Computational Insights on the Chemical Reactivity of Functionalized and Crosslinked Polyketones to Cu2+ Ion for Wastewater Treatment
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussions
3.1. Properties of XLPK Systems
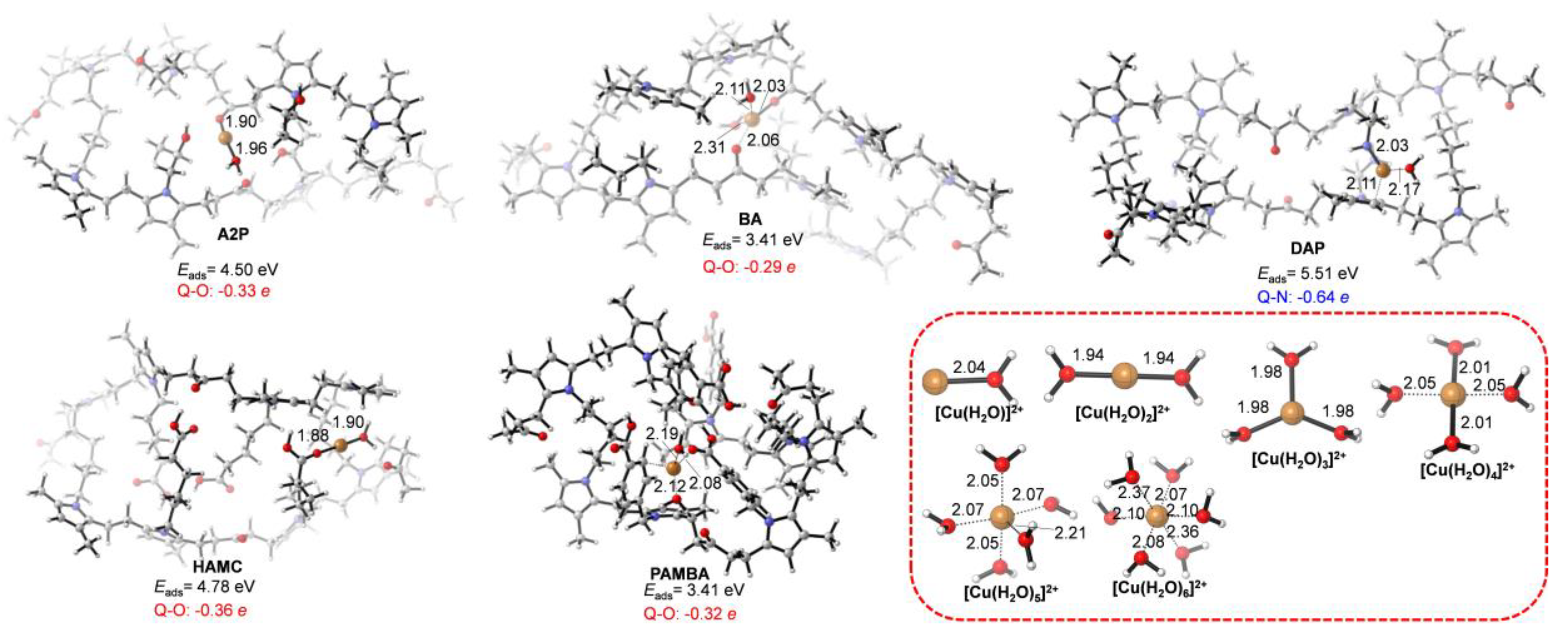
3.2. Adsorption Energies
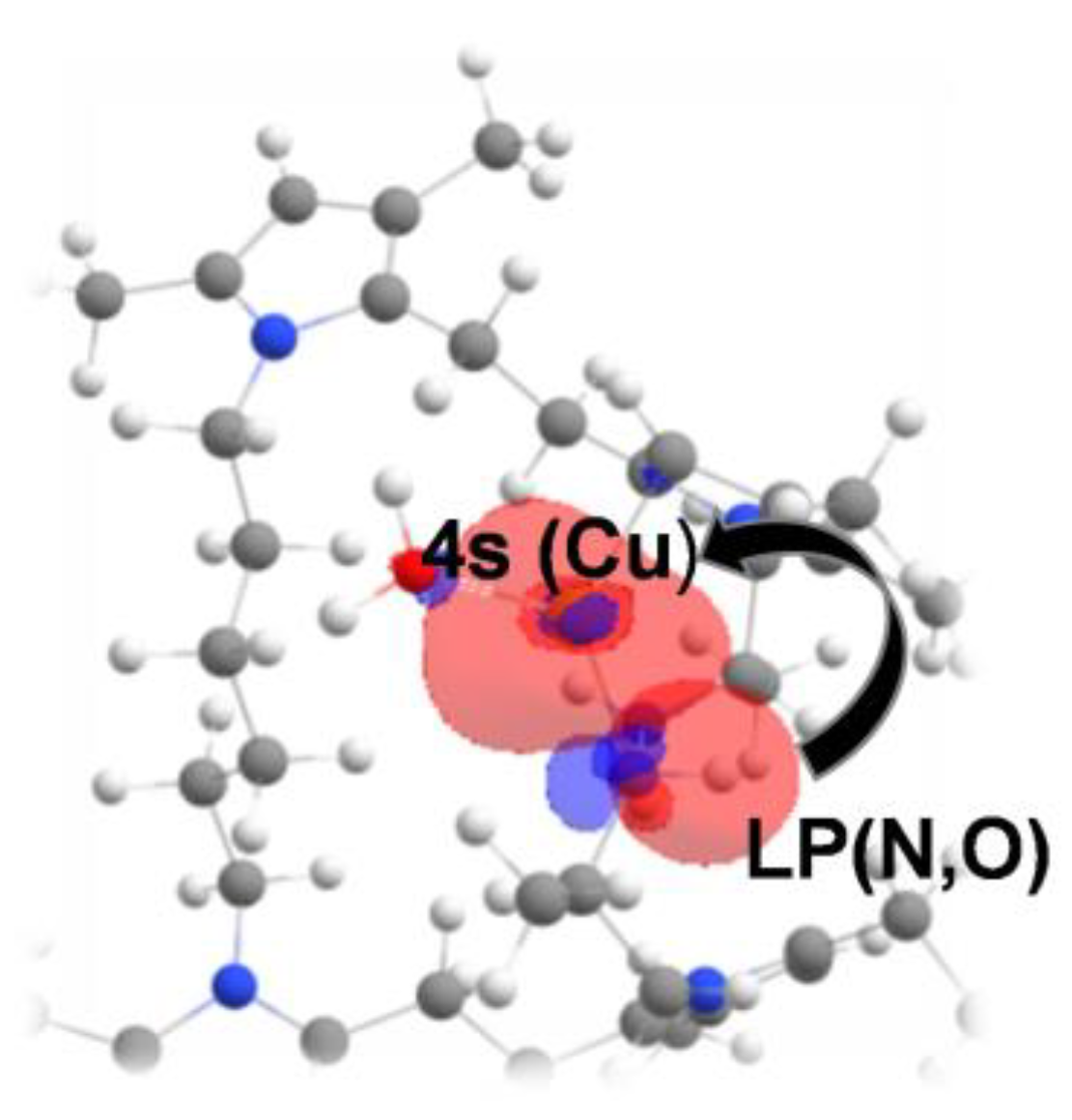
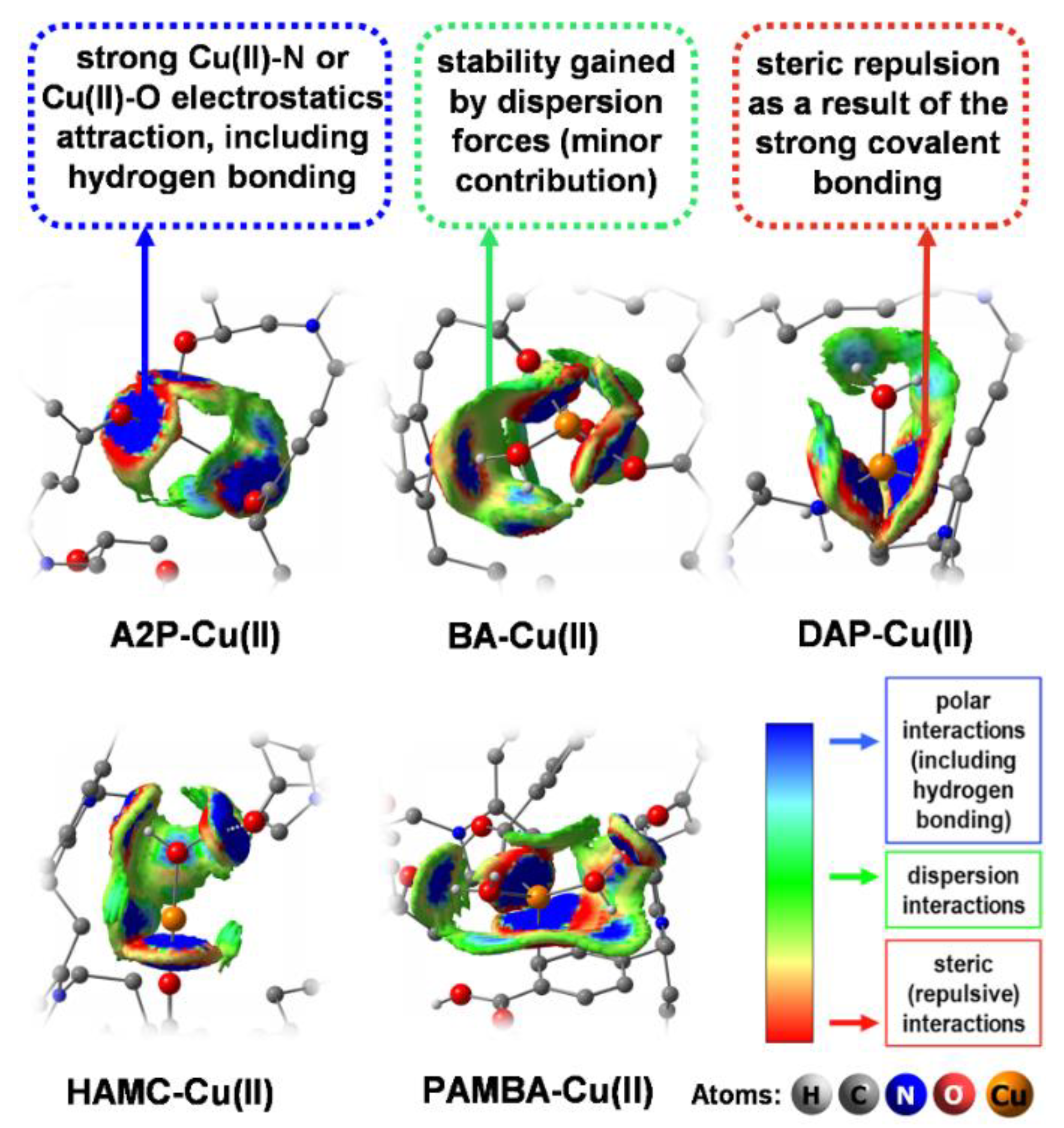
3.3. Adsorption Mechanism
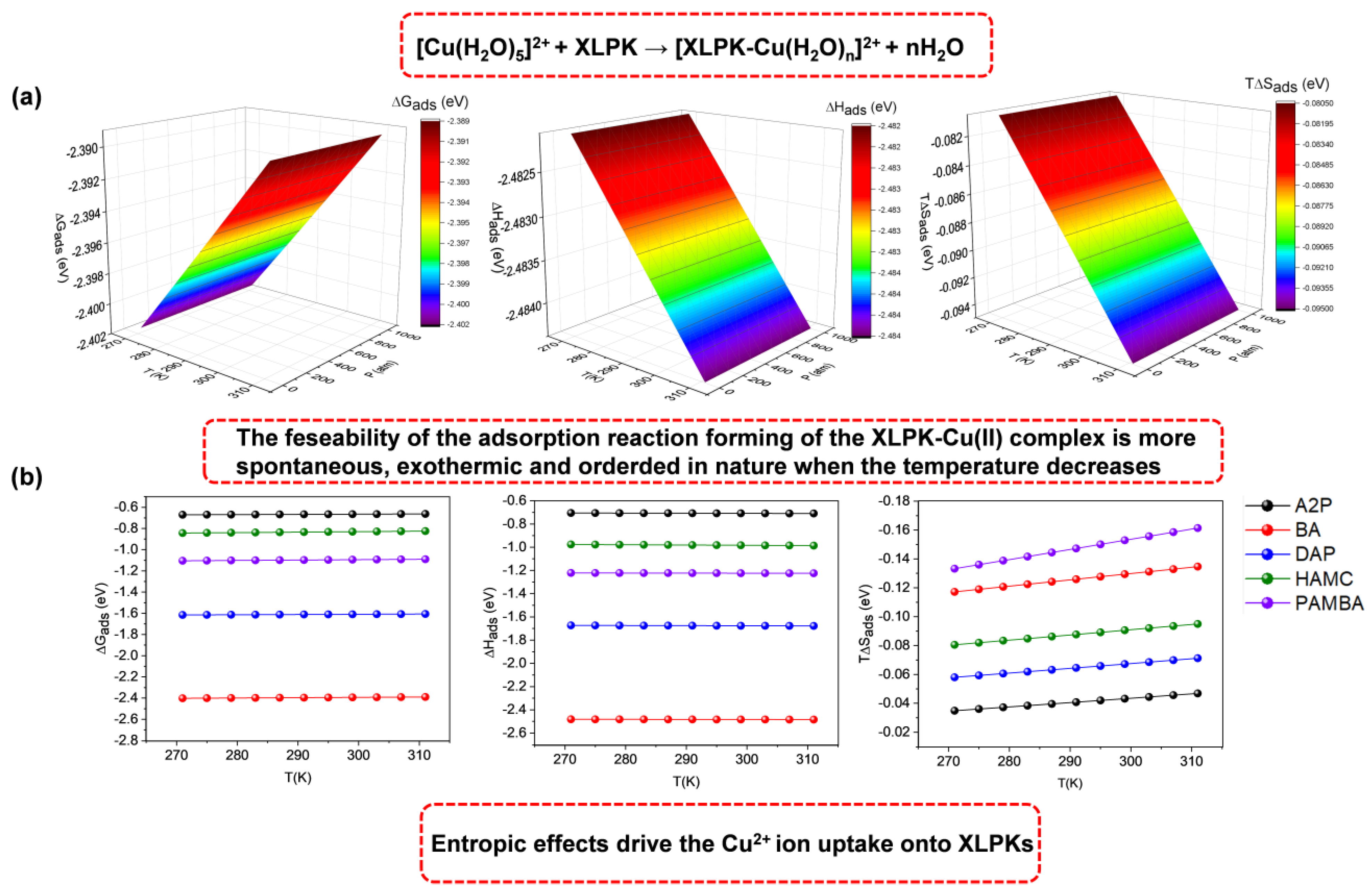
3.4. Thermochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Paswan, S.K.; Kumar, P.; Singh, R.K.; Kumar, L. Chapter 14—Heavy metal water pollution: An overview about remediation, removal and recovery of metals from contaminated water. In Metals in Water; Shukla, S.K., Kumar, S., Madhav, S., Mishra, P.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 263–284. [Google Scholar]
- Joseph, L.; Jun, B.-M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of Copper Ions from Wastewater: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Shao, H.; Yin, D.; Li, D.; Ma, Q.; Yu, W.; Dong, X. Simultaneous Visual Detection and Removal of Cu2+ with Electrospun Self-Supporting Flexible Amidated Polyacrylonitrile/Branched Polyethyleneimine Nanofiber Membranes. ACS Appl. Mater. Interfaces 2021, 13, 49288–49300. [Google Scholar] [CrossRef]
- Lv, X.; Hu, H.; Yao, L.; Deng, L.; Liu, X.; Yu, L.; He, H. Fabrication of surface ion imprinting rice husk-based polymer for selective detection and efficient adsorption of Cu2+ in lake water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 298, 122723. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpää, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Qi, J.; He, X.; Lu, Q. Novel chelating polyacrylonitrile membrane for efficient capture of Cu2+, Pb2+ and Fe3+. Chem. Eng. J. 2022, 450, 138203. [Google Scholar] [CrossRef]
