Analyses of Experimental Dental Adhesives Based on Zirconia/Silver Phosphate Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoparticles
2.2. Synthesis of Experimental Dental Adhesive
2.3. Characterizations
2.3.1. Antimicrobial and Anti-Biofilm Assessment
Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
Inhibition of Biofilm Formation
2.3.2. Structural and Degree of Conversion Analyses
2.3.3. Micro-Hardness Testing
2.3.4. Flexural Strength & Flexural Modulus
2.3.5. Bond Strength Analysis
2.3.6. Color Stability Measurement
2.4. Statistical Analysis
3. Results
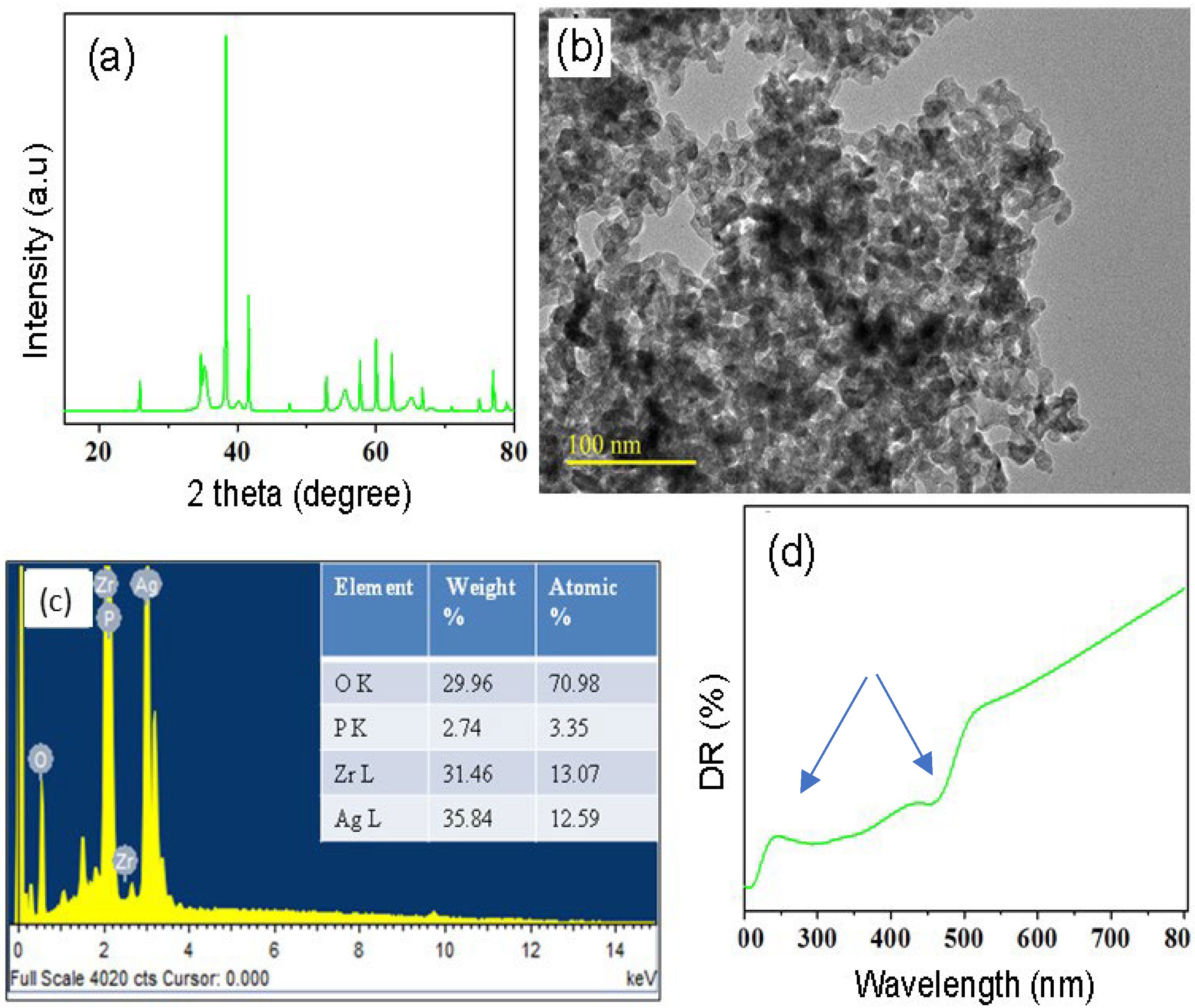
3.1. Nanoparticle Analyses
3.2. Antimicrobial Assessment
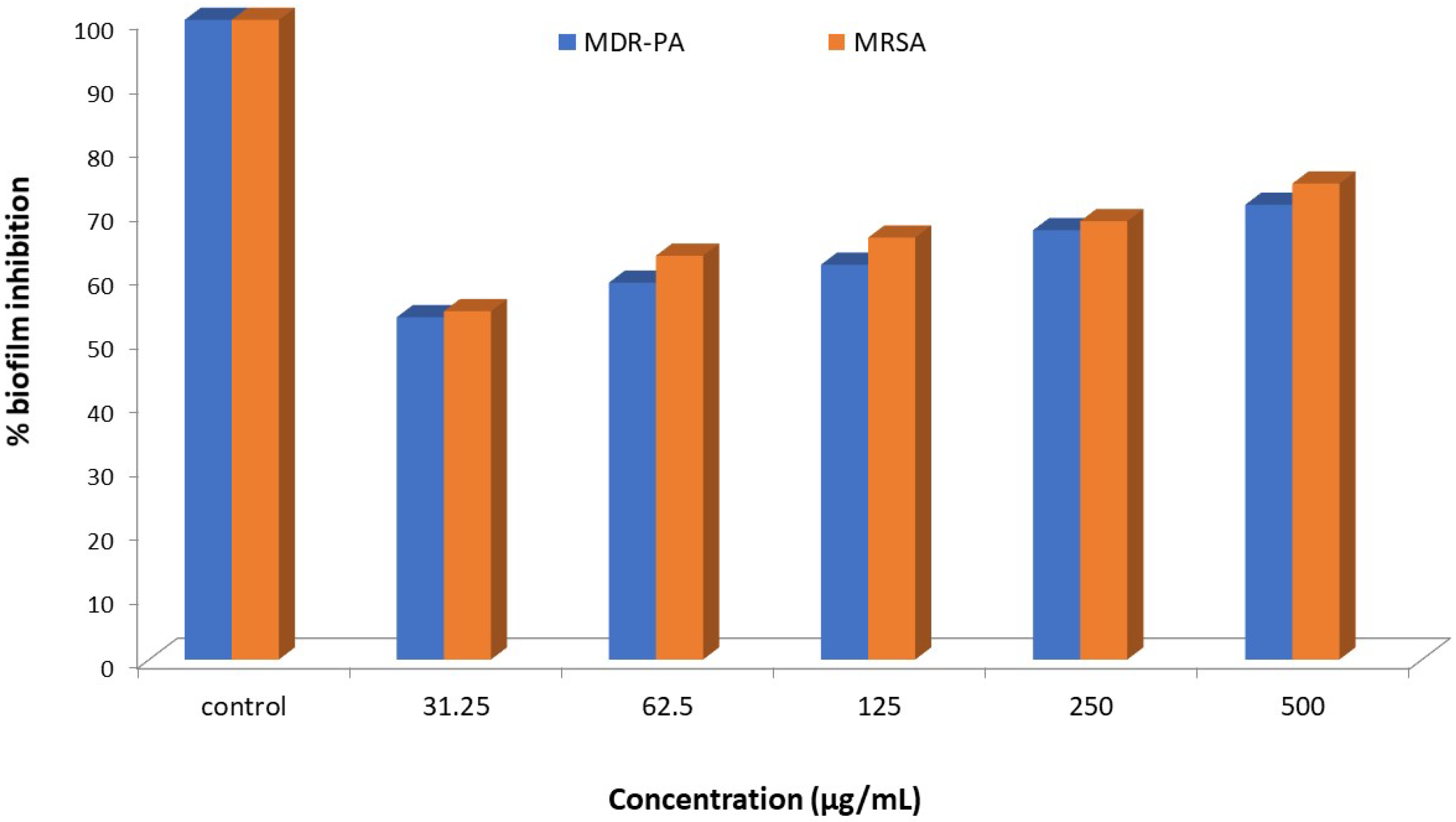
Biofilm Inhibition
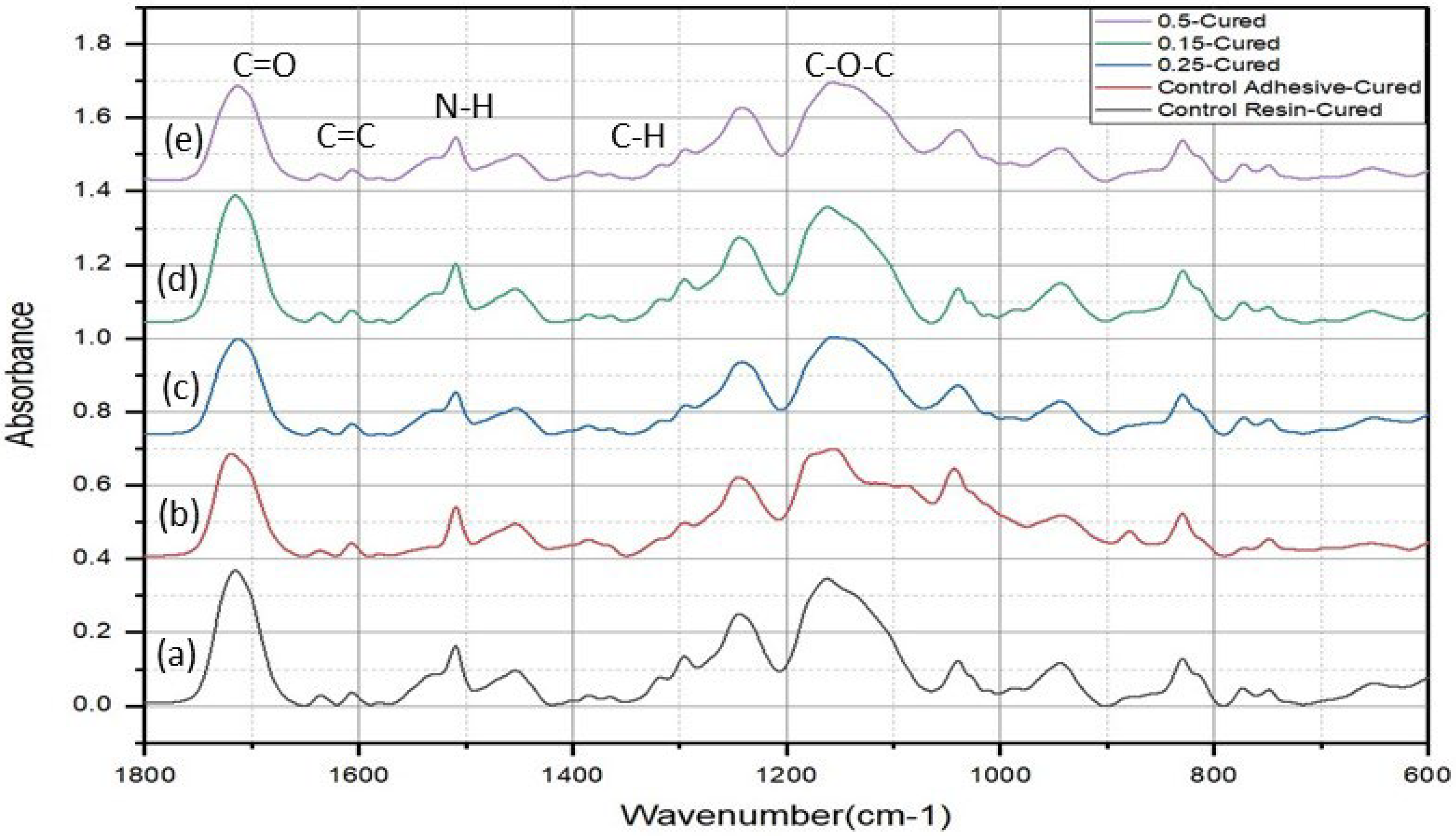
3.3. Structural and Degree of Conversion Analyses
3.4. Micro-Hardness Test
3.5. Flexural Strength and Modulus
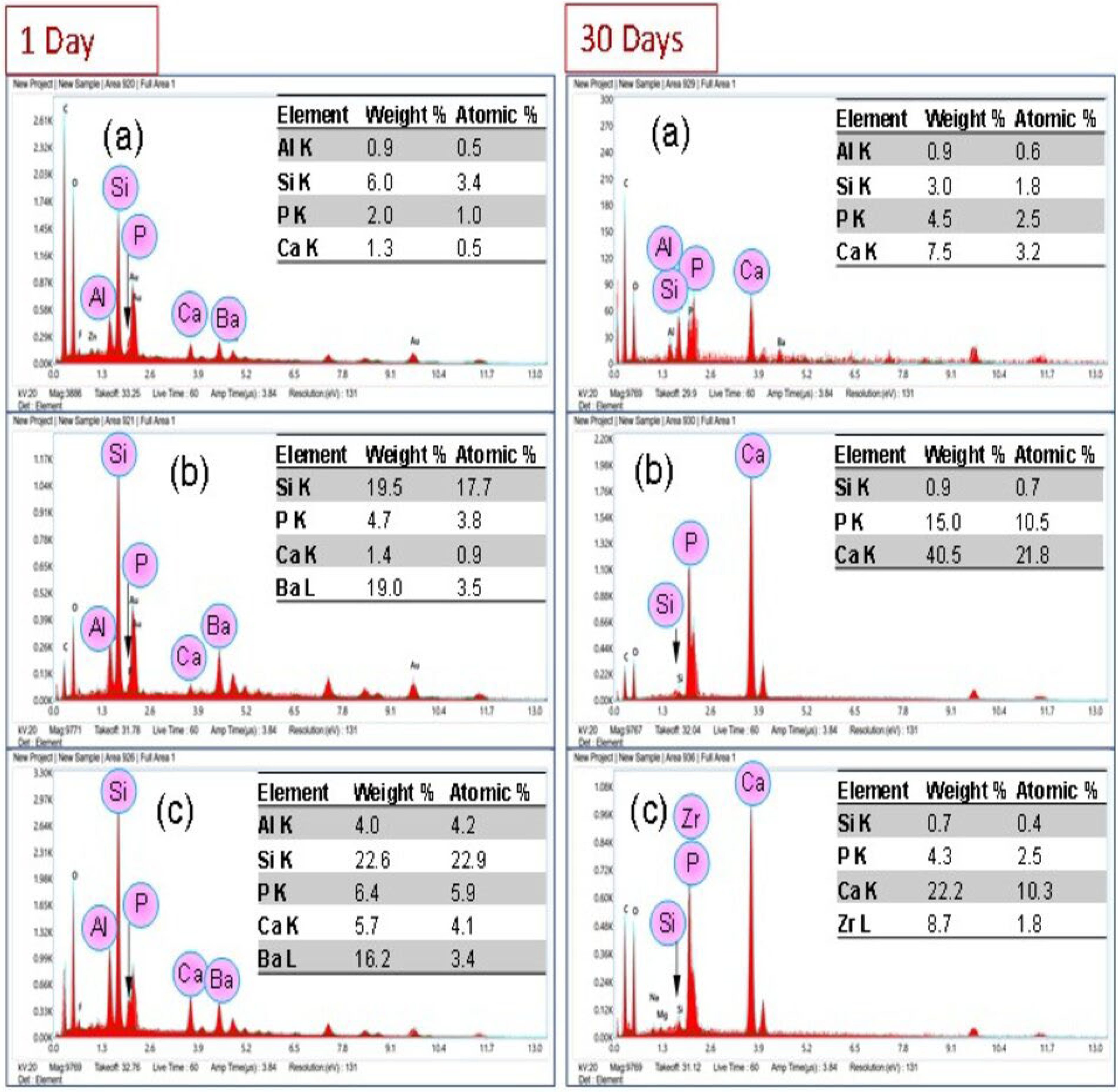
3.6. Bond Strength Analysis
3.7. Color Change Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demarco, F.F.; Collares, K.; Correa, M.B.; Cenci, M.S.; MORAES, R.R.d.; Opdam, N.J. Should my composite restorations last forever? Why are they failing? Braz. Oral Res. 2017, 31 (Suppl. S1), e56. [Google Scholar] [CrossRef]
- Zhang, Z.; Beitzel, D.; Mutluay, M.; Tay, F.R.; Pashley, D.H.; Arola, D. On the durability of resin–dentin bonds: Identifying the weakest links. Dent. Mater. 2015, 31, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying adhesive materials to improve the longevity of resinous restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef]
- Khan, A.S.; Ur Rehman, S.; AlMaimouni, Y.K.; Ahmad, S.; Khan, M.; Ashiq, M. Bibliometric analysis of literature published on antibacterial dental adhesive from 1996–2020. Polymers 2020, 12, 2848. [Google Scholar] [CrossRef]
- Vilde, T.; Stewart, C.A.; Finer, Y. Simulating the Intraoral Aging of Dental Bonding Agents: A Narrative Review. Dent. J. 2022, 10, 13. [Google Scholar] [CrossRef]
