Preparation and Thermal Properties of Propyl Palmitate-Based Phase Change Composites with Enhanced Thermal Conductivity for Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
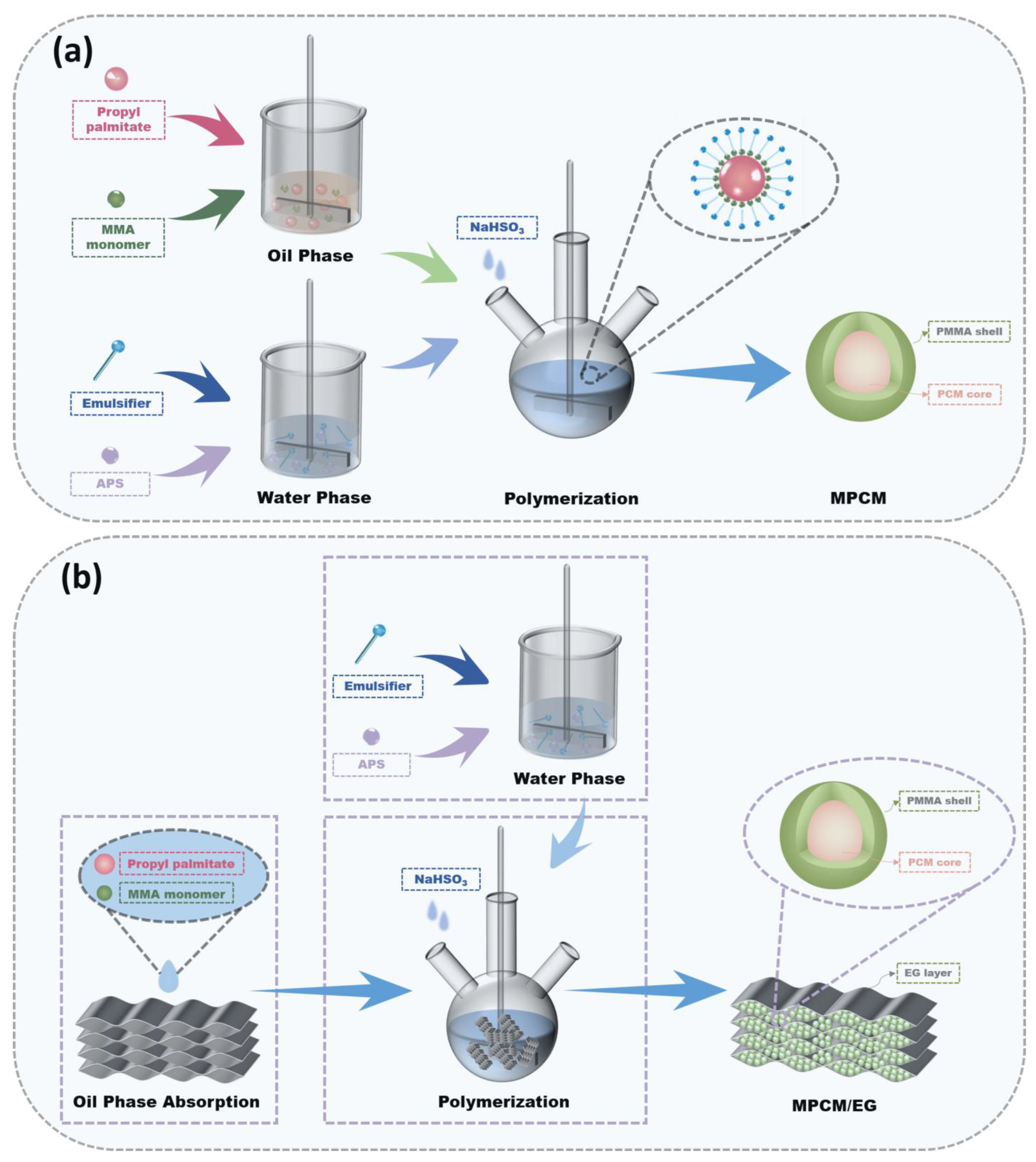
2.2. Preparation of Phase Change Composites
2.2.1. Preparation of Expanded Graphite (EG)
2.2.2. Preparation of PMMA/Propyl Palmitate Microcapsule (MPCM)
2.2.3. Preparation of MPCM/EG Phase Change Composites
2.3. Characterization
2.3.1. Scanning Electron Microscopy (SEM)
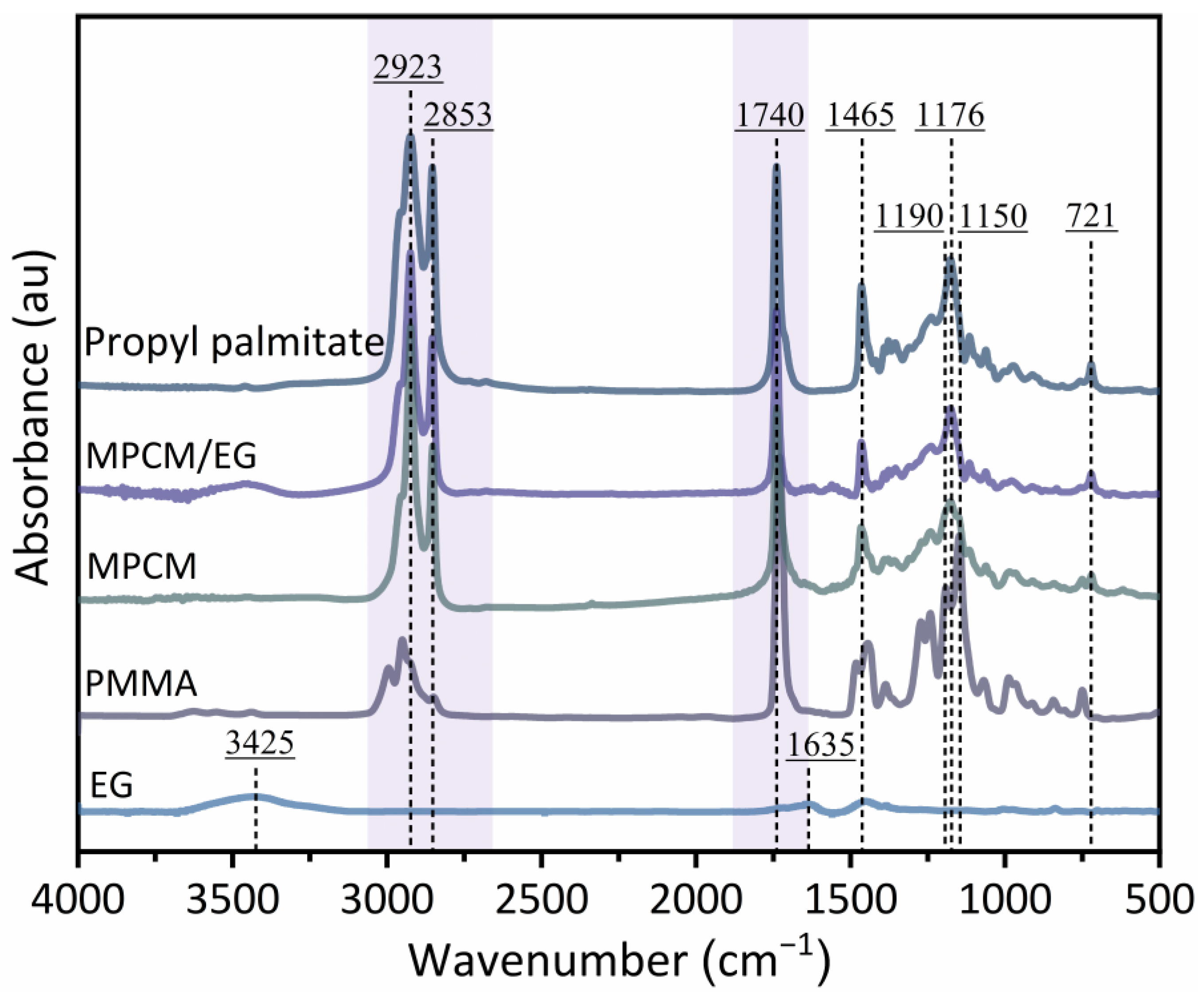
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Thermal Properties
2.3.4. Thermogravimetric Analysis (TGA)
2.3.5. Thermal Conductivity Analysis
2.3.6. Thermal Cycle Reliability
3. Results
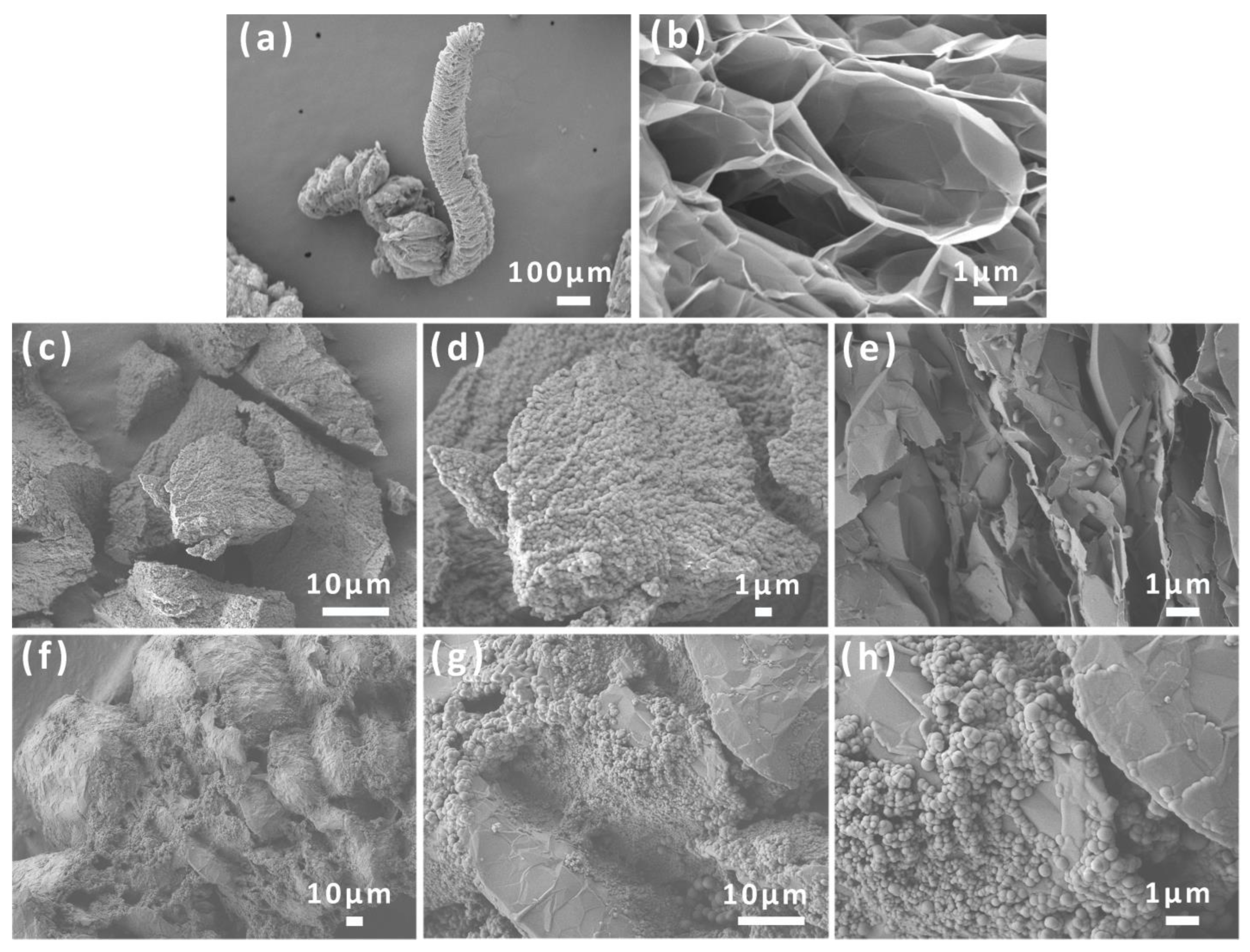
3.1. Morphological and Structural Analysis
3.2. Formation Mechanism
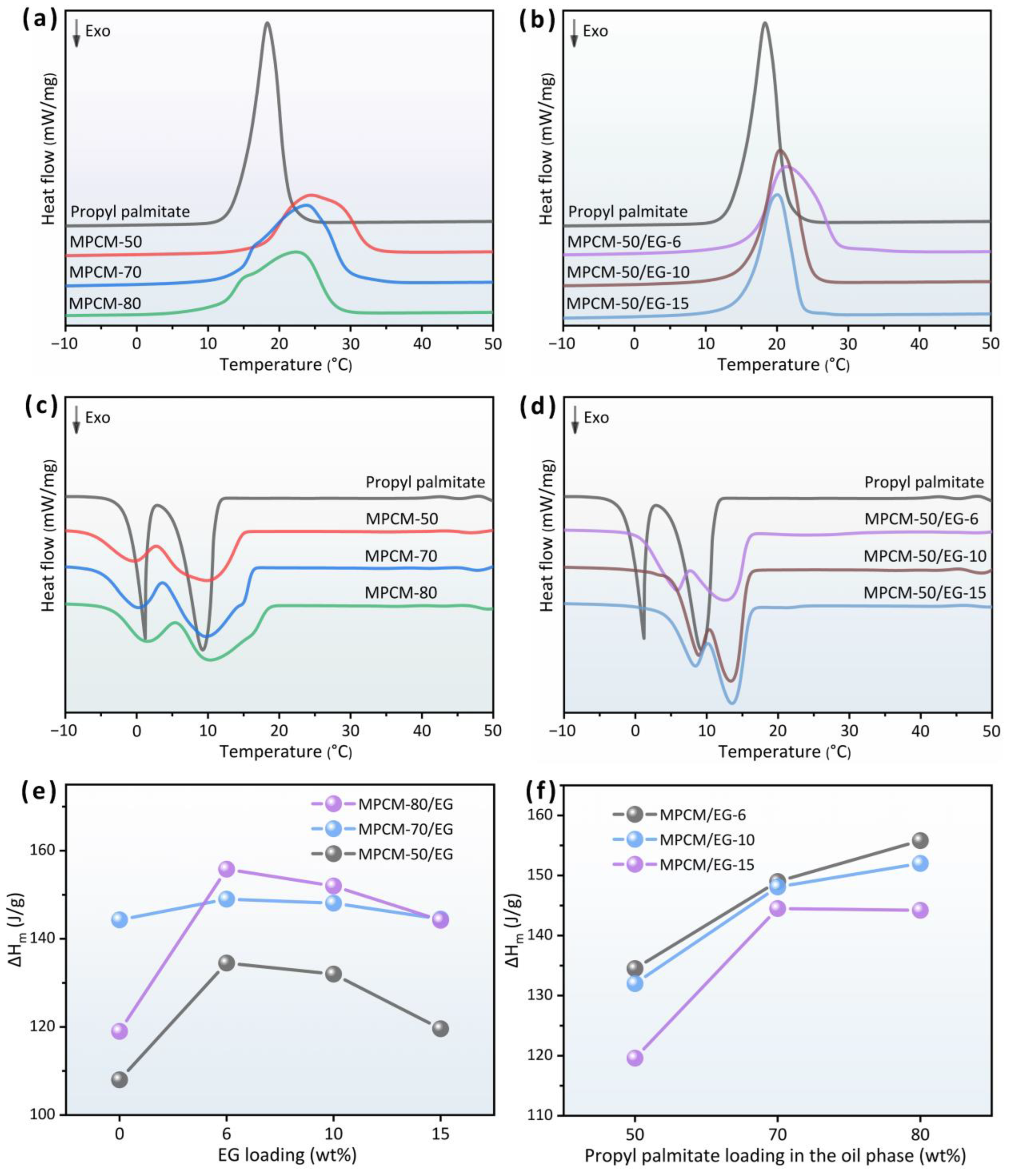
3.3. Thermal Properties
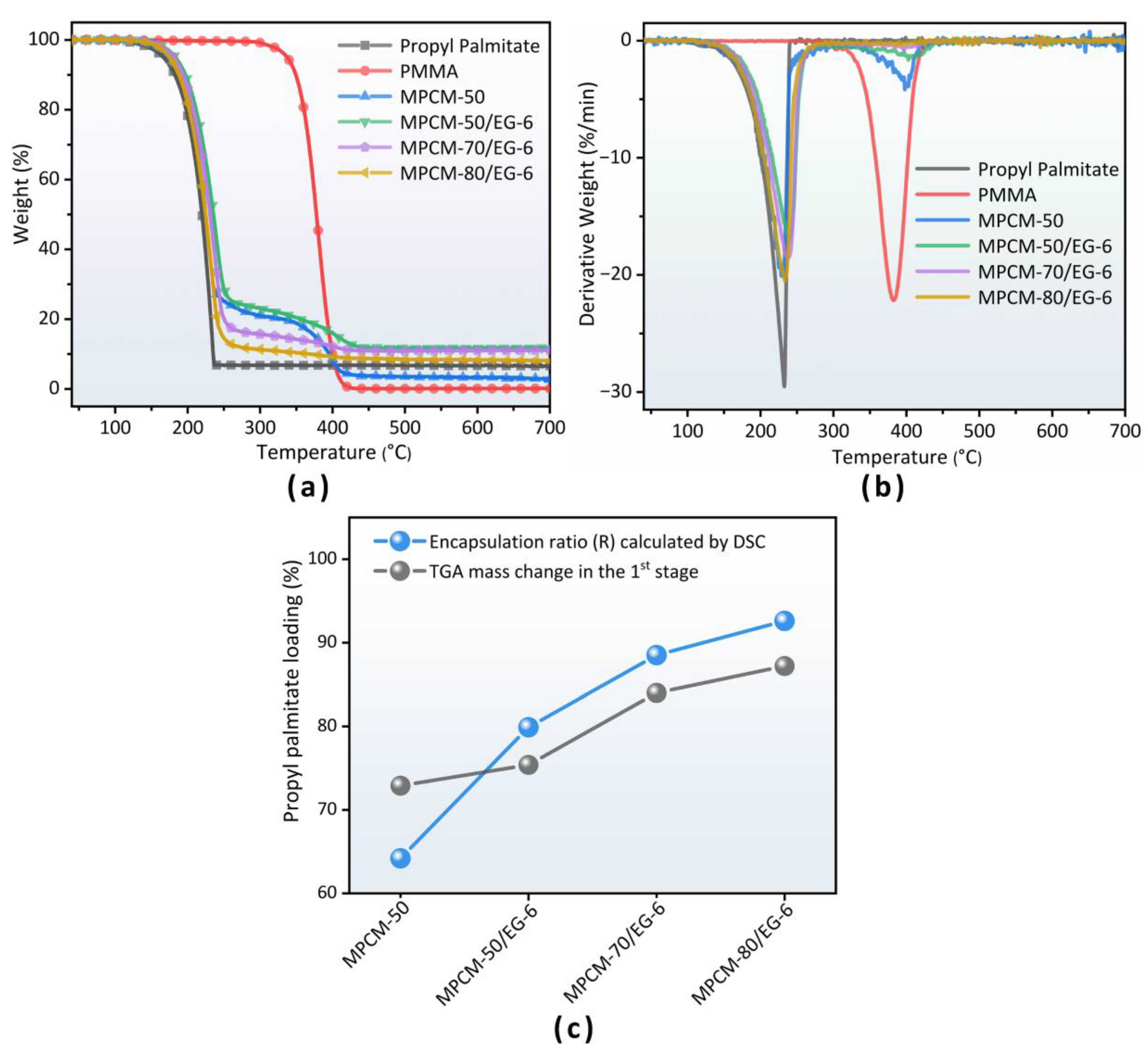
3.4. TGA Analysis
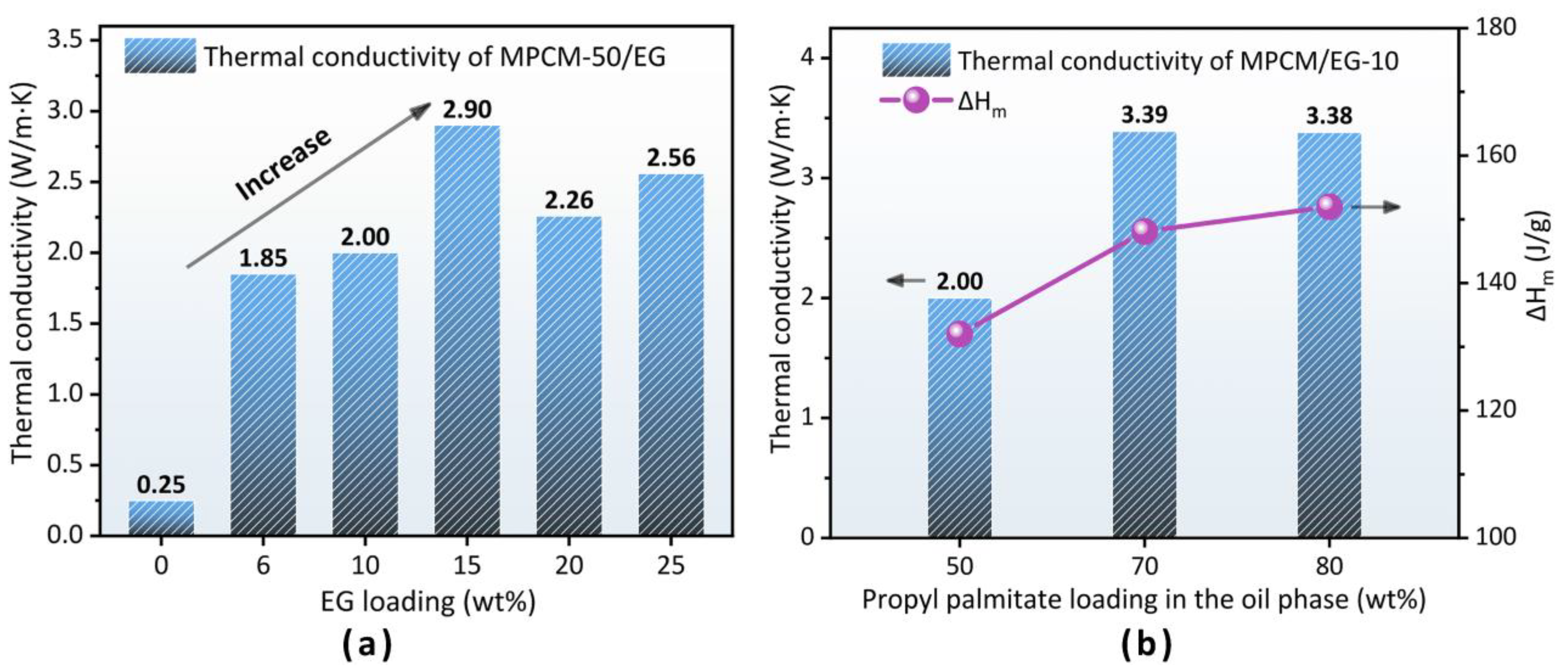
3.5. Thermal Conductivity Enhancement
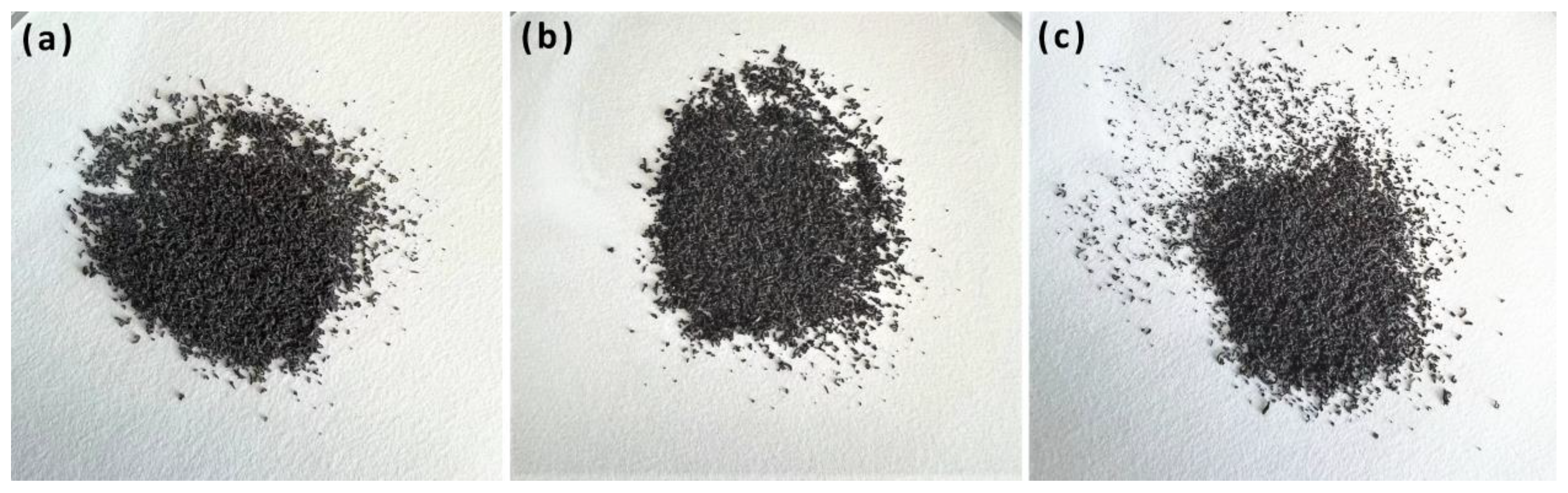
3.6. Leakage Tests
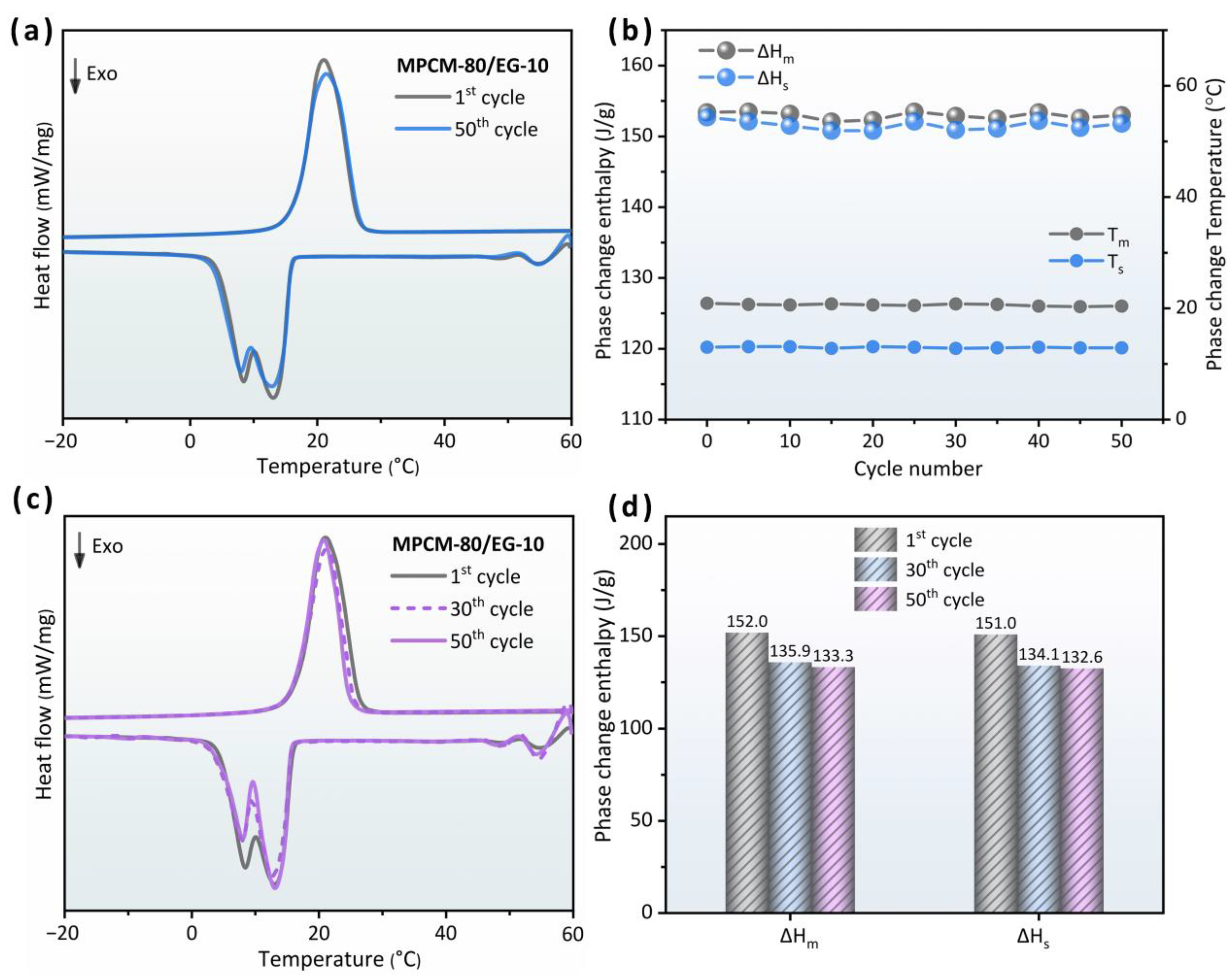
3.7. Thermal Cycle Reliability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gerkman, M.A.; Han, G.G.D. Toward controlled thermal energy storage and release in organic phase change materials. Joule 2020, 4, 1621–1625. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.X.; Zhang, Z.Y.; Li, T.; Wang, R.Z. Photoswitchable phase change materials for unconventional thermal energy storage and upgrade. Matter 2021, 4, 3385–3399. [Google Scholar] [CrossRef]
- Yang, A.S.; Cai, T.; Su, L.; Li, Y.; He, F.; Zhang, Q.P.; Zhou, Y.; He, R.; Zhang, K.; Yang, W. Review on organic phase change materials for sustainable energy storage. Sustain. Energ. Fuels 2022. [Google Scholar] [CrossRef]
- Li, Z.R.; Hu, N.; Fan, L.W. Nanocomposite phase change materials for high-performance thermal energy storage: A critical review. Energy Storage Mater. 2022. [Google Scholar] [CrossRef]
