Preparation and Application of Active Bionanocomposite Films Based on Sago Starch Reinforced with a Combination of TiO2 Nanoparticles and Penganum harmala Extract for Preserving Chicken Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Peganum Harmala Extract
2.3. Preparation of Bionanocomposite Films
2.4. Tests performed on Bionanocomposite Films
2.4.1. Measurement of the Film Thickness
2.4.2. Measurement of the Water Vapor Permeability
2.4.3. The Oxygen Permeability
2.4.4. Antibacterial Activity Assays
2.5. Preparation of Coated Chicken Fillets with Bionanocomposite Films
2.6. Tests Performed on Chicken Fillets
2.6.1. Measurement of pH
2.6.2. Measurement of Total Volatile Basic Nitrogen
2.6.3. Microbiological Analysis
2.7. Statistical Analysis
3. Results and Discussions
3.1. The Thickness of the Films
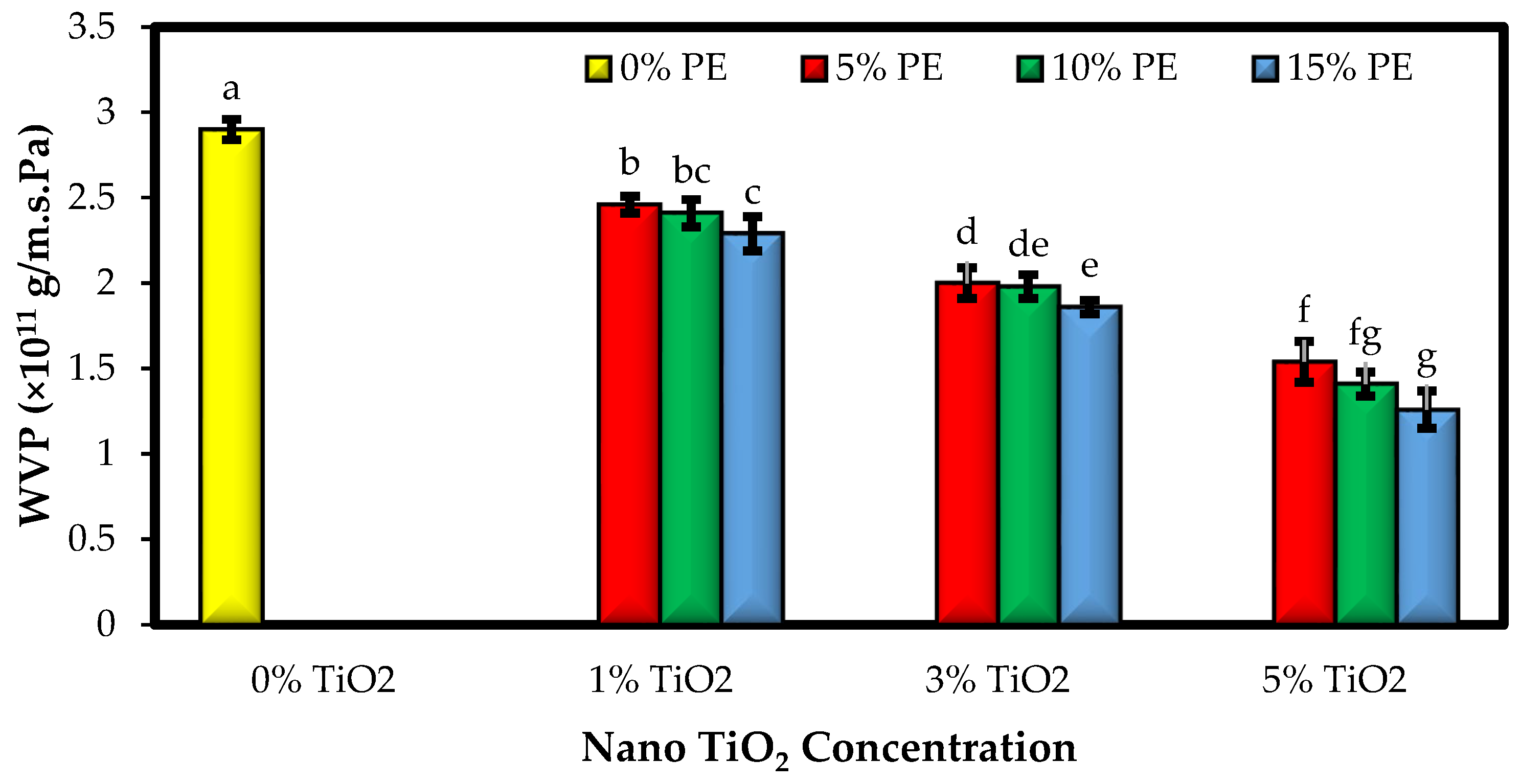
3.2. The Water Vapor Permeability of the Films (WVP)
3.3. The Oxygen Permeability of the Films (OP)
3.4. The Antibacterial Activity of the Films
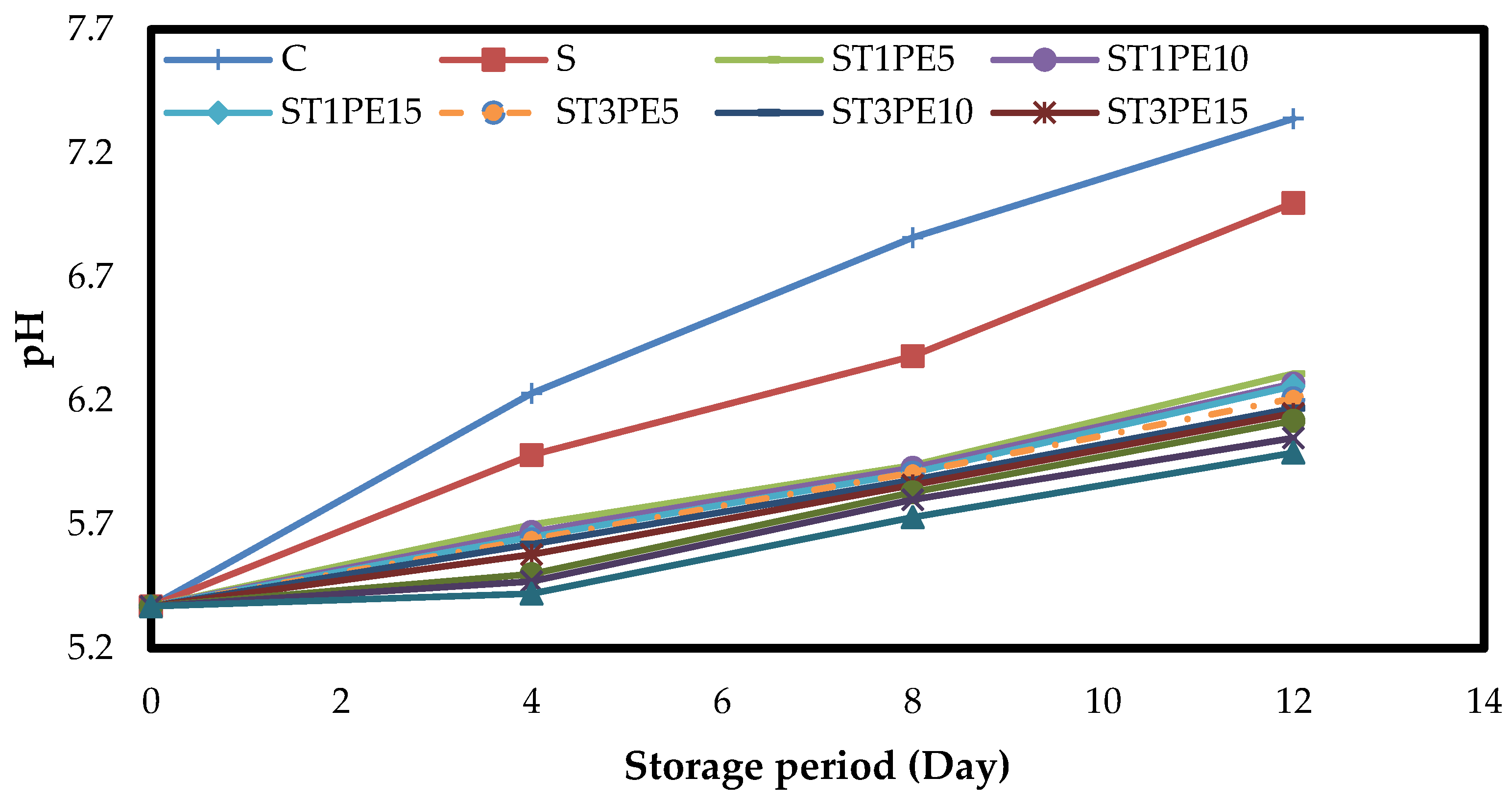