- Nassif, M.; El Askary, F. Nanotechnology and nanoparticles in contemporary dental adhesives. In Nanobiomaterials in Clinical Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 163–198. [Google Scholar]
- Zhang, J.; Zhao, Y.; Tian, Z.; Zhu, J.; Shi, Z.; Cui, Z.; Zhu, S. Enhancement performance of application mussel-biomimetic adhesive primer for dentin adhesives. RSC Adv. 2020, 10, 12035–12046. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Melo, M.A.; Weir, M.; Zhou, X.; Xu, H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef]
- Imzato, S. Immobilization of an antibacterial component in composite resin. Dent. Jpn. 1993, 30, 63–68. [Google Scholar]
- Mazloom-Jalali, A.; Taromi, F.A.; Atai, M.; Solhi, L. Dual modified nanosilica particles as reinforcing fillers for dental adhesives: Synthesis, characterization, and properties. J. Mech. Behav. Biomed. Mater. 2020, 110, 103904. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.C.; Meier, M.M.; Loguercio, A.D.; Cecchin, F.; Gomes, O.M.M.; Reis, A. Effects of zirconia nanoparticles addition to experimental adhesives on radiopacity and microhardness. Braz. J. Oral Sci. 2013, 12, 319–322. [Google Scholar] [CrossRef]
- Tao, S.; He, L.; Xu, H.H.; Weir, M.D.; Fan, M.; Yu, Z.; Zhang, M.; Zhou, X.; Liang, K.; Li, J. Dentin remineralization via adhesive containing amorphous calcium phosphate nanoparticles in a biofilm-challenged environment. J. Dent. 2019, 89, 103193. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Khalid, H.; AlMaimouni, Y.K.; Ikram, S.; Khan, M.; Din, S.U.; Talal, A.; Khan, A.S. Microwave assisted urethane grafted nano-apatites for dental adhesives. J. Bioact. Compat. Polym. 2020, 35, 479–490. [Google Scholar] [CrossRef]
- Al-Saleh, S.; Alateeq, A.; Alshaya, A.H.; Al-Qahtani, A.S.; Tulbah, H.I.; Binhasan, M.; Shabib, S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of TiO2 and ZrO2 nanoparticles on adhesive bond strength and viscosity of dentin polymer: A physical and chemical evaluation. Polymers 2021, 13, 3794. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Bermudez, J.; Dávila-Sánchez, A.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Hernández, M.; Astorga, J.; Reis, A.; Loguercio, A.D.; Farago, P.V. Zinc oxide and copper nanoparticles addition in universal adhesive systems improve interface stability on caries-affected dentin. J. Mech. Behav. Biomed. Mater. 2019, 100, 103366. [Google Scholar] [CrossRef] [PubMed]
