Special Packaging Materials from Recycled PET and Metallic Nano-Powders
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
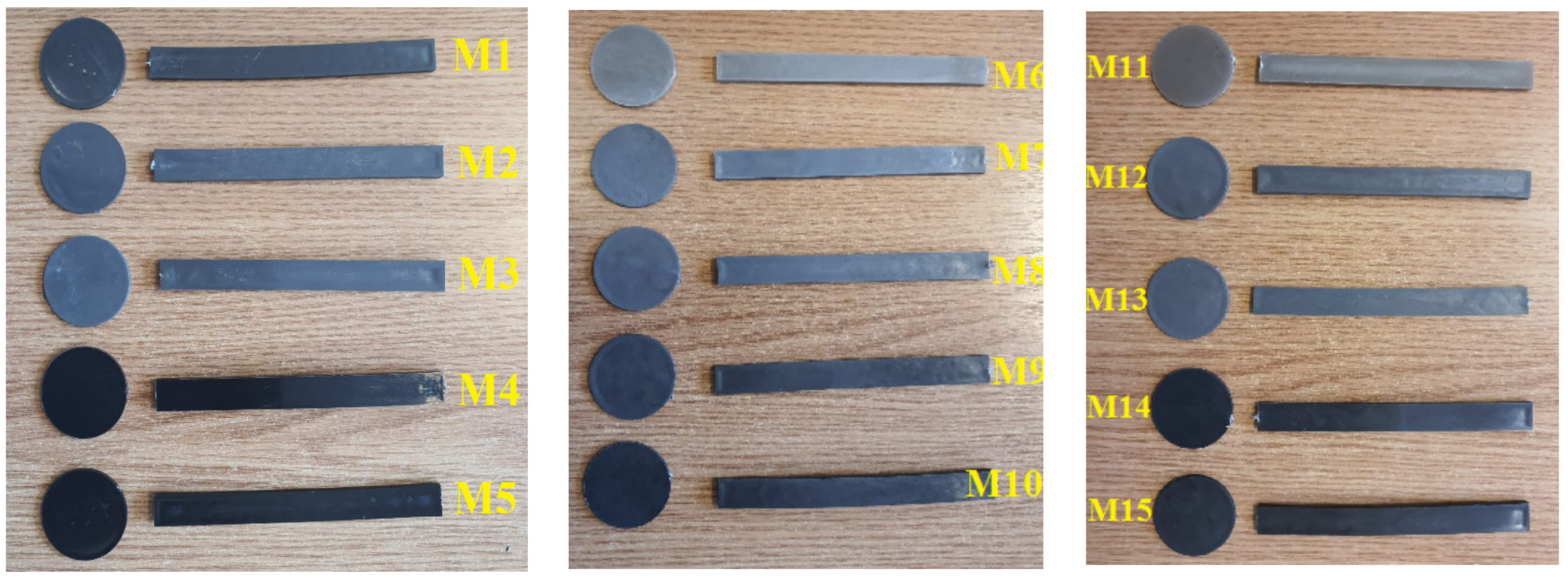
- 5 experimental models of composite materials using a PET polymer matrix and Al and Fe nanopowders reinforcers of 800 nm in grain size in concentrations of 5% and 8%, codified as follows:
- ○
- M1 recycled PET.
- ○
- M2 PET + 5% Al nanopowder.
- ○
- M3 PET + 8% Al nanopowder.
- ○
- M4 PET + 5% Fe nanopowder.
- ○
- M5 PET + 8% Fe nanopowder.
- 5 experimental models of composite materials using as a polymer matrix a mixture of PET and PP polymers and Al and Fe nanopowder reinforcers of 800 nm granulation in a concentration of 5% and 8%, codified as follows:
- ○
- M6 PET 70% + PP 30%.
- ○
- M7 PET + PP + 5% Al nanopowder.
- ○
- M8 PET + PP + 8% Al nanopowder.
- ○
- M9 PET + PP + 5% Fe nanopowder.
- ○
- M10 PET + PP + 8% Fe nanopowder.
- 5 experimental models of composite materials using as a polymer matrix a mixture of two polymers PET + HDPE (High-Density Polyethylene) and reinforcing Al and Fe nanopowders of 800 nm granulation in a concentration of 5% and 8%, coded as follows:
- ○
- M11 PET 70% + HDPE 30%.
- ○
- M12 PET + HDPE + 5% Al nanopowder.
- ○
- M13 PET + HDPE + 8% Al nanopowder.
- ○
- M14 PET + HDPE + 5% Fe nanopowder.
- ○
- M15 PET + HDPE + 8% Fe nanopowder.
2.3. Characterization Methods
3. Results and Discussions
3.1. FTIR Measumeremts
- (a)
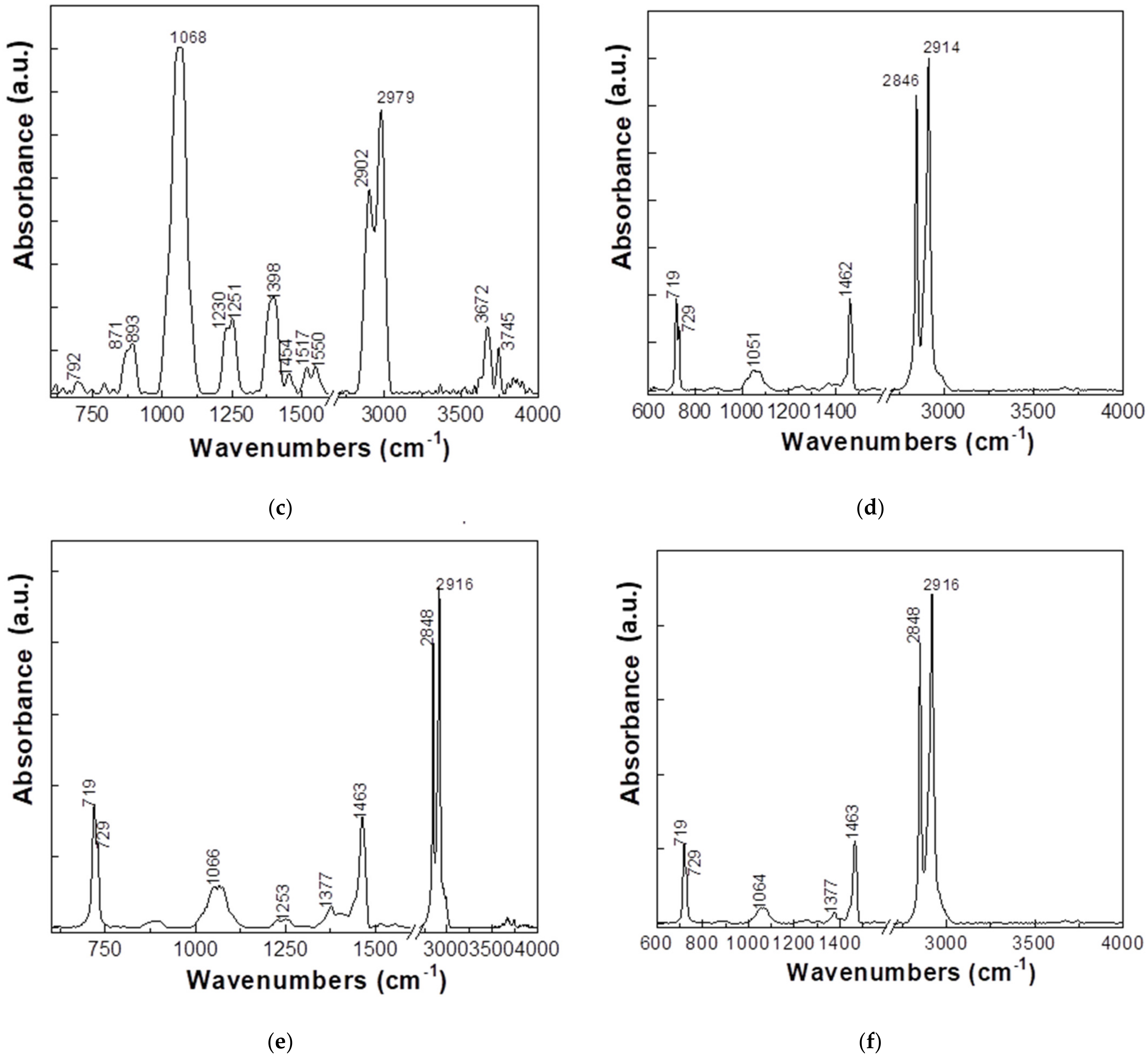
- HDPE + 8% Fe, 50 nm, has two IR bands with higher absorbance at approximately 1066 and 2900–2979 cm−1 being accompanied by other IR bands with lower absorbance having maxima at 879, 1251, 1400, 1454, 1515–1550, and 3678 cm−1.
- (b)
- HDPE + 8% Al, 50 nm, presents the following IR bands with maxima at 717, 729, 1053, 1462, 2846, and 2914 cm−1.
- (c)
- HDPE + 5% Al, 800 nm, shows two intense bands at 1068 and 2902–2979 cm−1, being accompanied by other IR bands with lower absorbance at approximately 871–893, 1230–1251, 1398, 1454, 1517–1550, and 3672–3745 cm−1.
- (d)
- HDPE + 3% Ferrite presents the following IR bands with maxima at approximately 719–729, 1051, 1462, and 2846–2914 cm−1.
- (e)
- LDPE + 5% Fe, 50 µm, presents IR bands with maxima at approximately 719–729, 1066, 1463, and 2848–2916 cm−1.