- Sikiru, S.; Oladosu, T.L.; Amosa, T.I.; Kolawole, S.Y.; Soleimani, H. Recent advances and impact of phase change materials on solar energy: A comprehensive review. J. Energy Storage 2022, 53, 105200. [Google Scholar] [CrossRef]
- Lamrani, B.; Johannes, K.; Kuznik, F. Phase change materials integrated into building walls: An updated review. Renew. Sust. Energ. Rev. 2021, 140, 110751. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.; Wang, H.; Lin, P.; Jia, L.; Li, L.; Chen, Y. Synthesis and properties of multifunctional microencapsulated phase change material for intelligent textiles. J. Mater. Sci. 2021, 56, 2176–2191. [Google Scholar] [CrossRef]
- Omara, A.A.M. Phase change materials for waste heat recovery in internal combustion engines: A review. J. Energy Storage 2021, 44, 103421. [Google Scholar] [CrossRef]
- Zou, L.; Lin, P.; Zhang, J.; Su, H.; Chen, Y. Highly-efficient thermal management of electronic devices enabled by boron nitride-incorporated phase change material gels. J. Mater. Sci. 2022, 57, 20268–20284. [Google Scholar] [CrossRef]
- Bao, J.; Tu, H.; Li, J.; Li, Y.; Yu, S.; Gao, J.; Lei, K.; Zhang, F.; Li, J. Applications of phase change materials in smart drug delivery for cancer treatment. Front. Bioeng. Biotech. 2022, 10, 991005. [Google Scholar] [CrossRef]
- Xue, F.; Qi, X.D.; Huang, T.; Tang, C.Y.; Zhang, N.; Wang, Y. Preparation and application of three-dimensional filler network towards organic phase change materials with high performance and multi-functions. Chem. Eng. J. 2021, 419, 129620. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Cao, X.; Du, Y.; Zhang, Z.; Gui, Y. Latent heat thermal energy storage systems with solid–liquid phase change materials: A review. Adv. Eng. Mater. 2018, 20, 1700753. [Google Scholar] [CrossRef]
- Wu, R.; Gao, W.; Zhou, Y.; Wang, Z.; Lin, Q. A novel three-dimensional network-based stearic acid/graphitized carbon foam composite as high-performance shape-stabilized phase change material for thermal energy storage. Compos. Part B-Eng. 2021, 225, 109318. [Google Scholar] [CrossRef]