- Atai, M.; Solhi, L.; Nodehi, A.; Mirabedini, S.M.; Kasraei, S.; Akbari, K.; Babanzadeh, S. PMMA-grafted nanoclay as novel filler for dental adhesives. Dent. Mater. 2009, 25, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, Y.; Fan, Y.; Ren, L.; Tang, X.; Meng, X. Application of silver nanoparticles in situ synthesized in dental adhesive resin. Int. J. Adhes. Adhes. 2021, 108, 102890. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Weir, M.D.; Liu, H.; Zhou, X.; Xu, H.H. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent. Mater. 2013, 29, 462–472. [Google Scholar] [CrossRef]
- Xue, J.; Zan, G.; Wu, Q.; Deng, B.; Zhang, Y.; Huang, H.; Zhang, X. Integrated nanotechnology for synergism and degradation of fungicide SOPP using micro/nano-Ag3PO4. Inorg. Chem. Front. 2016, 3, 354–364. [Google Scholar] [CrossRef]
- Panthi, G.; Ranjit, R.; Kim, H.-Y.; Mulmi, D.D. Size dependent optical and antibacterial properties of Ag3PO4 synthesized by facile precipitation and colloidal approach in aqueous solution. Optik 2018, 156, 60–68. [Google Scholar] [CrossRef]
- Nawaz, M.; Ansari, M.A.; Paz, A.P.; Hisaindee, S.; Qureshi, F.; Ul-Hamid, A.; Hakeem, A.S.; Taha, M. Sonochemical synthesis of ZnCo 2 O 4/Ag 3 PO 4 heterojunction photocatalysts for the degradation of organic pollutants and pathogens: A combined experimental and computational study. New J. Chem. 2022, 46, 14030–14042. [Google Scholar] [CrossRef]
- Qureshi, F.; Nawaz, M.; Ansari, M.A.; Khan, F.A.; Berekaa, M.M.; Abubshait, S.A.; Al-Mutairi, R.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M. Synthesis of M-Ag3PO4,(M=Se, Ag, Ta) Nanoparticles and Their Antibacterial and Cytotoxicity Study. Int. J. Mol. Sci. 2022, 23, 11403. [Google Scholar] [CrossRef] [PubMed]