- Vlachou, I.; Bokias, G. Investigation of Cross-Linked Chitosan-Based Membranes as Potential Adsorbents for the Removal of Cu2+ Ions from Aqueous Solutions. Materials 2023, 16, 1926. [Google Scholar] [CrossRef]
- Yang, L.; Hu, W.; Chang, Z.; Liu, T.; Fang, D.; Shao, P.; Shi, H.; Luo, X. Electrochemical recovery and high value-added reutilization of heavy metal ions from wastewater: Recent advances and future trends. Environ. Int. 2021, 152, 106512. [Google Scholar] [CrossRef]
- Zhong, W.; Zou, J.; Yu, Q.; Gao, Y.; Qu, F.; Liu, S.; Zhou, H.; Lu, L. Ultrasensitive indirect electrochemical sensing of thiabendazole in fruit and water by the anodic stripping voltammetry of Cu2+ with hierarchical Ti3C2Tx-TiO2 for signal amplification. Food Chem. 2023, 402, 134379. [Google Scholar] [CrossRef] [PubMed]
- Sagadevan, S.; Fatimah, I.; Egbosiuba, T.C.; Alshahateet, S.F.; Lett, J.A.; Weldegebrieal, G.K.; Le, M.-V.; Johan, M.R. Photocatalytic Efficiency of Titanium Dioxide for Dyes and Heavy Metals Removal from Wastewater. Bull. Chem. React. Eng. Catal. 2022, 21, 430–450. [Google Scholar] [CrossRef]
- Xu, G.-R.; An, Z.-H.; Xu, K.; Liu, Q.; Das, R.; Zhao, H.-L. Metal organic framework (MOF)-based micro/nanoscaled materials for heavy metal ions removal: The cutting-edge study on designs, synthesis, and applications. Coord. Chem. Rev. 2021, 427, 213554. [Google Scholar] [CrossRef]
