Fabrication, Structural Properties, and Electrical Characterization of Polymer Nanocomposite Materials for Dielectric Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atta, A.; Negm, H.; Abdeltwab, E.; Rabia, M.; Abdelhamied, M.M. Facile fabrication of polypyrrole/NiOx core-shell nanocomposites for hydrogen production from wastewater. Polym. Adv. Technol. 2023, 34, 1633–1641. [Google Scholar] [CrossRef]
- Shoaib, M.; Jamshaid, H.; Alshareef, M.; Alharthi, F.A.; Ali, M.; Waqas, M. Exploring the Potential of Alternate Inorganic Fibers for Automotive Composites. Polymers 2022, 14, 4946. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.N. Design of an Efficient PTB7: PC70BM-Based Polymer Solar Cell for 8% Efficiency. Polymers 2022, 14, 889. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elnaiem, A.M.; Salman, O.S.; Hakamy, A.; Hussein, S.I. Mechanical characteristics and thermal stability of hybrid epoxy and acrylic polymer coating/nanoclay of various thicknesses. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2094–2102. [Google Scholar] [CrossRef]
- Hassan, M.K.; Redhwi, A.M.N.; Mohamed, A.F.; Backar, A.H.; Abdellah, M.Y. Investigation of Erosion/Corrosion Behavior of GRP under Harsh Operating Conditions. Polymers 2022, 14, 5388. [Google Scholar] [CrossRef]
- Abdellah, M.Y.; Hassan, M.K.; Mohamed, A.F.; Khalil, K.A. A Novel and Highly Effective Natural Vibration Modal Analysis to Predict Nominal Strength of Open Hole Glass Fiber Reinforced Polymer Composites Structure. Polymers 2021, 13, 1251. [Google Scholar] [CrossRef]
- Ahmad Fauzi, A.A.; Osman, A.F.; Alrashdi, A.A.; Mustafa, Z.; Abdul Halim, K.A. On the Use of Dolomite as a Mineral Filler and Co-Filler in the Field of Polymer Composites: A Review. Polymers 2022, 14, 2843. [Google Scholar] [CrossRef]
