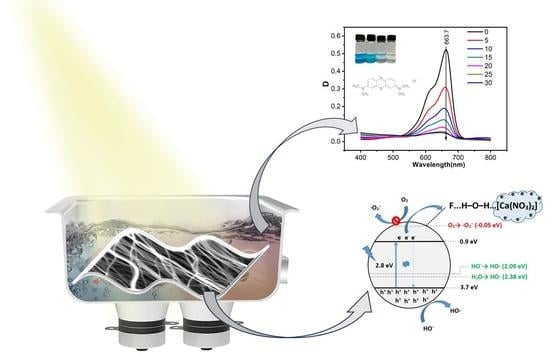
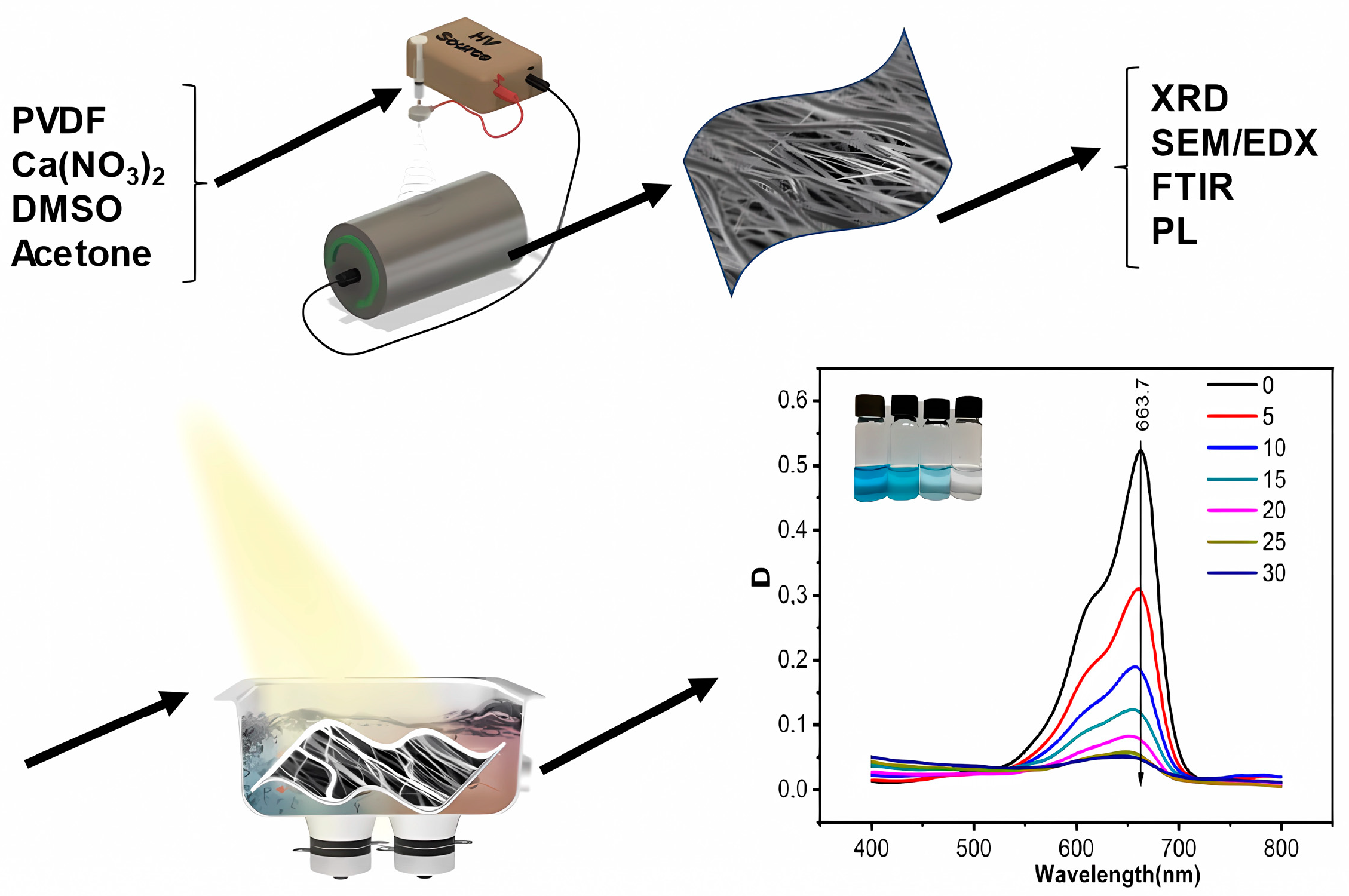
Hydrogen Bond-Induced Activation of Photocatalytic and Piezophotocatalytic Properties in Calcium Nitrate Doped Electrospun PVDF Fibers
Abstract
1. Introduction
2. Materials and Methods
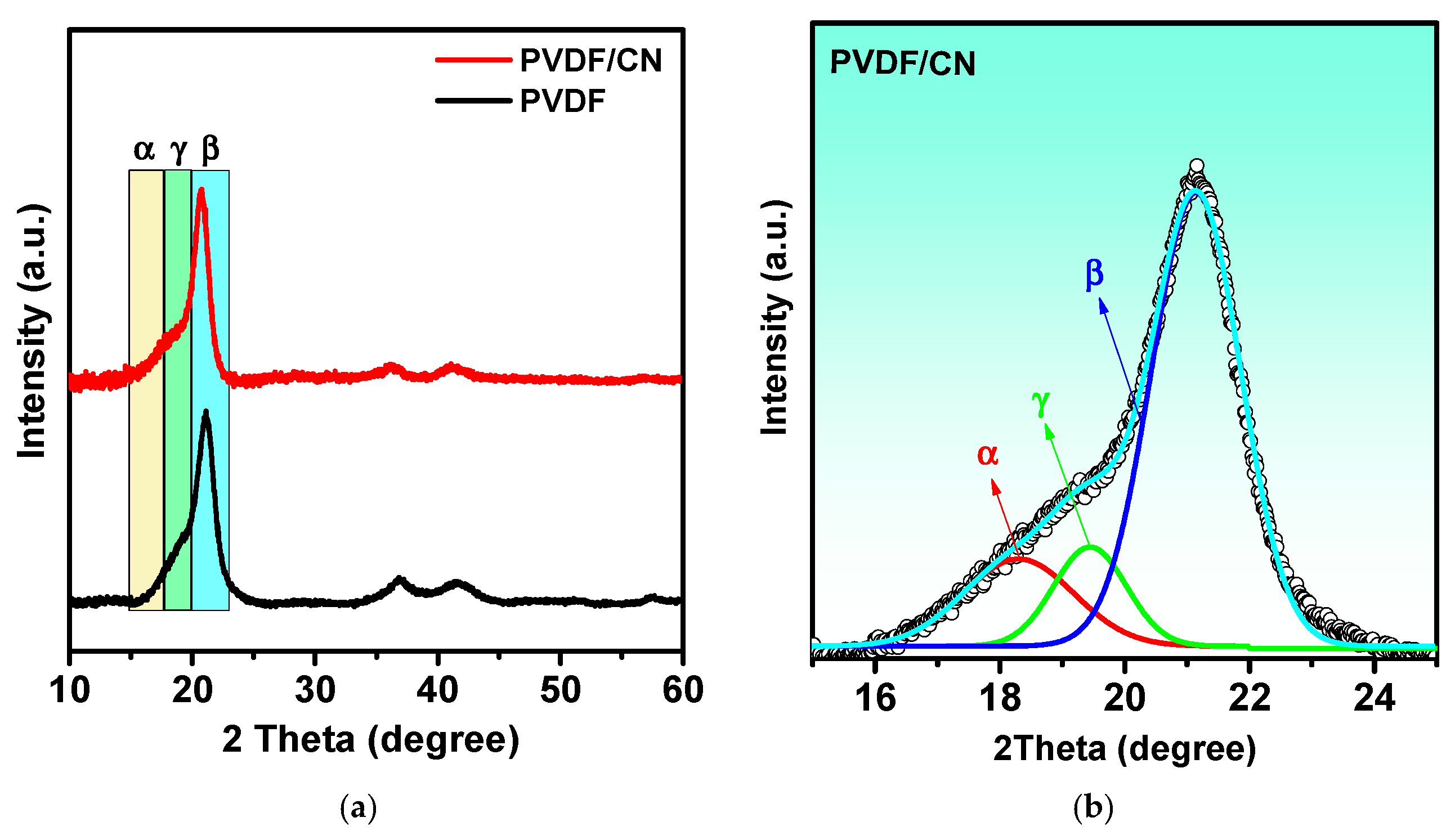
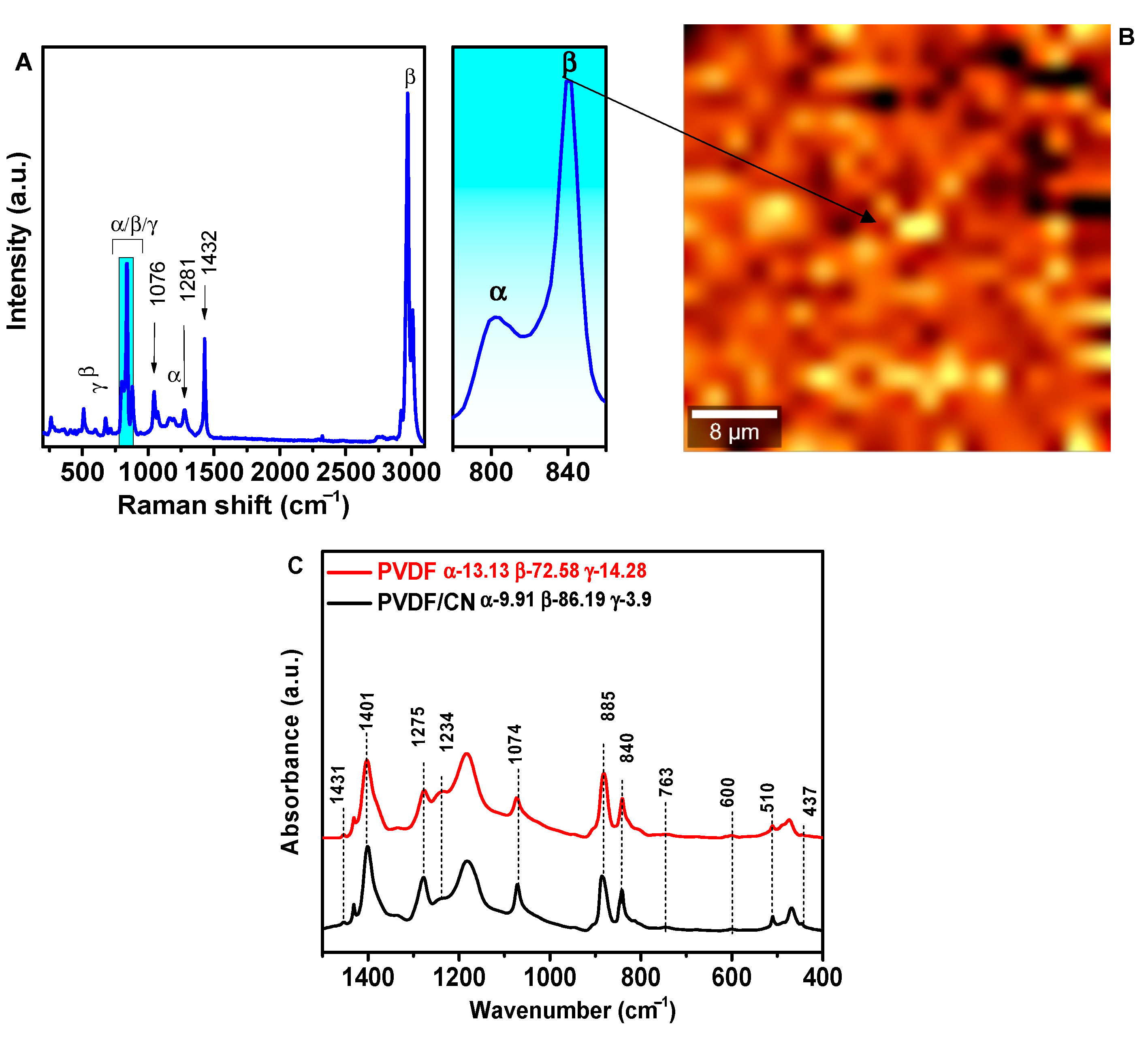
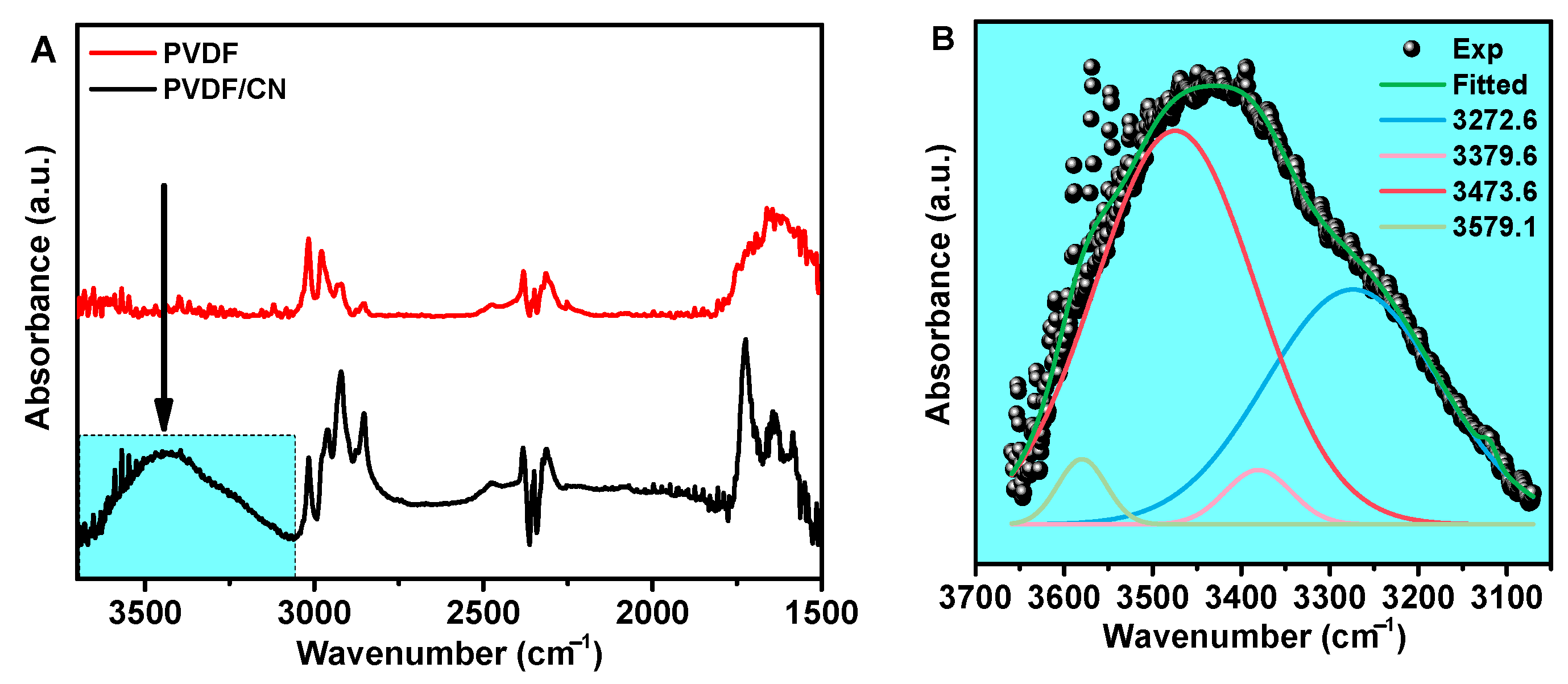
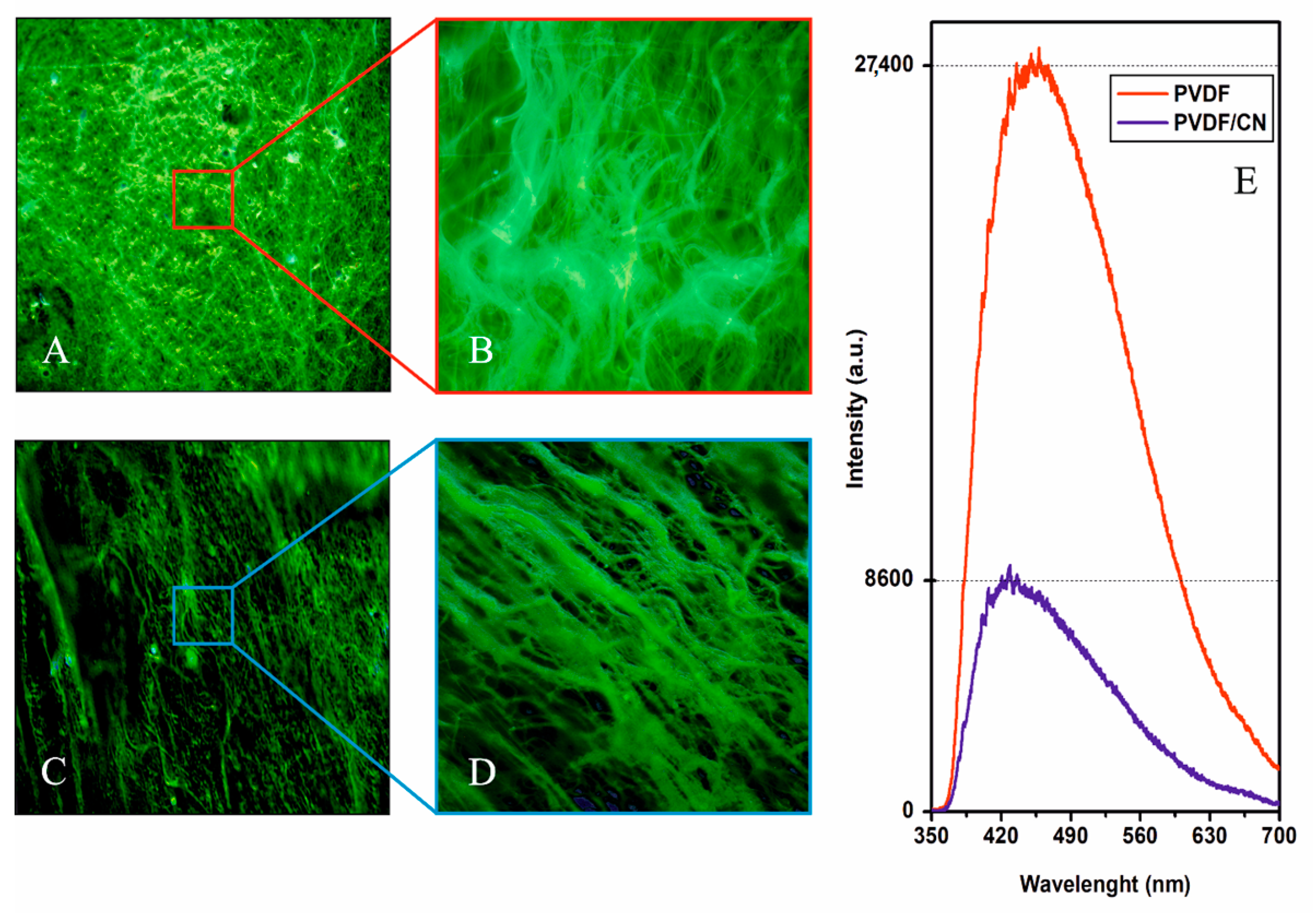
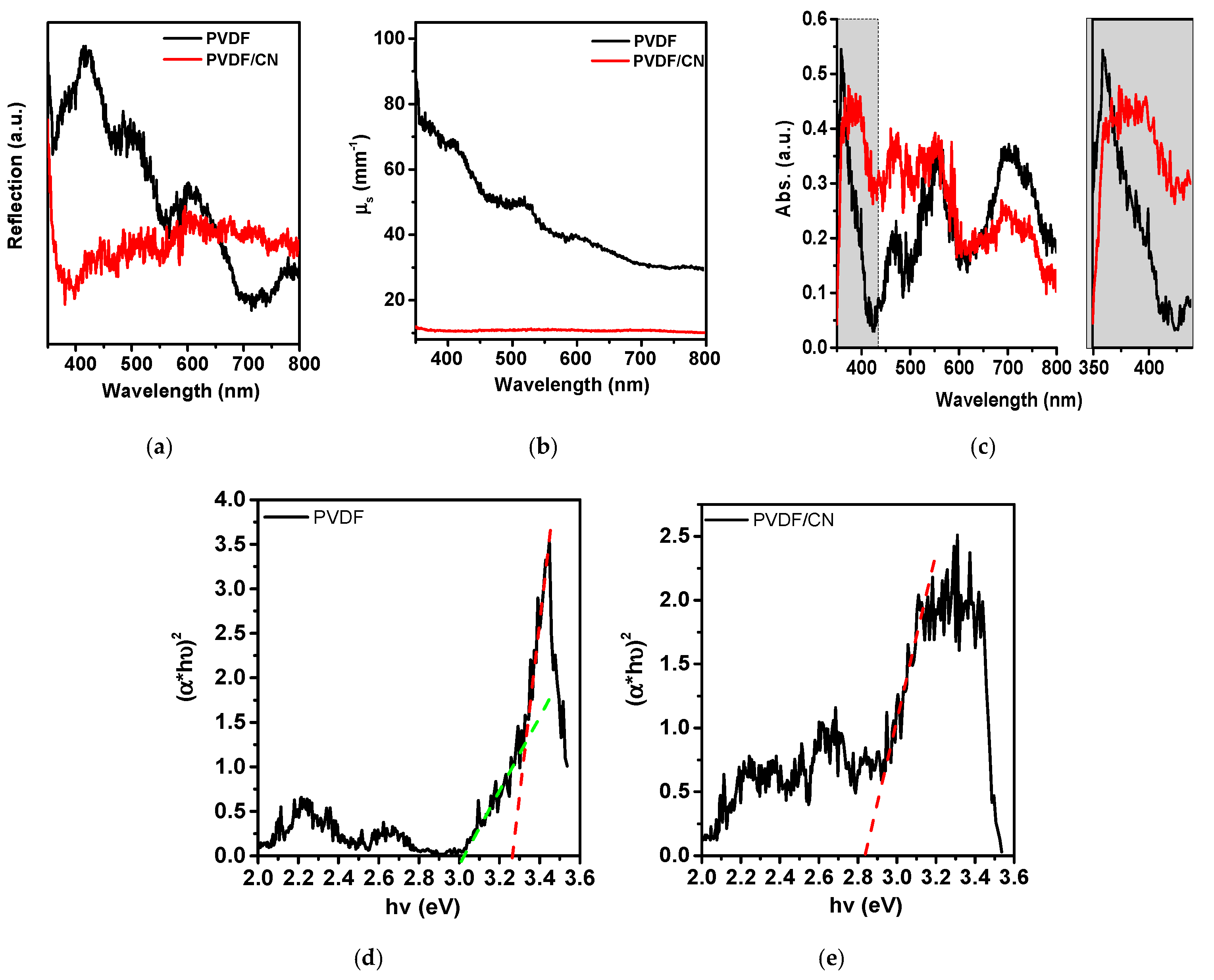
3. Results and Discussion
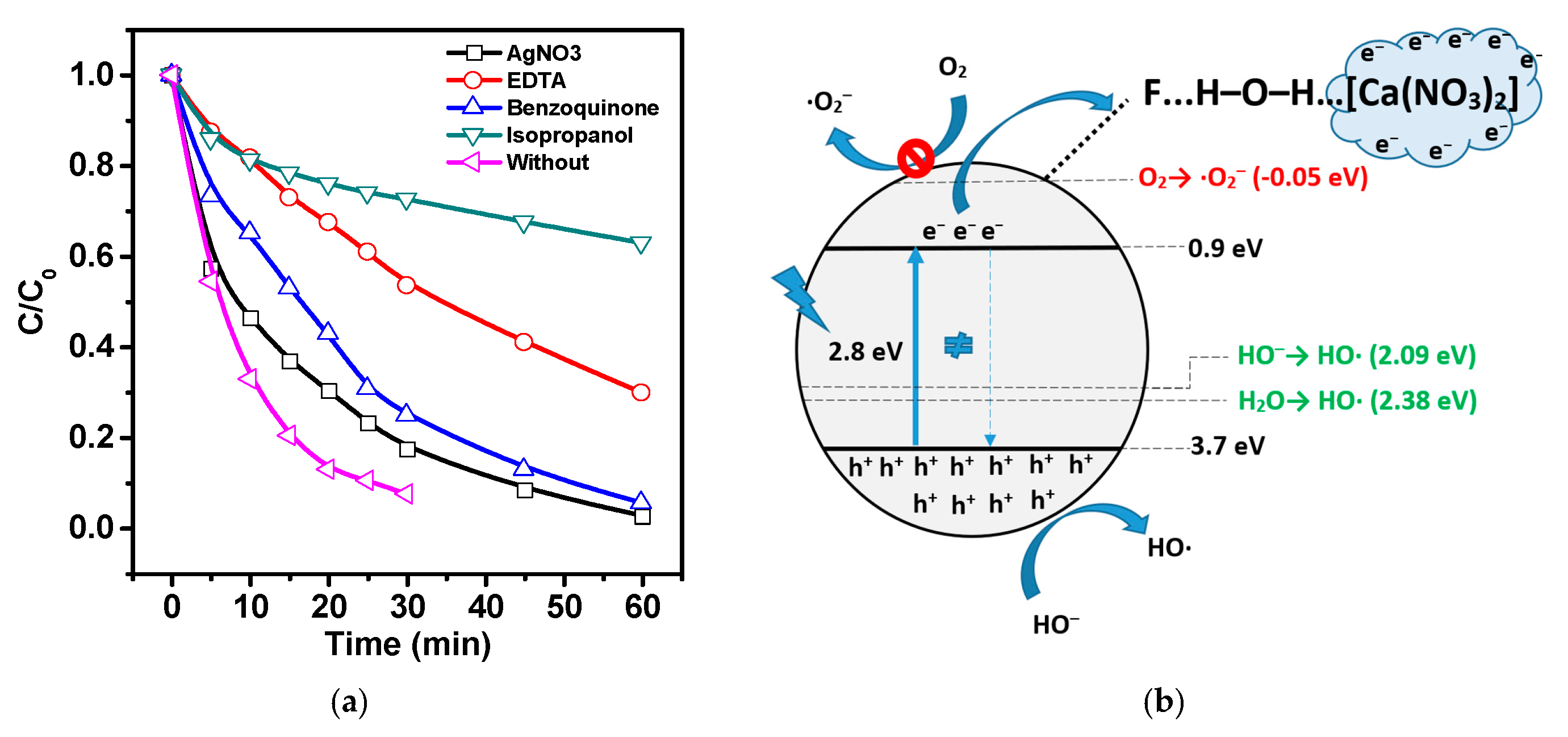
- Indirect sonolysis through cavitation pyrolysis of water, leading to the generation of short-lived hydroxyl radicals upon bubble formation and collapse;
- Photocatalytic mechanism (PCM), in which sonoluminescence during cavitation may contribute to the formation of electron–hole pairs;
- Thermocatalytic mechanism (TCM), in which, according to the hot spot theory, local high temperature during cavitation may promote the formation of electron–hole pairs through thermo-induced thermal excitation of the semiconductor.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, N.N.; Escribà-Gelonch, M.; Sarafraz, M.M.; Pho, Q.H.; Sagadevan, S.; Hessel, V. Process Technology and Sustainability Assessment of Wastewater Treatment. Ind. Eng. Chem. Res. 2023, 62, 1195–1214. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Su, X.; Suss, M.E.; Tian, H.; Guyes, E.N.; Shocron, A.N.; Conforti, K.M.; De Souza, J.P.; Kim, N.; Tedesco, M.; et al. Electrochemical Methods for Water Purification, Ion Separations, and Energy Conversion. Chem. Rev. 2022, 122, 13547–13635. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced Oxidation Processes for Water and Wastewater Viral Disinfection. A Systematic Review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Z.; Abramova, A.V.; Cravotto, G. Sonochemical Processes for the Degradation of Antibiotics in Aqueous Solutions: A Review. Ultrason. Sonochem. 2021, 74, 105566. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent Advances in Nano-Fenton Catalytic Degradation of Emerging Pharmaceutical Contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Chauhan, R.; Dinesh, G.K.; Alawa, B.; Chakma, S. A Critical Analysis of Sono-Hybrid Advanced Oxidation Process of Ferrioxalate System for Degradation of Recalcitrant Pollutants. Chemosphere 2021, 277, 130324. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Zhang, G.; Wang, P. A Review on Fenton-like Processes for Organic Wastewater Treatment. J. Environ. Chem. Eng. 2016, 4, 762–787. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Santana, C.S.; Velloso, C.C.V.; da Silva, A.H.M.; Magalhães, F.; Aguiar, A. A Review on the Treatment of Textile Industry Effluents through Fenton Processes. Process Saf. Environ. Prot. 2021, 155, 366–386. [Google Scholar] [CrossRef]
- Verinda, S.B.; Muniroh, M.; Yulianto, E.; Maharani, N.; Gunawan, G.; Amalia, N.F.; Hobley, J.; Usman, A.; Nur, M. Degradation of Ciprofloxacin in Aqueous Solution Using Ozone Microbubbles: Spectroscopic, Kinetics, and Antibacterial Analysis. Heliyon 2022, 8, e10137. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Elahinia, A.; Vasseghian, Y.; Dragoi, E.N.; Omidi, F.; Mousavi Khaneghah, A. A Review on Pollutants Removal by Sono-Photo -Fenton Processes. J. Environ. Chem. Eng. 2020, 8, 104330. [Google Scholar] [CrossRef]
- Lv, S.; Du, Y.; Wu, F.; Cai, Y.; Zhou, T. Review on LSPR Assisted Photocatalysis: Effects of Physical Fields and Opportunities in Multifield Decoupling. Nanoscale Adv. 2022, 4, 2608–2631. [Google Scholar] [CrossRef]
- Retamoso, C.; Escalona, N.; González, M.; Barrientos, L.; Allende-González, P.; Stancovich, S.; Serpell, R.; Fierro, J.L.G.; Lopez, M. Effect of Particle Size on the Photocatalytic Activity of Modified Rutile Sand (TiO2) for the Discoloration of Methylene Blue in Water. J. Photochem. Photobiol. A Chem. 2019, 378, 136–141. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J. A Review on Particle Size Effect in Metal-Catalyzed Heterogeneous Reactions. Chin. J. Chem. 2020, 38, 1422–1444. [Google Scholar] [CrossRef]
- Ahmad, I.; Zou, Y.; Yan, J.; Liu, Y.; Shukrullah, S.; Naz, M.Y.; Hussain, H.; Khan, W.Q.; Khalid, N.R. Semiconductor Photocatalysts: A Critical Review Highlighting the Various Strategies to Boost the Photocatalytic Performances for Diverse Applications. Adv. Colloid Interface Sci. 2023, 311, 102830. [Google Scholar] [CrossRef]