- (f)
- LDPE + 5% Ferrite, the position of the main IR bands is approximately 719–729, 1064, 1463, and 2848–2916 cm−1.
- (g)
- LDPE + 8% Al, presents IR bands with maxima at approximately 719–729, 1066, 1463, and 2848–2914 cm−1.
- (h)
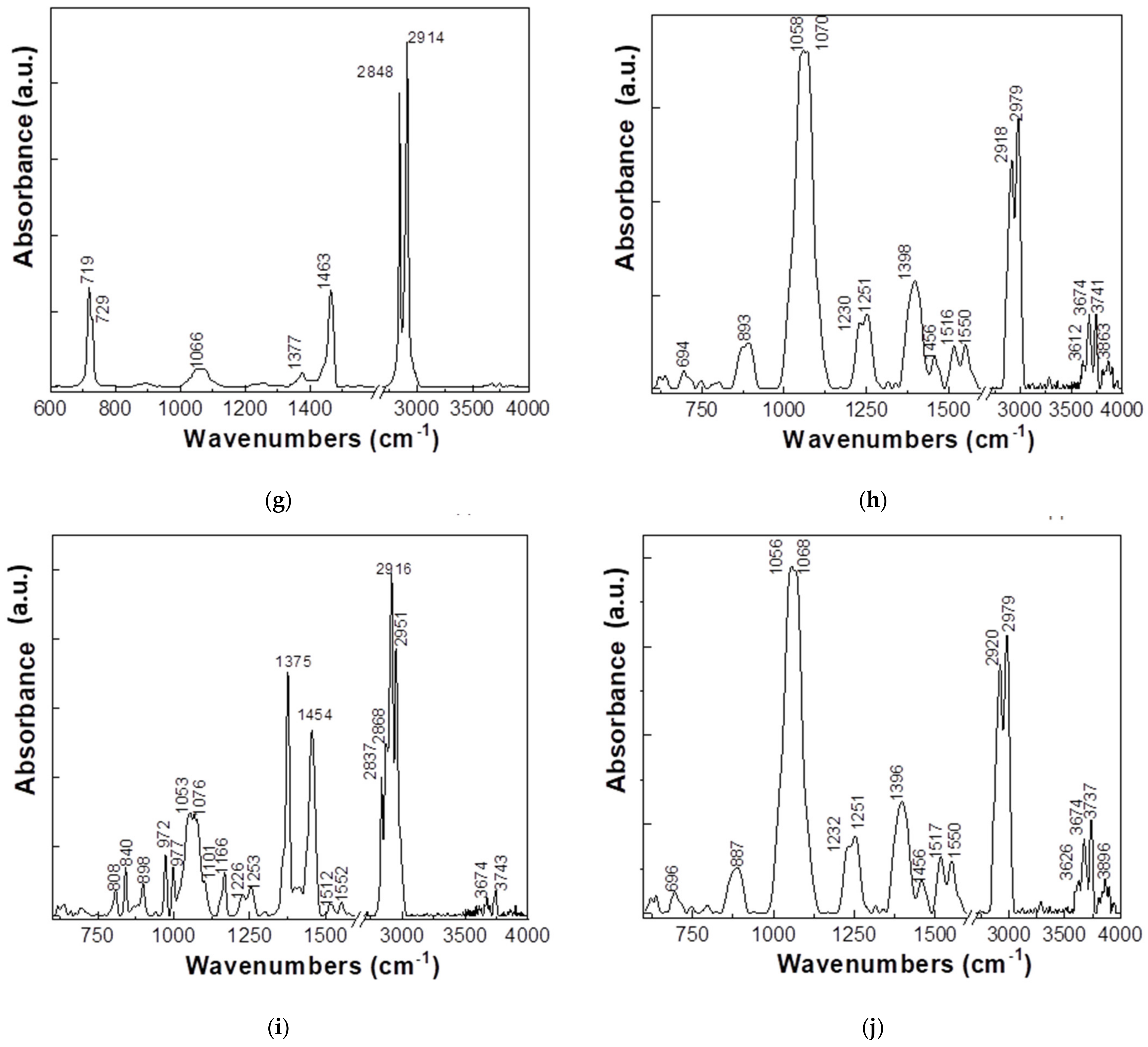
- PP + 8% Ferrite, presents IR bands with maxima at approximately 893, 1058–1070, 1230–1251, 1398, 1454, 1516–1550, 2918–2979, and 3612–3674–3741 cm−1.
- (i)
- PP + 8% Fe, 800 nm, shows IR bands with maxima at approximately 808, 840, 898, 977, 