- Wu, Y.; Ying, Y.; Liu, Y.; Zhang, H.; Huang, J. Preparation of chitosan/poly vinyl alcohol films and their inhibition of biofilm formation against Pseudomonas aeruginosa PAO1. Int. J. Biol. Macromol. 2018, 118, 2131–2137. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Hou, L. The effect of glycerol on properties of chitosan/poly (vinyl alcohol) films with AlCl3· 6H2O aqueous solution as the solvent for chitosan. Carbohydr. Polym. 2016, 135, 191–198. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, G.; Yang, W.; Jing, Q.; Bai, P.; Yang, Y.; Wang, Z.L. Harmonic-resonator-based triboelectric nanogenerator as a sustainable power source and a self-powered active vibration sensor. Adv. Mater. 2013, 25, 6094–6099. [Google Scholar] [CrossRef]
- Ashour, G.; Hussein, M.; Sobahi, T. Nanocomposite containing polyamide and GNS for enhanced properties. Synthesis and characterization. J. Umm Al-Qura Univ. Appl. Sci. 2021, 7, 1–6. [Google Scholar]
- El-Gammam, Y.A. Effect of Fast Neutron Irradiation on Optical Properties of Lead Borate Glass. J. Umm Al-Qura Univ. Appl. Sci. 2020, 6, 21–24. [Google Scholar]
- Abdelhamied, M.M.; Atta, A.; Abdelreheem, A.M.; Farag, A.T.M.; El Okr, M.M. Synthesis and optical properties of PVA/PANI/Ag nanocomposite films. J. Mater. Sci. Mater. Electron. 2020, 31, 22629–22641. [Google Scholar] [CrossRef]
- Xu, N.; Chen, J.; Wei, Q.; Ding, E.; Zeng, X.; Xue, F.; Shang, J. Preparation of polyvinyl alcohol/two-dimensional transition metal dichalcogenides composites by high-pressure homogenization. J. Appl. Polym. Sci. 2020, 137, 48487. [Google Scholar] [CrossRef]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132, 42004. [Google Scholar] [CrossRef]
- Abdel-Razik, H.H.; Asghar, B.H.; Kenawy, E. Synthesis, characterization and spectroscopic investigation of pyrazinoporphyrazine network polymer-supported metal (II)-based catalysts. Chin. J. Polym. Sci. 2013, 31, 242–250. [Google Scholar] [CrossRef]
- Tangpasuthadol, V.; Pongchaisirikul, N.; Hoven, V.P. Surface modification of chitosan films: Effects of hydrophobicity on protein adsorption. Carbohydr. Res. 2003, 338, 937–942. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Cheng, S.; Huang, N.; Leng, Y. Syntheses of novel chitosan derivative with excellent solubility, anticoagulation, and antibacterial property by chemical modification. J. Appl. Polym. Sci. 2012, 124, 2641–2648. [Google Scholar] [CrossRef]