- Joseph, A.; Vijayanandan, A. Review on Support Materials Used for Immobilization of Nano-Photocatalysts for Water Treatment Applications. Inorganica Chim. Acta 2023, 545, 121284. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of Photocatalysis and Their Coping Strategies. Chem. Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Ferreira, L.F.; Silva, V.C. Magnetic Materials for Photocatalytic Applications—A Review. J. Solgel. Sci. Technol. 2020, 96, 1–14. [Google Scholar] [CrossRef]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Sheikh Abdul Kadir, S.H.; Kurniawan, T.A.; Jilani, A. Immobilization Techniques of a Photocatalyst into and onto a Polymer Membrane for Photocatalytic Activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef] [PubMed]
- Orudzhev, F.F.; Alikhanov, N.M.-R.; Rabadanov, M.K.; Ramazanov, S.M.; Isaev, A.B.; Gadzhimagomedov, S.K.; Aliyev, A.S.; Abdullaev, V.R. Synthesis and study of the properties of magnetically separable nanophotocatalyst BiFeO3. Chemical Probl. 2018, 16, 484–495. [Google Scholar] [CrossRef]
- Ghosh, S.; Kouamé, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.H.; Remita, H. Conducting Polymer Nanostructures for Photocatalysis under Visible Light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Podjaski, F.; Kröger, J.; Biswal, B.P.; Lotsch, B.V. Polymer Photocatalysts for Solar-to-Chemical Energy Conversion. Nat. Rev. Mater. 2020, 6, 168–190. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated Polymers for Visible-Light-Driven Photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Sobola, D.; Orudzhev, F.; Ramazanov, S.; Trčka, T. Brief Review of PVDF Properties and Applications Potential. Polymers 2022, 14, 4793. [Google Scholar] [CrossRef]
- Dong, C.; Fu, Y.; Zang, W.; He, H.; Xing, L.; Xue, X. Self-Powering/Self-Cleaning Electronic-Skin Basing on PVDF/TiO2 Nanofibers for Actively Detecting Body Motion and Degrading Organic Pollutants. Appl. Surf. Sci. 2017, 416, 424–431. [Google Scholar] [CrossRef]
- Cauda, V.; Stassi, S.; Bejtka, K.; Canavese, G. Nanoconfinement: An Effective Way to Enhance PVDF Piezoelectric Properties. ACS Appl. Mater. Interfaces 2013, 5, 6430–6437. [Google Scholar] [CrossRef]
- Hafiza, N.; Norharyati, W.; Salleh, W.; Rosman, N.; Asikin, N. PVDF/Fe2O3 Mixed Matrix Membrane for Oily Wastewater Treatment. Malays. J. Fundam. Appl. Sci. 2019, 15, 703–707. [Google Scholar]