- Diamantis, S.A.; Pournara, A.D.; Koutsouroubi, E.D.; Moularas, C.; Deligiannakis, Y.; Armatas, G.S.; Hatzidimitriou, A.G.; Manos, M.J.; Lazarides, T. Detection and Sorption of Heavy Metal Ions in Aqueous Media by a Fluorescent Zr(IV) Metal–Organic Framework Functionalized with 2-Picolylamine Receptor Groups. Inorg. Chem. 2022, 61, 7847–7858. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Mishra, A.; Pande, P.P. A review natural polymeric coagulants in wastewater treatment. Mater. Today Proc. 2021, 46, 6113–6117. [Google Scholar] [CrossRef]
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Wu, S.; Xiong, W.; Li, H. Insights into the Fe oxidation state of sphere-like Fe2O3 nanoparticles for simultaneous Pb2+ and Cu2+ detection. J. Alloys Compd. 2023, 934, 167863. [Google Scholar] [CrossRef]
- Si, R.; Chen, Y.; Wang, D.; Yu, D.; Ding, Q.; Li, R.; Wu, C. Nanoarchitectonics for High Adsorption Capacity Carboxymethyl Cellulose Nanofibrils-Based Adsorbents for Efficient Cu2+ Removal. Nanomaterials 2022, 12, 160. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, N.; Ullah, N.; Falath, W. Removal of hazardous dyes, toxic metal ions and organic pollutants from wastewater by using porous hyper-cross-linked polymeric materials: A review of recent advances. J. Environ. Manag. 2021, 287, 112360. [Google Scholar] [CrossRef]
- Masoumi, H.; Ghaemi, A.; Gilani, H.G. Evaluation of hyper-cross-linked polymers performances in the removal of hazardous heavy metal ions: A review. Sep. Purif. Technol. 2021, 260, 118221. [Google Scholar] [CrossRef]