- Alotaibi, B.M.; Atta, A.; Atta, M.R.; Abdeltwab, E.; Abdel-Hamid, M.M. Low energy irradiation induced effects on the surface characteristics of polydimethylsiloxane polymeric films. Macromol. Res. 2023, 31, 1–11. [Google Scholar] [CrossRef]
- Alotaibi, B.M.; Atta, M.R.; Abdeltwab, E.; Atta, A.; Abdel-Hamid, M.M. Surface modifications and optical studies of irradiated flexible PDMS materials. Surf. Innov. 2023, 40, 1–11. [Google Scholar] [CrossRef]
- Atta, A.; Abdelhamied, M.M.; Essam, D.; Shaban, M.; Alshammari, A.H.; Rabia, M. Structural and physical properties of polyaniline/silver oxide/silver nanocomposite electrode for supercapacitor applications. Int. J. Energy Res. 2021, 5, 6702–6710. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wang, H.; Xiong, M.; Yang, T.; Zakharova, G.S. Highly sensitive and selective ammonia gas sensors based on PbS quantum dots/TiO2 nanotube arrays at room temperature. Sens. Actuators B Chem. 2016, 236, 529–536. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Murakami, M.; Shono, T.; Hasegawa, T.; Fukumura, T.; Kawasaki, M.; Ahmet, P.; Chikyow, T.; Koshihara, S.-Y.; Koinuma, H. Room-temperature ferromagnetism in transparent transition metal-doped titanium dioxide. Science 2001, 291, 854–856. [Google Scholar] [CrossRef]
- Alotaibi, B.M.; Atta, A.; Atta, M.R.; Abdeltwab, E.; Abdel-Hamid, M.M. Modifying the optical properties of hydrogen-beam-irradiated flexible PVA polymeric films. Surf. Innov. 2023, 40, 1–12. [Google Scholar] [CrossRef]
- Lewis, S.; Haynes, V.; Wheeler-Jones, R.; Sly, J.; Perks, R.M.; Piccirillo, L. Surface characterization of poly (methylmethacrylate) based nanocomposite thin films containing Al2O3 and TiO2 nanoparticles. Thin Solid Film. 2010, 518, 2683–2687. [Google Scholar] [CrossRef]
- Lavacchi, A.; Bellini, M.; Berretti, E.; Chen, Y.; Marchionni, A.; Miller, H.A.; Vizza, F. Titanium dioxide nanomaterials in electrocatalysis for energy. Curr. Opin. Electrochem. 2021, 28, 100720. [Google Scholar] [CrossRef]
