Triumfetta cordifolia Gum as a Promising Bio-Ingredient to Stabilize Emulsions with Potentials in Cosmetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Protocol for Emulsion Formulation
2.3. Analytical Methods
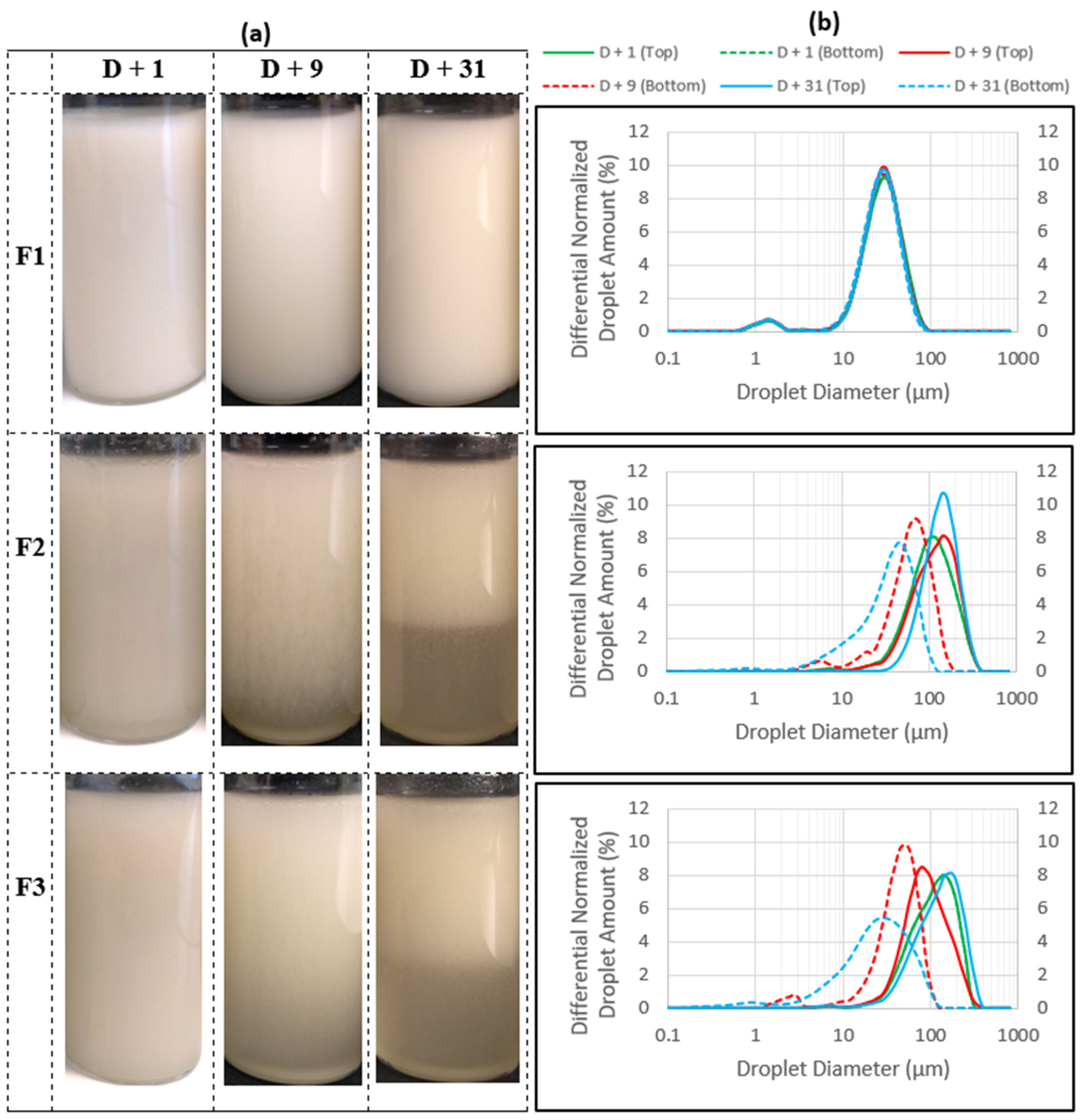
2.3.1. Visual Observation
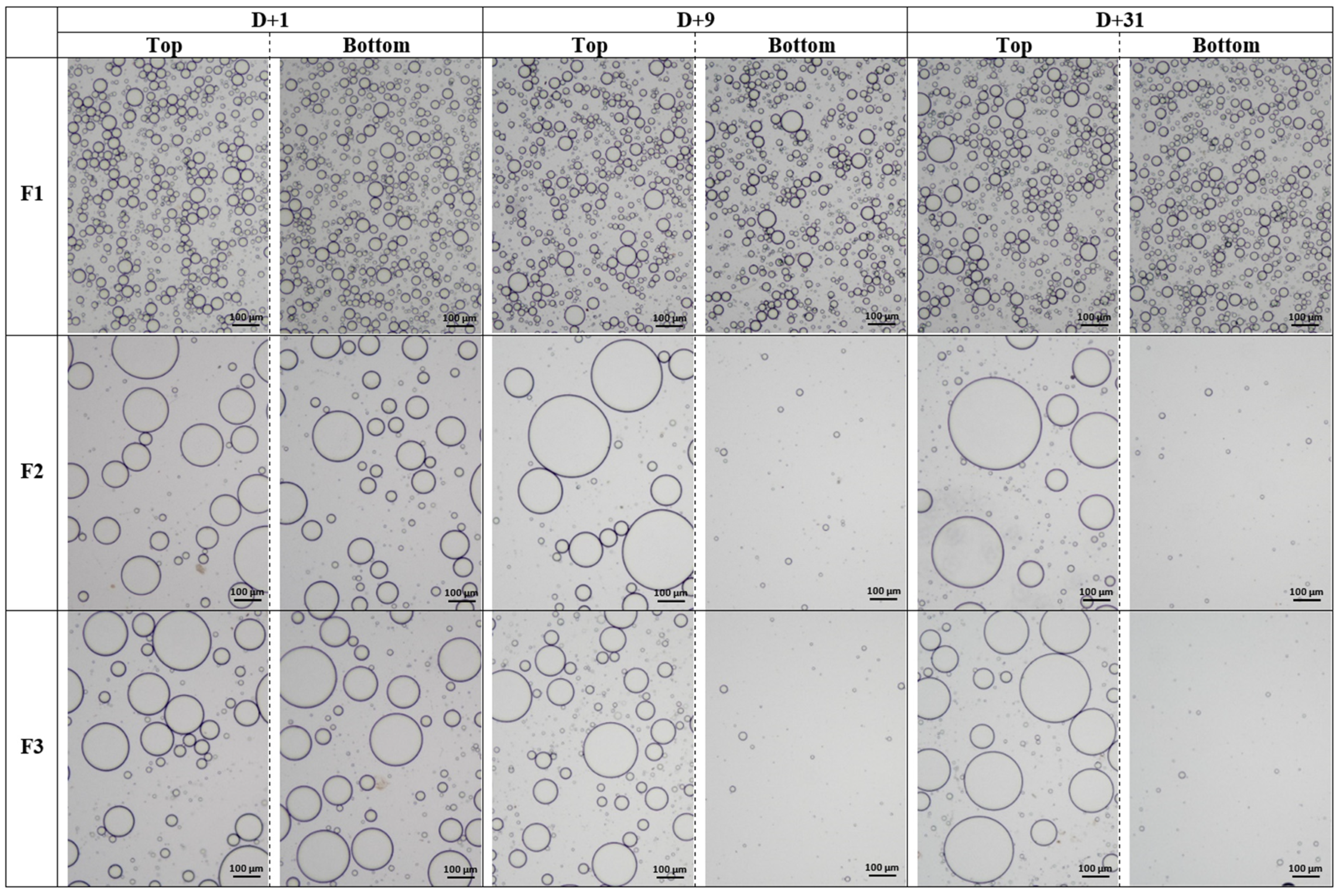
2.3.2. Optical Microscopy
2.3.3. Droplet Size Distribution
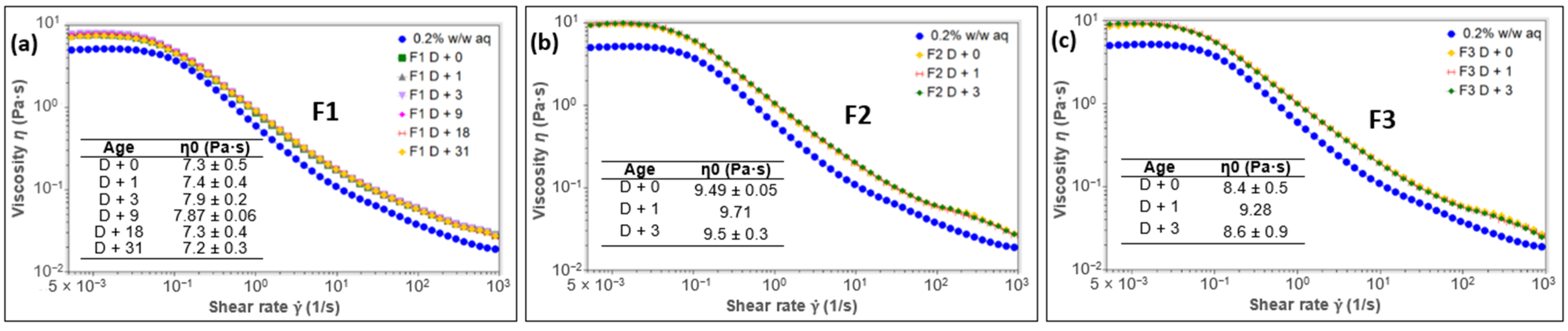
2.3.4. Rheology
2.3.5. pH Monitoring
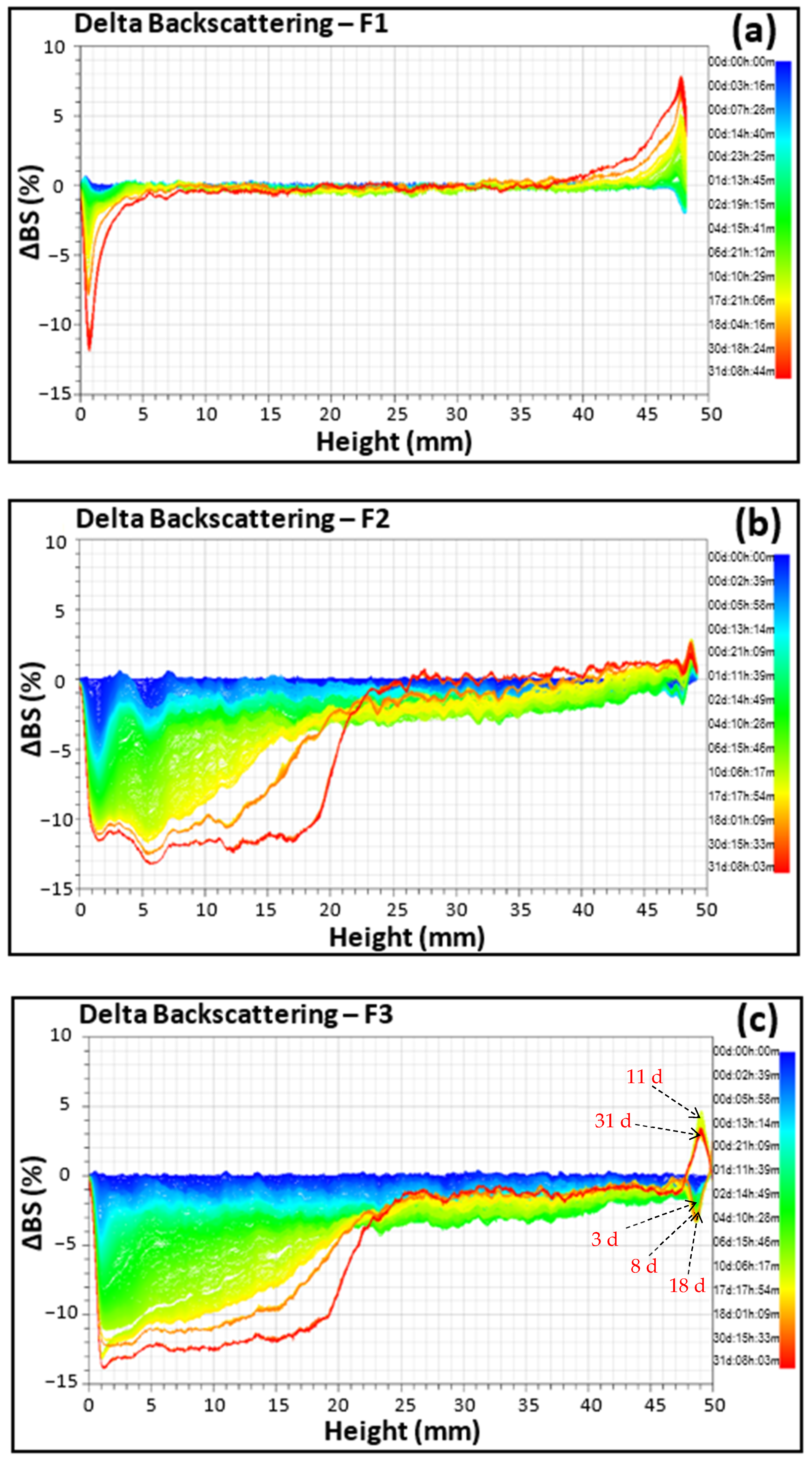
2.3.6. Stability Monitoring of Emulsions Using the SMLS Technique