- Trench, A.B.; Machado, T.R.; Gouveia, A.F.; Foggi, C.C.; Teodoro, V.; Sánchez-Montes, I.; Teixeira, M.M.; da Trindade, L.G.; Jacomaci, N.; Perrin, A. Rational design of W-doped Ag3PO4 as an efficient antibacterial agent and photocatalyst for organic pollutant degradation. ACS Omega 2020, 5, 23808–23821. [Google Scholar] [CrossRef]
- Shao, J.; Ma, J.; Lin, L.; Wang, B.; Jansen, J.A.; Walboomers, X.F.; Zuo, Y.; Yang, F. Three-dimensional printing of drug-loaded scaffolds for antibacterial and analgesic applications. Tissue Eng. Part C Methods 2019, 25, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Hu, R.; Yang, Y.; Li, P.; Wu, Q. Bifunctional nano-Ag 3 PO 4 with capabilities of enhancing ceftazidime for sterilization and removing residues. RSC Adv. 2019, 9, 17913–17920. [Google Scholar] [CrossRef] [PubMed]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A.S. Influence of incorporation of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int. J. Nanomed. 2016, 11, 5633. [Google Scholar] [CrossRef]
- Yang, J.; Shen, J.; Wu, X.; He, F.; Xie, H.; Chen, C. Effects of nano-zirconia fillers conditioned with phosphate ester monomers on the conversion and mechanical properties of Bis-GMA-and UDMA-based resin composites. J. Dent. 2020, 94, 103306. [Google Scholar] [CrossRef]
- Paik, Y.; Kim, J.-H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, Y.-I. Dentin Biomodification with Flavonoids and Calcium Phosphate Ion Clusters to Improve Dentin Bonding Stability. Materials 2022, 15, 1494. [Google Scholar] [CrossRef]
- Sano, H.; Chowdhury, A.F.M.A.; Saikaew, P.; Matsumoto, M.; Hoshika, S.; Yamauti, M. The microtensile bond strength test: Its historical background and application to bond testing. Jpn. Dent. Sci. Rev. 2020, 56, 24–31. [Google Scholar] [CrossRef]
- AlSubaie, A.A.; Sarfraz, Z.; AlAli, A.A.; AlEssa, A.E.; Subaie, H.A.A.; Shah, A.T.; Khan, A.S. Effect of nano-zinc oxide and fluoride-doped bioactive glass-based dentifrices on esthetic restorations. Dent. Med. Probl. 2019, 56, 59–65. [Google Scholar] [CrossRef]