1101, 1053–1076, 1166, 1226–1253, 1375, 1454, 2837–2868–2916–2951, and 3674–3743 cm−1.
- (j)
- PP + 8% Al, 50 nm, presents IR bands with maxima at 887, 1056–1068, 1232–1251, 1396, 1454, 1517–1550, 2920–2979, and 3626–3674–3737 cm−1.
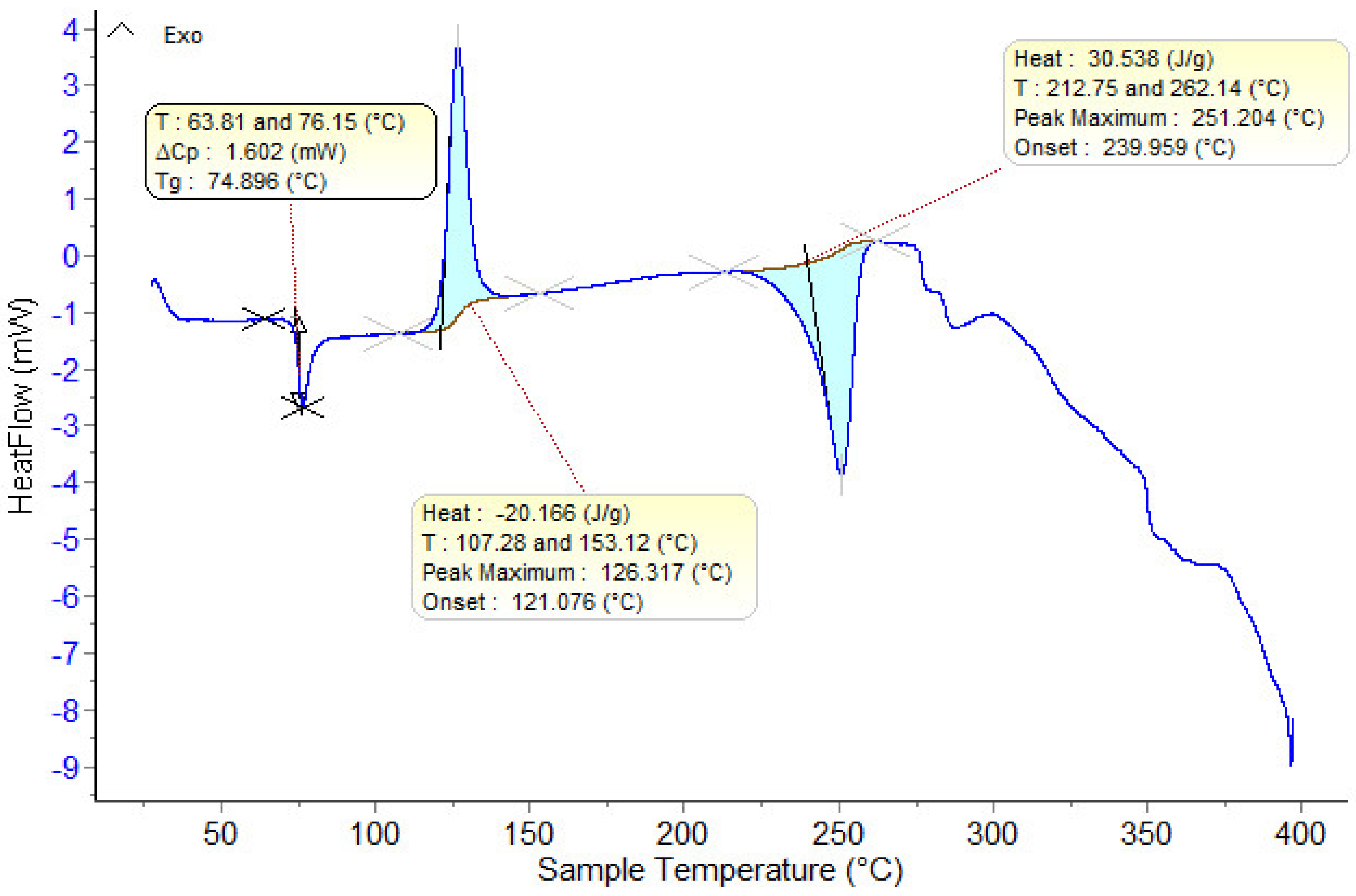
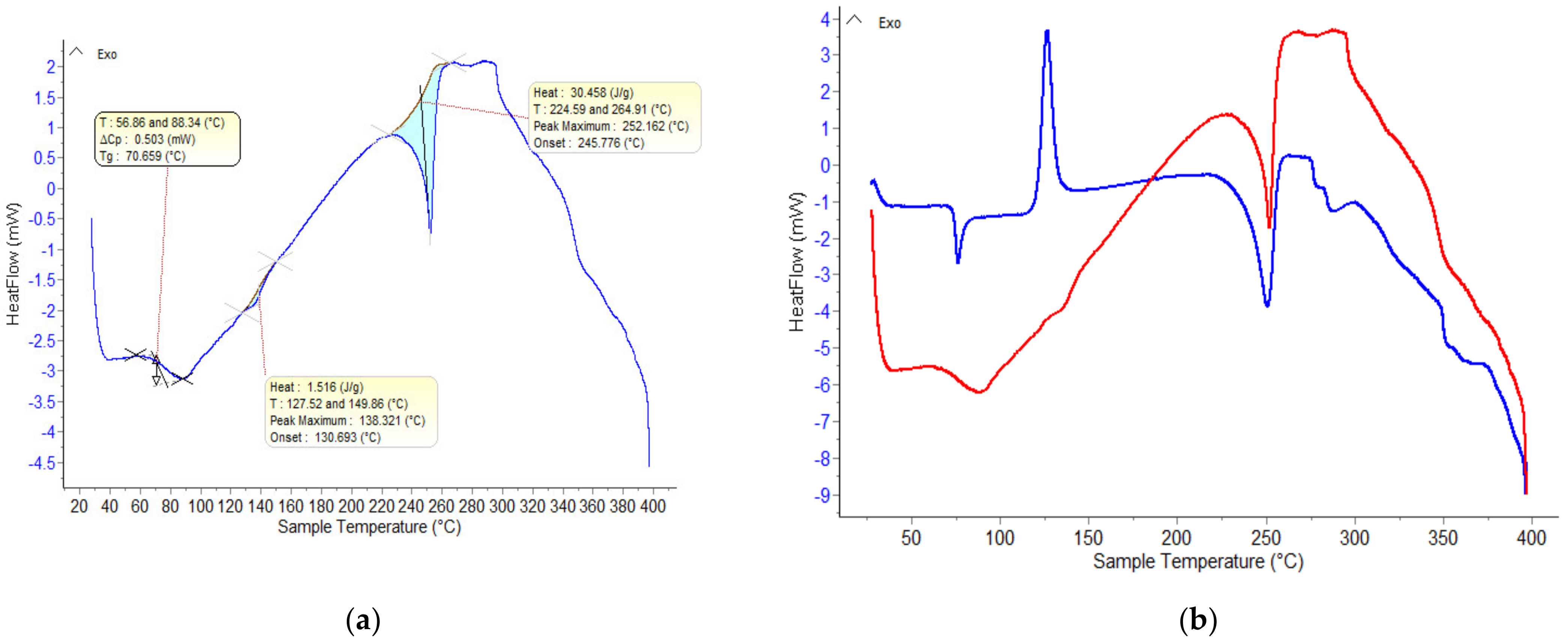
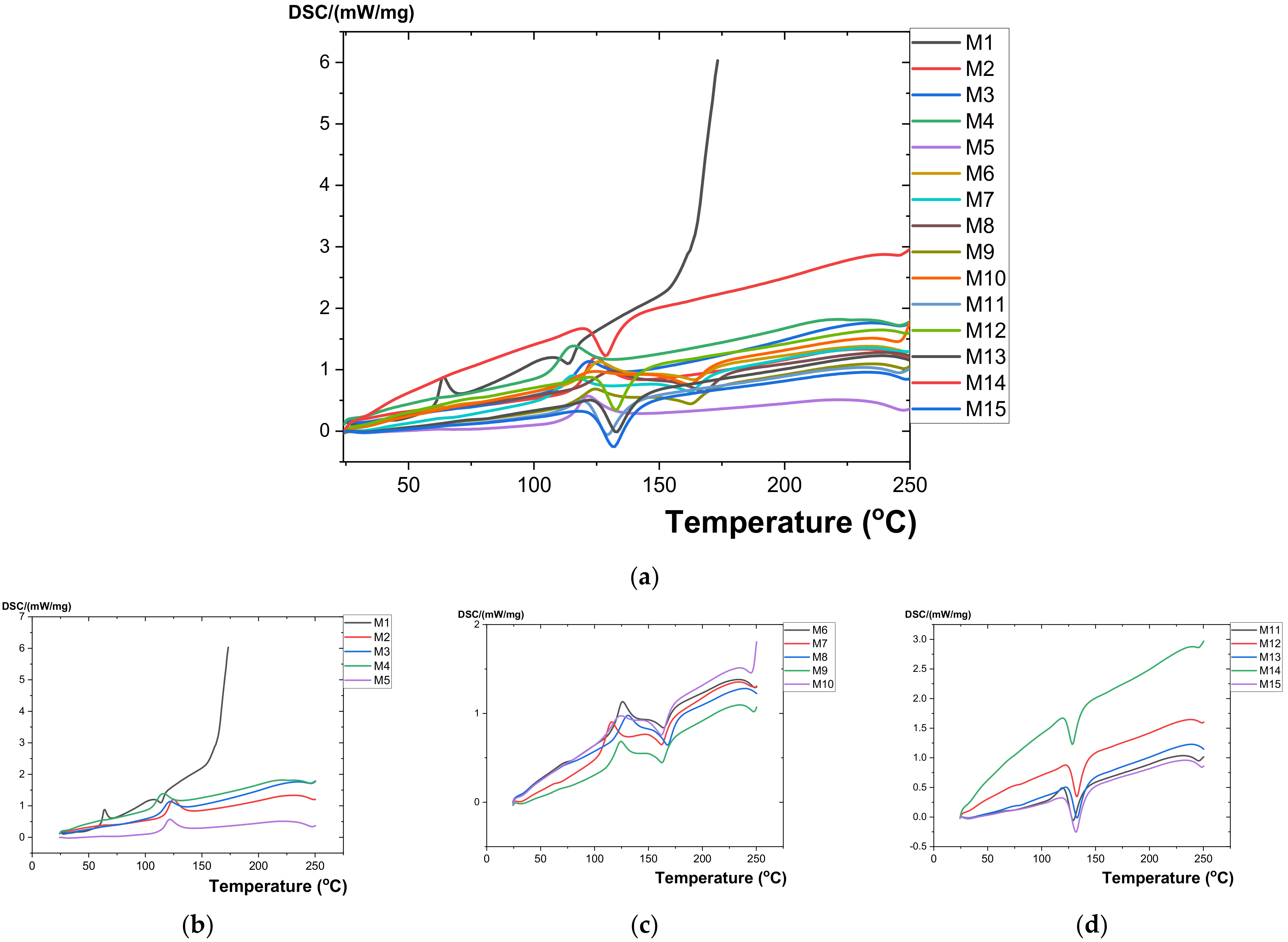
3.2. DSC Measurements
3.3. Measurements of Hydrostatic Density
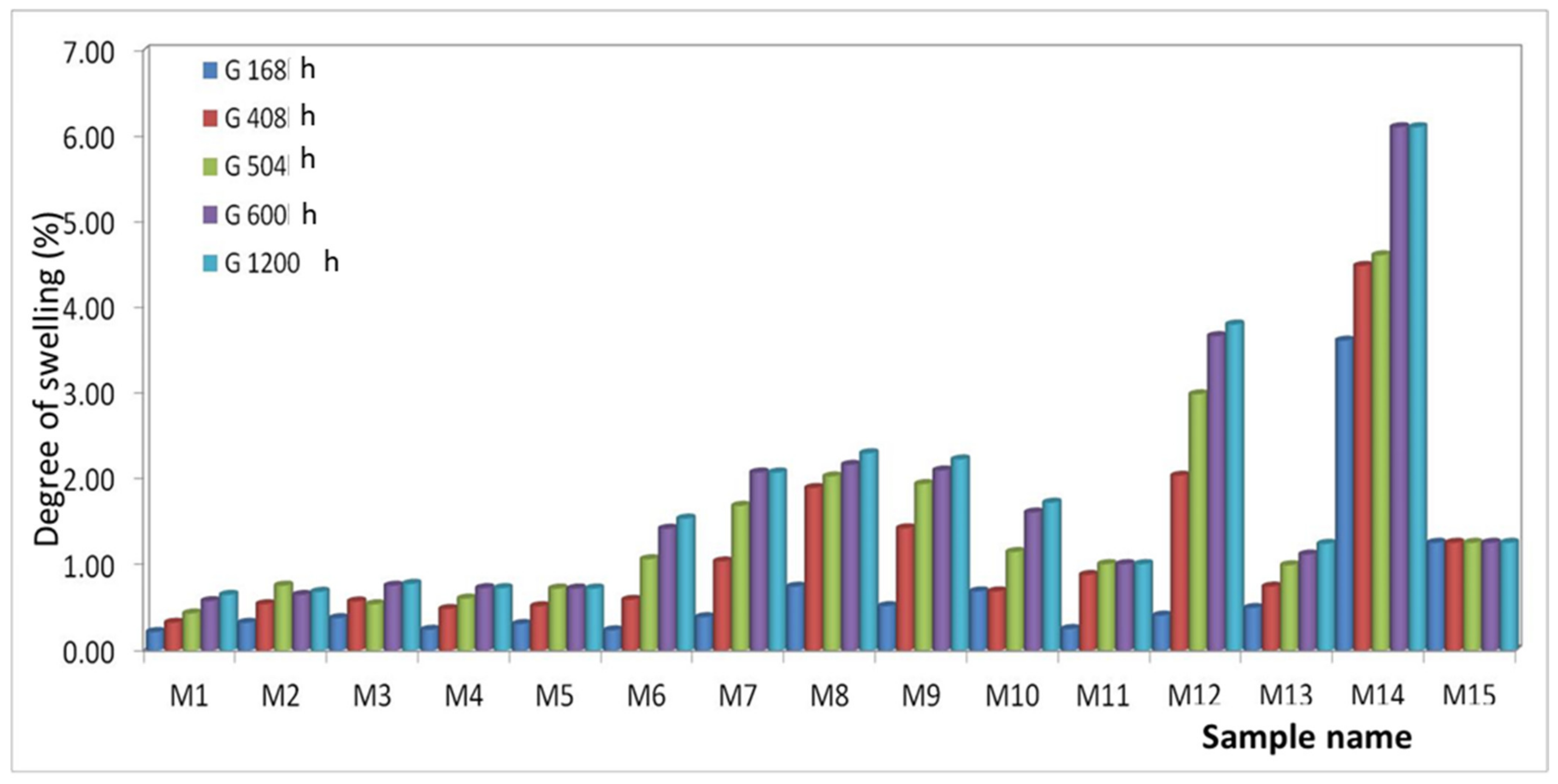
3.4. Measurements of Swelling Capacity
- Q—degree of swelling.