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
| Emulsion | Date | |||||
|---|---|---|---|---|---|---|
| D = 0 | D + 1 | D + 3 | D + 9 | D + 18 | D + 31 | |
| F1 | 4.4 | 4.6 | 4.5 | 4.7 | 4.4 | 4.8 |
| F2 | 4.3 | 4.7 | 4.6 | 4.7 | 4.4 | 4.7 |
| F3 | 4.4 | 4.4 | 4.4 | 4.4 | 4.3 | 4.6 |
| Emulsion | D + 1 | D + 9 | D + 31 | |||
|---|---|---|---|---|---|---|
| Top | Bottom | Top | Bottom | Top | Bottom | |
| F1 | 22.9 ± 0.3 | 22.5 ± 0.3 | 22.1 ± 0.3 | 22.3 ± 0.3 | 22.1 ± 0.3 | |
| F2 | 92.8 ± 0.3 | 102.4 ± 0.3 | 50.3 ± 0.3 | 121.5 ± 0.2 | 26.9 ± 0.4 | |
| F3 | 94.4 ± 0.3 | 80.4 ± 0.3 | 34.8 ± 0.3 | 109.9 ± 0.3 | 19.4 ± 0.5 | |
| D + 1 | D + 9 | D + 31 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Top | Middle | Bottom | Global | Top | Middle | Bottom | Global | Top | Middle | Bottom | Global | |
| F1 | 0.4 | 0.2 | 0.4 | 0.3 | 1.4 | 0.7 | 1.3 | 0.9 | 3.6 | 1.1 | 2.9 | 2.0 |
| F2 | 1.2 | 1.6 | 3.1 | 1.9 | 2.7 | 3.4 | 8.0 | 4.2 | 4.1 | 6.7 | 12.5 | 7.9 |
| F3 | 1.5 | 2.3 | 3.1 | 2.3 | 2.8 | 4.2 | 9.3 | 5.2 | 6.9 | 6.5 | 15.2 | 8.8 |
References
- Draelos, Z.D. The use of cosmetic products to improve self esteem & quality of life. In Essential Psychiatry for the Aesthetic Practitioner; Rieder, E.A., Fried, R.G., Eds.; John Wiley & Sons Ltd.: Oxford, UK, 2021; pp. 34–41. [Google Scholar] [CrossRef]
- Cosmetics Market Size, Share & COVID-19 Impact Analysis, by Category (Hair Care, Skin Care, Makeup and Others), by Gender (Men and Women), by Distribution Channel (Specialty Stores, Hypermarkets/Supermarkets, Online Channels, and Other), and Regional Forecasts, 2012–2028. Available online: https://www.fortunebusinessinsights.com/cosmetics-market-102614 (accessed on 11 September 2022).