- Prasad, G.; Sathiyanathan, P.; Prabu, A.A.; Kim, K.J. Piezoelectric Characteristics of Electrospun PVDF as a Function of Phase-Separation Temperature and Metal Salt Content. Macromol. Res. 2017, 25, 981–988. [Google Scholar] [CrossRef]
- Wu, W.; Yin, X.; Dai, B.; Kou, J.; Ni, Y.; Lu, C. Water Flow Drived Piezo-Photocatalytic Flexible Films: Bi-Piezoelectric Integration of ZnO Nanorods and PVDF. Appl. Surf. Sci. 2020, 517, 146119. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, L.; Zheng, J.; Wang, Z.; Li, B.; Yan, Y.; Meng, M. Synergistic Interaction of Z-Scheme 2D/3D g-C3N4/BiOI Heterojunction and Porous PVDF Membrane for Greatly Improving the Photodegradation Efficiency of Tetracycline. J. Colloid Interface Sci. 2021, 586, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Huang, H.; Wang, W.; Chen, Y.; Lu, C.; Kou, J.; Wang, L.; Wang, F.; Xu, Z. Greatly Enhanced Photocatalytic Activity by Organic Flexible Piezoelectric PVDF Induced Spatial Electric Field. Catal. Sci. Technol. 2017, 7, 5594–5601. [Google Scholar] [CrossRef]
- Rabadanova, A.; Abdurakhmanov, M.; Gulakhmedov, R.; Shuaibov, A.; Selimov, D.; Sobola, D.; Částková, K.; Ramazanov, S.; Orudzhev, F. Piezo-, Photo- and Piezophotocatalytic Activity of Electrospun Fibrous PVDF/CTAB Membrane. Chim. Techno Acta 2022, 9, 20229420. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Dang, T.; Wang, M.; Liu, J.; Yan, Z.; Xie, H. Hydrogen-Bond Based Charge Bridge in a Heterojunction System for the Synergistic Degradation and Detoxification of Two PPCPs. Chem. Eng. J. 2023, 454, 140018. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, J.; Wang, G.; Cao, Q.; Zhang, C.; Li, M.; Shao, J.; Xu, Y.; Li, H.; Lu, J. Construction of Tri-Functional HOFs Material for Efficient Selective Adsorption and Photodegradation of Bisphenol A and Hydrogen Production. Adv. Funct. Mater. 2023, 33, 2300954. [Google Scholar] [CrossRef]
- Sarkar, R.; Kundu, T.K. Hydrogen Bond Interactions of Hydrated Aluminum Nitrate with PVDF, PVDF-TrFE, and PVDF-HFP: A Density Functional Theory-Based Illustration. Int. J. Quantum. Chem. 2020, 120, e26328. [Google Scholar] [CrossRef]
