Modeling of Poly(Ethylene Terephthalate) Homogeneous Glycolysis Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Glycolytic Agents
2.3. Homogeneous Glycolysis
2.3.1. Homogeneous Glycolysis under the Action of Ethylene Glycol in Solution
2.3.2. Homogeneous Melt Glycolysis under the Action of BHET
2.3.3. Homogeneous Melt Glycolysis under the Action of OET
2.3.4. Step-By-Step Homogeneous Melt Glycolysis under the Action of OET, BHET, and Ethylene Glycol
2.4. Characterization of PET, Glycolytic Agents, and Glycolysis Products
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Gel Permeation Chromatography (GPC)
2.4.3. Determination of Bis(2-Hydroxyethyl) Terephthalate Content
3. Results and Discussion
3.1. PET Flakes and Glycolytic Agents Characterization
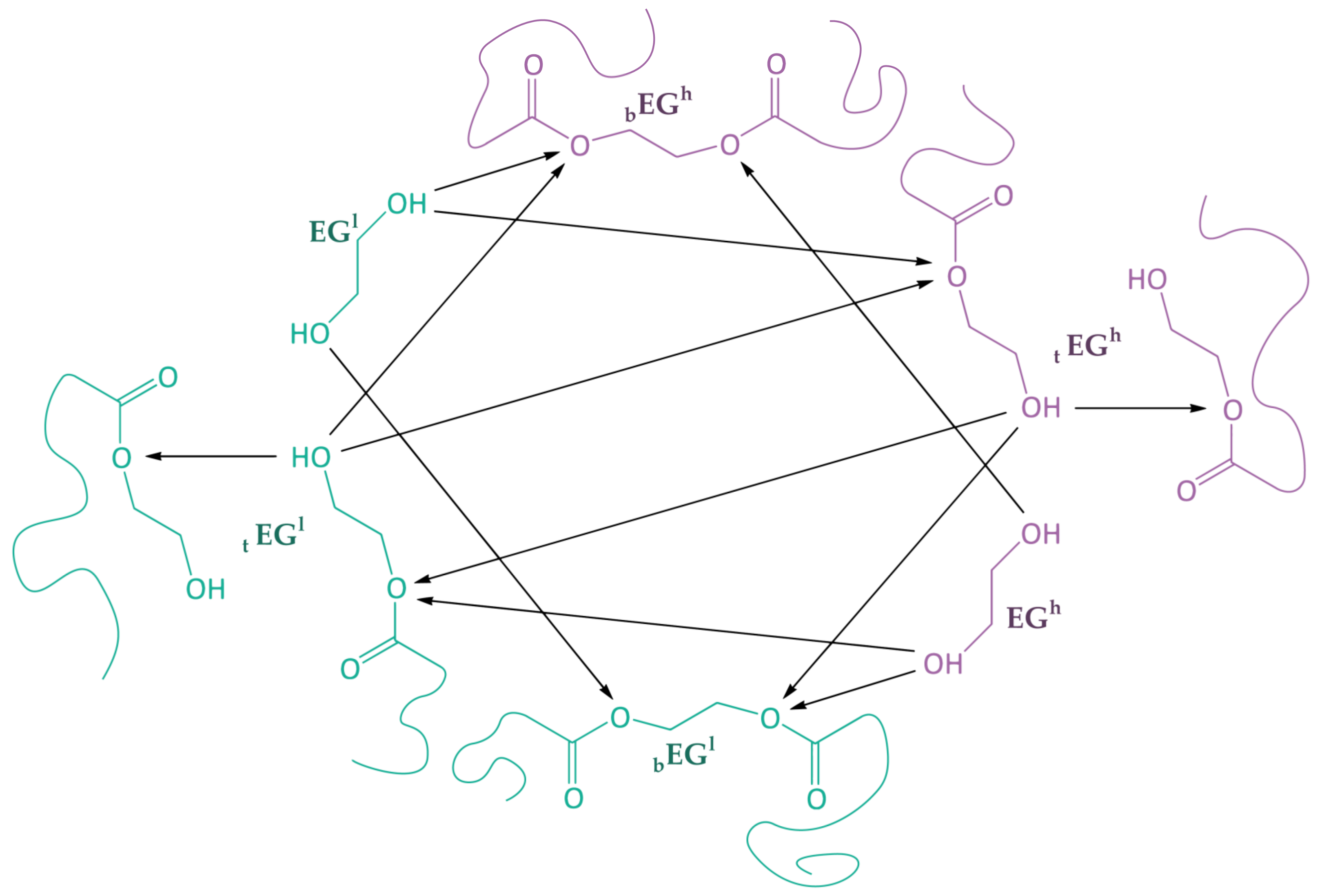
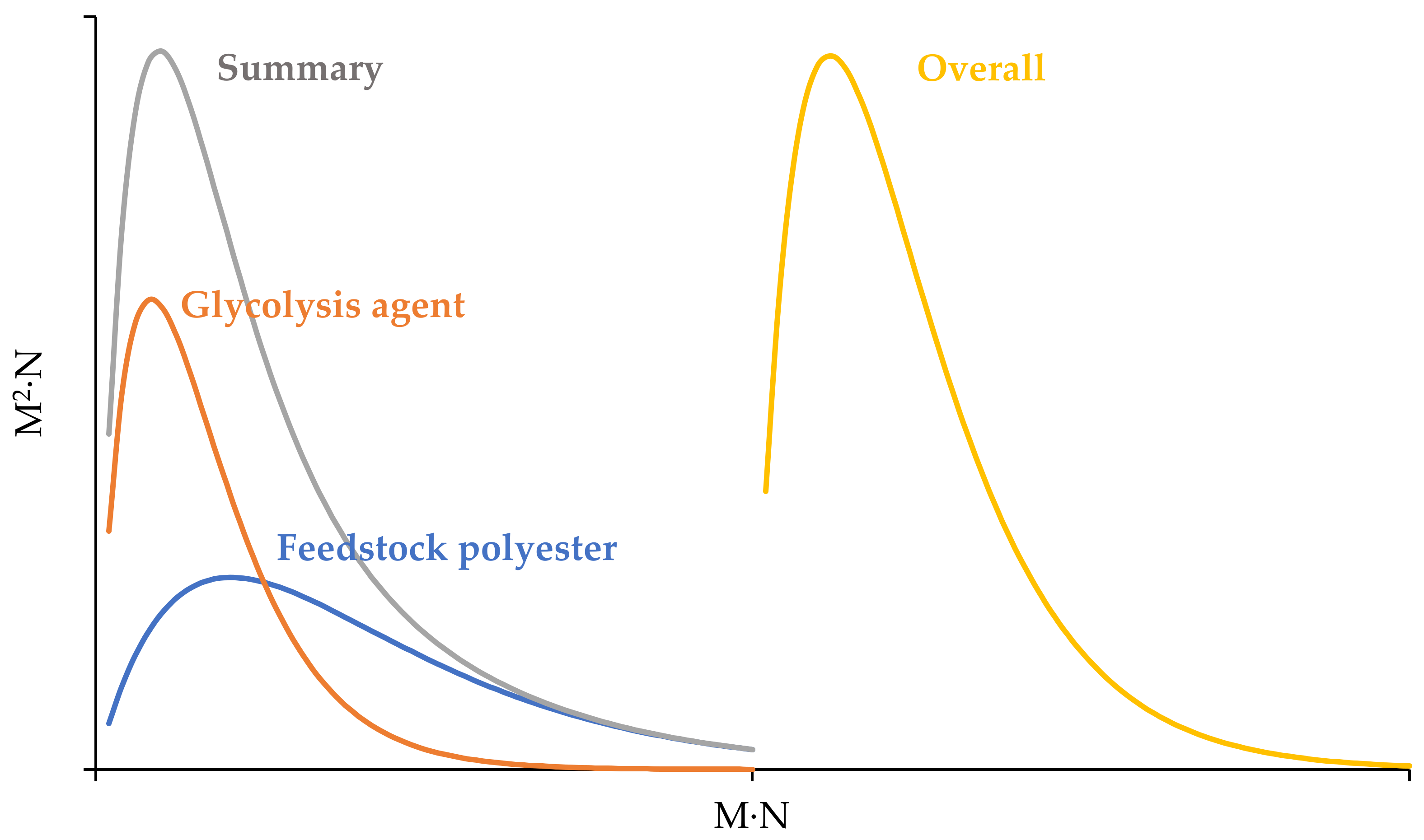
3.2. Kinetic Model Development
- 1.
- Polyesters within the same source (feedstock or agent) are in equilibrium. Therefore, the reactions occurring in them do not lead to a change in the molecular weight distribution. Under this assumption, the polydispersity index of polyester from a single source corresponds to the polyester obtained via ideal step-growth polymerization (Flory-Schulz distribution). Thus, the following reactions are not taken into account in the model:
- 2.