- Sikora, E. Cosmetic Emulsions: Monograph; Wydawnictwo PK: Kraków, Poland, 2019; p. 10. [Google Scholar]
- Zhang, W.; Xu, X.; Zhao, X.; Zhou, G. Insight into the oil polarity impact on interfacial properties of myofibrillar protein. Food Hydrocoll. 2022, 128, 107563. [Google Scholar] [CrossRef]
- Chanamai, R.; Horn, G.; McClements, D.J. Influence of Oil Polarity on Droplet Growth in Oil-in-Water Emulsions Stabilized by a Weakly Adsorbing Biopolymer or a Nonionic Surfactant. J. Colloid Interface Sci. 2002, 247, 167–176. [Google Scholar] [CrossRef]
- Nash, J.J.; Erk, K. Stability and Interfacial Viscoelasticity of Oil-Water Nanoemulsions Stabilized by Soy Lecithin and Tween 20 for the Encapsulation of Bioactive Carvacrol. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 517, 1–11. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsions: Formation, Stability, Industrial Applications; De Gruyter: Berlin, Germany; Boston, MA, USA, 2016; pp. 1–195. [Google Scholar] [CrossRef]
- Stojiljković, D.; Arsić, I.; Tasic, M. The influence of polar and non-polar emollients on the structure and skin moisturizing potential of the emulsions stabilized by mixed emulsifier. Acta Med. Median. 2016, 55, 25–30. [Google Scholar] [CrossRef]
- Savary, G.; Grisel, M.; Picard, C. Impact of emollients on the spreading properties of cosmetic products: A combined sensory and instrumental characterization. Colloids Surf. B 2013, 102, 371–378. [Google Scholar] [CrossRef]
- Terescenco, D.; Savary, G.; Picard, C.; Clemenceau, F.; Merat, E.; Grisel, M. Influence of the emollient on emulsions containing lamellar liquid crystals: From molecular organization towards applicative properties. Int. J. Cosmet. Sci. 2018, 40, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Global Cosmetic Ingredients Market 2019-2023 | Rising Prominence of Clean Labeling in Cosmetic Formulations to Boost Growth. Available online: https://apnews.com/press-release/pr-businesswire/1eaab62c99c4493d8c2b0c2dfa026f88 (accessed on 29 May 2021).