- Ma, X.; Wang, C.; Wu, F.; Guan, Y.; Xu, G. TiO2 nanomaterials in photoelectrochemical and electrochemiluminescent biosensing. In Surface-modified Nanobiomaterials for Electrochemical and Biomedicine Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–17. [Google Scholar]
- Du, H.; Xue, Y.; Wang, C.; Jie, G. ZnIn2S4 QDs@ TiO2 nanosphere-BiOI double heterojunction combined with unique tripod DNA walker amplification for photoelectrochemical biosensing of microRNA-21. Sens. Actuators B Chem. 2022, 373, 132704. [Google Scholar] [CrossRef]
- Alotaibi, B.M.; Al-Yousef, H.A.; Alsaif, N.A.; Atta, A. Oxygen beam induced modifications on the structural characteristics and physico-chemical properties of PANI/lead sulfide composite films. Inorg. Chem. Commun. 2022, 144, 109904. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, G.; Wu, Z.; Pan, W.; Fan, X. Liquefied petroleum gas sensing properties of ZnO/PPy/PbS QDs nanocomposite prepared by self-assembly combining with SILAR method. IEEE Sens. J. 2018, 19, 2855–2862. [Google Scholar] [CrossRef]
- Althubiti, N.A.; Atta, A.; Al-Harbi, N.; Sendi, R.K.; Abdelhamied, M.M. Structural, characterization and linear/nonlinear optical properties of oxygen beam irradiated PEO/NiO composite films. Opt. Quantum Electron. 2023, 55, 348. [Google Scholar] [CrossRef]
- Althubiti, N.A.; Al-Harbi, N.; Sendi, R.K.; Atta, A.; Henaish, A.M. Surface Characterization and Electrical Properties of Low Energy Irradiated PANI/PbS Polymeric Nanocomposite Materials. Inorganics 2023, 11, 74. [Google Scholar] [CrossRef]
- Al-Yousef, H.A.; Alotaibi, B.M.; Atta, A.; Abdel-Hamid, M.M. Characterization and dielectric studies of hydrogen-beam-irradiated PDMS polymeric materials. Macromol. Res. 2023, 1–10. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Shen, J.; Zhu, Z.; Liu, Y. Synthesis of BiOI nanosheet/coarsened TiO2 nanobelt heterostructures for enhancing visible light photocatalytic activity. RSC Adv. 2016, 6, 30037–30047. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Morad, I.; Wasfy, M.H.; Mansour, A.F. Synthesis, structural and electrical properties of PVA/TiO2 nanocomposite films with different TiO2 phases prepared by sol–gel technique. J. Mater. Sci. Mater. Electron. 2020, 31, 17574–17584. [Google Scholar] [CrossRef]