- Almusa, A.; Delgado, A.H.S.; Ashley, P.; Young, A.M. Determination of Dental Adhesive Composition throughout Solvent Drying and Polymerization Using ATR-FTIR Spectroscopy. Polymers 2021, 13, 3886. [Google Scholar] [CrossRef]
- Syed, M.R.; Bano, N.Z.; Ghafoor, S.; Khalid, H.; Zahid, S.; Siddiqui, U.; Hakeem, A.S.; Asif, A.; Kaleem, M.; Khan, A.S. Synthesis and characterization of bioactive glass fiber-based dental restorative composite. Ceram. Int. 2020, 46, 21623–21631. [Google Scholar] [CrossRef]
- Delgado, A.H.; Young, A.M. Modelling ATR-FTIR spectra of dental bonding systems to investigate composition and polymerisation kinetics. Materials 2021, 14, 760. [Google Scholar] [CrossRef]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 2017, 52, 507–540. [Google Scholar] [CrossRef]
- Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.-A.; Popa, M.; Desbrieres, J. The benefits of smart nanoparticles in dental applications. Int. J. Mol. Sci. 2021, 22, 2585. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.; Collares, F.M.; Melo, M.A.S. Antibacterial response of oral microcosm biofilm to nano-zinc oxide in adhesive resin. Dent. Mater. 2021, 37, e182–e193. [Google Scholar] [CrossRef]
- Oltramare, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Short- and Long-Term Dentin Bond Strength of Bioactive Glass-Modified Dental Adhesives. Nanomaterials 2021, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Souza, V.S.; Scholten, J.D.; Collares, F.M. Quantum Dots of Tantalum Oxide with an Imidazolium Ionic Liquid as Antibacterial Agent for Adhesive Resin. J. Adhes. Dent. 2020, 22, 207–214. [Google Scholar] [CrossRef]
- Dias, P.G.; da Silva, E.M.; Carvalho, C.M.; Miranda, M.; Portela, M.B.; Amaral, C.M. Characterization and Antibacterial Effect of an Experimental Adhesive Containing Different Concentrations of Proanthocyanidin. J. Adhes. Dent. 2020, 22, 139–147. [Google Scholar] [CrossRef]
- Fujimura, Y.; Weerasinghe, D.; Kawashima, M. Development of an antibacterial bioactive dental adhesive: Simplicity and innovation. Am. J. Dent. 2018, 31, 13b–16b. [Google Scholar]