- The concentration of the catalyst in the reaction mixture is assumed to be constant. If this assumption is violated, the actual conversion of the process will be lower than the one calculated by the model. The effect of the catalyst is included in the effective constants [42,43] in Equations (2) and (3) (see below). The model was verified using the values for the antimony trioxide catalyst used;
- 3.
- All reactions under the action of terminal hydroxyethyl groups () proceed at the same rate, regardless of the chain lengths. These reactions have a rate constant (Figure 2, Table 3). Similarly, all reactions under the action of ethylene glycol (EG) proceed at the same rate, with these reactions having a rate constant (Figure 2, Table 3). The constants are taken equal to the constants used for calculations in the path of PET synthesis.The effective polycondensation rate constant can be determined from Equation (2):where is the effective polycondensation rate constant; k1 is the polycondensation rate constant; —catalyst concentration, mol/L; A is the pre-exponential factor, 5.66 × 108 L2/(mol2·min1) [42,43]; Ea—activation energy, 18,500 cal/mol [42,43]; R is the universal gas constant, 1987 cal/mol·K, and T is temperature, K.
- 4.
- The volume of the reaction mixture is assumed to be constant, since densities of polyethylene terephthalate, oligoethylene terephthalates, and bis(2-hydroxyethyl) terephthalate are close in magnitude;
- 5.
- Mass transfer processes, including the presence of ethylene glycol in the gas phase, are not taken into account. This assumption can be made since the reactors in which PET glycolysis is carried out are usually equipped with reflux condensers, which return ethylene glycol back to the reaction mixture. However, violation of this assumption can lead to an underestimation of the conversion by the model relative to one in the real process, since the reactions involving ethylene glycol are at equilibrium;
- 6.
- The concentration of terminal acid groups is taken equal to 0 for all samples. The calculation was made for polyesters and oligoethers with solely hydroxyl end groups;
- 7.
- Ester exchange reactions are not taken into account, since their occurrence at the temperatures used makes a significantly smaller contribution than the occurrence of reactions under the action of hydroxyl groups [46];
- 8.
- Reactions for the formation of ethers (DEG units), degradation reactions (formation of vinyl groups, aldehydes, and chromophore groups) are not taken into account.
3.3. Model Calculations
3.4. Homogeneous Glycolysis under the Action of Ethylene Glycol in Solution
3.5. Homogeneous Melt Glycolysis under the Action of BHET
3.6. Homogeneous Melt Glycolysis under the Action of OET
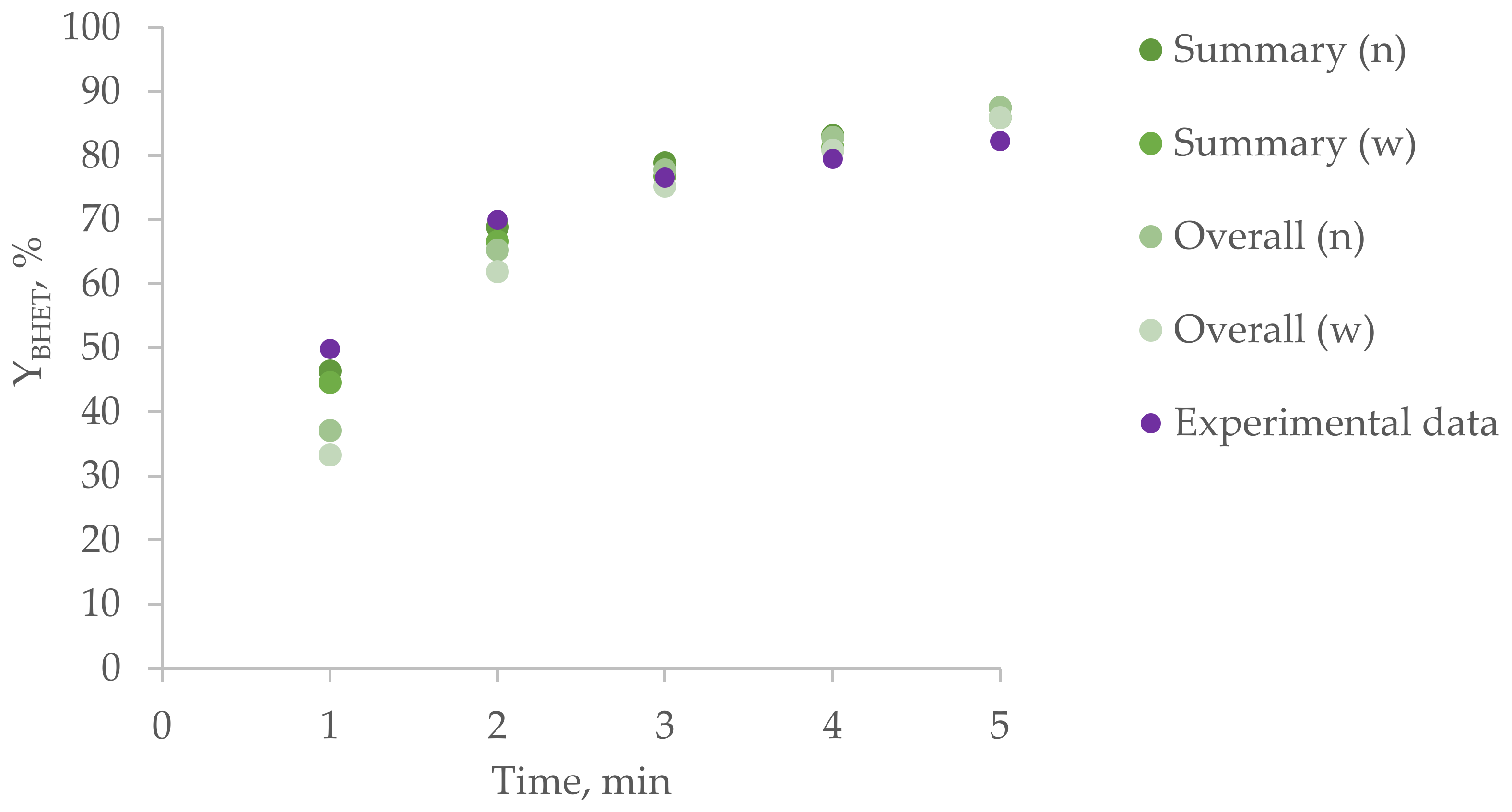