- Althubiti, N.A.; Atta, A.; Abdeltwab, E.; Al-Harbi, N.; Abdel-Hamid, M.M. Structural characterization and dielectric properties of low energy hydrogen beam irradiated PVA/ZnO nanocomposite materials. Inorg. Chem. Commun. 2023, 153, 110779. [Google Scholar] [CrossRef]
- Gottam, R.; Srinivasan, P. Composite electrode material of MoO3-MC-SiO2-PANI: Aqueous supercapacitor cell with high energy density, 1 V and 250,000 CD cycles. Polym. Adv. Technol. 2021, 32, 2465–2475. [Google Scholar] [CrossRef]
- Nahar, K.; Aziz, S.; Bashar, M.; Haque, M.; Al-Reza, S.M. Synthesis and characterization of Silver nanoparticles from Cinnamomum tamala leaf extract and its antibacterial potential. Int. J. Nano Dimens. 2020, 11, 88–98. [Google Scholar]
- Ishaq, S.; Kanwal, F.; Atiq, S.; Moussa, M.; Azhar, U.; Gul, I.; Losic, D. Dielectric and impedance spectroscopic studies of three phase graphene/titania/poly (vinyl alcohol) nanocomposite films. Results Phys. 2018, 11, 540–548. [Google Scholar] [CrossRef]
- Abutalib, M.; Rajeh, A. Preparation and characterization of polyaniline/sodium alginate-doped TiO2 nanoparticles with promising mechanical and electrical properties and antimicrobial activity for food packaging applications. J. Mater. Sci. Mater. Electron. 2020, 31, 9430–9442. [Google Scholar] [CrossRef]
- Atta, A. Enhanced dielectric properties of flexible Cu/polymer nanocomposite films. Surf. Innov. 2020, 9, 17–24. [Google Scholar] [CrossRef]
- Agrawal, A.; Satapathy, A. Thermal and dielectric behaviour of polypropylene composites reinforced with ceramic fillers. J. Mater. Sci. Mater. Electron. 2015, 26, 103–112. [Google Scholar] [CrossRef]
- Mahendia, S.; Tomar, A.; Kumar, S. Electrical conductivity and dielectric spectroscopic studies of PVA–Ag nanocomposite films. J. Alloys Compd. 2010, 508, 406–411. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.; Mohamed, P.A.; Kadir, M.; Hamsan, M.; Abdulwahid, R.T.; Woo, H. Increase of metallic silver nanoparticles in Chitosan: AgNt based polymer electrolytes incorporated with alumina filler. Results Phys. 2019, 13, 102326. [Google Scholar] [CrossRef]