- Fortunato, M.; Chandraiahgari, C.R.; De Bellis, G.; Ballirano, P.; Sarto, F.; Tamburrano, A.; Sarto, M.S. Piezoelectric Effect and Electroactive Phase Nucleation in Self-Standing Films of Unpoled PVDF Nanocomposite Films. Nanomaterials 2018, 8, 743. [Google Scholar] [CrossRef]
- Yuennana, J.; Muensit, N. Fabrication and Properties of Flexible CaCl2/P(VDF-HFP) Composite Films. J. Phys. Conf. Ser. 2019, 1380, 012118. [Google Scholar] [CrossRef]
- Sukwisute, P.; Yuennan, J.; Muensit, N. Effects of the Electric Field and AlCl3·6H2O Salt on the Crystal, Morphology and Dielectric Properties of P(VDF-HFP) Fibres. J. Phys. Conf. Ser. 2018, 1144, 012179. [Google Scholar] [CrossRef]
- Xue, W.; Lv, C.; Jing, Y.; Chen, F.; Fu, Q. Fabrication of Electrospun PVDF Nanofibers with Higher Content of Polar β Phase and Smaller Diameter by Adding a Small Amount of Dioctadecyl Dimethyl Ammonium Chloride. Chin. J. Polym. Sci. 2017, 35, 992–1000. [Google Scholar] [CrossRef]
- Eleshmawi, I.S. Effect of LiCl Filler on the Structure and Morphology of PVDF Films. J. Elastomers Plast. 2008, 40, 211–221. [Google Scholar] [CrossRef]
- Tawansi, A.; Oraby, A.H.; Abdelrazek, E.M.; Ayad, M.I.; Abdelaziz, M. Effect of Local Structure of MnCl2-Filled PVDF Films on Their Optical, Electrical, Electron Spin Resonance, and Magnetic Properties. J. Appl. Polym. Sci. 1998, 70, 1437–1445. [Google Scholar] [CrossRef]
- Tawansi, A.; Ayad, M.I.; Abdel-Razek, E.M. Effect of Valence Electron Spin Polarization on the Physical Properties of CuCl2-Filled Poly(Vinylidene Fluoride) as a Microwave Modulator. J. Appl. Polym. Sci. 1999, 72, 771–781. [Google Scholar] [CrossRef]
- Tawansi, A.; Oraby, A.H.; Badr, S.I.; Elashmawi, I.S. Physical Properties and β-Phase Increment of AgNO3-Filled Poly(Vinylidene Fluoride) Films. Polym. Int. 2004, 53, 370–377. [Google Scholar] [CrossRef]
- Hakeem, N.A.; Abdelkader, H.I.; El-sheshtawi, N.A.; Eleshmawi, I.S. Spectroscopic, Thermal, and Electrical Investigations of PVDF Films Filled with BiCl3. J. Appl. Polym. Sci. 2006, 102, 2125–2131. [Google Scholar] [CrossRef]
- Elashmawi, I.S. Effect of NaCl filler on ferroelectric phase and polaron configurations of PVDF films. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2007, 42, 389–393. [Google Scholar] [CrossRef]