- Tao, J.; Luan, J.; Liu, Y.; Qu, D.; Yan, Z.; Ke, X. Technology development and application prospects of organic-based phase change materials: An overview. Renew. Sust. Energ. Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- Shen, Z.; Kwon, S.; Lee, H.L.; Toivakka, M.; Oh, K. Cellulose nanofibril/carbon nanotube composite foam-stabilized paraffin phase change material for thermal energy storage and conversion. Carbohyd. Polym. 2021, 273, 118585. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Zhang, G.; Kan, Y.; Li, C.; Deng, J.; Wang, C. Flexible composite phase change material with anti-leakage and anti-vibration properties for battery thermal management. Appl. Energ. 2022, 309, 118434. [Google Scholar] [CrossRef]
- Shchukina, E.M.; Graham, M.; Zheng, Z.; Shchukin, D.G. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.; Hu, M.; Wang, L.; Kokogiannakis, G.; Chen, J.; Gao, L.; Li, A.; Xu, C. Microencapsulated phase change materials with graphene-based materials: Fabrication, characterisation and prospects. Renew. Sust. Energ. Rev. 2022, 168, 112806. [Google Scholar] [CrossRef]
- Liu, L.; Alva, G.; Huang, X.; Fang, G. Preparation, heat transfer and flow properties of microencapsulated phase change materials for thermal energy storage. Renew. Sust. Energ. Rev. 2016, 66, 399–414. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Chen, X.; Wang, G.; Huang, X.; Li, A.; Dong, W. Nanoconfinement effects on thermal properties of nanoporous shape-stabilized composite PCMs: A review. Nano Energy 2018, 53, 769–797. [Google Scholar] [CrossRef]
- Huang, X.; Chen, X.; Li, A.; Atinafu, D.; Gao, H.; Dong, W.; Wang, G. Shape-stabilized phase change materials based on porous supports for thermal energy storage applications. Chem. Eng. J. 2019, 356, 641–661. [Google Scholar] [CrossRef]
- Hamidi, E.; Ganesan, P.B.; Sharma, R.K.; Yong, K.W. Computational study of heat transfer enhancement using porous foams with phase change materials: A comparative review. Renew. Sust. Energ. Rev. 2023, 176, 113196. [Google Scholar] [CrossRef]
- Yu, C.; Park, J.; Youn, J.R.; Song, Y.S. Integration of form-stable phase change material into pyroelectric energy harvesting system. Appl. Energ. 2022, 307, 118212. [Google Scholar] [CrossRef]