- Deng, S.; Djukic, L.; Paton, R.; Ye, L. Thermoplastic–epoxy interactions and their potential applications in joining composite structures—A review. Compos. Part A Appl. Sci. Manuf. 2015, 68, 121–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; He, Y.; Nawaz, F.; Liu, S.; Zhang, H.; Xiao, F.-S. Solvothermal synthesis of carboxyl and amido functionalized mesoporous resins for water treatments. J. Mater. Chem. 2010, 20, 4609–4614. [Google Scholar] [CrossRef]
- Chwastowska, J.; Rogowska, A.; Sterlińska, E.; Dudek, J. Chelating 2-mercaptobenzothiazole loaded resin. Application to the separation of inorganic and alkylmercury species for their atomic absorption spectrometry determination in natural waters. Talanta 1999, 49, 837–842. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Heavy metals adsorption on some iminodiacetate chelating resins as a function of the adsorption parameters. React. Funct. Polym. 2008, 68, 1346–1354. [Google Scholar] [CrossRef]
- Ji, C.; Song, S.; Wang, C.; Sun, C.; Qu, R.; Wang, C.; Chen, H. Preparation and adsorption properties of chelating resins containing 3-aminopyridine and hydrophilic spacer arm for Hg(II). Chem. Eng. J. 2010, 165, 573–580. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Mudhoo, A.; Sillanpää, M. Aminopolycarboxylic acid functionalized adsorbents for heavy metals removal from water. Water Res. 2013, 47, 4812–4832. [Google Scholar] [CrossRef]
- Toncelli, C.; Haijer, A.; Alberts, F.; Broekhuis, A.A.; Picchioni, F. The Green Route from Carbon Monoxide Fixation to Functional Polyamines: A Class of High-Performing Metal Ion Scavengers. Ind. Eng. Chem. Res. 2015, 54, 9450–9457. [Google Scholar] [CrossRef]