3.7. Step-By-Step Homogeneous Melt Glycolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirshanov, K.; Toms, R.; Aliev, G.; Naumova, A.; Melnikov, P.; Gervald, A. Recent Developments and Perspectives of Recycled Poly(Ethylene Terephthalate)-Based Membranes: A Review. Membranes 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Investigation of Polyester Tire Cord Glycolysis Accompanied by Rubber Crumb Devulcanization. Polymers 2022, 14, 684. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Toms, R.V.; Gerval’d, A.Y. Prospects of Polyester Tire Cord Waste Utilization. Kauchuk I Rezina 2022, 81, 148–154. [Google Scholar]
- Suhaimi, N.A.S.; Muhamad, F.; Abd Razak, N.A.; Zeimaran, E. Recycling of Polyethylene Terephthalate Wastes: A Review of Technologies, Routes, and Applications. Polym. Eng. Sci. 2022, 62, 2355–2375. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Unsaturated Polyester Resin Nanocomposites Based on Post-Consumer Polyethylene Terephthalate. Polymers 2022, 14, 1602. [Google Scholar] [CrossRef]
- Singh, A.K.; Bedi, R.; Kaith, B.S. Composite Materials Based on Recycled Polyethylene Terephthalate and Their Properties—A Comprehensive Review. Compos. B Eng. 2021, 219, 108928. [Google Scholar] [CrossRef]
- Dasan, K.P. PET Nanocomposites: Preparation and Characterization. In Poly(Ethylene Terephthalate) Based Blends, Composites and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2015; pp. 99–111. [Google Scholar]
- Celik, Y.; Shamsuyeva, M.; Endres, H.J. Thermal and Mechanical Properties of the Recycled and Virgin PET—Part I. Polymers 2022, 14, 1326. [Google Scholar] [CrossRef]
- Quintero, Y.G.; Figueroa, D.R.; Gil, H.; Zuleta, A.A. Physical and Mechanical Properties of Recycled Pet Composites. Stavební Obz.-Civ. Eng. J. 2019, 28. [Google Scholar] [CrossRef]
- Rusu, M.A.A.; Radu, S.-A.; Moldovan, C.; Sarosi, C.; Mazilu (Moldovan), I.A.; Rusu, L.M. Mechanical and Structural Properties of Composites Made from Recycled and Virgin Polyethylene Terephthalate (PET) and Metal Chip or Mesh Wire. MATEC Web Conf. 2019, 299, 06007. [Google Scholar] [CrossRef][Green Version]
- Velásquez, E.J.; Garrido, L.; Guarda, A.; Galotto, M.J.; López de Dicastillo, C. Increasing the Incorporation of Recycled PET on Polymeric Blends through the Reinforcement with Commercial Nanoclays. Appl. Clay Sci. 2019, 180, 105185. [Google Scholar] [CrossRef]
- Gomzyak, V.I.; Bychkov, N.V.; Aduev, A.S.; Ivanova, V.A.; Koshelev, A.D.; Chvalun, S.N. Polymerization of D,L-Lactide in the Presence of BoltornTM Polyester Polyol. Fine Chem. Technol. 2022, 17, 242–252. [Google Scholar] [CrossRef]
- Barnard, E.; Rubio Arias, J.J.; Thielemans, W. Chemolytic Depolymerisation of PET: A Review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Ghasemi, M.H.; Neekzad, N.; Ajdari, F.B.; Kowsari, E.; Ramakrishna, S. Mechanistic Aspects of Poly(Ethylene Terephthalate) Recycling–toward Enabling High Quality Sustainability Decisions in Waste Management. Environ. Sci. Pollut. Res. 2021, 28, 43074–43101. [Google Scholar] [CrossRef] [PubMed]