- Yin, G.Z.; Hobson, J.; Duan, Y.; Wang, D.Y. Polyrotaxane: New generation of sustainable, ultra-flexible, form-stable and smart phase change materials. Energy Storage Mater. 2021, 40, 347–357. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Ji, J.; Liu, L.; Wu, Y.; Yang, M.; Lu, D.; Zheng, H. Development of flexible form-stable phase change material with enhanced electrical resistance for thermal management. J. Clean. Prod. 2021, 311, 127517. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sust. Energ. Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Paneliya, S.; Khanna, S.; Singh, A.P.; Patel, Y.K.; Vanpariya, A.; Makani, N.H.; Banerjee, R.; Mukhopadhyay, I. Core shell paraffin/silica nanocomposite: A promising phase change material for thermal energy storage. Renew. Energ. 2021, 167, 591–599. [Google Scholar] [CrossRef]
- Rodriguez-Cumplido, F.; Pabon-Gelves, E.; Chejne-Jana, F. Recent developments in the synthesis of microencapsulated and nanoencapsulated phase change materials. J. Energy Storage 2019, 24, 100821. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Alkan, C.; Özcan, A.N. Thermal energy storage characteristics of myristic acid-palmitic eutectic mixtures encapsulated in PMMA shell. Sol. Energy Mater. Sol. Cells 2019, 193, 1–6. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Li, Y.; Zhao, L.; Wang, H.; Song, G.; Tang, G. Microencapsulated phase change materials with TiO2-doped PMMA shell for thermal energy storage and UV-shielding. Sol. Energy Mater. Sol. Cells 2017, 168, 62–68. [Google Scholar] [CrossRef]
- Gao, Y.; Geng, X.; Wang, X.; Han, N.; Zhang, X.; Li, W. Synthesis and characterization of microencapsulated phase change materials with chitosan-based polyurethane shell. Carbohyd. Polym. 2021, 273, 118629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Z.; Kapar, S.; Ataeian, P.; Da Silva Bernardes, J.; Berry, R.; Zhao, W.; Zhou, G.; Tam, K.C. Microencapsulation of phase change materials with polystyrene/cellulose nanocrystal hybrid shell via Pickering emulsion polymerization. ACS Sustain. Chem. Eng. 2019, 7, 17756–17767. [Google Scholar] [CrossRef]