- X2,3,4,…—mass of swollen polymer (after each 168-h cycle).
- X1—dry polymer mass.
- -
- PET (M1–M5): It is found that the highest water absorption is presented by M3 and the lowest by M1. This can be justified by the fact that the percentage of 8% Al nanopowder creates the most voids in the mixing process in the melt, which creates the possibility of inserting water. The values of the degree of swelling are very close due to the majority concentration of the polymer. The classification of materials is as follows: Q M1 < Q M4 < Q M5 < Q M2 < Q M3;
- -
- PET + PP (M6–M10): It is found that M8 has the highest water absorption and M1 has the lowest. Here it can be seen that the Al nanopowder creates more voids than the Fe one. The classification of materials is as follows: Q M6 < Q M7 < Q M9 < Q M10 < Q M8;
- -
- PET + HDPE (M11–M15): It is found that M14 has the highest water absorption and M11 has the lowest. In this case, it is observed that the materials containing Al nanopowder have a lower degree of swelling than those with Fe nanopowder. It can be said that in the PET + HDPE polymer mixture, Al nanopowder is better homogenized than Fe. The classification of materials is as follows: Q M11 < Q M12 < Q M13 < Q M15 < Q M14;
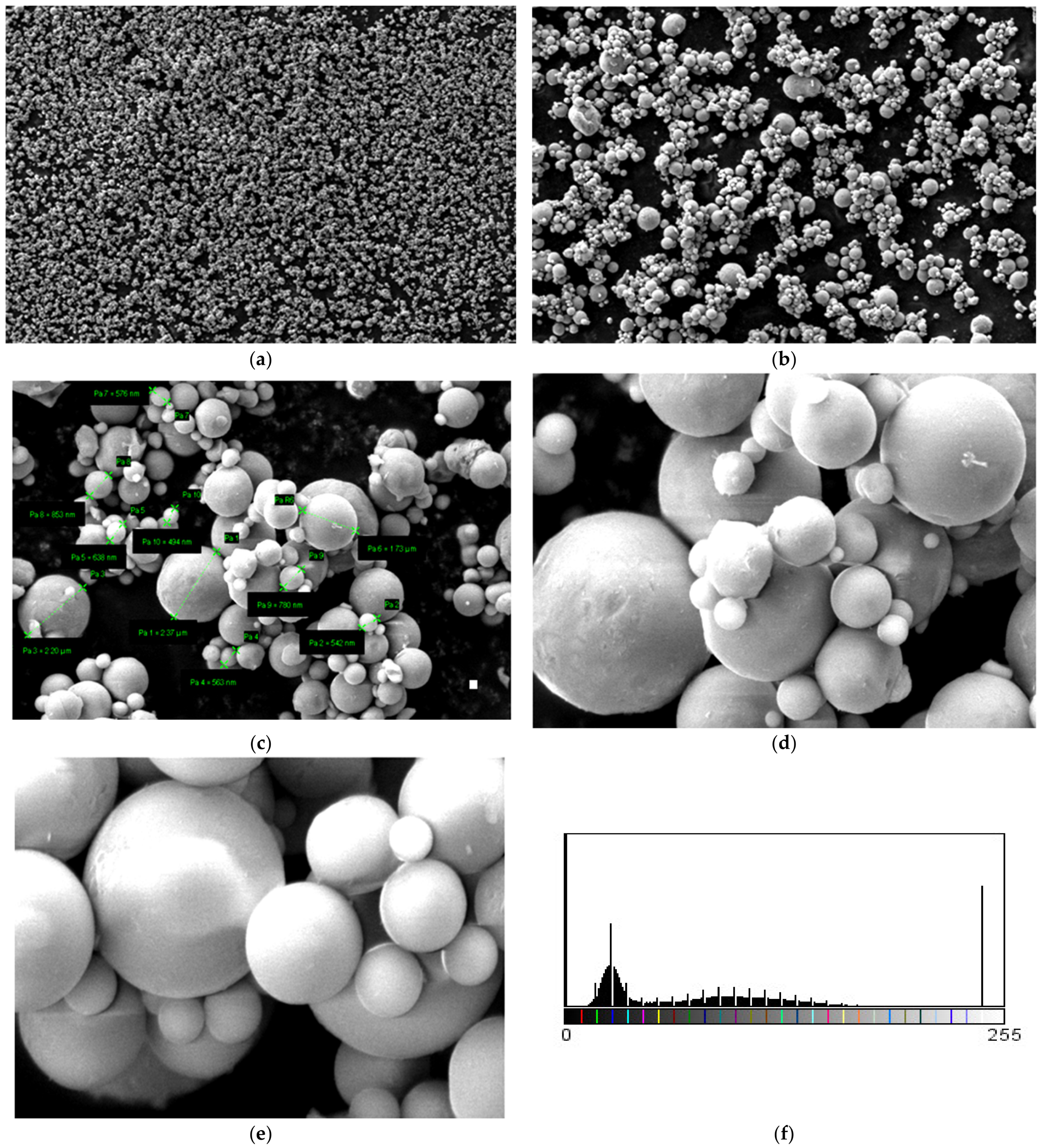
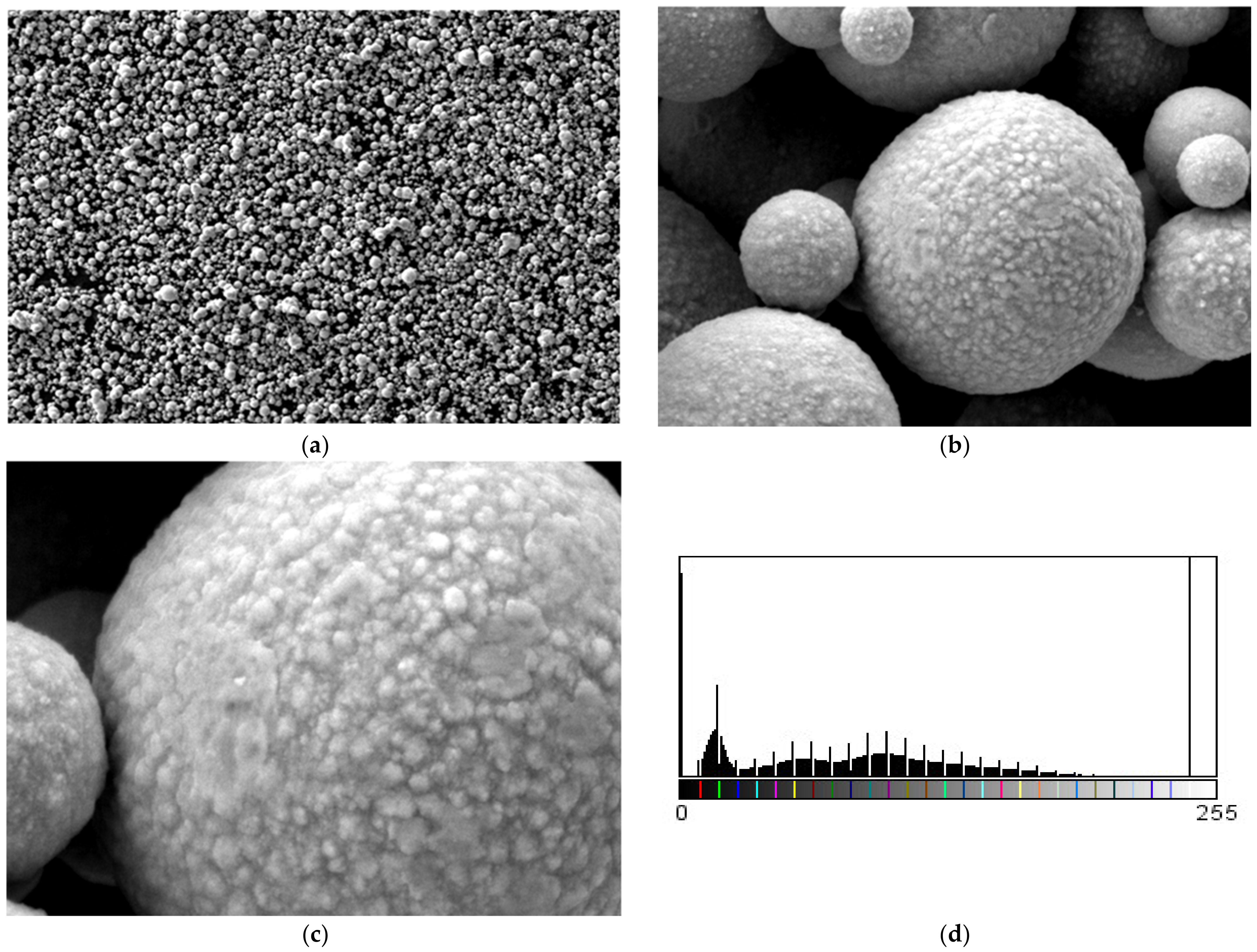
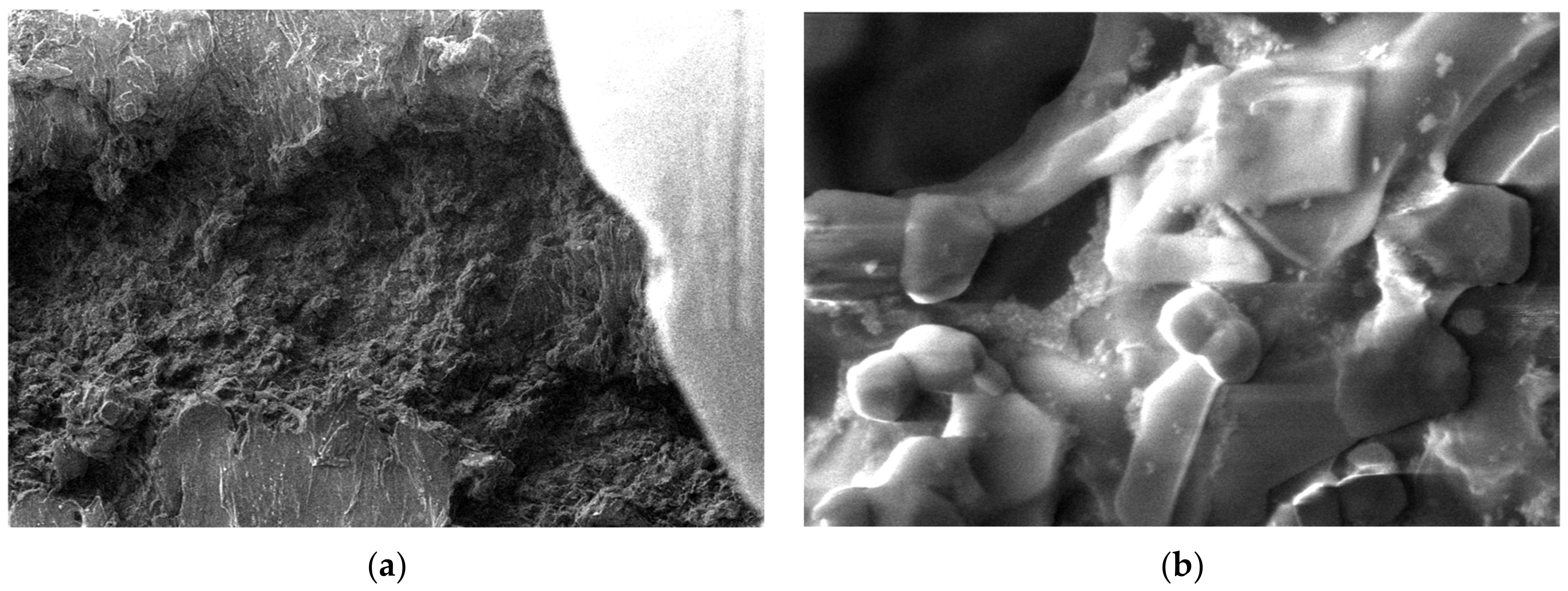
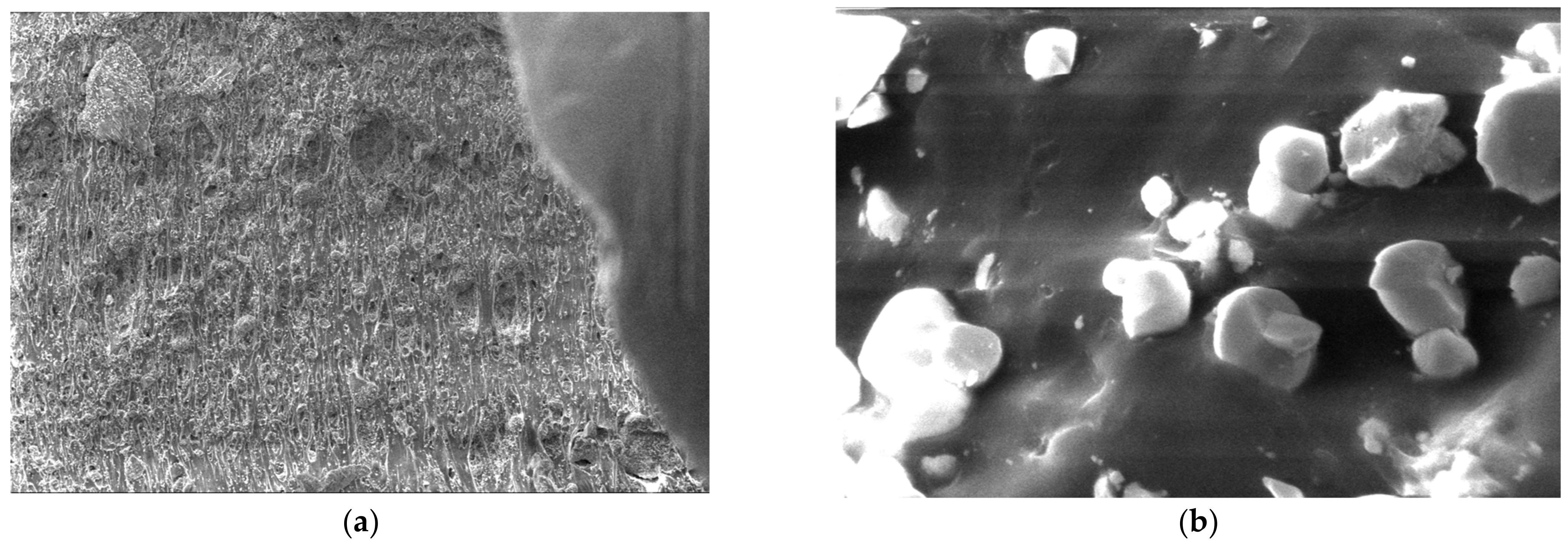
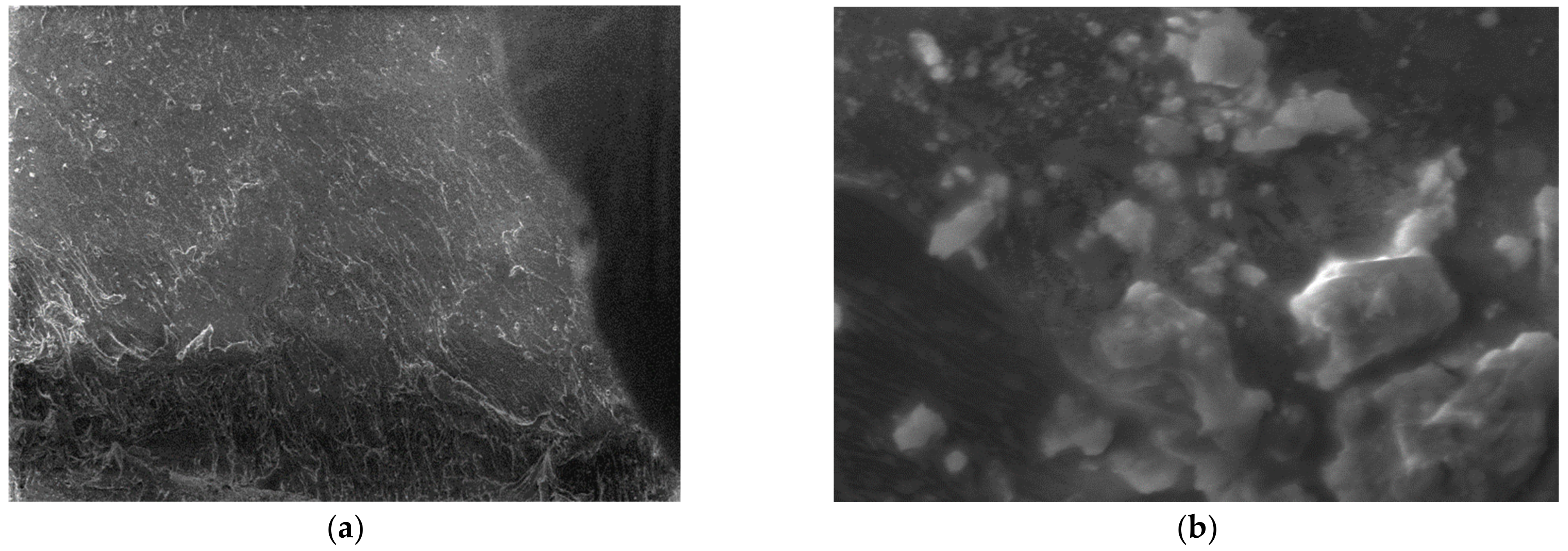
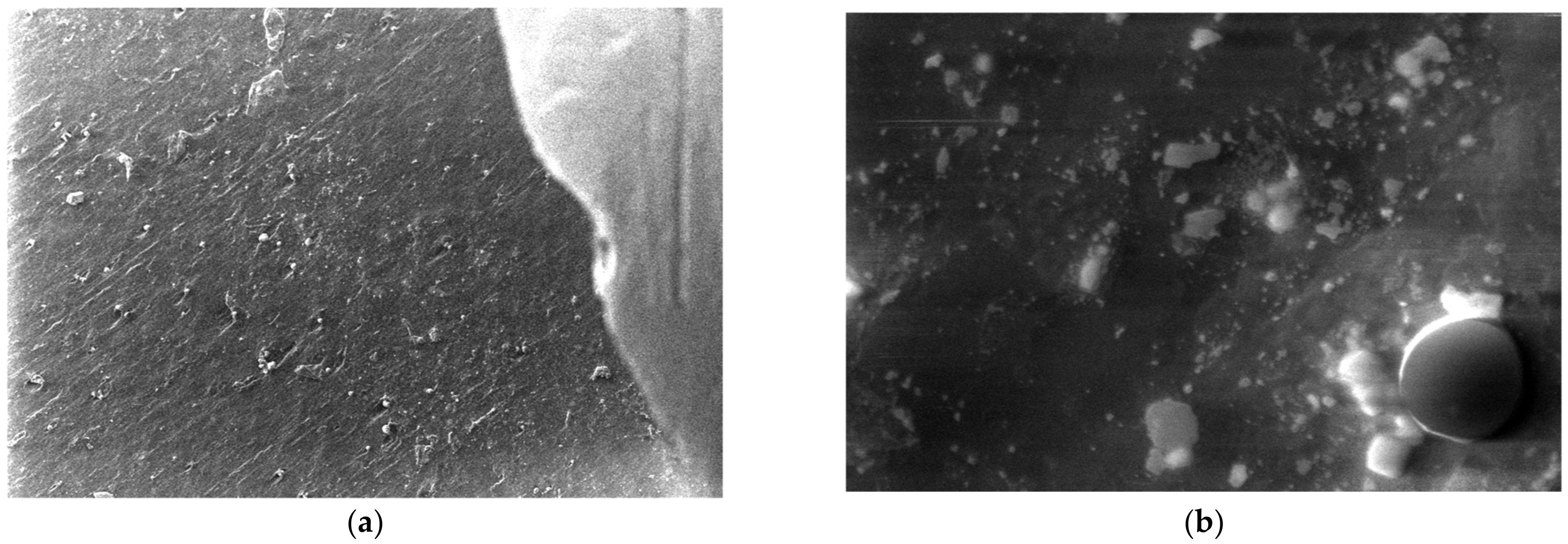
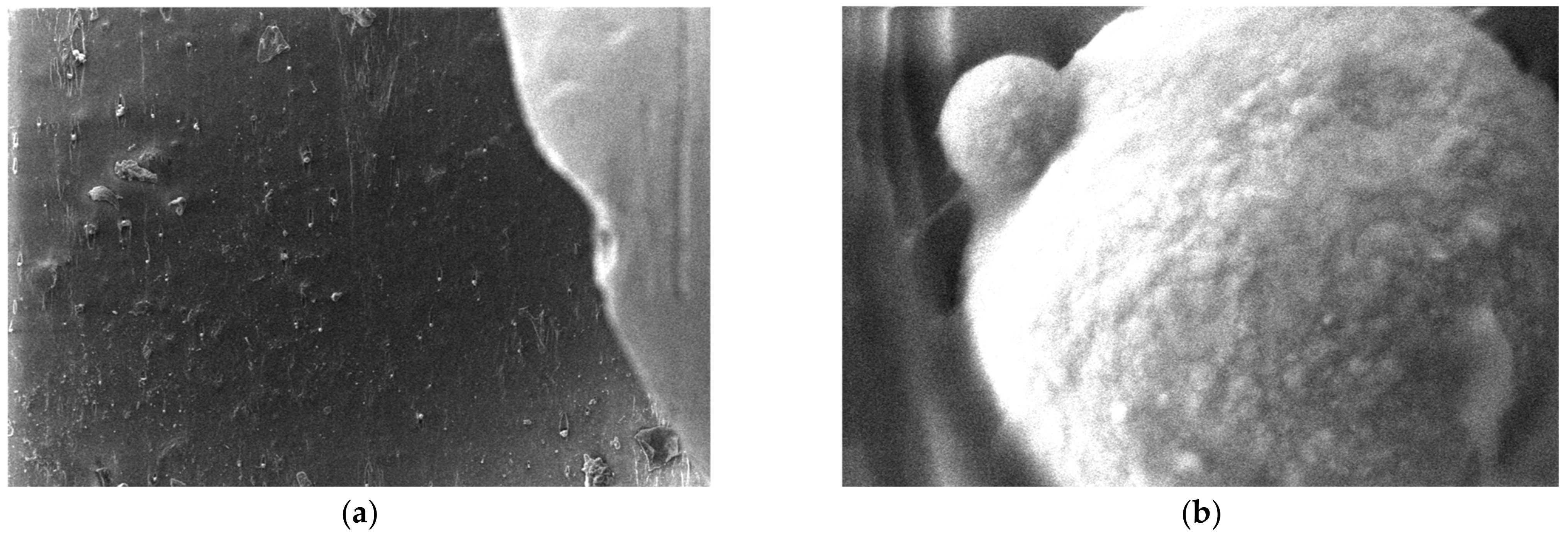
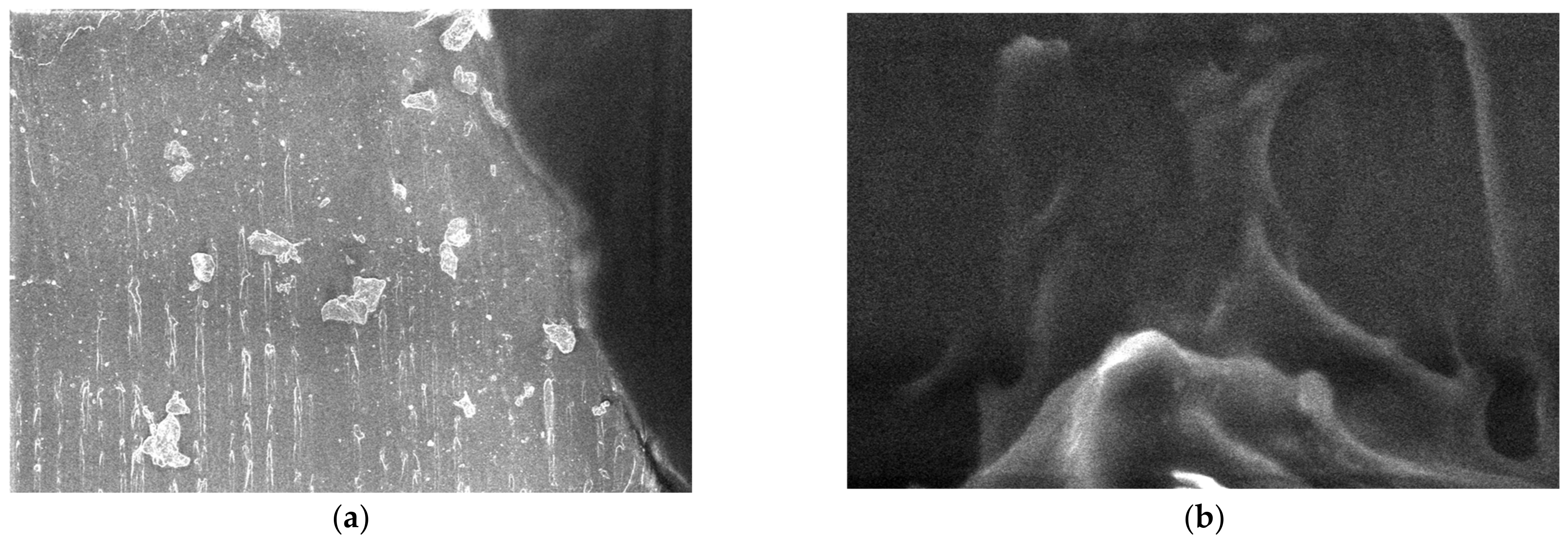
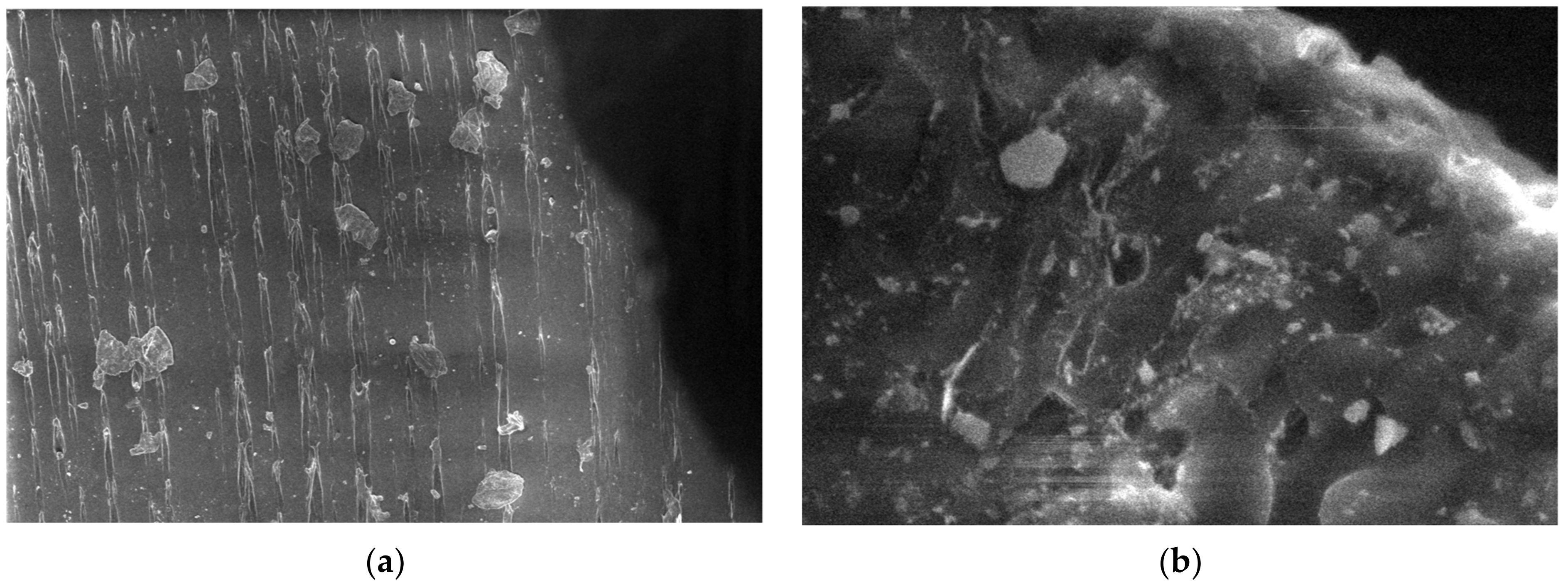
3.5. SEM Measurements
- -
- It can be observed from the composition that for M7 and M8, the smooth powder particles are specific to aluminum, and for M8 and M10, the rough spherical particles are specific to iron.