- Figaroa, P.A.; Miedema, H.; Euverink, G.-J.; Picchioni, F. Functional polyketones for the removal of calcium and magnesium from water (part I): Synthesis and chemical characterization. Pure Appl. Chem. 2017, 89, 41–50. [Google Scholar] [CrossRef]
- Figaroa, P.A.; Miedema, H.; Euverink, G.-J.; Picchioni, F. Functional polyketones for the removal of calcium and magnesium from water (Part II): Cross-linking and functional characterization. Pure Appl. Chem. 2017, 89, 51–60. [Google Scholar] [CrossRef]
- Zong, Y.; Li, Q.; Mu, H.; Jian, Z. Palladium Promoted Copolymerization of Carbon Monoxide with Polar or Non-polar Olefinic Monomers. Curr. Org. Chem. 2021, 25, 287–300. [Google Scholar] [CrossRef]
- Guan, K.; Zhang, L.; Wang, S.; Takagi, R.; Matsuyama, H. Controlling the formation of porous polyketone membranes via a cross-linkable alginate additive for oil-in-water emulsion separations. J. Membr. Sci. 2020, 611, 118362. [Google Scholar] [CrossRef]
- Toncelli, C.; De Reus, D.C.; Picchioni, F.; Broekhuis, A.A. Properties of Reversible Diels–Alder Furan/Maleimide Polymer Networks as Function of Crosslink Density. Macromol. Chem. Phys. 2012, 213, 157–165. [Google Scholar] [CrossRef]
- Reis, D.T.; Ribeiro, I.H.S.; Pereira, D.H. DFT study of the application of polymers cellulose and cellulose acetate for adsorption of metal ions (Cd2+, Cu2+ and Cr3+) potentially toxic. Polym. Bull. 2020, 77, 3443–3456. [Google Scholar] [CrossRef]
- Ribeiro, I.H.S.; Reis, D.T.; Pereira, D.H. A DFT-based analysis of adsorption of Cd2+, Cr3+, Cu2+, Hg2+, Pb2+, and Zn2+, on vanillin monomer: A study of the removal of metal ions from effluents. J. Mol. Model. 2019, 25, 267. [Google Scholar] [CrossRef]