- Huo, J.H.; Peng, Z.G.; Feng, Q. Synthesis and properties of microencapsulated phase change material with a urea–formaldehyde resin shell and paraffin wax core. J. Appl. Polym. Sci. 2020, 137, 48578. [Google Scholar] [CrossRef]
- Dixit, P.; Parvate, S.; Reddy, V.J.; Singh, J.; Maiti, T.K.; Dasari, A.; Chattopadhyay, S. Effect of surfactants on encapsulation of hexadecane phase change material in calcium carbonate shell for thermal energy storage. J. Energy Storage 2022, 55, 105491. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Cao, L.; Tang, F.; Fang, G. Synthesis and characterization of microencapsulated paraffin with titanium dioxide shell as shape-stabilized thermal Energy Storage Mater. in buildings. Energy and Buildings 2014, 72, 31–37. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wu, D. Design and synthesis of magnetic microcapsules based on n-eicosane core and Fe3O4/SiO2 hybrid shell for dual-functional phase change materials. Appl. Energ. 2014, 134, 456–468. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Zhao, Y.; Ling, Z.; Zhang, Z.; Fang, X. Compatible paraffin@ SiO2 microcapsules/polydimethylsiloxane composites with heat storage capacity and enhanced thermal conductivity for thermal management. Compos. Sci. Technol. 2022, 218, 109192. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Alva, G.; Fang, G. Palmitic acid/polyvinyl butyral/expanded graphite composites as form-stable phase change materials for solar thermal energy storage. Appl. Energ. 2018, 228, 1801–1809. [Google Scholar] [CrossRef]
- Li, F.; Zhen, H.; Li, L.; Li, Y.; Wang, Q.; Cheng, X. A template-method synthesis of mesoporous-MgO/expanded graphite for enhancing thermal properties of methyl palmitate-lauric acid phase change materials. Mater. Today Energy 2022, 26, 100999. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, C.; Fang, G. Preparation and thermal properties of n-eicosane/nano-SiO2/expanded graphite composite phase-change material for thermal energy storage. Mater. Chem. Phys. 2020, 240, 122178. [Google Scholar] [CrossRef]