- Lotfy, S.; Atta, A.; Abdeltwab, E. Comparative study of gamma and ion beam irradiation of polymeric nanocomposite on electrical conductivity. J. Appl. Polym. Sci. 2018, 135, 46146. [Google Scholar] [CrossRef]
- Latif, I.; AL-Abodi, E.E.; Badri, D.H.; Al Khafagi, J. Preparation, characterization and electrical study of (carboxymethylated polyvinyl alcohol/ZnO) nanocomposites. Am. J. Polym. Sci. 2012, 2, 135–140. [Google Scholar] [CrossRef]
- Amin, G.; Abd-El Salam, M. Optical, dielectric and electrical properties of PVA doped with Sn nanoparticles. Mater. Res. Express 2014, 1, 025024. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Choudhary, S.; Sengwa, R. Dielectric and optical properties of alumina and silica nanoparticles dispersed poly (methyl methacrylate) matrix-based nanocomposites for advanced polymer technologies. J. Polym. Res. 2021, 28, 1–15. [Google Scholar] [CrossRef]
- Thangarasu, R.; Senthilkumar, N.; Babu, B.; Mohanraj, K.; Chandrasekaran, J.; Balasundaram, O. Structural, Optical, Morphological and Electrical Properties of V2O5 Nanorods and Its Ag/n-V2O5/p-Si/Ag Diode Application. J. Adv. Phys. 2018, 7, 312–318. [Google Scholar] [CrossRef]
- Abdelhamied, M.; Abdelreheem, A.; Atta, A. Influence of ion beam and silver nanoparticles on dielectric properties of flexible PVA/PANI polymer composite films. Plast. Rubber Compos. 2021, 51, 1–12. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, R. Influence of low energy ion beam implantation on Cu nanowires synthesized using scaffold-based electrodeposition. Nano-Struct. Nano-Objects 2019, 18, 100318. [Google Scholar] [CrossRef]
- Sahu, G.; Das, M.; Yadav, M.; Sahoo, B.P.; Tripathy, J. Dielectric relaxation behavior of silver nanoparticles and graphene oxide embedded poly (vinyl alcohol) nanocomposite film: An effect of ionic liquid and temperature. Polymers 2020, 12, 374. [Google Scholar] [CrossRef]