- APG II. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003, 141, 399–436. [Google Scholar] [CrossRef]
- Lay, K. The American species of Triumfetta L. Ann. Mo. Bot. Gard. 1950, 37, 317–318. [Google Scholar] [CrossRef]
- Jiofack, R.B.T. Triumfetta cordifolia. In Plant Resources of Tropical Africa 16. Fibres; Brink, M., Achigan-Dako, E.G., Eds.; PROTA Foundation/CTA: Wageningen, The Nederlands, 2012; pp. 440–443. [Google Scholar]
- Rye, B.L. A contribution to the taxonomy of the Tiliaceae of Western Australia. Nuytsia 1998, 9, 415–420. [Google Scholar] [CrossRef]
- Carney, J.A. African traditional plant knowledge in the Circum-Caribbean region. J. Ethnobiol. 2003, 23, 167–185. [Google Scholar]
- Hequet, V.; Le Corre, M.; Rigault, F.; Blanfort, V. Les Espèces Exotiques Envahissantes de Nouvelle-Calédonie; IRD, AMAP: Nouméa, Nouvelle-Calédonie, 2009; pp. 17–61. [Google Scholar]
- Ruffo, C.K.; Birnie, A.; Tengnäs, B. Edible Wild Plants of Tanzania; RELMA/Sida: Nairobi, Kenya, 2002; pp. 668–669. [Google Scholar]
- Leung, T.W.; Busson, F.; Jardin, C. Food Composition Table for Use in Africa; FAO: Rome, Italy, 1968. [Google Scholar]
- Latham, P.; Mbuta, A.K.K. Useful Plants of Bas-Congo province, Democratic Republic of Congo, 2nd ed.; The Salvation Army: Kinshasa, Democratic Republic of Congo, 2014; p. 512. [Google Scholar]
- Saidou, C. Propriétés physico-chimiques et fonctionnelles des gommes hydrocolloïdes des écorces de Triumfetta cordifolia (Tiliacée) et de Bridelia thermifolia (Euphorbiacée). Ph.D. Thesis, Université de Grenoble, Grenoble, France, 2012. [Google Scholar]
- Woguia, A.L.; Ngondi, J.L.; Boudjeko, T.; Rihouey, C.; Oben, E.J. Hypolipidemic and antioxidative effects of Dika Nut (Irvingia gabonensis) seeds and Nkui (Trimphetta cordifolia) stem bark mucilages in Triton WR-1339 induced hyperlipidemic Rats. Food Sci. Biotechnol. 2012, 21, 1715–1721. [Google Scholar] [CrossRef]
- Idris, O.M.; Williams, P.A.; Phillips, G.O. Characterisation of gum from Acacia senegal trees of different age and location using multi-detection gel permeation chromatography. Food Hydrocoll. 1998, 12, 379–388. [Google Scholar] [CrossRef]
- Al-Assaf, S.; Phillips, G.O.; Amar, V. Gum Ghatti. In Handbook of hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 477–494. [Google Scholar]
- Saidou, C.; Tchatchueng, J.B.; Ndjouenkeu, R.; Roux, D.C.D. Extraction and Partial Characterisation of Hydrocolloid Gums from Some African Legumes. J. Food Eng. 2011, 7, 1898–1905. [Google Scholar] [CrossRef]
- Myritol® 331. Available online: https://carecreations.basf.us/files/pdf/Myrtiol-331-benchmarking-data.pdf (accessed on 6 May 2023).
- Terescenco, D.; Picard, C.; Clemenceau, F.; Grisel, M.; Savary, G. Influence of the emollient structure on the properties of cosmetic emulsion containing lamellar liquid crystals. Colloids Surf. A 2018, 536, 10–19. [Google Scholar] [CrossRef]
- Rojas, E.E.G.; Coimbra, J.S.R.; Telis-Romero, J. Thermophysical Properties of Cotton, Canola, Sunflower and Soybean Oils as a Function of Temperature. Int. J. Food Prop. 2013, 16, 1620–1629. [Google Scholar] [CrossRef]
- Naddaf, M.; Alzier, A.; Allaham, H. Surface tension and hydroperoxide content of vegetable oils: Effect of repeated deep frying. Rev. Roum. Chim. 2021, 66, 931–939. [Google Scholar]
- Product Specification. Available online: https://shop.chemsupply.com.au/documents/PL041.pdf (accessed on 6 May 2023).