- Wang, W.; Tang, B.; Ju, B.; Gao, Z.; Xiu, J.; Zhang, S. Fe3O4-functionalized graphene nanosheet embedded phase change material composites: Efficient magnetic-and sunlight-driven energy conversion and storage. J. Mater. Chem. A 2017, 5, 958–968. [Google Scholar] [CrossRef]
- Prado, J.I.; Lugo, L. Enhancing the thermal performance of a stearate phase change material with graphene nanoplatelets and MgO nanoparticles. ACS Appl. Mater. Inter. 2020, 12, 39108–39117. [Google Scholar] [CrossRef] [PubMed]
- Paneliya, S.; Khanna, S.; Makani, N.H.; Banerjee, R.; Mukhopadhyay, I. Highly stable n-hexacosane loaded exfoliated graphite nanosheets for enhanced thermal energy storage application. J. Energy Storage 2022, 48, 103903. [Google Scholar] [CrossRef]
- Qiu, J.; Fan, X.; Shi, Y.; Zhang, S.; Jin, X.; Wang, W.; Tang, B. PEG/3D graphene oxide network form-stable phase change materials with ultrahigh filler content. J. Mater. Chem. A 2019, 7, 21371–21377. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Wang, H.; Du, Q. Encapsulated phase change materials stabilized by modified graphene oxide. J. Mater. Chem. A 2014, 2, 5304–5314. [Google Scholar] [CrossRef]
- Shen, S.; Tan, S.; Wu, S.; Guo, C.; Liang, J.; Yang, Q.; Xu, G.; Deng, J. The effects of modified carbon nanotubes on the thermal properties of erythritol as phase change materials. Energ. Convers. Manage. 2018, 157, 41–48. [Google Scholar] [CrossRef]
- Niu, S.; Kang, M.; Liu, Y.; Lin, W.; Liang, C.; Zhao, Y.; Cheng, J. The preparation and characterization of phase change material microcapsules with multifunctional carbon nanotubes for controlling temperature. Energy 2023, 126652. [Google Scholar] [CrossRef]
- Shahid, U.B.; Abdala, A. A critical review of phase change material composite performance through Figure-of-Merit analysis: Graphene vs Boron Nitride. Energy Storage Mater. 2021, 34, 365–387. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Saidur, R.; Tyagi, V.V. Energizing organic phase change materials using silver nanoparticles for thermal energy storage. J. Energy Storage 2023, 58, 106361. [Google Scholar] [CrossRef]
- Chung, D.D.L. A review of exfoliated graphite. J. Mater. Sci. 2016, 51, 554–568. [Google Scholar] [CrossRef]