- -
- Among these experimental models, it is found that the most homogeneous is M7 (the agglomerations are less), making it optimal for the production of special packaging to be used in the food and pharmaceutical industries.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. A European Strategy for Plastics in a Circular Economy. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. European Commission: Brussels, Belgium, 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2018%3A28%3AFIN (accessed on 16 January 2018).
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the Implementation of the Circular Economy Action Plan. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52019DC0190 (accessed on 2 June 2023).
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- IK Industrievereinigung Kunststoffverpackungen e.V.; Plastics Europe Deutschland e.V. The Plastic Packaging Industry’s Path to a Circular Economy. Available online: https://newsroom.kunststoffverpackungen.de/en/2020/06/18/the-plastic-packaging-industrys-path-to-a-circular-economy/ (accessed on 25 June 2023).
- Plastics Europe. Plastics—The Facts 2015 an Analysis of European Plastics Production, Demand and Waste Data. 2015. Available online: http://www.plasticseurope.org/download_file/view/479/179 (accessed on 25 June 2023).
- Kamble, A.; Tanwar, S.; Shanbhag, T. Isolation of plastic degrading micro-organisms from soil samples collected at various locations in Mumbai, India. Int. Res. J. Environment Sci. 2015, 4, 77–85. [Google Scholar]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Driedger, A.G.J.; Dürr, H.H.; Mitchell, K.; Van Cappellen, P. Plastic debris in the Laurentian Great Lakes: A review. J. Great Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Eshaq, G.; Rabie, A.M.; ElMetwally, A.E. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef]
- Harper, C.A. Modern Plastics Handbook, 1st ed.; McGraw Hill: New York, NY, USA, 1999. [Google Scholar]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Sepperumal, U.; Markandan, M.; Palraja, I. Micromorphological and chemical changes during biodegradation of Polyethylene terephthalate (PET) by Penicillium sp. J. Microbiol. Biotechnol. 2013, 3, 47–53. [Google Scholar]
- Koshti, R.; Mehta, L.; Samarth, N. Biological Recycling of Polyethylene Terephthalate: A Mini-Review. J. Polym. Environ. 2018, 26, 3520–3529. [Google Scholar] [CrossRef]
- Munoz, M.; Ortiz, D.; Nieto-Sandoval, J.; de Pedro, Z.M.; Casas, J.A. Adsorption of micropollutants onto realistic microplastics: Role of microplastic nature, size, age, and NOM fouling. Chemosphere 2021, 283, 131085. [Google Scholar] [CrossRef]
- Aflori, M.; Drobota, M. Combined treatments for the improving of the PET surfaces hydrophilicity. Dig. J. Nanomat. Biostruct. 2015, 10, 587–593. [Google Scholar]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. ChemistryOpen 2021, 10, 1202–1226. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.; Lavender Law, K. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrieres, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Sci. Rep. 2019, 19, 12–21. [Google Scholar]
- Barthelemy, E.; Spyropoulos, D.; Milana, M.R.; Pfaff, K.; Gontard, N.; Lampi, E.; Castle, L. Safety evaluation of mechanical recycling processes used to produce polyethylene terephthalate (PET) intended for food contact applications. Food Addit. Contam. 2014, 31, 490–497. [Google Scholar] [CrossRef]
- Dodi, G.; Popescu, D.; Cojocaru, F.D.; Aradoaei, M.; Ciobanu, R.C.; Mihai, C.T. Use of Fourier-Transform Infrared Spectroscopy for DNA Identification on Recycled PET Composite Substrate. Appl. Sci. 2022, 12, 4371. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Hamid, M.K.A.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Mancini, S.D.; Schwartzman, J.A.S.; Rodriques Nogueira, A.; Kagohara, D.A.; Zanin, M. Additional steps in mechanical recycling of PET. J. Clean. Prod. 2010, 18, 92–100. [Google Scholar] [CrossRef]
- Silano, V.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Mortensen, A.; Riviere, G.; et al. Safety evaluation of the food enzyme pectinesterase from the genetically modified Aspergillus oryzae strain AR-962. EFSA J. 2023, 21, e07832. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Contamination Levels in Recollected PET Bottles from Non-Food Applications and their Impact on the Safety of Recycled PET for Food Contact. Molecules 2020, 25, 4998. [Google Scholar] [CrossRef]
- Welle, F. Decontamination efficiency of a new post-consumer poly (ethylene terephthalate) (PET) recycling concept. Food Addit. Contam. 2008, 25, 123–131. [Google Scholar] [CrossRef]
- Dudler, V.; Milana, M.R.; Papaspyrides, C.; PoCas, M.F.T.; Lioupis, A.; Volk, K.; Lampi, E. Safety assessment of the process RE-PET, based on EREMA Basic technology, used to recycle post-consumer PET into food contact materials. EFSA J. 2020, 18, e06049. [Google Scholar] [CrossRef]
- Hossain, S.; Mozumder, S.I. Post-consumer polyethylene terephthalate (PET) recycling in Bangladesh through optimization of hot washing parameters. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 40, 62–76. [Google Scholar]
- Roungpaisan, N.; Srisawat, N.; Rungruangkitkrai, N.; Chartvivatpornchai, N.; Boonyarit, J.; Kittikorn, T.; Chollakup, R. Effect of Recycling PET Fabric and Bottle Grade on r-PET Fiber Structure. Polymers 2023, 15, 2330. [Google Scholar] [CrossRef] [PubMed]
- Félix, J.S.; Alfaro, P.; Nerín, C. Pros and cons of analytical methods to quantify surrogate contaminants from the challenge test in recycled polyethylene terephthalate. Anal. Chim. Acta 2011, 687, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Mihai, C.-T.; Cojocaru, F.D.; Uritu, C.M.; Dodi, G.; Botezat, D.; Gardikiotis, I. Vibrational Spectroscopy Fingerprinting in Medicine: From Molecular to Clinical Practice. Materials 2019, 12, 2884. [Google Scholar] [CrossRef]
- Mizera, A.; Manas, M.; Stoklasek, P. Effect of Temperature Ageing on Injection Molded High-Density Polyethylene Parts Modified by Accelerated Electrons. Materials 2022, 15, 742. [Google Scholar] [CrossRef]
- Cabanes, A.; Fullana, A. New methods to remove volatile organic compounds from post-comsumer plastic waste. Sci. Total Environ. 2021, 758, 144066. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal Oxide Nanoparticles for Safe Active and Intelligent Food Packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Lee, Y.S. Oxygen scavenging films in food packaging. Environ. Chem. Lett. 2018, 16, 523–538. [Google Scholar] [CrossRef]
- Epure, E.L.; Cojocaru, F.D.; Aradoaei, M.; Ciobanu, R.C.; Dodi, G. Exploring the Surface Potential of Recycled Polyethylene Terephthalate Composite Supports on the Collagen Contamination Level. Polymers 2023, 15, 776. [Google Scholar] [CrossRef] [PubMed]
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene Characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Da Silva, D.J.; Wiebeck, H. CARS-PLS regression and ATR-FTIR spectroscopy for eco-friendly and fast composition analyses of LDPE/HDPE blends. J. Polym. Res. 2018, 25, 112. [Google Scholar] [CrossRef]
- Castell, P.; Wouters, M.; de With, G.; Fischer, H.; Huijs, F. Surface modification of poly(propylene) by photoinitiators: Improvement of adhesion and wettability. J. Appl. Polym. Sci. 2004, 92, 2341–2350. [Google Scholar] [CrossRef]
- Available online: https://www.en-standard.eu/une-en-iso-175-2011-plastics-methods-of-test-for-the-determination-of-the-effects-of-immersion-in-liquid-chemicals-iso-175-2010/ (accessed on 25 June 2023).