- Maldonado-Valderrama, J.; Miller, R.; Fainerman, V.B.; Wilde, P.J.; Morris, V.J. Effect of gastric conditions on β-lactoglobulin interfacial networks: Influence of the oil phase on protein structure. Langmuir ACS J. Surf. Colloids 2010, 26, 15901–15908. [Google Scholar] [CrossRef]
- El-Mahrab-Robert, M.; Rosilio, V.; Bolzinger, M.A.; Chaminade, P.; Grossiord, J.L. Assessment of oil polarity: Comparison of evaluation methods. Int. J. Pharm. 2008, 348, 89–94. [Google Scholar] [CrossRef]
- Bergfreund, J.; Siegenthaler, S.; Lutz-Bueno, V.; Bertsch, P.; Fischer, P. Surfactant Adsorption to Different Fluid Interfaces. Langmuir 2021, 37, 6722–6727. [Google Scholar] [CrossRef]
- Fraunhofer Diffraction Theory and Mie Scattering Theory. Available online: https://www.shimadzu.com/an/service-support/technical-support/analysis-basics/lesson13.html (accessed on 4 November 2022).
- Anderson, D.S. The acid-base balance of the skin. Br. J. Dermatol. 1951, 63, 283–296. [Google Scholar] [CrossRef]
- Du Plessis, J.L.; Stefaniak, A.B.; Wilhelm, K.P. Measurement of Skin Surface pH. Curr. Probl. Dermatol. 2018, 54, 19–25. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Bancroft, W.D. The Theory of Emulsification, I.J. Phys. Chem 1912, 16, 177–233. [Google Scholar] [CrossRef]
- Bancroft, W.D. The Theory of Emulsification, V.J. Phys. Chem 1913, 17, 501–519. [Google Scholar] [CrossRef]
- Atgié, M.; Masbernat, O.; Roger, K. Emulsions Stabilized by Gum Arabic: Composition and Packing within Interfacial Films. Langmuir 2019, 35, 962–972. [Google Scholar] [CrossRef]
- Garti, N.; Leser, M.E. Emulsification Properties of Hydrocolloids. Polym. Adv. Technol. 2001, 12, 123–135. [Google Scholar] [CrossRef]
- Randall, C.; Phillips, G.O.; Williams, P.A. The role of the proteinaceous component on the emulsifying properties of gum Arabic. Food Hydrocoll. 1988, 2, 131–140. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial interactions and the stability of oil-in-water emulsions. Pure Appl. Chem. 1992, 64, 1721–1724. [Google Scholar] [CrossRef]
- Weiss, J.; Muschiolik, G. Factors Affecting the Droplet Size of Water-in-Oil Emulsions (W/O) and the Oil Globule Size in Water-in-Oil-in-Water Emulsions (W/O/W). J. Dispers. Sci. Technol. 2007, 28, 703–716. [Google Scholar] [CrossRef]
- Hadnađev, T.D.; Dokić, P.; Krstonošić, V.; Hadnađev, M. Influence of oil phase concentration on droplet size distribution and stability of oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2013, 115, 313–321. [Google Scholar] [CrossRef]
- Dong, B.; Qin, Z.; Wang, Y.; Zhang, J.; Xu, Z.; Liu, A.; Guo, X. Investigating the Rheology and Stability of Heavy Crude Oil-in-Water Emulsions Using APG08 Emulsifiers. ACS Omega 2022, 7, 37736–37747. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T.G. Rhéologie Appliquée: Sur la route de la Rhéologie avec Joe Flow, 3rd ed.; Anton Paar GmbH: Graz, Autriche, 2020; pp. 31–46. [Google Scholar]
- TRIOS Online Help. Available online: http://www.ifug.ugto.mx/~labblanda/manuales/TRIOS_Help_DHR-AR.htm (accessed on 20 February 2022).