- Damayanti; Wu, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Krisbiantoro, P.A.; Chiao, Y.-W.; Liao, W.; Sun, J.-P.; Tsutsumi, D.; Yamamoto, H.; Kamiya, Y.; Wu, K.C.-W. Catalytic Glycolysis of Polyethylene Terephthalate (PET) by Solvent-Free Mechanochemically Synthesized MFe2O4 (M = Co, Ni, Cu and Zn) Spinel. Chem. Eng. J. 2022, 450, 137926. [Google Scholar] [CrossRef]
- Du, J.-T.; Sun, Q.; Zeng, X.-F.; Wang, D.; Wang, J.-X.; Chen, J.-F. ZnO Nanodispersion as Pseudohomogeneous Catalyst for Alcoholysis of Polyethylene Terephthalate. Chem. Eng. Sci. 2020, 220, 115642. [Google Scholar] [CrossRef]
- Jeong, J.-M.; Jin, S.B.; Son, S.G.; Suh, H.; Moon, J.-M.; Choi, B.G. Fast and Facile Synthesis of Two-Dimensional Fe III Nanosheets Based on Fluid-Shear Exfoliation for Highly Catalytic Glycolysis of Poly(Ethylene Terephthalate). React. Chem. Eng. 2021, 6, 297–303. [Google Scholar] [CrossRef]
- Jeong, J.; Jin, S.B.; Park, H.J.; Park, S.H.; Jeon, H.; Suh, H.; Park, Y.; Seo, D.; Hwang, S.Y.; Kim, D.H.; et al. Large-Scale Fast Fluid Dynamic Processes for the Syntheses of 2D Nanohybrids of Metal Nanoparticle-Deposited Boron Nitride Nanosheet and Their Glycolysis of Poly(Ethylene Terephthalate). Adv. Mater Interfaces 2020, 7, 2000599. [Google Scholar] [CrossRef]
- Fehér, Z.; Kiss, J.; Kisszékelyi, P.; Molnár, J.; Huszthy, P.; Kárpáti, L.; Kupai, J. Optimisation of PET Glycolysis by Applying Recyclable Heterogeneous Organocatalysts. Green Chem. 2022, 24, 8447–8459. [Google Scholar] [CrossRef]
- Lalhmangaihzuala, S.; Laldinpuii, Z.; Lalmuanpuia, C.; Vanlaldinpuia, K. Glycolysis of Poly(Ethylene Terephthalate) Using Biomass-Waste Derived Recyclable Heterogeneous Catalyst. Polymers 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, X.; Ju, Z.; Sun, P.; Xin, J.; Yao, X.; Zhou, Q.; Zhang, S. Ultrafast Homogeneous Glycolysis of Waste Polyethylene Terephthalate via a Dissolution-Degradation Strategy. Ind. Eng. Chem. Res. 2018, 57, 16239–16245. [Google Scholar] [CrossRef]
- Moncada, J.; Dadmun, M.D. The Structural Evolution of Poly(Ethylene Terephthalate) Oligomers Produced via Glycolysis Depolymerization. J. Mater. Chem. A Mater. 2023, 11, 4679–4690. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Zhang, X.; Qian, J.; Xing, X.; Wang, X. Synthesis of Poly(Ethylene Terephthalate) Based on Glycolysis of Waste PET Fiber. J. Macromol. Sci. Part A 2020, 57, 430–438. [Google Scholar] [CrossRef]
- Mohammadi, S.; Enayati, M. Dual Catalytic Activity of Antimony (III) Oxide: The Polymerization Catalyst for Synthesis of Polyethylene Terephthalate Also Catalyze Depolymerization. Polym. Degrad. Stab. 2022, 206, 110180. [Google Scholar] [CrossRef]
- Biros, S.M.; Bridgewater, B.M.; Villeges-Estrada, A.; Tanski, J.M.; Parkin, G. Antimony Ethylene Glycolate and Catecholate Compounds: Structural Characterization of Polyesterification Catalysts. Inorg. Chem. 2002, 41, 4051–4057. [Google Scholar] [CrossRef]