- Zhao, M.; Ye, Y.; Yang, R. Absorption-polymerization method for synthesizing phase change composites with high enthalpy and thermal conductivity for efficient thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 248, 112027. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Cao, J. An energy-efficient composite by using expanded graphite stabilized paraffin as phase change material. Compos. Part A-Appl. S. 2018, 107, 83–93. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, R. N-octanoic acid-based phase change composites synthesized by absorption polymerization for efficient thermal energy storage. J. Energy Storage 2023, 64, 107169. [Google Scholar] [CrossRef]
- Shtein, M.; Nadiv, R.; Buzaglo, M.; Kahil, K.; Regev, O. Thermally conductive graphene-polymer composites: Size, percolation, and synergy effects. Chem. Mater. 2015, 27, 2100–2106. [Google Scholar] [CrossRef]
- Chen, L.; Zou, R.; Xia, W.; Liu, Z.; Shang, Y.; Zhu, J.; Wang, Y.; Lin, J.; Xia, D.; Cao, A. Electro-and photodriven phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 2012, 6, 10884–10892. [Google Scholar] [CrossRef] [PubMed]
| Sample | mPCM/(mPCM + mMMA) (wt%) | EG (wt%) |
|---|---|---|
| MPCM-50 | 50 | — |
| MPCM-70 | 70 | — |
| MPCM-80 | 80 | — |
| MPCM-50/EG-6 | 50 | 6 |
| MPCM-70/EG-6 | 70 | 6 |
| MPCM-80/EG-6 | 80 | 6 |
| MPCM-50/EG-10 | 50 | 10 |
| MPCM-70/EG-10 | 70 | 10 |
| MPCM-80/EG-10 | 80 | 10 |
| MPCM-50/EG-15 | 50 | 15 |
| MPCM-70/EG-15 | 70 | 15 |
| MPCM-80/EG-15 | 80 | 15 |
| MPCM-50/EG-20 | 50 | 20 |
| MPCM-50/EG-25 | 50 | 25 |
| MPCM-50@EG-6 | 50 | 6 |
| Sample | ΔHm (J/g) | ΔHs (J/g) | Tm (°C) | Ts (°C) | φPropyl palmitate 1 (%) | Encapsulation Ratio 2 (%) | Encapsulation Efficiency 3 (%) |
|---|---|---|---|---|---|---|---|
| Propyl palmitate | 168.3 | 165.7 | 18.3 | 9.2 | — | — | — |
| MPCM-50 | 108.0 | 106.2 | 24.5 | 9.8 | 50.0 | 64.2 | 128.3 |
| MPCM-70 | 144.3 | 141.0 | 23.8 | 9.8 | 70.0 | 85.7 | 122.5 |
| MPCM-80 | 119.0 | 118.3 | 22.4 | 10.3 | 80.0 | 70.7 | 88.4 |
| MPCM-50/EG-6 | 134.5 | 133.1 | 21.4 | 12.6 | 47.0 | 79.9 | 170.0 |