- Cruzetta, L.; Garcia, I.M.; de Souza Balbinot, G.; Motta, A.S.; Collares, F.M.; Sauro, S.; Leitune, V.C.B. Evaluation of the Physicochemical and Antibacterial Properties of Experimental Adhesives Doped with Lithium Niobate. Polymers 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Kalachandra, S. Influence of fillers on the water sorption of composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Shah, P.K.; Stansbury, J.W. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, 586–593. [Google Scholar] [CrossRef]
- Brik, M.; Srivastava, A.; Popov, A. A few common misconceptions in the interpretation of experimental spectroscopic data. Opt. Mater. 2022, 127, 112276. [Google Scholar] [CrossRef]
- Savoini, B.; Ballesteros, C.; Santiuste, J.E.M.; González, R.; Popov, A.I.; Chen, Y. Copper and iron precipitates in thermochemically reduced yttria-stabilized zirconia crystals. Philos. Mag. Lett. 2001, 81, 555–561. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.; Xu, J.; Leblanc, R.; Wang, D.; Li, Y.; Guo, H.; Zhang, J. Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties. Cryst. Growth Des. 2009, 9, 3874–3880. [Google Scholar] [CrossRef]
- Ananchenko, D.V.; Nikiforov, S.V.; Sobyanin, K.V.; Konev, S.F.; Dauletbekova, A.K.; Akhmetova-Abdik, G.; Akilbekov, A.T.; Popov, A.I. Paramagnetic Defects and Thermoluminescence in Irradiated Nanostructured Monoclinic Zirconium Dioxide. Materials 2022, 15, 8624. [Google Scholar] [CrossRef]
- Eraiah, B. Optical Properties of Silver-Vanadium-Phosphate Glasses. Mapana J. Sci. 2017, 16, 1–7. [Google Scholar] [CrossRef]
- Cocco, A.R.; Lima, G.S.; Leal, F.B.; Munchow, E.A.; Ogliari, F.A.; Piva, E. Addition of nanoparticles for development of radiopaque dental adhesives. Int. J. Adhes. Adhes. 2018, 80, 122–127. [Google Scholar] [CrossRef]
- Ito, S.; Hashimoto, M.; Wadgaonkar, B.; Svizero, N.; Carvalho, R.M.; Yiu, C.; Rueggeberg, F.A.; Foulger, S.; Saito, T.; Nishitani, Y.; et al. Effects of resin hydrophilicity on water sorption and changes in modulus of elasticity. Biomaterials 2005, 26, 6449–6459. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; Peumans, M.; De Munck, J.; Lambrechts, P.; Van Meerbeek, B. The role of HEMA in one-step self-etch adhesives. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ahmed, M.H.; Yao, C.; Mercelis, B.; Yoshihara, K.; Peumans, M.; Van Meerbeek, B. Experimental two-step universal adhesives bond durably in a challenging high C-factor cavity model. Dent. Mater. 2022, 39, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Zubaidah, N.; Effendy, R.; Soetojo, A.; Estiyaningsih, T.; Tanzil, M.I.; Khotimah, K. Difference of Chemical Bonds Between UDMA Bonding Agents with Ethanol Solvent and Acetone Solvent on Dentin Collagen. Pesqui. Bras. Odontopediatria Clínica Integr. 2021, 21, e0116. [Google Scholar] [CrossRef]
- Borges, B.C.; Souza-Junior, E.J.; Brandt, W.C.; Loguercio, A.D.; Montes, M.A.; Puppin-Rontani, R.M.; Sinhoreti, M.A. Degree of conversion of simplified contemporary adhesive systems as influenced by extended air-activated or passive solvent volatilization modes. Oper. Dent. 2012, 37, 246–252. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Tichy, A.; Hosaka, K.; Abdou, A.; Nakajima, M.; Tagami, J. Degree of Conversion Contributes to Dentin Bonding Durability of Contemporary Universal Adhesives. Oper. Dent. 2020, 45, 556–566. [Google Scholar] [CrossRef]
- Younas, B.; Khan, A.S.; Muzaffar, D.; Hussain, I.; Anwar Chaudhry, A.; Ur Rehman, I. In situ reaction kinetic analysis of dental restorative materials. Eur. Phys. J. Appl. Phys. 2013, 64, 30701. [Google Scholar] [CrossRef]
- Leitune, V.C.; Collares, F.M.; Trommer, R.M.; Andrioli, D.G.; Bergmann, C.P.; Samuel, S.M. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013, 41, 321–327. [Google Scholar] [CrossRef]
- Gioka, C.; Bourauel, C.; Hiskia, A.; Kletsas, D.; Eliades, T.; Eliades, G. Light-cured or chemically cured orthodontic adhesive resins? A selection based on the degree of cure, monomer leaching, and cytotoxicity. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 413–419; quiz 516. [Google Scholar] [CrossRef]