- Napper, D.H. The Role of Polymers in the Stabilization of Disperse Systems. In Chemistry and Technology of Water-Soluble Polymers; Finch, C.A., Ed.; Springer: Boston, MA, USA, 1983; pp. 233–248. [Google Scholar] [CrossRef]
- Napper, D.H. Polymeric Stabilization of Colloidal Dispersions; Academic Press: New York, NY, USA, 1984; pp. 18–30. [Google Scholar]
- Blijdenstein, T.B.J.; van Winden, A.J.M.; van Vlieta, T.; van der Linden, E.; van Aken, G.A. Serum separation and structure of depletion- and bridging-flocculated emulsions: A comparison. Colloids Surf. A Physicochem. Eng. Asp. 2004, 245, 41–48. [Google Scholar] [CrossRef]
- Dickinson, E. A Model of a Concentrated Dispersion Exhibiting Bridging Flocculation and Depletion Flocculation. J. Colloid Interface Sci. 1989, 132, 274–278. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]


| Trade Name | INCI Name | Composition | Density at 22 °C (g·cm−3) * | Viscosity at 20 °C (mPa·s) | Surface Tension at 25 °C (mN·m−1) | Interfacial Tension versus Water at 25 °C (mN·m−1) |
|---|---|---|---|---|---|---|
| Myritol 331 | Cocoglycerides | Blend of mono-, di and triglycerides ** R-OOC-CH2-CH(OH)-CH2-OH R-OOC-CH2-CH(COOR)-CH2-OH R-OOC-CH2-CH(COOR)-CH2-COOR | 0.93 | 43–48 [26] | 29.54 ± 0.01 [27] | 8.37 ± 0.36 [27] |
| Sunflower oil *** | Helianthus annuus seed oil | Fatty acid triglycerides R-OOC-CH2-CH(COOR)-CH2-COOR with residual free fatty acids | 0.91 | 63.9 [28] | 35.1 ± 0.34 [29] | 27.1 [29] |
| Paraffin oil Codex AAB2 | Paraffinum liquidum | Alkanes -R | 0.83 | 32 [30] | 30.02 ± 0.02 [27] | 54.00 ± 0.56 [27] |
| Phase | Ingredient (INCI Name) | Function | Weight Percentage (%) |
|---|---|---|---|
| Aqueous phase | Ultrapure water | Solvent | 78.8 |
| Polymer (T. cordifolia) | Emulsifying, stabilizing and texturizing agent | 0.2 | |
| Dehydroacetic acid (and) benzyl alcohol | Preservative | 1.0 | |
| Fatty phase (code of emulsion) | Cocoglycerides (F1) | Emollient | 20.0 |
| Helianthus annuus seed oil (F2) | |||
| Paraffinum liquidum (F3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanwa, M.N.; Malhiac, C.; Hucher, N.; Cheumani, A.M.Y.; Ndikontar, M.K.; Grisel, M. Triumfetta cordifolia Gum as a Promising Bio-Ingredient to Stabilize Emulsions with Potentials in Cosmetics. Polymers 2023, 15, 2828. https://doi.org/10.3390/polym15132828
Fanwa MN, Malhiac C, Hucher N, Cheumani AMY, Ndikontar MK, Grisel M. Triumfetta cordifolia Gum as a Promising Bio-Ingredient to Stabilize Emulsions with Potentials in Cosmetics. Polymers. 2023; 15(13):2828. https://doi.org/10.3390/polym15132828
Chicago/Turabian StyleFanwa, Michèle N., Catherine Malhiac, Nicolas Hucher, Arnaud M. Y. Cheumani, Maurice K. Ndikontar, and Michel Grisel. 2023. "Triumfetta cordifolia Gum as a Promising Bio-Ingredient to Stabilize Emulsions with Potentials in Cosmetics" Polymers 15, no. 13: 2828. https://doi.org/10.3390/polym15132828
APA StyleFanwa, M. N., Malhiac, C., Hucher, N., Cheumani, A. M. Y., Ndikontar, M. K., & Grisel, M. (2023). Triumfetta cordifolia Gum as a Promising Bio-Ingredient to Stabilize Emulsions with Potentials in Cosmetics. Polymers, 15(13), 2828. https://doi.org/10.3390/polym15132828