- Chen, C.; Bai, Z.; Cao, Y.; Dong, M.; Jiang, K.; Zhou, Y.; Tao, Y.; Gu, S.; Xu, J.; Yin, X.; et al. Enhanced Piezoelectric Performance of BiCl3/PVDF Nanofibers-Based Nanogenerators. Compos. Sci. Technol. 2020, 192, 108100. [Google Scholar] [CrossRef]
- Dhakras, D.; Borkar, V.; Ogale, S.; Jog, J. Enhanced Piezoresponse of Electrospun PVDF Mats with a Touch of Nickel Chloride Hexahydrate Salt. Nanoscale 2012, 4, 752–756. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Biswas, A.; Sen, S.; Das, C.; Henkel, K.; Schmeisser, D.; Mandal, D. Yb3+ Assisted Self-Polarized PVDF Based Ferroelectretic Nanogenerator: A Facile Strategy of Highly Efficient Mechanical Energy Harvester Fabrication. Nano Energy 2016, 30, 621–629. [Google Scholar] [CrossRef]
- Orudzhev, F.; Alikhanov, N.; Amirov, A.; Rabadanova, A.; Selimov, D.; Shuaibov, A.; Gulakhmedov, R.; Abdurakhmanov, M.; Magomedova, A.; Ramazanov, S.; et al. Porous Hybrid PVDF/BiFeO3 Smart Composite with Magnetic, Piezophotocatalytic, and Light-Emission Properties. Catalysts 2023, 13, 874. [Google Scholar] [CrossRef]
- Orudzhev, F.; Ramazanov, S.; Sobola, D.; Kaspar, P.; Trčka, T.; Částková, K.; Kastyl, J.; Zvereva, I.; Wang, C.; Selimov, D.; et al. Ultrasound and Water Flow Driven Piezophototronic Effect in Self-Polarized Flexible α-Fe2O3 Containing PVDF Nanofibers Film for Enhanced Catalytic Oxidation. Nano Energy 2021, 90, 106586. [Google Scholar] [CrossRef]
- Singh, P.; Borkar, H.; Singh, B.P.; Singh, V.N.; Kumar, A. Ferroelectric Polymer-Ceramic Composite Thick Films for Energy Storage Applications. AIP Adv. 2014, 4, 87117. [Google Scholar] [CrossRef]
- Lotfian, S.; Giraudmaillet, C.; Yoosefinejad, A.; Thakur, V.K.; Nezhad, H.Y. Electrospun Piezoelectric Polymer Nanofiber Layers for Enabling in Situ Measurement in High-Performance Composite Laminates. ACS Omega 2018, 3, 8891–8902. [Google Scholar] [CrossRef] [PubMed]
- Hartono, A.; Satira, S.; Djamal, M.; Ramli, R.; Bahar, H.; Sanjaya, E.; Hartono, A.; Satira, S.; Djamal, M.; Ramli, R.; et al. Effect of Mechanical Treatment Temperature on Electrical Properties and Crystallite Size of PVDF Film. Adv. Mater. Phys. Chem. 2013, 3, 71–76. [Google Scholar] [CrossRef]