- Atiq, S.; Majeed, M.; Ahmad, A.; Abbas, S.K.; Saleem, M.; Riaz, S.; Naseem, S. Synthesis and investigation of structural, morphological, magnetic, dielectric and impedance spectroscopic characteristics of Ni-Zn ferrite nanoparticles. Ceram. Int. 2017, 43, 2486–2494. [Google Scholar] [CrossRef]
- Abdel Reheem, A.; Atta, A.; Afify, T. Optical and electrical properties of argon ion beam irradiated PVA/Ag nanocomposites. Surf. Rev. Lett. 2017, 24, 1750038. [Google Scholar] [CrossRef]
- Jilani, W.; Fourati, N.; Zerrouki, C.; Gallot-Lavallée, O.; Guermazi, H. Optical, dielectric properties and energy storage efficiency of ZnO/epoxy nanocomposites. J. Inorg. Organomet. Polym. Mater. 2019, 29, 456–464. [Google Scholar] [CrossRef]
- Ahmed, H.; Hashim, A. Fabrication of PVA/NiO/SiC nanocomposites and studying their dielectric properties for antibacterial applications. Egypt. J. Chem. 2020, 63, 805–811. [Google Scholar] [CrossRef]
- Bouaamlat, H.; Hadi, N.; Belghiti, N.; Sadki, H.; Naciri Bennani, M.; Abdi, F.; Lamcharfi, T.-d.; Bouachrine, M.; Abarkan, M. Dielectric properties, AC conductivity, and electric modulus analysis of bulk ethylcarbazole-terphenyl. Adv. Mater. Sci. Eng. 2020, 2020, 8689150. [Google Scholar] [CrossRef]
- Hashim, A.; Hadi, A. Novel pressure sensors made from nanocomposites (biodegradable polymers–metal oxide nanoparticles): Fabrication and characterization. Ukr. J. Phys. 2018, 63, 754. [Google Scholar] [CrossRef]
- Raghu, S.; Archana, K.; Sharanappa, C.; Ganesh, S.; Devendrappa, H. Electron beam and gamma ray irradiated polymer electrolyte films: Dielectric properties. J. Radiat. Res. Appl. Sci. 2016, 9, 117–124. [Google Scholar] [CrossRef]
- Atta, A.; Lotfy, S.; Abdeltwab, E. Dielectric properties of irradiated polymer/multiwalled carbon nanotube and its amino functionalized form. J. Appl. Polym. Sci. 2018, 135, 46647. [Google Scholar] [CrossRef]
- Ebrahim, S.; Kashyout, A.-H.; Soliman, M. Ac and Dc conductivities of polyaniline/poly vinyl formal blend films. Curr. Appl. Phys. 2009, 9, 448–454. [Google Scholar] [CrossRef]

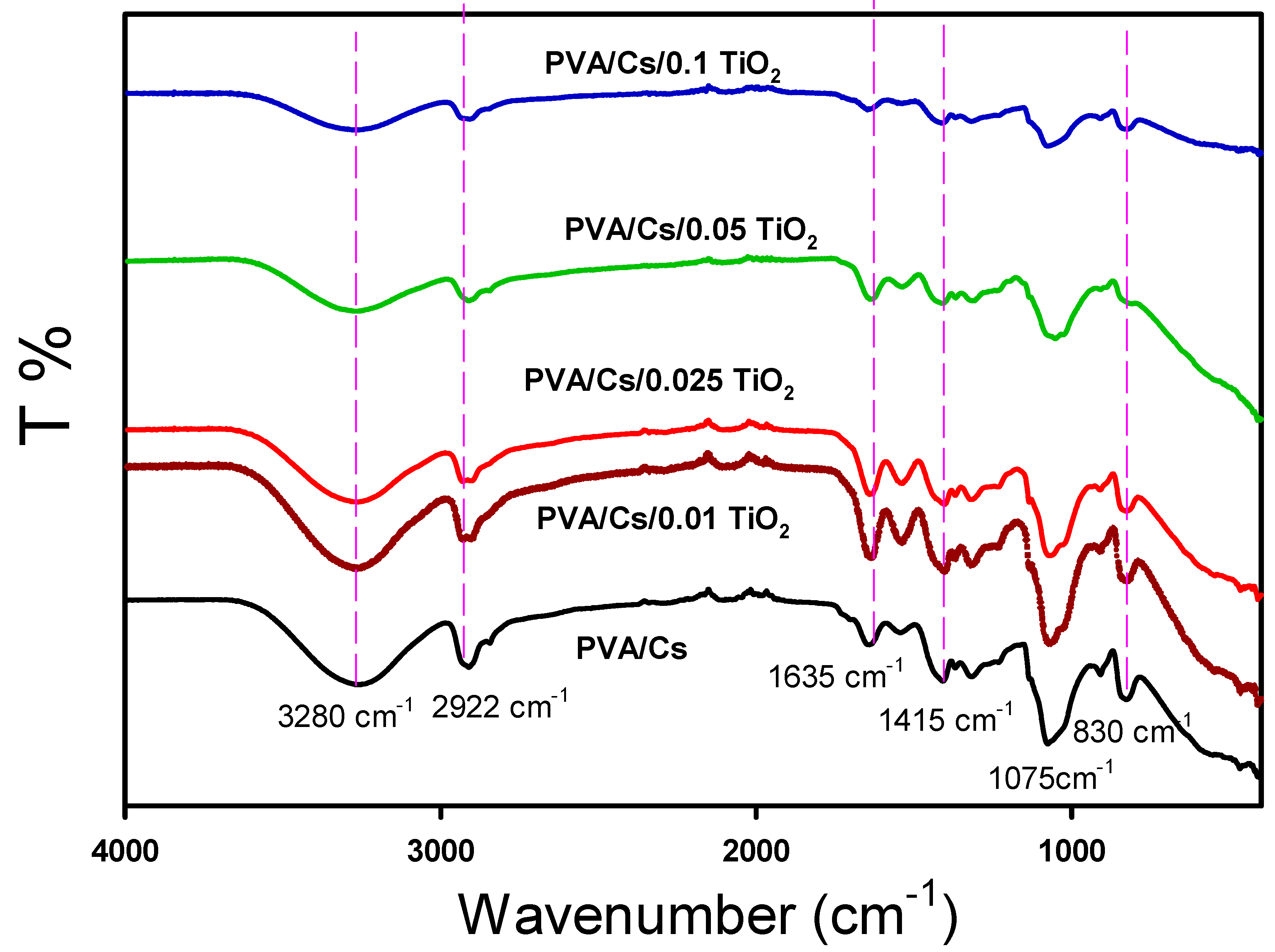