- Bashir, A.; Manzoor, T.; Malik, L.A.; Qureashi, A.; Pandith, A.H. Enhanced and Selective Adsorption of Zn(II), Pb(II), Cd(II), and Hg(II) Ions by a Dumbbell- and Flower-Shaped Potato Starch Phosphate Polymer: A Combined Experimental and DFT Calculation Study. ACS Omega 2020, 5, 4853–4867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, S.; Rong, C.; Zhong, A.; Liu, S. Toward Understanding the Isomeric Stability of Fullerenes with Density Functional Theory and the Information-Theoretic Approach. ACS Omega 2018, 3, 17986–17990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Rong, C.; Zhong, A.; Lu, T.; Liu, S. Molecular acidity: An accurate description with information-theoretic approach in density functional reactivity theory. J. Comput. Chem. 2018, 39, 117–129. [Google Scholar] [CrossRef]
- Wu, J.; Yu, D.; Liu, S.; Rong, C.; Zhong, A.; Chattaraj, P.K.; Liu, S. Is It Possible To Determine Oxidation States for Atoms in Molecules Using Density-Based Quantities? An Information-Theoretic Approach and Conceptual Density Functional Theory Study. J. Phys. Chem. A 2019, 123, 6751–6760. [Google Scholar] [CrossRef]
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the origin and nature of internal methyl rotation barriers: An information-theoretic approach study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
- Casewit, C.J.; Colwell, K.S.; Rappe, A.K. Application of a universal force field to organic molecules. J. Am. Chem. Soc. 1992, 114, 10035–10046. [Google Scholar] [CrossRef]
- BIOVIA Materials Studio 2022, version 22.1.0.3462; Dassault Systèmes Forcite: San Diego, CA, USA, 2022.