- Constantino, C.J.L.; Job, A.E.; Simões, R.D.; Giacometti, J.A.; Zucolotto, V.; Oliveira, O.N.; Gozzi, G.; Chinaglia, D.L. Phase Transition in Poly(Vinylidene Fluoride) Investigated with Micro-Raman Spectroscopy. Appl. Spectrosc. 2005, 59, 275–279. [Google Scholar] [CrossRef]
- Barnakov, Y.A.; Paul, O.; Joaquim, A.; Falconer, A.; Barnakov, V.Y.; Dikin, D.; Petranovskii, V.P.; Zavalin, A.; Ueda, A.; Williams, F.; et al. Light Intensity-Induced Phase Transitions in Graphene Oxide Doped Polyvinylidene Fluoride. Opt. Mater. Express 2018, 8, 2579–2585. [Google Scholar] [CrossRef]
- Chen, S.; Yao, K.; Tay, F.E.H.; Liow, C.L. Ferroelectric Poly(Vinylidene Fluoride) Thin Films on Si Substrate with the Β Phase Promoted by Hydrated Magnesium Nitrate. J. Appl. Phys. 2007, 102, 104108. [Google Scholar] [CrossRef]
- Hare, D.E.; Sorensen, C.M. Interoscillator Coupling Effects on the OH Stretching Band of Liquid Water. J. Chem. Phys. 1992, 96, 13–22. [Google Scholar] [CrossRef]
- Carey, D.M.; Korenowski, G.M. Measurement of the Raman Spectrum of Liquid Water. J. Chem. Phys. 1998, 108, 2669–2675. [Google Scholar] [CrossRef]
- Brooksby, P.A.; Ronald Fawcett, W. Infrared (ATR) Study of Hydrogen Bonding in Solutions Containing Water and Ethylene Carbonate. J. Phys. Chem. A 2000, 104, 8307–8314. [Google Scholar] [CrossRef]
- Mohammadi Ghaleni, M.; Al Balushi, A.; Kaviani, S.; Tavakoli, E.; Bavarian, M.; Nejati, S. Fabrication of Janus Membranes for Desalination of Oil-Contaminated Saline Water. ACS Appl. Mater. Interfaces 2018, 10, 44871–44879. [Google Scholar] [CrossRef]
- Mi, C.; Ren, Z.; Li, H.; Yan, S.; Sun, X. Synergistic Effect of Hydrogen Bonds and Diffusion on the β-Crystallization of Poly(Vinylidene Fluoride) on Poly(Methyl Methacrylate) Interface. Ind. Eng. Chem. Res. 2019, 58, 7389–7396. [Google Scholar] [CrossRef]
- Jana, S.; Garain, S.; Sen, S.; Mandal, D. The Influence of Hydrogen Bonding on the Dielectric Constant and the Piezoelectric Energy Harvesting Performance of Hydrated Metal Salt Mediated PVDF Films. Phys. Chem. Chem. Phys. 2015, 17, 17429–17436. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, S.; Zhang, X.; Xie, Y. Excitonic Effects in Polymeric Photocatalysts. Angew. Chem. Int. Ed. 2020, 59, 22828–22839. [Google Scholar] [CrossRef]