| MPCM-70/EG-6 | 149.0 | 145.0 | 21.5 | 12.4 | 65.8 | 88.5 | 134.5 |
| MPCM-80/EG-6 | 155.8 | 154.6 | 21.4 | 12.3 | 75.2 | 92.6 | 123.1 |
| MPCM-50/EG-10 | 132.0 | 133.8 | 20.4 | 13.4 | 45.0 | 78.4 | 174.3 |
| MPCM-70/EG-10 | 148.1 | 146.1 | 20.7 | 13.2 | 63.0 | 88.0 | 139.7 |
| MPCM-80/EG-10 | 152.0 | 151.0 | 20.8 | 13.0 | 72.0 | 90.3 | 125.4 |
| MPCM-50/EG-15 | 119.6 | 118.2 | 20.0 | 13.5 | 42.5 | 71.1 | 167.2 |
| MPCM-70/EG-15 | 144.5 | 142.9 | 20.4 | 13.5 | 59.5 | 85.9 | 144.3 |
| MPCM-80/EG-15 | 144.2 | 143.5 | 20.3 | 13.3 | 68.0 | 85.7 | 126.0 |
| MPCM-50@EG-6 | 24.1 | 22.2 | 18.0 | 14.5 | 47.0 | 14.3 | 30.5 |
| Sample | T5% °C | Tmax1 °C | Tmax2 °C | Mass Change (35–250 °C) (%) | Residue at 700 °C (%) |
|---|---|---|---|---|---|
| Propyl palmitate | 167.0 | 232.6 | — | 93.2 | 6.8 |
| PMMA | 337.5 | — | 382.3 | 0.3 | 0.2 |
| MPCM-50 | 173.4 | 229.8 | 396.6 | 72.9 | 3.5 |
| MPCM-50/EG-6 | 183.3 | 239.5 | 403.3 | 75.4 | 11.6 |
| MPCM-70/EG-6 | 180.4 | 237.5 | 396.0 | 84.0 | 9.4 |
| MPCM-80/EG-6 | 176.7 | 233.3 | 395.8 | 87.2 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Zhao, M.; Yang, R. Preparation and Thermal Properties of Propyl Palmitate-Based Phase Change Composites with Enhanced Thermal Conductivity for Thermal Energy Storage. Polymers 2023, 15, 3192. https://doi.org/10.3390/polym15153192
Yin L, Zhao M, Yang R. Preparation and Thermal Properties of Propyl Palmitate-Based Phase Change Composites with Enhanced Thermal Conductivity for Thermal Energy Storage. Polymers. 2023; 15(15):3192. https://doi.org/10.3390/polym15153192
Chicago/Turabian StyleYin, Linzhi, Min Zhao, and Rui Yang. 2023. "Preparation and Thermal Properties of Propyl Palmitate-Based Phase Change Composites with Enhanced Thermal Conductivity for Thermal Energy Storage" Polymers 15, no. 15: 3192. https://doi.org/10.3390/polym15153192
APA StyleYin, L., Zhao, M., & Yang, R. (2023). Preparation and Thermal Properties of Propyl Palmitate-Based Phase Change Composites with Enhanced Thermal Conductivity for Thermal Energy Storage. Polymers, 15(15), 3192. https://doi.org/10.3390/polym15153192