- Abdullah, M.M.S.; Al-Lohedan, H.A. Demulsification of Water in Heavy Crude Oil Emulsion Using a New Amphiphilic Ionic Liquid Based on the Glycolysis of Polyethylene Terephthalate Waste. J. Mol. Liq. 2020, 307, 112928. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Yao, H.; Zhou, Q.; Xin, J.; Lu, X.; Zhang, S. Degradation of Poly(Ethylene Terephthalate) Catalyzed by Metal-Free Choline-Based Ionic Liquids. Green Chem. 2020, 22, 3122–3131. [Google Scholar] [CrossRef]
- Shuangjun, C.; Weihe, S.; Haidong, C.; Hao, Z.; Zhenwei, Z.; Chaonan, F. Glycolysis of Poly(Ethylene Terephthalate) Waste Catalyzed by Mixed Lewis Acidic Ionic Liquids. J. Therm. Anal Calorim. 2021, 143, 3489–3497. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, C.; Hao, Z.; Jiang, W.; Zhang, L.; Zeng, G.; Sun, P.; Zhang, Q. Molecular Mechanism of Waste Polyethylene Terephthalate Recycling by the 1,5,7-Triazabicyclo[4.4.0]Decium Acetate/Zinc Acetate Deep Eutectic Solvent: The Crucial Role of 1,5,7-Triazabicyclo[4.4.0]Decium Cation. Appl. Catal. A Gen. 2022, 641, 118681. [Google Scholar] [CrossRef]
- Marullo, S.; Rizzo, C.; Dintcheva, N.T.; D’Anna, F. Amino Acid-Based Cholinium Ionic Liquids as Sustainable Catalysts for PET Depolymerization. ACS Sustain. Chem. Eng. 2021, 9, 15157–15165. [Google Scholar] [CrossRef]
- Park, R.; Sridhar, V.; Park, H. Taguchi Method for Optimization of Reaction Conditions in Microwave Glycolysis of Waste PET. J. Mater. Cycles Waste Manag. 2020, 22, 664–672. [Google Scholar] [CrossRef]
- Zahova, S.; Tsacheva, I.; Troev, K.; Mitova, V. Conventional and MW Assisted PET Glycolysis Promoted by Titanium Based Catalyst. Polym. Degrad. Stab. 2023, 212, 110353. [Google Scholar] [CrossRef]
- Bahramian, A. Synergistic Effects of Gamma Irradiation on the PET Surface and Heat Treatment of Hydrotalcite Catalyst Supported by Pt/TiO2 Nanoparticles on PET Depolymerization Rate. Surf. Interface Anal. 2021, 53, 215–229. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Flores-Giraldo, L.; Gutiérrez-Ortiz, J.I. Kinetics of Catalytic Glycolysis of PET Wastes with Sodium Carbonate. Chem. Eng. J. 2011, 168, 312–320. [Google Scholar] [CrossRef]
- Viana, M.E.; Riul, A.; Carvalho, G.M.; Rubira, A.F.; Muniz, E.C. Chemical Recycling of PET by Catalyzed Glycolysis: Kinetics of the Heterogeneous Reaction. Chem. Eng. J. 2011, 173, 210–219. [Google Scholar] [CrossRef]
- Sangalang, A.; Bartolome, L.; Kim, D.H. Generalized Kinetic Analysis of Heterogeneous PET Glycolysis: Nucleation-Controlled Depolymerization. Polym. Degrad. Stab. 2015, 115, 45–53. [Google Scholar] [CrossRef]
- El Mejjatti, A.; Harit, T.; Riahi, A.; Khiari, R.; Bouabdallah, I.; Malek, F. Chemical Recycling of Poly(Ethylene Terephthalate). Application to the Synthesis of Multiblock Copolyesters. Express Polym. Lett. 2014, 8, 544–553. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gervald, A.Y.; Toms, R.V.; Lobanov, A.N. Obtaining Phthalate Substituted Post-Consumer Polyethylene Terephthalate and Its Isothermal Crystallization. Fine Chem. Technol. 2022, 17, 164–171. [Google Scholar] [CrossRef]