3.5. The pH of Chicken Fillets
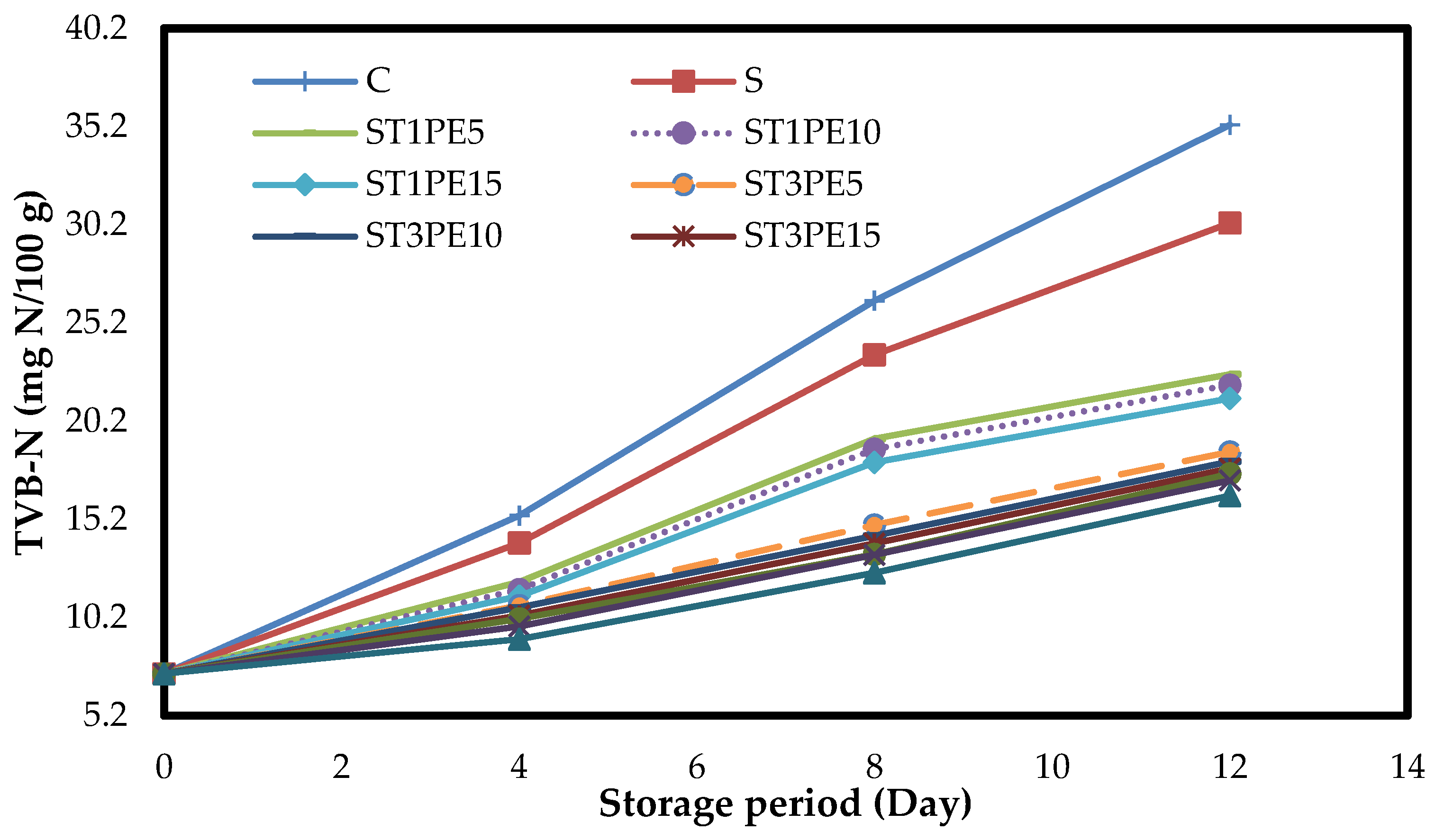
3.6. The Total Volatile Basic Nitrogen of Chicken Fillets (TVB-N)
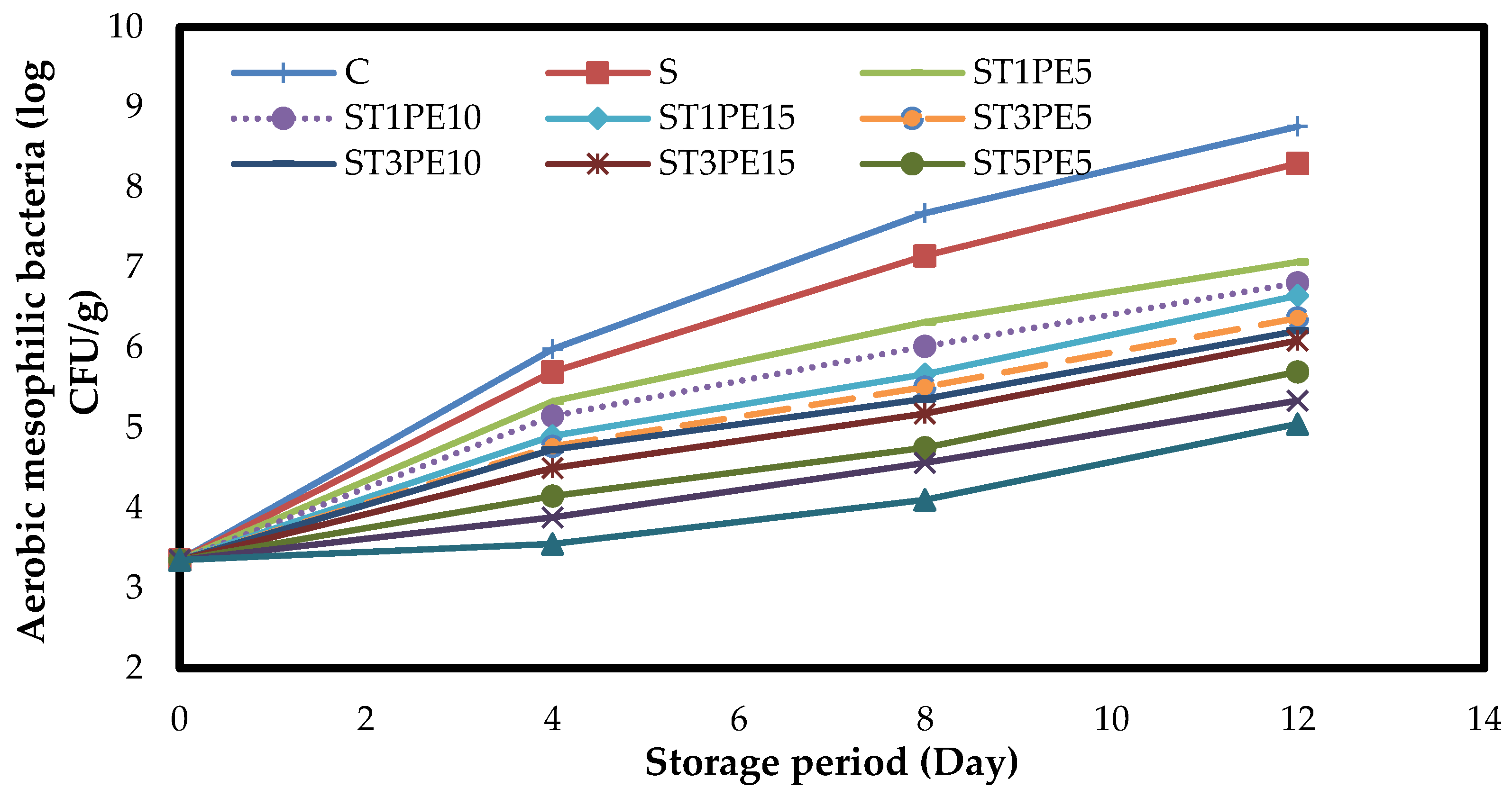
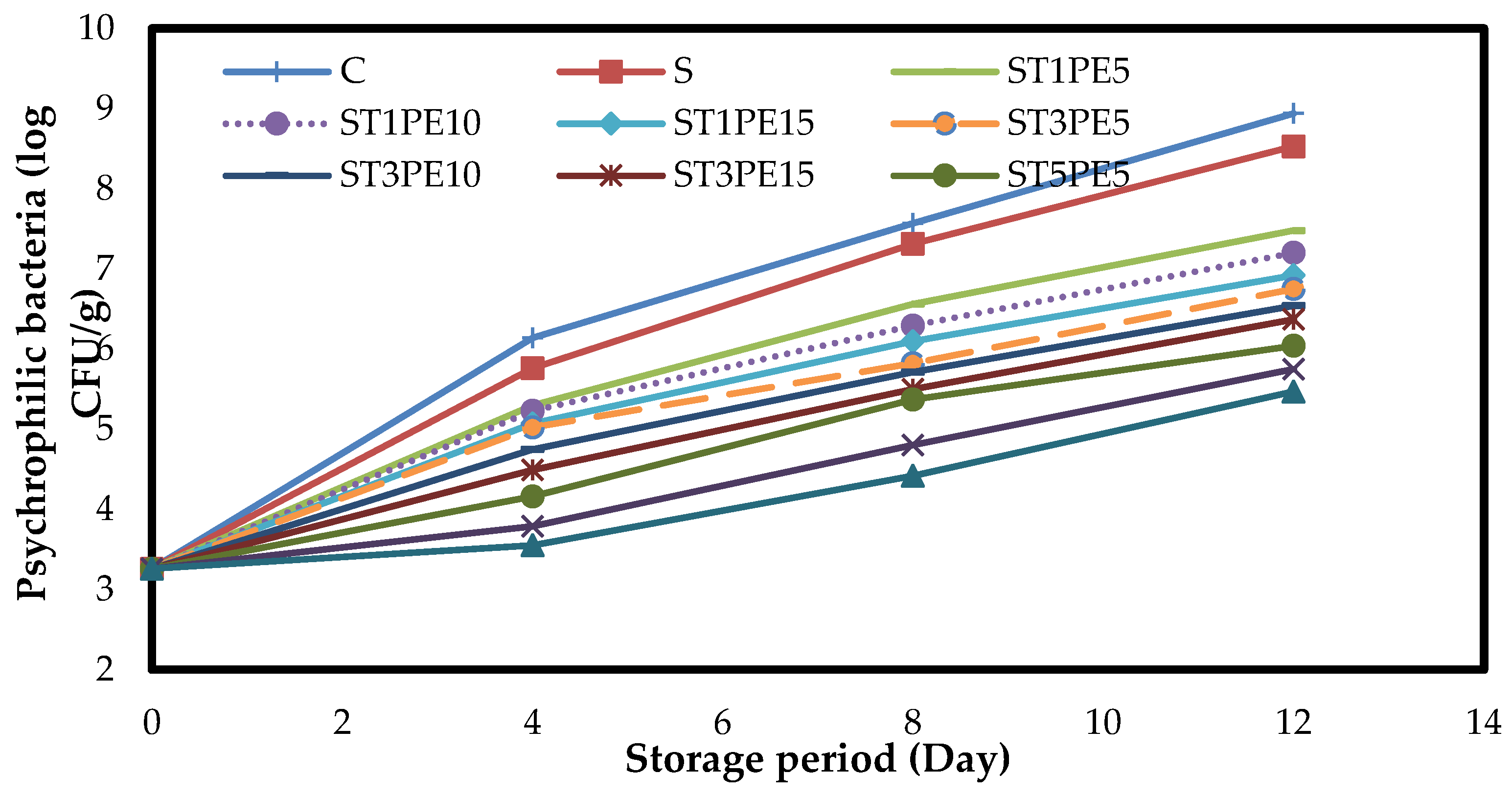
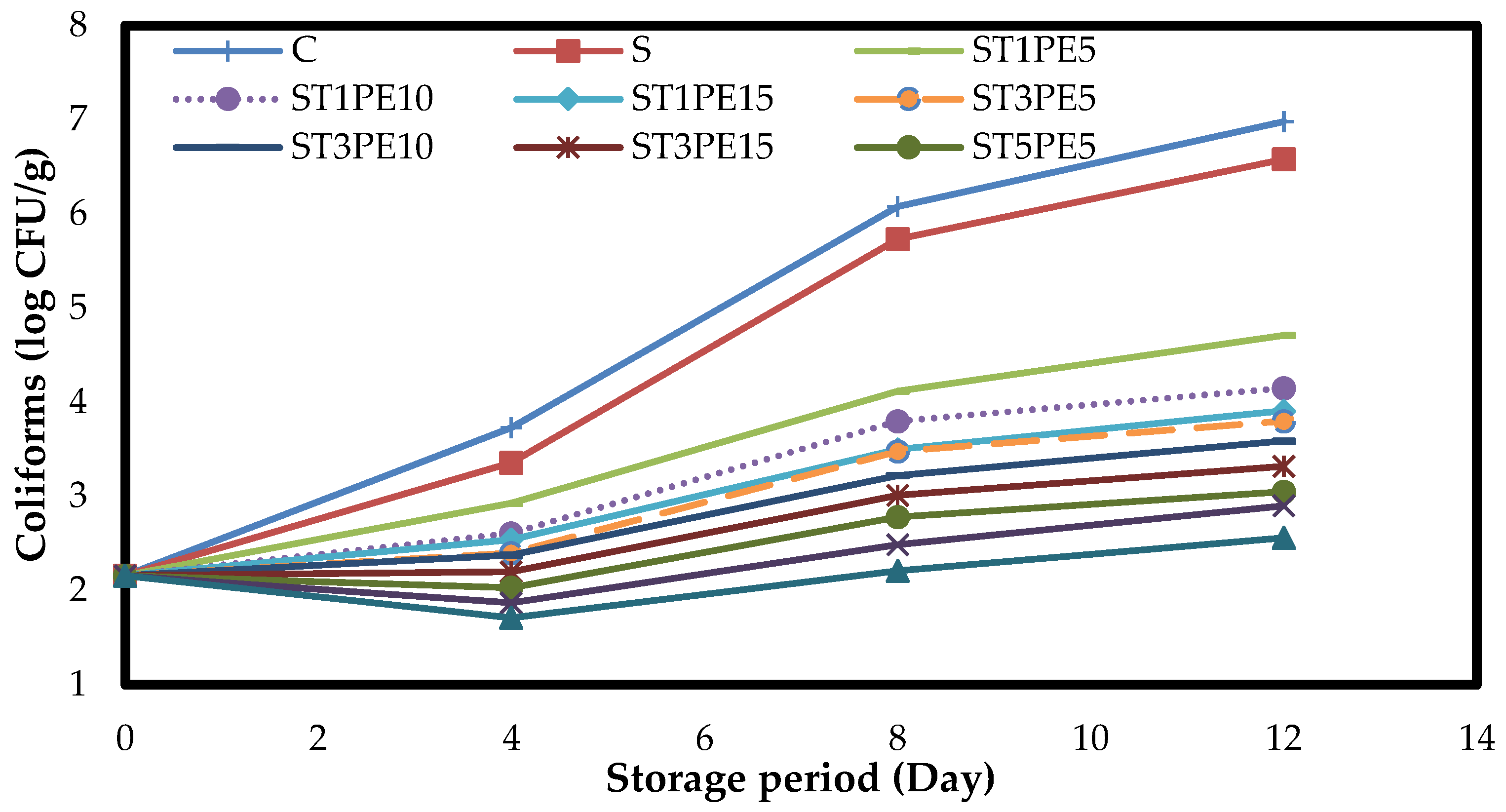
3.7. Microbiology of Chicken Fillets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Koshy, J.T.; Vasudevan, D.; Sangeetha, D.; Prabu, A.A. Biopolymer Based Multifunctional Films Loaded with Anthocyanin Rich Floral Extract and ZnO Nano Particles for Smart Packaging and Wound Healing Applications. Polymers 2023, 15, 2372. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, A.; Mohammadi Nafchi, A.; Baghaei, H.; Nouri, L. Fabrication and characterization of a smart film based on cassava starch and pomegranate peel powder for monitoring lamb meat freshness. Food Sci. Nutr. 2022, 10, 3293–3301. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.; Kuskov, T.; Matveeva, A.; Ulihin, A.; Bychkov, A.; Lomovskiy, I.; Polienko, Y. Disordering of Starch Films as a Factor Influencing the Release Rate of Biologically Active Substances. Polymers 2023, 15, 2303. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Ayaseh, A.; Alirezalu, K.; Nemati, Z.; Pateiro, M.; Lorenzo, J.M. Effect of chitosan coating incorporated with Artemisia fragrans essential oil on fresh chicken meat during refrigerated storage. Polymers 2021, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, V.; Mohammadi Nafchi, A.; Bolandi, M.; Baghaei, H. Fabrication and characterization of a pH-sensitive indicator film by purple basil leaves extract to monitor the freshness of chicken fillets. Food Packag. Shelf Life 2022, 34, 100946. [Google Scholar] [CrossRef]
- Afshar Mehrabi, F.; Sharifi, A.; Ahvazi, M. Effect of chitosan coating containing Nepeta pogonosperma extract on shelf life of chicken fillets during chilled storage. Food Sci. Nutr. 2021, 9, 4517–4528. [Google Scholar] [CrossRef]
- Alessandroni, L.; Caprioli, G.; Faiella, F.; Fiorini, D.; Galli, R.; Huang, X.; Marinelli, G.; Nzekoue, F.; Ricciutelli, M.; Scortichini, S.; et al. A shelf-life study for the evaluation of a new biopackaging to preserve the quality of organic chicken meat. Food Chem. 2022, 371, 131134. [Google Scholar] [CrossRef]
- Othman, S.H.; Wane, B.M.; Nordin, N.; Noor Hasnan, N.Z.; Talib, R.A.; Karyadi, J.N.W. Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers 2021, 13, 4060. [Google Scholar] [CrossRef]
- Noorian, S.; Mohammadi Nafchi, A.; Bolandi, M.; Jokar, M. Effects of Nano-Titanium Dioxide and Mentha piperita Essential Oil on Physicochemical, Mechanical, and Optical Properties of Cassava Starch Film. Starch-Stärke 2022, 74, 2200090. [Google Scholar] [CrossRef]
- Wang, C.; Gong, C.; Qin, Y.; Hu, Y.; Jiao, A.; Jin, Z.; Qiu, C.; Wang, J. Bioactive and functional biodegradable packaging films reinforced with nanoparticles. J. Food Eng. 2022, 312, 110752. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Villagrán-de la Mora, Z.; Ruvalcaba-Gómez, J.M.; Romero-Toledo, R.; Sandoval-Contreras, T.; Aguilera-Aguirre, S.; Montalvo-González, E.; Pérez-Larios, A. Use of titanium dioxide (TiO2) nanoparticles as reinforcement agent of polysaccharide-based materials. Processes 2020, 8, 1395. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef] [PubMed]
- Shaili, T.; Abdorreza, M.N.; Fariborz, N. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr. Polym. 2015, 134, 726–731. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Improving health benefits with considering traditional and modern health benefits of Peganum harmala. Clin. Phytosci. 2021, 7, 18. [Google Scholar] [CrossRef]
- Hemmati, C.; Nikooei, M. Peganum harmala is a new plant host of ‘Candidatus Phytoplasma aurantifolia’. J. Plant Pathol. 2019, 101, 1287. [Google Scholar] [CrossRef]
- Mohsenipour, Z.; Hassanshahian, M. Antibacterial activity of Espand (Peganum harmala) alcoholic extracts against six pathogenic bacteria in planktonic and biofilm forms. Biol. J. Microorg. 2016, 4, 109–120. [Google Scholar]
- Allaq, A.A.; Sidik, N.J.; Abdul-Aziz, A.; Ahmed, I.A. Antioxidant, antibacterial, and phytochemical screening of ethanolic crude extracts of Libyan Peganum harmala seeds. J. Pharm. Res. Int. 2021, 33, 74–82. [Google Scholar] [CrossRef]
- Tasallot Maraghi, E.; Kashef, N.; Gohari, A.R.; Fekrirad, Z. Antimicrobial Activity of Extracts from Satureja khuzistanica, Peganum harmala, Satureja sahendica on Planktonic Growth and Biofilm Formation of Staphylococcus aureus. J. Sabzevar Univ. Med. Sci. 2021, 28, 556–568. [Google Scholar]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, physicochemical and biological evaluation of quercetin based chitosan-gelatin film for food packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3985-17; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2017.
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Anon. AOAC 985.14: Moisture in Meat and Poultry Products Rapid Microwave Drying Method. In AOAC Official Methods of Analysis; AOAC: Gaithersburg, MD, USA, 1995; Chapter 39; pp. 79–80. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&cPath=1&products_id=2029 (accessed on 25 June 2023).