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA Materials Studio 2022, version 22.1.0.3462; Dassault Systèmes Adsorption Locator: San Diego, CA, USA, 2022.
- Horn, P.R.; Mao, Y.; Head-Gordon, M. Probing non-covalent interactions with a second generation energy decomposition analysis using absolutely localized molecular orbitals. Phys. Chem. Chem. Phys. 2016, 18, 23067–23079. [Google Scholar] [CrossRef] [PubMed]
- Epifanovsky, E.; Gilbert, A.T.B.; Feng, X.; Lee, J.; Mao, Y.; Mardirossian, N.; Pokhilko, P.; White, A.F.; Coons, M.P.; Dempwolff, A.L.; et al. Software for the frontiers of quantum chemistry: An overview of developments in the Q-Chem 5 package. J. Chem. Phys. 2021, 155, 084801. [Google Scholar] [CrossRef]
- The Nature Education: Key Physical Variables in the Ocean: Temperature, Salinity, and Density. Available online: https://www.nature.com/scitable/knowledge/library/key-physical-variables-in-the-ocean-temperature-102805293/ (accessed on 6 July 2013).
- Pearson, R.G. The electronic chemical potential and chemical hardness. J. Mol. Struct. THEOCHEM 1992, 255, 261–270. [Google Scholar] [CrossRef]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Mulliken, R.S. A New Electroaffinity Scale; Together with Data on Valence States and on Valence Ionization Potentials and Electron Affinities. J. Chem. Phys. 2004, 2, 782–793. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.v.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Cárdenas-Jirón, G.I.; Zagal, J.H. Donor–acceptor intermolecular hardness on charge transfer reactions of substituted cobalt phthalocyanines. J. Electroanal. Chem. 2001, 497, 55–60. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bryantsev, V.S.; Diallo, M.S.; van Duin, A.C.T.; Goddard, W.A., III. Hydration of Copper(II): New Insights from Density Functional Theory and the COSMO Solvation Model. J. Phys. Chem. A 2008, 112, 9104–9112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquarello, A.; Petri, I.; Salmon, P.S.; Parisel, O.; Car, R.; Tóth, É.; Powell, D.H.; Fischer, H.E.; Helm, L.; Merbach, A.E. First Solvation Shell of the Cu(II) Aqua Ion: Evidence for Fivefold Coordination. Science 2001, 291, 856–859. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, B.; Niu, Y.; Chen, H.; Liu, Y.; Wang, A.; Bai, L. Synthesis of silica supported thiosemicarbazide for Cu(II) and Zn(II) adsorption from ethanol: A comparison with aqueous solution. Fuel 2021, 286, 119287. [Google Scholar] [CrossRef]
- Pawlaczyk, M.; Schroeder, G. Adsorption studies of Cu(II) ions on dendrimer-grafted silica-based materials. J. Mol. Liq. 2019, 281, 176–185. [Google Scholar] [CrossRef]
- Sayed, M.M.; Abd El-Hamid, I.S.; M. El-Bery, H.; Farrag, M.; Abdelhakiem, A.K.; Aly, K.I. Synthesis, characterization and application of high adsorption performance of novel 1,4-polyketone. Sci. Rep. 2022, 12, 16317. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Cho, Y.S.; Kim, T.; Jung, Y.S.; Hwang, T.S. Synthesis of Highly Durable Sulfonated Polyketone Fibers by Direct Sulfonation Reaction and Their Adsorption Properties for Heavy Metals. Macromol. Res. 2020, 28, 336–342. [Google Scholar] [CrossRef]