| Encode | The Temperatures in the Heating Zones (°C) | ||||
|---|---|---|---|---|---|
| M1 | 300 | 295 | 290 | 285 | 280 |
| M2 | 260 | 255 | 250 | 245 | 240 |
| M3 | 260 | 255 | 250 | 245 | 240 |
| M4 | 270 | 265 | 260 | 255 | 250 |
| M5 | 270 | 265 | 260 | 255 | 250 |
| M6 | 260 | 255 | 250 | 245 | 240 |
| M7 | 260 | 255 | 250 | 245 | 240 |
| M8 | 260 | 255 | 250 | 245 | 240 |
| M9 | 260 | 255 | 250 | 245 | 240 |
| M10 | 260 | 255 | 250 | 245 | 240 |
| M11 | 260 | 255 | 250 | 245 | 240 |
| M12 | 250 | 245 | 240 | 235 | 230 |
| M13 | 250 | 245 | 240 | 235 | 230 |
| M14 | 250 | 245 | 240 | 235 | 230 |
| M15 | 250 | 245 | 240 | 235 | 230 |
| Sample Code | Process I | Process II | Complex Melting Process | |||||
|---|---|---|---|---|---|---|---|---|
| Vitreous Transition | Crystallization | Melting I | Melting II | |||||
| Tonset, °C | ΔCp, J/g·K | Tonset, °C | Tmax, °C | Tonset, °C | Tmin, °C | Tonset, °C | Tmin, °C | |
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| M1 | 64.9 | 0.032 | 117.7 | 123.3 | 239.1 | 249 | - | - |
| M2 | 64.7 | 0.076 | 117.1 | 124.5 | 237.8 | 249.9 | - | - |
| M3 | 63.7 | 0.001 | 114.8 | 123.3 | 238.9 | 249.5 | - | - |
| M4 | 64.2 | 0.051 | 108.3 | 117.3 | 236.1 | 248.9 | - | - |
| M5 | 65.5 | 0.028 | 115.9 | 123.5 | 236.0 | 250.1 | - | - |
| M6 | 72.4 | 0.044 | 116.6 | 124.1 | 153.3 | 162.9 | 237.9 | 247.4 |
| M7 | 64.7 | 0.046 | 107.6 | 116.3 | 153.3 | 163.8 | 237.4 | 254.1 |
| M8 | 74.5 | 0.010 | 114.9 | 126.1 | 153.7 | 163.6 | 238.5 | 252.0 |
| M9 | 69.6 | 0.009 | 114.7 | 124.2 | 153.7 | 162.9 | 239.7 | 248.8 |
| M10 | 73.7 | 0.001 | 111.9 | 123.4 | 151.4 | 163.2 | 236.8 | 247.1 |
| M11 | 73.7 | 0.006 | - | - | 123.6 | 130.8 | 238.0 | 247.4 |
| M12 | 74.0 | 0.045 | - | - | 123.6 | 130.4 | 238.6 | 247.0 |
| M13 | 72.6 | 0.068 | - | - | 123.1 | 130.3 | 242.8 | 248.4 |
| M14 | 72.2 | 0.006 | - | - | 122.7 | 129.3 | 240.1 | 246.9 |
| M15 | 68.6 | 0.003 | - | - | 123.3 | 131.7 | 239.4 | 249.0 |
| ME | Recycled PET (g) | Al Powder 800 nm (g) | Fe Powder 800 nm (g) | PP (g) | HDPE (g) | Total |
|---|---|---|---|---|---|---|
| M1 | 200 | 0 | 0 | 0 | 0 | 200 |
| M2 | 190 | 10 | 0 | 0 | 0 | 200 |
| M3 | 184 | 16 | 0 | 0 | 0 | 200 |
| M4 | 190 | 0 | 10 | 0 | 0 | 200 |
| M5 | 184 | 0 | 16 | 0 | 0 | 200 |
| M6 | 140 | 0 | 0 | 60 | 0 | 200 |
| M7 | 133 | 10 | 0 | 57 | 0 | 200 |
| M8 | 129 | 16 | 0 | 55 | 0 | 200 |
| M9 | 133 | 0 | 10 | 57 | 0 | 200 |
| M10 | 129 | 0 | 16 | 55 | 0 | 200 |
| M11 | 140 | 0 | 0 | 0 | 60 | 200 |
| M12 | 133 | 10 | 0 | 0 | 57 | 200 |
| M13 | 129 | 16 | 0 | 0 | 55 | 200 |
| M14 | 133 | 0 | 10 | 0 | 57 | 200 |
| M15 | 129 | 0 | 16 | 0 | 55 | 200 |
| TOTAL RAW MATERIALS | 2275.2 | 78 | 78 | 284.4 | 284.4 | 3000 |
| Code | Average Mass (Mass) | Hydrostatic Density [g/cm3] |
|---|---|---|
| M1 | 1.755 | 1.318 ± 0.0004 |
| M2 | 1.809 | 1.347 ± 0.0009 |
| M3 | 1.923 | 1.382 ± 0.0011 |
| M4 | 1.773 | 1.317 ± 0.0018 |
| M5 | 1.845 | 1.381 ± 0.0004 |
| M6 | 1.545 | 1.186 ± 0.0016 |
| M7 | 1.546 | 1.395 ± 0.2833 |
| M8 | 1.579 | 1.207 ± 0.0013 |
| M9 | 1.701 | 1.306 ± 0.0000 |
| M10 | 1.507 | 1.827 ± 0.6088 |
| M11 | 1.517 | 1.180 ± 0.0004 |
| M12 | 1.558 | 1.210 ± 0.0000 |
| M13 | 1.577 | 1.219 ± 0.0004 |
| M14 | 1.583 | 1.228 ± 0.0004 |
| M15 | 1.840 | 1.318 ± 0.0004 |
| Code | m0 | m1 | Q 168 h | m2 | Q 408 h | m3 | Q 504 h | m4 | Q 600 h | m5 | Q 1200 h |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 0.0926 | 0.0928 | 0.22 | 0.093 | 0.32 | 0.0930 | 0.43 | 0.0931 | 0.58 | 0.0932 | 0.65 |
| M2 | 0.0927 | 0.0930 | 0.32 | 0.0932 | 0.54 | 0.0934 | 0.76 | 0.0933 | 0.65 | 0.0933 | 0.68 |
| M3 | 0.0928 | 0.0932 | 0.38 | 0.0933 | 0.57 | 0.0933 | 0.54 | 0.0935 | 0.75 | 0.0935 | 0.78 |
| M4 | 0.0826 | 0.0828 | 0.24 | 0.0830 | 0.48 | 0.0831 | 0.61 | 0.0832 | 0.73 | 0.0832 | 0.73 |
| M5 | 0.0970 | 0.0973 | 0.31 | 0.0975 | 0.52 | 0.0977 | 0.72 | 0.0977 | 0.72 | 0.0977 | 0.72 |
| M6 | 0.0847 | 0.0849 | 0.24 | 0.0852 | 0.59 | 0.0856 | 1.06 | 0.0859 | 1.42 | 0.0860 | 1.53 |
| M7 | 0.0772 | 0.0775 | 0.39 | 0.0780 | 1.04 | 0.0785 | 1.68 | 0.0788 | 2.07 | 0.0788 | 2.07 |
| M8 | 0.0740 | 0.0746 | 0.74 | 0.0754 | 1.89 | 0.0755 | 2.03 | 0.0756 | 2.16 | 0.0757 | 2.30 |
| M9 | 0.0774 | 0.0778 | 0.52 | 0.0785 | 1.42 | 0.0789 | 1.94 | 0.0790 | 2.10 | 0.0791 | 2.22 |
| M10 | 0.0872 | 0.0878 | 0.69 | 0.0878 | 0.69 | 0.0882 | 1.15 | 0.0886 | 1.61 | 0.0887 | 1.72 |
| M11 | 0.0796 | 0.0798 | 0.25 | 0.0803 | 0.88 | 0.0804 | 1.01 | 0.0804 | 1.01 | 0.0804 | 1.01 |
| M12 | 0.0737 | 0.0740 | 0.41 | 0.0752 | 2.04 | 0.0759 | 2.99 | 0.0764 | 3.66 | 0.0765 | 3.80 |
| M13 | 0.0806 | 0.0810 | 0.50 | 0.0812 | 0.74 | 0.0814 | 0.99 | 0.0815 | 1.12 | 0.0816 | 1.24 |
| M14 | 0.0803 | 0.0832 | 3.61 | 0.0839 | 4.48 | 0.0840 | 4.61 | 0.0852 | 6.10 | 0.0852 | 6.10 |
| M15 | 0.1038 | 0.1051 | 1.25 | 0.1051 | 1.25 | 0.1051 | 1.25 | 0.1051 | 1.25 | 0.1051 | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, R.C.; Aradoaei, M.; Caramitu, A.R.; Ion, I.; Schreiner, C.M.; Tsakiris, V.; Marinescu, V.; Hitruc, E.G.; Aflori, M. Special Packaging Materials from Recycled PET and Metallic Nano-Powders. Polymers 2023, 15, 3161. https://doi.org/10.3390/polym15153161
Ciobanu RC, Aradoaei M, Caramitu AR, Ion I, Schreiner CM, Tsakiris V, Marinescu V, Hitruc EG, Aflori M. Special Packaging Materials from Recycled PET and Metallic Nano-Powders. Polymers. 2023; 15(15):3161. https://doi.org/10.3390/polym15153161
Chicago/Turabian StyleCiobanu, Romeo C., Mihaela Aradoaei, Alina R. Caramitu, Ioana Ion, Cristina M. Schreiner, Violeta Tsakiris, Virgil Marinescu, Elena Gabriela Hitruc, and Magdalena Aflori. 2023. "Special Packaging Materials from Recycled PET and Metallic Nano-Powders" Polymers 15, no. 15: 3161. https://doi.org/10.3390/polym15153161
APA StyleCiobanu, R. C., Aradoaei, M., Caramitu, A. R., Ion, I., Schreiner, C. M., Tsakiris, V., Marinescu, V., Hitruc, E. G., & Aflori, M. (2023). Special Packaging Materials from Recycled PET and Metallic Nano-Powders. Polymers, 15(15), 3161. https://doi.org/10.3390/polym15153161