- Javed, S.; Fisse, J.; Vogt, D. Optimization and Kinetic Evaluation for Glycolytic Depolymerization of Post-Consumer PET Waste with Sodium Methoxide. Polymers 2023, 15, 687. [Google Scholar] [CrossRef]
- Ha, K.-S.; Rhee, H.-K. Optimal Reaction Conditions for the Minimization of Energy Consumption and By-Product Formation in a Poly(Ethylene Terephthalate) Process. J. Appl. Polym. Sci. 2002, 86, 993–1008. [Google Scholar] [CrossRef]
- Kim, I.S.; Woo, B.G.; Choi, K.Y.; Kiang, C. Two-Phase Model for Continuous Final-Stage Melt Polycondensation of Poly(Ethylene Terephthalate). III. Modeling of Multiple Reactors with Multiple Reaction Zones. J. Appl. Polym. Sci. 2003, 90, 1088–1095. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, H.-Y.; Yeo, Y.-K. Identification of Kinetics of Direct Esterification Reactions for PET Synthesis Based on a Genetic Algorithm. Korean J. Chem. Eng. 2001, 18, 432–441. [Google Scholar] [CrossRef]
- Liu, T.; Gu, X.; Wang, J.; Feng, L. Modeling and Analysis of New Reactor Concepts for Poly(Ethylene Terephthalate) Esterification Process. Chem. Eng. Process.-Process Intensif. 2019, 135, 217–226. [Google Scholar] [CrossRef]
- Collins, S.; Peace, S.K.; Richards, R.W.; MacDonald, W.A.; Mills, P.; King, S.M. Transesterification in Poly(Ethylene Terephthalate). Molecular Weight and End Group Effects. Macromolecules 2000, 33, 2981–2988. [Google Scholar] [CrossRef]



| Quantity | Reaction Step 1 | Reaction Step 2 | Reaction Step 3 |
|---|---|---|---|
| PET loading, relative mass fraction | 100 | 430 | 820 |
| OET-1 loading, relative mass fraction | 330 | ||
| BHET-1 loading, relative mass fraction | 0 | 390 | |
| EG loading, relative mass fraction | 0 | 0 | 100 |
| Sb2O3 + EG loading, relative mass fraction | 0.86 | 1.64 | 1.84 |
| Temperature, °C | 250 | 220 | 190 |
| Name of the Reacting Component | Abbreviation | Source of the Reacting Component | Concentration, g/L |
|---|---|---|---|
| Bonded ethylene glycol | Feedstock: Poly(ethylene terephthalate), oligo(ethylene terephthalate), bis(2-hydroxyethyl) terephthalate, and/or ethylene glycol) | c1 | |
| Terminal ethylene glycol | c2 | ||
| Ethylene glycol | c3 | ||
| Bonded ethylene glycol | Glycolysis agent: Oligo(ethylene terephthalate), bis(2-hydroxyethyl) terephthalate, and/or ethylene glycol) | c4 | |
| Terminal ethylene glycol | c5 | ||
| Ethylene glycol | c6 |
| Reaction (Figure 2) | Reaction Rate Constant |
|---|---|
| n ≥ 1, m ≥ 0 | |
| n = 0, m ≥ 0 |
| № | Reaction | Reaction Rate Equation |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 7 | ||
| 8 | ||
| 9 | ||
| 10 | ||
| 11 | ||
| 12 |
| Corresponding Experiment | c1, g/L | c2, g/L | c3, g/L | c4, g/L | c5, g/L | c6, g/L |