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Ghazihoseini, S.; Alipoormazandarani, N.; Nafchi, A.M. The Effects of Nano-SiO2 on Mechanical, Barrier, and Moisture Sorption Isotherm Models of Novel Soluble Soybean Polysaccharide Films. Int. J. Food Eng. 2015, 11, 833–840. [Google Scholar] [CrossRef]
- Rohn, S.; Rawel, H.M.; Kroll, J. Antioxidant activity of protein-bound quercetin. J. Agric. Food Chem. 2004, 52, 4725–4729. [Google Scholar] [CrossRef] [PubMed]
- Sani, I.K.; Pirsa, S.; Tağı, Ş. Preparation of chitosan/zinc oxide/Melissa officinalis essential oil nano-composite film and evaluation of physical, mechanical and antimicrobial properties by response surface method. Polym. Test. 2019, 79, 106004. [Google Scholar] [CrossRef]
- Suput, D.; Lazic, V.; Pezo, L.; Markov, S.; Vastag, Z.; Popovic, L.; Rudulovic, A.; Ostojic, S.; Zlatanovic, S.; Popovic, S. Characterization of starch edible films with different essential oils addition. Pol. J. Food Nutr. Sci. 2016, 66, 277–286. [Google Scholar] [CrossRef]
- Asdagh, A.; Karimi Sani, I.; Pirsa, S.; Amiri, S.; Shariatifar, N.; Eghbaljoo–Gharehgheshlaghi, H.; Shabahang, Z.; Taniyan, A. Production and Characterization of Nanocomposite Film Based on Whey Protein Isolated/Copper Oxide Nanoparticles Containing Coconut Essential Oil and Paprika Extract. J. Polym. Environ. 2021, 29, 335–349. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Tanomand, A.; Kazeminava, F.; Kamounah, F.S.; Ayaseh, A.; Ganbarov, K.; Yousefi, M.; Katourani, A.; Yousefi, B.; Kafil, H.S. Fabrication and characterization of a titanium dioxide (TiO2) nanoparticles reinforced bio-nanocomposite containing Miswak (Salvadora persica L.) extract—The antimicrobial, thermo-physical and barrier properties. Int. J. Nanomed. 2019, 14, 3439. [Google Scholar] [CrossRef]
- Pirsa, S.; Karimi Sani, I.; Khodayvandi, S. Design and fabrication of starch-nano clay composite films loaded with methyl orange and bromocresol green for determination of spoilage in milk package. Polym. Adv. Technol. 2018, 29, 2750–2758. [Google Scholar] [CrossRef]
- D’hooge, D.R.; Van Steenberge, P.H.M.; Reyniers, M.-F.; Marin, G.B. The strength of multi-scale modeling to unveil the complexity of radical polymerization. Prog. Polym. Sci. 2016, 58, 59–89. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Kim, Y.-T. Chapter 17—Biopolymer-Based Composite Packaging Materials with Nanoparticles. In Innovations in Food Packaging, 2nd ed.; Han, J.H., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 413–442. [Google Scholar]
- Hu, X.; Jia, X.; Zhi, C.; Jin, Z.; Miao, M. Improving the properties of starch-based antimicrobial composite films using ZnO-chitosan nanoparticles. Carbohydr. Polym. 2019, 210, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, K.; Asadpour, G.; Sharifi, H. Effect of ZnO nanoparticles on the mechanical, barrier and optical properties of thermoplastic cationic starch/montmorillonite biodegradable films. Int. J. Biol. Macromol. 2019, 124, 519–529. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films. Carbohydr. Polym. 2016, 147, 372–378. [Google Scholar] [CrossRef]
- Babapour, H.; Jalali, H.; Mohammadi Nafchi, A. The synergistic effects of zinc oxide nanoparticles and fennel essential oil on physicochemical, mechanical, and antibacterial properties of potato starch films. Food Sci. Nutr. 2021, 9, 3893–3905. [Google Scholar] [CrossRef]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Jokar, M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1–7. [Google Scholar] [CrossRef]
- Tamimi, N.; Mohammadi Nafchi, A.; Hashemi-Moghaddam, H.; Baghaie, H. The effects of nano-zinc oxide morphology on functional and antibacterial properties of tapioca starch bionanocomposite. Food Sci. Nutr. 2021, 9, 4497–4508. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.S.; Parveen, A.; Koppalkar, A.R.; Prasad, M.A. Effect of nano-titanium dioxide with different antibiotics against methicillin-resistant Staphylococcus aureus. J. Biomater. Nanobiotechnol. 2010, 1, 37. [Google Scholar] [CrossRef]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Ramezanloo, F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr. J. Pharm. Pharmacol. 2012, 6, 1573–1580. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Scherrer, R.; Gerhardt, P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J. Bacteriol. 1971, 107, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Kadam, D.M.; Wang, C.; Wang, S.; Grewell, D.; Lamsal, B.; Yu, C. Microstructure and antimicrobial functionality of nano-enhanced protein-based biopolymers. Trans. ASABE 2014, 57, 1141–1150. [Google Scholar]
- Din, M.I.; Sehar, R.; Hussain, Z.; Khalid, R.; Shah, A.T. Synthesis of biodegradable semolina starch plastic films reinforced with biogenically synthesized ZnO nanoparticles. Inorg. Nano-Met. Chem. 2021, 51, 985–994. [Google Scholar] [CrossRef]
- Lian, R.; Cao, J.; Jiang, X.; Rogachev, A.V. Physicochemical, antibacterial properties and cytocompatibility of starch/chitosan films incorporated with zinc oxide nanoparticles. Mater. Today Commun. 2021, 27, 102265. [Google Scholar] [CrossRef]