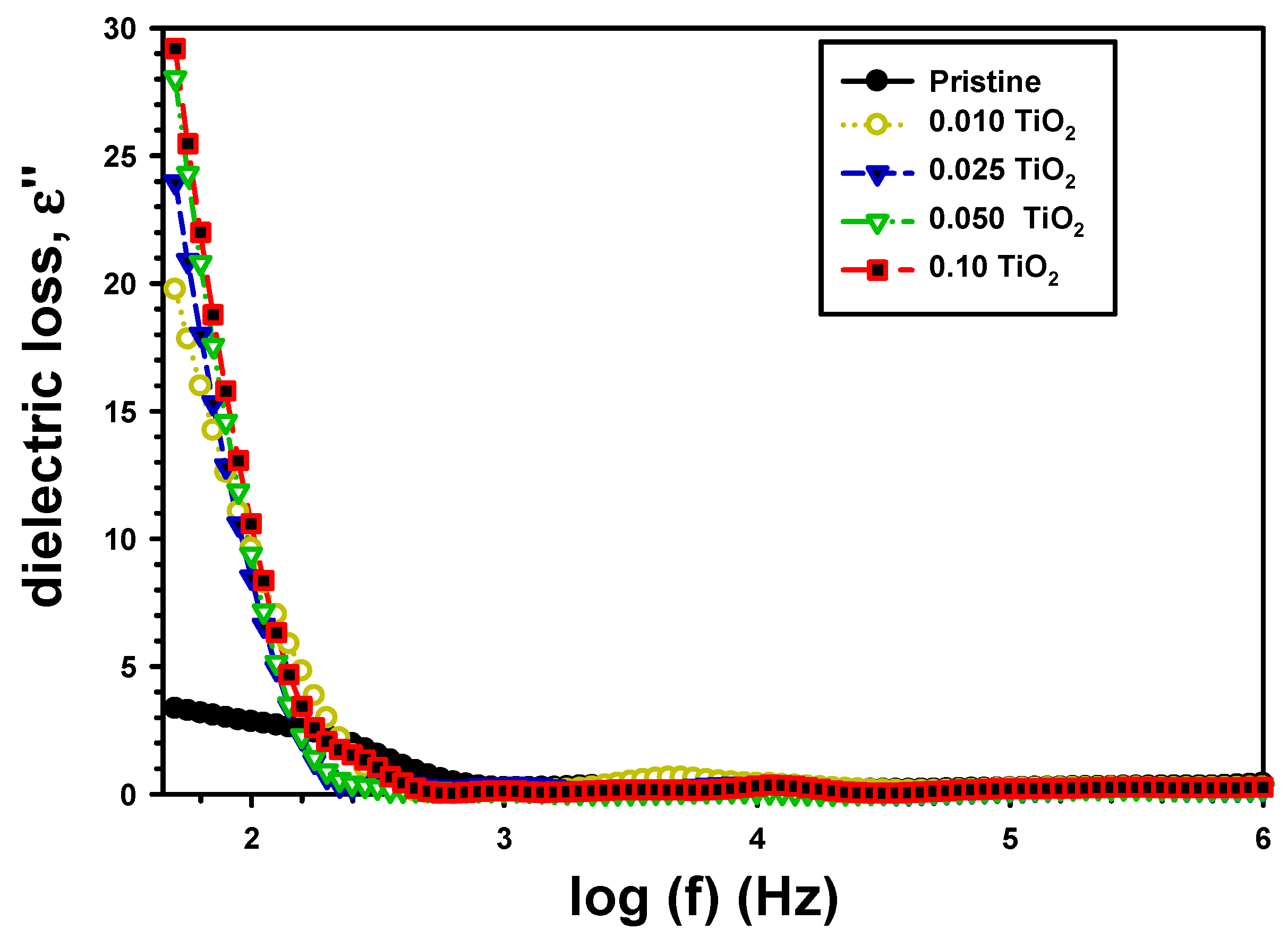
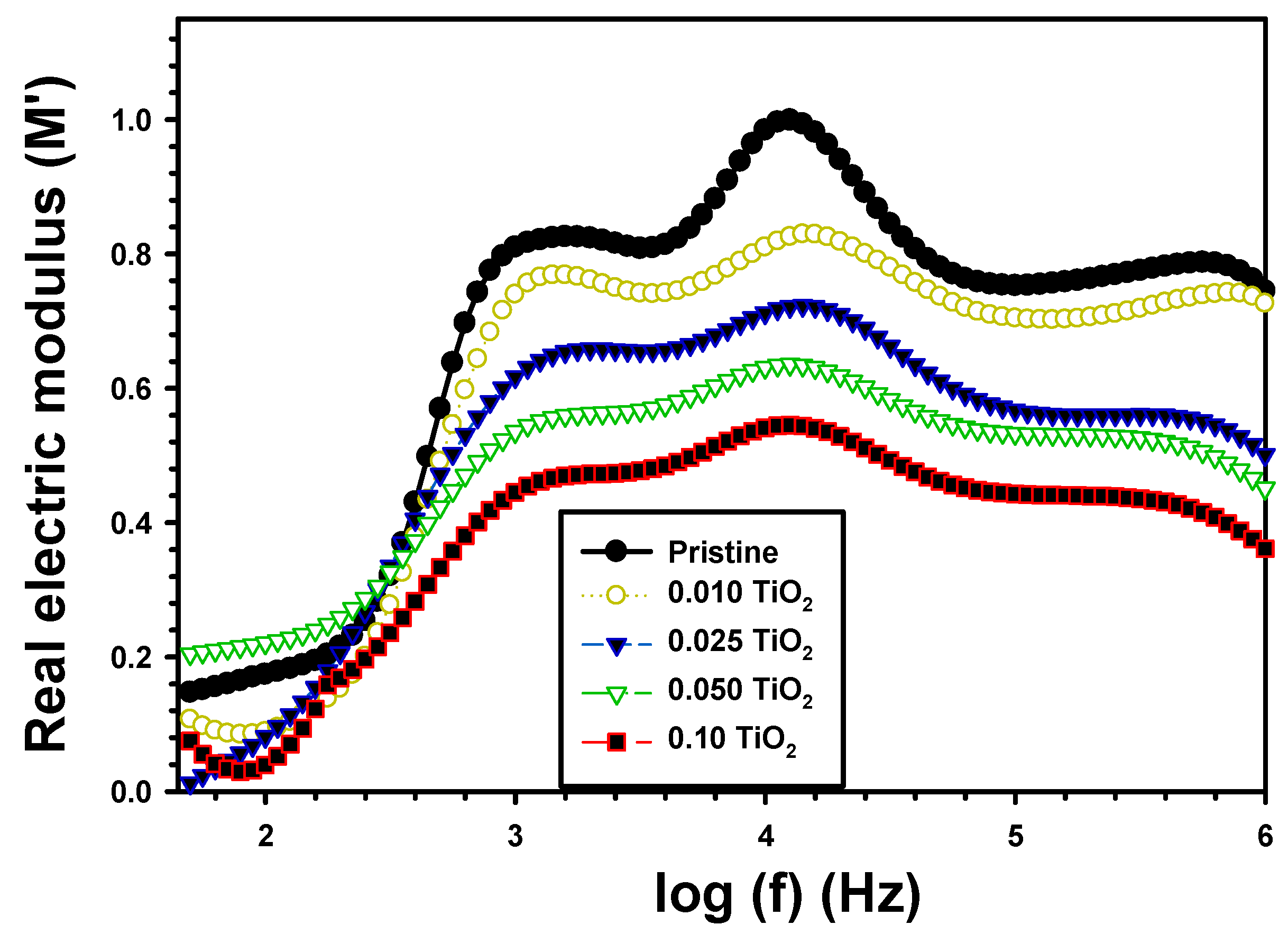
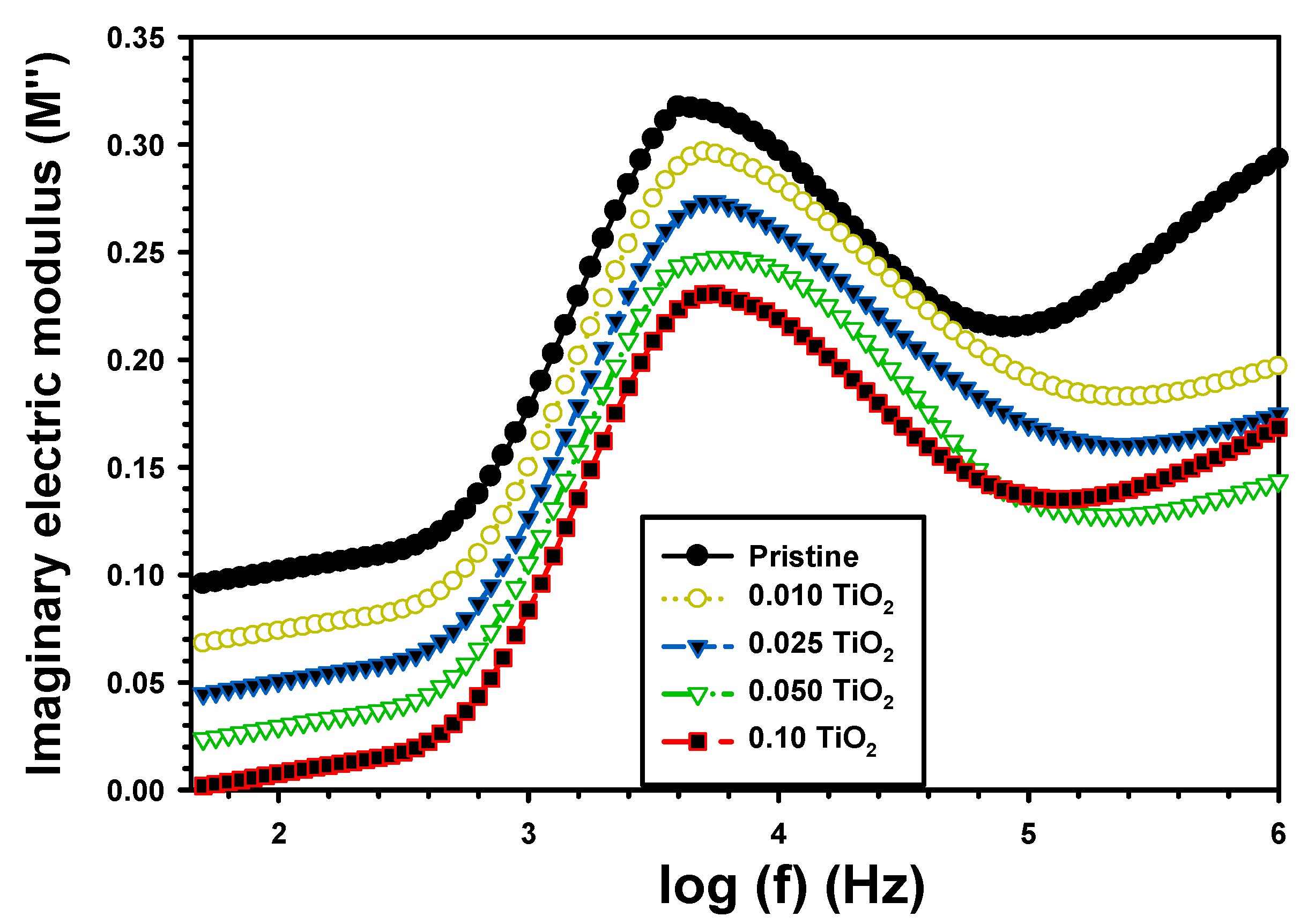
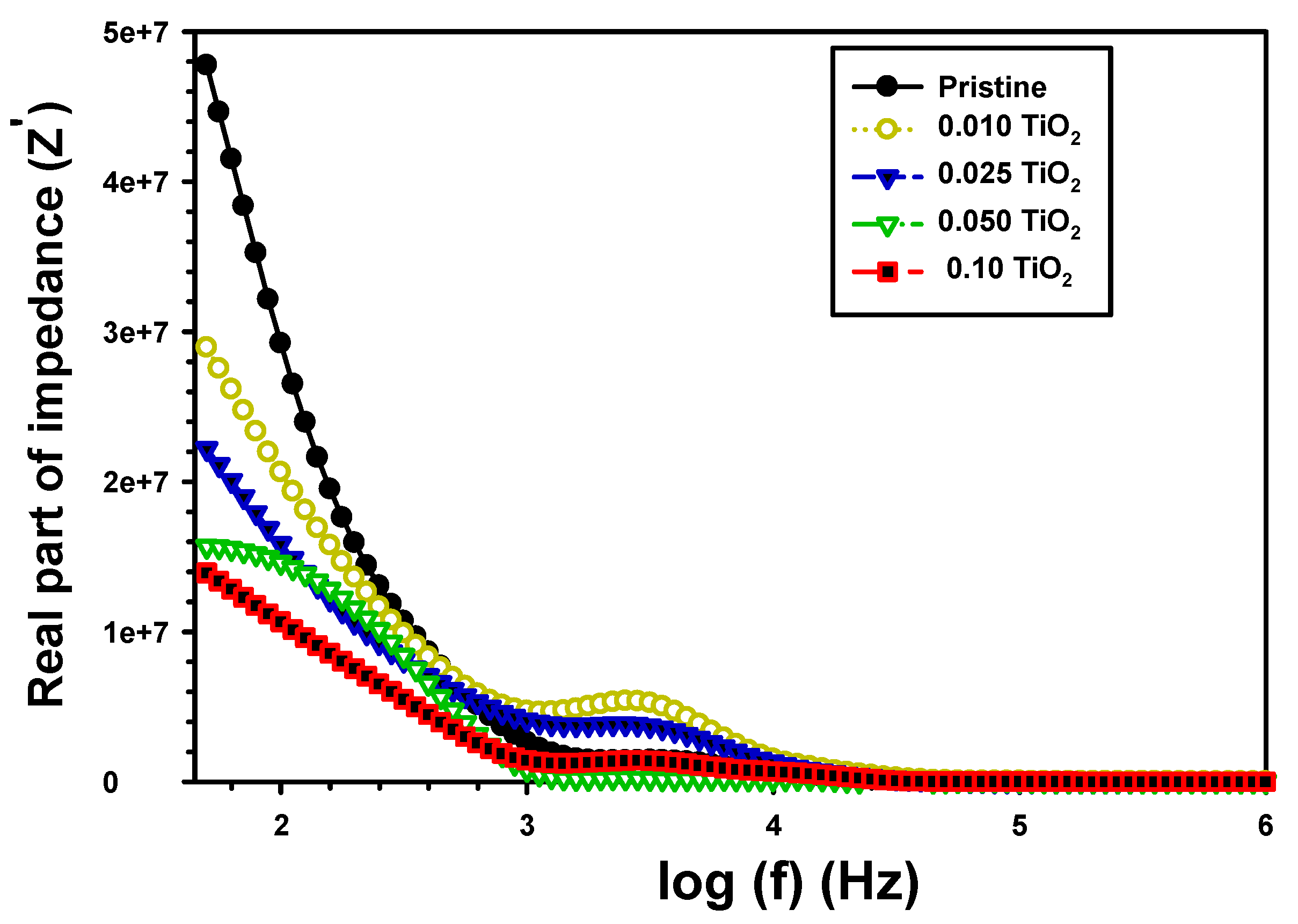
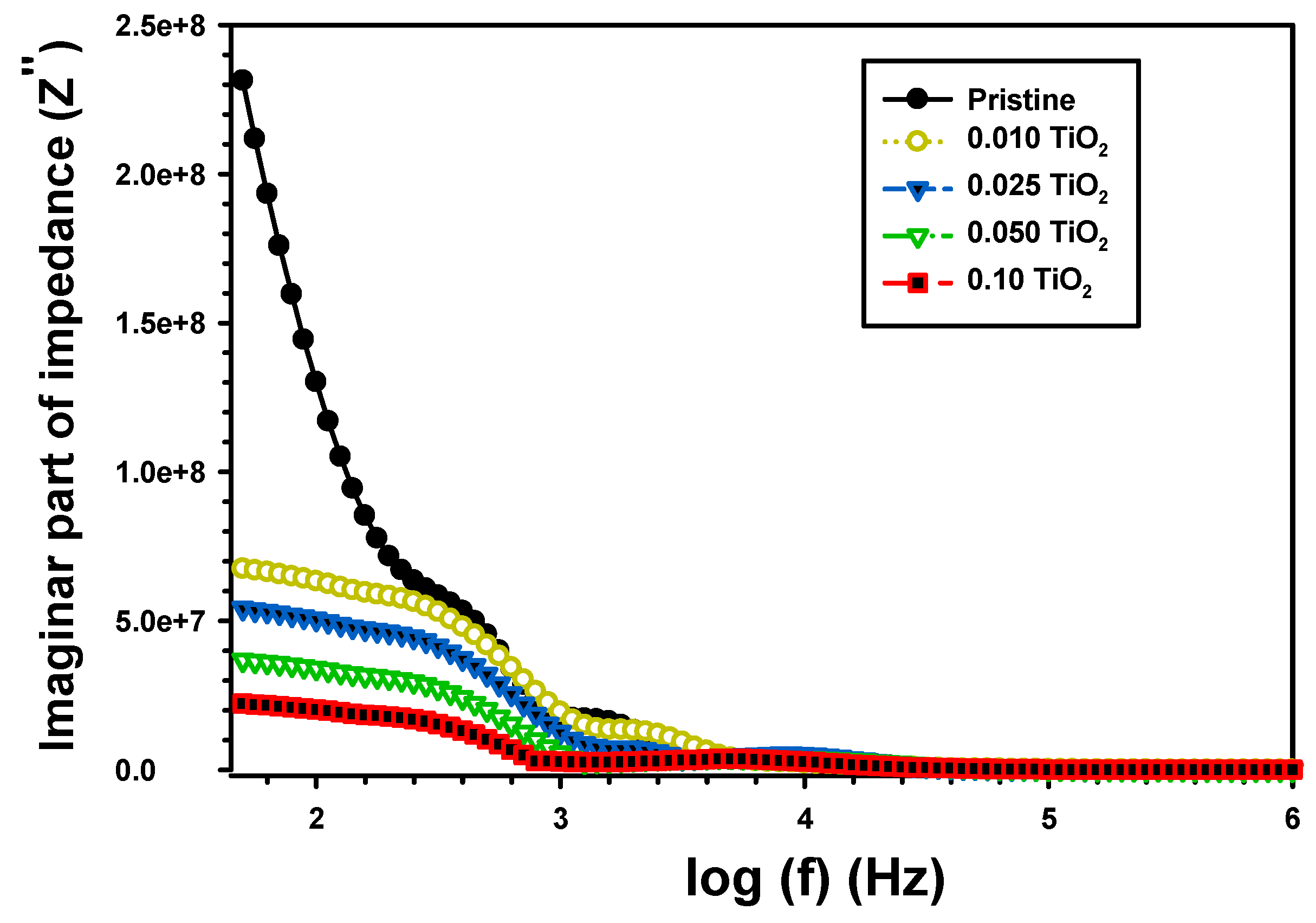
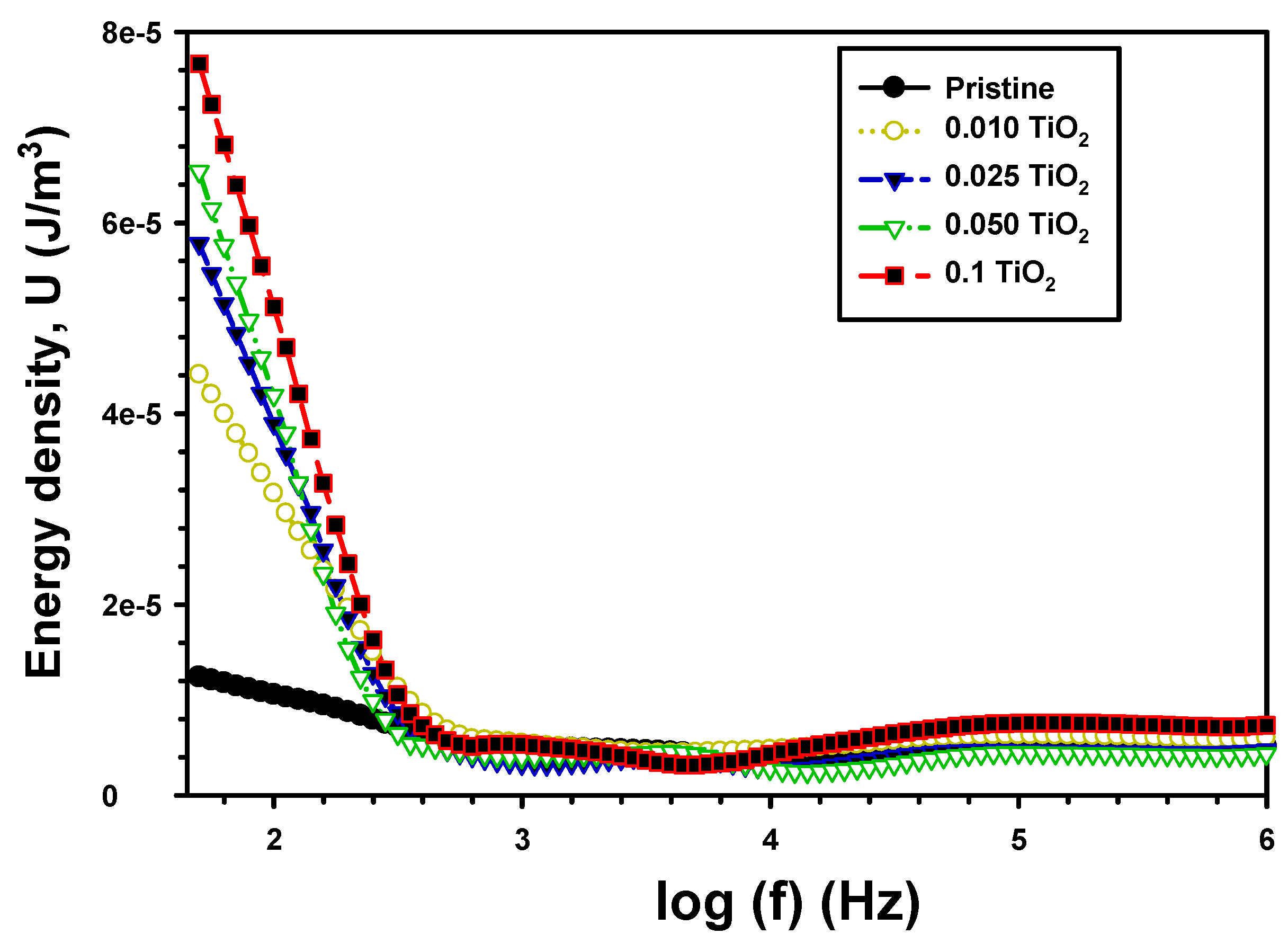
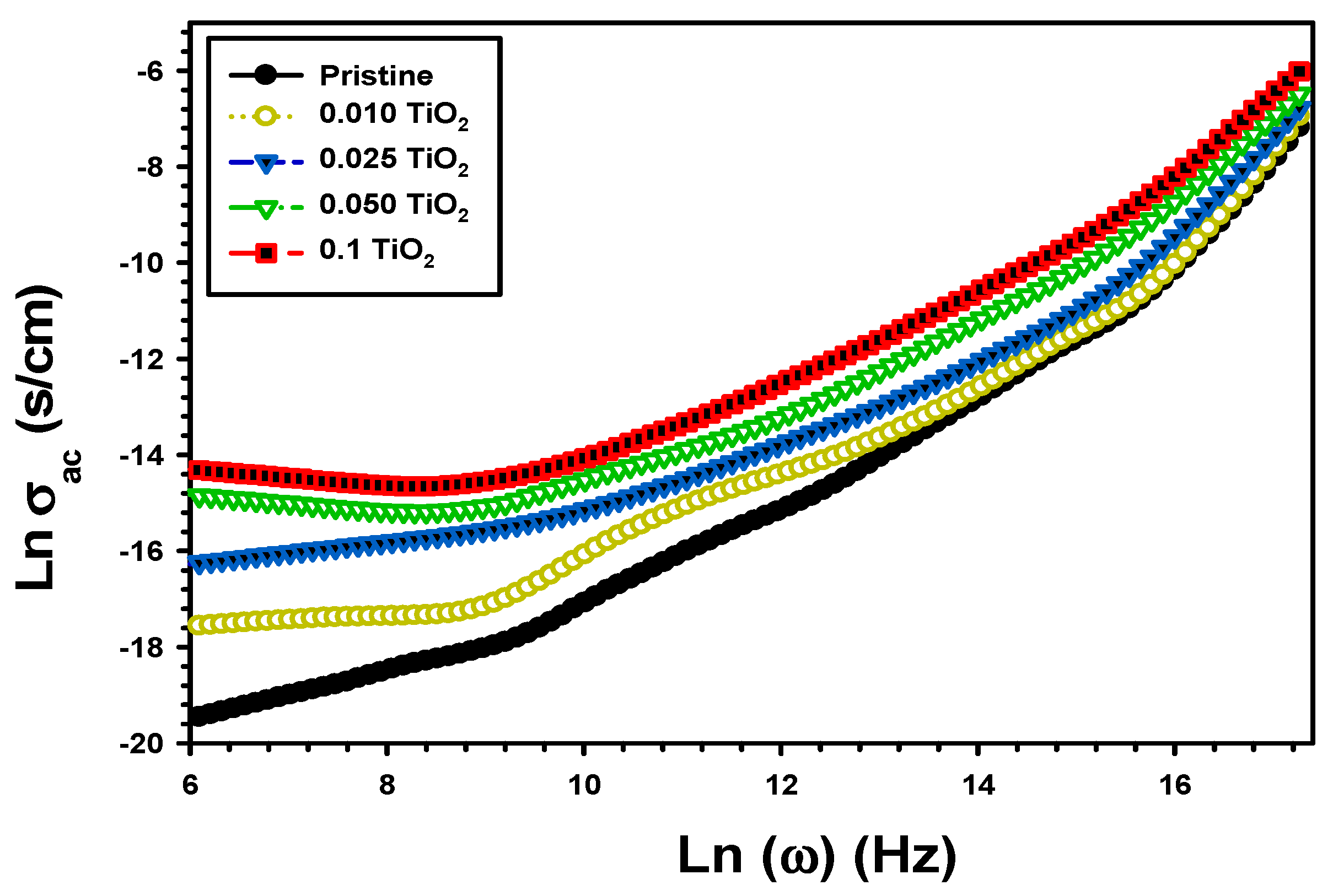

| Z’ | Z” | M′ | M″ | σac (S/cm) | U (J/m3) | |||
|---|---|---|---|---|---|---|---|---|
| PVA/Cs | 2.46 | 2.83 | 2.65 × 107 | 13 × 107 | 0.174 | 0.101 | 0.04 × 10−7 | 1.05 × 10−5 |
| 0.01%TiO2 | 7.38 | 9.61 | 2.07 × 107 | 6.5 × 107 | 0.089 | 0.073 | 0.25 × 10−7 | 3.16 × 10−5 |
| 0.025%TiO2 | 9.07 | 8.48 | 1.59 × 107 | 5.2 × 107 | 0.081 | 0.050 | 0.96 × 10−7 | 3.89 × 10−5 |
| 0.050%TiO2 | 9.76 | 9.37 | 1.45 × 107 | 3.6 × 107 | 0.221 | 0.029 | 3.38 × 10−7 | 4.18 × 10−5 |
| 0.1%TiO2 | 11.93 | 10.58 | 1.06 × 107 | 2.3 × 107 | 0.039 | 0.007 | 5.75 × 10−7 | 5.12 × 10- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, A.; Alotiby, M.F.; Al-Harbi, N.; El-Aassar, M.R.; Uosif, M.A.M.; Rabia, M. Fabrication, Structural Properties, and Electrical Characterization of Polymer Nanocomposite Materials for Dielectric Applications. Polymers 2023, 15, 3067. https://doi.org/10.3390/polym15143067
Atta A, Alotiby MF, Al-Harbi N, El-Aassar MR, Uosif MAM, Rabia M. Fabrication, Structural Properties, and Electrical Characterization of Polymer Nanocomposite Materials for Dielectric Applications. Polymers. 2023; 15(14):3067. https://doi.org/10.3390/polym15143067
Chicago/Turabian StyleAtta, Ali, Mohammed F. Alotiby, Nuha Al-Harbi, Mohamed R. El-Aassar, Mohamed A. M. Uosif, and Mohamed Rabia. 2023. "Fabrication, Structural Properties, and Electrical Characterization of Polymer Nanocomposite Materials for Dielectric Applications" Polymers 15, no. 14: 3067. https://doi.org/10.3390/polym15143067
APA StyleAtta, A., Alotiby, M. F., Al-Harbi, N., El-Aassar, M. R., Uosif, M. A. M., & Rabia, M. (2023). Fabrication, Structural Properties, and Electrical Characterization of Polymer Nanocomposite Materials for Dielectric Applications. Polymers, 15(14), 3067. https://doi.org/10.3390/polym15143067