|---|---|---|---|---|---|---|
| 2.3.1, in DMSO | 0.71 | 0.01 | 0 | 0 | 0 | 4.38 |
| 2.3.1, in NMP | 0.43 | 0.01 | 0 | 0 | 0 | 3.60 |
| PET:BHET Molar Ratio | c1, g/L | c2, g/L | c3, g/L | c4, g/L | c5, g/L | c6, g/L |
|---|---|---|---|---|---|---|
| 1:1 | 3.08 | 0.05 | 0 | 0 | 6.16 | 0 |
| 1:3 | 1.44 | 0.02 | 0 | 0 | 8.63 | 0 |
| PET:BHET Molar Ratio | Mn | Mw | PDI | YBHET, % | |
|---|---|---|---|---|---|
| 1:1 | Experimental data | 530 | 850 | 1.60 | 20.3 |
| Simulation result | 563 | 839 | 1.49 | 14.66 (w) 17.26 (n) | |
| 1:3 | Experimental data | 410 | 630 | 1.50 | 33.8 |
| Simulation result | 446 | 611 | 1.37 | 25.02 (w) 28.50 (n) | |
| PET:OET Molar Ratio | c1, g/L | c2, g/L | c3, g/L | c4, g/L | c5, g/L | c6, g/L |
|---|---|---|---|---|---|---|
| 1:1 | 3.41 | 0.05 | 0 | 2.27 | 2.27 | 0 |
| 1:3 | 1.66 | 0.03 | 0 | 3.33 | 3.33 | 0 |
| PET:OET Molar Ratio | Mn | Mw | PDI | YBHET, % | |
|---|---|---|---|---|---|
| 1:1 | Experimental data | 1520 | 3050 | 2.00 | 4.2 |
| Simulation result | 1295 | 2287 | 1.77 | 2.42 (w) 3.05 (n) | |
| 1:3 | Experimental data | 890 | 1580 | 1.80 | 5.7 |
| Simulation result | 933 | 1567 | 1.68 | 4.86 (w) 6.00 (n) | |
| Reaction Step | c1, g/L | c2, g/L | c3, g/L | c4, g/L | c5, g/L | c6, g/L |
|---|---|---|---|---|---|---|
| 1 | 1.66 | 0.03 | 0 | 3.33 | 3.33 | 0 |
| 2 | 0.58 | 0.33 | 0.02 | 2.26 | 6.46 | 0.09 |
| 3 | 0.31 | 0.44 | 0.08 | 2.96 | 4.20 | 3.07 |
| Reaction Step | Mn | Mw | PDI | YBHET, % | |
|---|---|---|---|---|---|
| 1 | Experimental data | 890 | 1590 | 1.80 | 5.7 |
| Simulation result | 933 | 1567 | 1.68 | 4.86 (w) 6.00 (n) | |
| 2 | Experimental data | 560 | 840 | 1.50 | 20.0 |
| Simulation result | 524 | 763 | 1.45 | 17.24 (w) 20.11 (n) | |
| 3 | Experimental data | 320 | 450 | 1.40 | 41.6 |
| Simulation result | 393 | 511 | 1.30 | 33.56 (w) 37.40 (n) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirshanov, K.A.; Toms, R.V.; Balashov, M.S.; Golubkov, S.S.; Melnikov, P.V.; Gervald, A.Y. Modeling of Poly(Ethylene Terephthalate) Homogeneous Glycolysis Kinetics. Polymers 2023, 15, 3146. https://doi.org/10.3390/polym15143146
Kirshanov KA, Toms RV, Balashov MS, Golubkov SS, Melnikov PV, Gervald AY. Modeling of Poly(Ethylene Terephthalate) Homogeneous Glycolysis Kinetics. Polymers. 2023; 15(14):3146. https://doi.org/10.3390/polym15143146
Chicago/Turabian StyleKirshanov, Kirill A., Roman V. Toms, Mikhail S. Balashov, Sergey S. Golubkov, Pavel V. Melnikov, and Alexander Yu. Gervald. 2023. "Modeling of Poly(Ethylene Terephthalate) Homogeneous Glycolysis Kinetics" Polymers 15, no. 14: 3146. https://doi.org/10.3390/polym15143146
APA StyleKirshanov, K. A., Toms, R. V., Balashov, M. S., Golubkov, S. S., Melnikov, P. V., & Gervald, A. Y. (2023). Modeling of Poly(Ethylene Terephthalate) Homogeneous Glycolysis Kinetics. Polymers, 15(14), 3146. https://doi.org/10.3390/polym15143146