- Goulas, A.E.; Kontominas, M.G. Effect of modified atmosphere packaging and vacuum packaging on the shelf-life of refrigerated chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Eur. Food Res. Technol. 2007, 224, 545–553. [Google Scholar] [CrossRef]
- Khezrian, A.; Shahbazi, Y. Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. Int. J. Biol. Macromol. 2018, 106, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti Khiabani, M.; Akhondzadeh Basti, A.; Abdulkhani, A. The effects of gelatin-CMC films incorporated with chitin nanofiber and Trachyspermum ammi essential oil on the shelf life characteristics of refrigerated raw beef. Int. J. Food Microbiol. 2020, 318, 108493. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Sensory and biochemical aspects of quality of whole bigeye tuna (Thunnus obesus) during bulk storage in controlled atmospheres. Food Chem. 2005, 89, 347–354. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, F.; Ahmed, I.; Li, Z.; Lin, H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control 2018, 92, 37–46. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009, 82, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Russo, F.; Torrieri, E.; Masi, P.; Villani, F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 2006, 72, 4663–4671. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Román, F. Antimicrobial nanomaterials as water disinfectant: Applications, limitations and future perspectives. Sci. Total Environ. 2014, 466–467, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Rsaissi, N.; Kamili, E.; Bencharki, B.; Hillali, L.; Bouhache, M. Antimicrobial activity of fruits extracts of the wild jujube “Ziziphus lotus (L.) Desf”. Int. J. Sci. Eng. Res. 2013, 4, 1521–1528. [Google Scholar]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Abderrahim, L.A.; Taïbi, K.; Ait Abderrahim, C. Assessment of the Antimicrobial and Antioxidant Activities of Ziziphus lotus and Peganum harmala. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 409–414. [Google Scholar] [CrossRef]
- Darabpour, E.; Bavi, A.P.; Motamedi, H.; Nejad, S.M.S. Antibacterial activity of different parts of Peganum harmala L. growing in Iran against multi-drug resistant bacteria. EXCLI J. 2011, 10, 252. [Google Scholar]
- Arfat, Y.A.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Yarnpakdee, S. Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. J. Food Sci. Technol. 2015, 52, 6182–6193. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Qin, Y.; Ye, Q. Effect of nano-TiO2-LDPE packaging on microbiological and physicochemical quality of Pacific white shrimp during chilled storage. Int. J. Food Sci. Technol. 2015, 50, 1567–1573. [Google Scholar] [CrossRef]



| Film Samples | E. coli | S. aureus | L. monocytogenes | S. entritidis |
|---|---|---|---|---|
| Control | ND | ND | ND | ND |
| 1% TiO2 + 5%PE | 48.52 ± 0.21 i | 63.75 ± 0.25 i | 47.32 ± 0.48 i | 42.15 ± 0.23 i |
| 1% TiO2 + 10%PE | 51.74 ± 0.17 h | 66.43 ± 0.19 h | 50.29 ± 0.34 h | 45.17 ± 0.30 h |
| 1% TiO2 + 15%PE | 56.31 ± 0.32 g | 69.49 ± 0.28 g | 55.57 ± 0.20 g | 49.37 ± 0.16 g |
| 3% TiO2 + 5%PE | 79.41 ± 0.25 f | 92.31 ± 0.45 f | 78.31 ± 0.18 f | 75.58 ± 0.22 f |
| 3% TiO2 + 10%PE | 83.18 ± 0.30 e | 94.59 ± 0.36 e | 81.98 ± 0.41 e | 80.33 ± 0.39 e |
| 3% TiO2 + 15%PE | 86.90 ± 0.09 d | 97.13 ± 0.41 d | 85.37 ± 0.15 d | 84.52 ± 0.17 d |
| 5% TiO2 + 5%PE | 93.61 ± 0.29 c | 107.83 ± 0.33 c | 92.82 ± 0.24 c | 90.88 ± 0.14 c |
| 5% TiO2 + 10%PE | 97.59 ± 0.16 b | 109.11 ± 0.22 b | 96.33 ± 0.19 b | 94.07 ± 0.43 b |
| 5% TiO2 + 15%PE | 100.82 ± 0.41 a | 112.50 ± 0.47 a | 100.14 ± 0.20 a | 99.14 ± 0.25 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagher Abiri, A.; Baghaei, H.; Mohammadi Nafchi, A. Preparation and Application of Active Bionanocomposite Films Based on Sago Starch Reinforced with a Combination of TiO2 Nanoparticles and Penganum harmala Extract for Preserving Chicken Fillets. Polymers 2023, 15, 2889. https://doi.org/10.3390/polym15132889
Bagher Abiri A, Baghaei H, Mohammadi Nafchi A. Preparation and Application of Active Bionanocomposite Films Based on Sago Starch Reinforced with a Combination of TiO2 Nanoparticles and Penganum harmala Extract for Preserving Chicken Fillets. Polymers. 2023; 15(13):2889. https://doi.org/10.3390/polym15132889
Chicago/Turabian StyleBagher Abiri, Alireza, Homa Baghaei, and Abdorreza Mohammadi Nafchi. 2023. "Preparation and Application of Active Bionanocomposite Films Based on Sago Starch Reinforced with a Combination of TiO2 Nanoparticles and Penganum harmala Extract for Preserving Chicken Fillets" Polymers 15, no. 13: 2889. https://doi.org/10.3390/polym15132889
APA StyleBagher Abiri, A., Baghaei, H., & Mohammadi Nafchi, A. (2023). Preparation and Application of Active Bionanocomposite Films Based on Sago Starch Reinforced with a Combination of TiO2 Nanoparticles and Penganum harmala Extract for Preserving Chicken Fillets. Polymers, 15(13), 2889. https://doi.org/10.3390/polym15132889