- Levine, D.S.; Head-Gordon, M. Energy decomposition analysis of single bonds within Kohn–Sham density functional theory. Proc. Natl. Acad. Sci. USA 2017, 114, 12649–12656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razak, M.R.; Yusof, N.A.; Aris, A.Z.; Nasir, H.M.; Haron, M.J.; Ibrahim, N.A.; Johari, I.S.; Kamaruzaman, S. Phosphoric acid modified kenaf fiber (K-PA) as green adsorbent for the removal of copper (II) ions towards industrial waste water effluents. React. Funct. Polym. 2020, 147, 104466. [Google Scholar] [CrossRef]
- van Duijnen, P.T.; Swart, M. Molecular and Atomic Polarizabilities: Thole’s Model Revisited. J. Phys. Chem. A 1998, 102, 2399–2407. [Google Scholar] [CrossRef]
- Duan, G.; Li, X.; Ma, X.; Zhong, W.; Wang, S. High-efficiency adsorption removal for Cu(II) and Ni(II) using a novel acylamino dihydroxamic acid chelating resin. Sci. Total Environ. 2023, 864, 160984. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chai, Y.; Zeng, L.; Gao, Z.; Zhang, J.; Ji, H. Efficient Removal of Copper Ion from Wastewater Using a Stable Chitosan Gel Material. Molecules 2019, 24, 4205. [Google Scholar] [CrossRef] [Green Version]
- Cegłowski, M.; Schroeder, G. Preparation of porous resin with Schiff base chelating groups for removal of heavy metal ions from aqueous solutions. Chem. Eng. J. 2015, 263, 402–411. [Google Scholar] [CrossRef]



| System | μ (eV)/|Δμ| a (eV) | η(eV)/ηDA b (eV) | Ω (eV) | QCu2+|e| |
|---|---|---|---|---|
| A2P | −3.16/3.90 a | 1.18/0.08 b | 4.23 | 0.48 c |
| BA | −2.96/2.81 a | 1.32/0.84 b | 3.33 | 0.51 c |
| DAP | −3.04/4.01 a | 1.29/0.08 b | 3.59 | 0.46 c |
| HAMC | −3.12/3.94 a | 1.19/0.07 b | 4.08 | 0.50 c |
| PAMBA | −3.33/2.44 a | 1.02/0.88 b | 5.43 | 0.52 c |
| [Cu(H2O)]2+ | −7.05 | 2.88 | 5.23 | 1.36 |
| [Cu(H2O)2]2+ | −5.77 | 3.18 | 8.63 | 1.08 |
| [Cu(H2O)5]2+ | −4.20 | 3.09 | 2.85 | 0.88 |
| [Cu(H2O)6]2+ | −3.72 | 2.57 | 2.68 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, D.E.; Cortés-Arriagada, D.; Araya-Hermosilla, R. Computational Insights on the Chemical Reactivity of Functionalized and Crosslinked Polyketones to Cu2+ Ion for Wastewater Treatment. Polymers 2023, 15, 3157. https://doi.org/10.3390/polym15153157
Ortega DE, Cortés-Arriagada D, Araya-Hermosilla R. Computational Insights on the Chemical Reactivity of Functionalized and Crosslinked Polyketones to Cu2+ Ion for Wastewater Treatment. Polymers. 2023; 15(15):3157. https://doi.org/10.3390/polym15153157
Chicago/Turabian StyleOrtega, Daniela E., Diego Cortés-Arriagada, and Rodrigo Araya-Hermosilla. 2023. "Computational Insights on the Chemical Reactivity of Functionalized and Crosslinked Polyketones to Cu2+ Ion for Wastewater Treatment" Polymers 15, no. 15: 3157. https://doi.org/10.3390/polym15153157
APA StyleOrtega, D. E., Cortés-Arriagada, D., & Araya-Hermosilla, R. (2023). Computational Insights on the Chemical Reactivity of Functionalized and Crosslinked Polyketones to Cu2+ Ion for Wastewater Treatment. Polymers, 15(15), 3157. https://doi.org/10.3390/polym15153157