- Bakulin, A.A.; Rao, A.; Pavelyev, V.G.; Van Loosdrecht, P.H.M.; Pshenichnikov, M.S.; Niedzialek, D.; Cornil, J.; Beljonne, D.; Friend, R.H. The Role of Driving Energy and Delocalized States for Charge Separation in Organic Semiconductors. Science 2012, 335, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Balzer, D.; Kassal, I. Even a Little Delocalization Produces Large Kinetic Enhancements of Charge-Separation Efficiency in Organic Photovoltaics. Sci. Adv. 2022, 8, 9692. [Google Scholar] [CrossRef]
- Ononye, A.I.; McIntosh, A.R.; Bolton, J.R. Mechanism of the Photochemistry of P-Benzoquinone in Aqueous Solutions. 1. Spin Trapping and Flash Photolysis Electron Paramagnetic Resonance Studies. J. Phys. Chem. 1986, 90, 6266–6270. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orudzhev, F.F.; Sobola, D.S.; Ramazanov, S.M.; Častková, K.; Selimov, D.A.; Rabadanova, A.A.; Shuaibov, A.O.; Gulakhmedov, R.R.; Abdurakhmanov, M.G.; Giraev, K.M. Hydrogen Bond-Induced Activation of Photocatalytic and Piezophotocatalytic Properties in Calcium Nitrate Doped Electrospun PVDF Fibers. Polymers 2023, 15, 3252. https://doi.org/10.3390/polym15153252
Orudzhev FF, Sobola DS, Ramazanov SM, Častková K, Selimov DA, Rabadanova AA, Shuaibov AO, Gulakhmedov RR, Abdurakhmanov MG, Giraev KM. Hydrogen Bond-Induced Activation of Photocatalytic and Piezophotocatalytic Properties in Calcium Nitrate Doped Electrospun PVDF Fibers. Polymers. 2023; 15(15):3252. https://doi.org/10.3390/polym15153252
Chicago/Turabian StyleOrudzhev, F. F., D. S. Sobola, Sh. M. Ramazanov, K. Častková, D. A. Selimov, A. A. Rabadanova, A. O. Shuaibov, R. R. Gulakhmedov, M. G. Abdurakhmanov, and K. M. Giraev. 2023. "Hydrogen Bond-Induced Activation of Photocatalytic and Piezophotocatalytic Properties in Calcium Nitrate Doped Electrospun PVDF Fibers" Polymers 15, no. 15: 3252. https://doi.org/10.3390/polym15153252
APA StyleOrudzhev, F. F., Sobola, D. S., Ramazanov, S. M., Častková, K., Selimov, D. A., Rabadanova, A. A., Shuaibov, A. O., Gulakhmedov, R. R., Abdurakhmanov, M. G., & Giraev, K. M. (2023). Hydrogen Bond-Induced Activation of Photocatalytic and Piezophotocatalytic Properties in Calcium Nitrate Doped Electrospun PVDF Fibers. Polymers, 15(15), 3252. https://doi.org/10.3390/polym15153252