- Khalid, H.; Syed, M.R.; Rahbar, M.I.; Iqbal, H.; Ahmad, S.; Kaleem, M.; Matinlinna, J.P.; Khan, A.S. Effect of nano-bioceramics on monomer leaching and degree of conversion of resin-based composites. Dent. Mater. J. 2018, 37, 940–949. [Google Scholar] [CrossRef]
- Kreutz, M.; Kreutz, C.; Kanzow, P.; Tauböck, T.T.; Burrer, P.; Noll, C.; Bader, O.; Rohland, B.; Wiegand, A.; Rizk, M. Effect of Bioactive and Antimicrobial Nanoparticles on Properties and Applicability of Dental Adhesives. Nanomaterials 2022, 12, 3862. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.Y.; Sarfraz, Z.; Habib, A.; Khan, A.S.; Matinlinna, J.P. Effect of silanization of hydroxyapatite fillers on physical and mechanical properties of a bis-GMA based resin composite. J. Mech. Behav. Biomed. Mater. 2016, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Abed, Y.A.; Sabry, H.A.; Alrobeigy, N.A. Degree of conversion and surface hardness of bulk-fill composite versus incremental-fill composite. Tanta Dent. J. 2015, 12, 71–80. [Google Scholar] [CrossRef]
- Pomacóndor-Hernández, C.; Osorio, R.; Aguilera, F.; Cabello, I.; Goes, M.; Toledano, M. Effect of zinc-doping in physicochemical properties of dental adhesives. Am. J. Dent. 2015, 28, 292–296. [Google Scholar] [PubMed]
- Alsharif, S.O.; Bin Md Akil, H.; Abbas Abd El-Aziz, N.; Arifin Bin Ahmad, Z. Effect of alumina particles loading on the mechanical properties of light-cured dental resin composites. Mater. Des. 2014, 54, 430–435. [Google Scholar] [CrossRef]
- Carvalho, R.M.; Manso, A.P.; Geraldeli, S.; Tay, F.R.; Pashley, D.H. Durability of bonds and clinical success of adhesive restorations. Dent. Mater. 2012, 28, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.M.; Elsayed, H.Y.; Abdalla, A.I.; Darrag, A.M. The effect of water storage on micro-shear bond strength of contemporary composite resins using different dentin adhesive systems. Tanta Dent. J. 2014, 11, 47–55. [Google Scholar] [CrossRef]
- Heintze, S.D.; Rousson, V.; Mahn, E. Bond strength tests of dental adhesive systems and their correlation with clinical results–a meta-analysis. Dent. Mater. 2015, 31, 423–434. [Google Scholar] [CrossRef]
- Ismail, A.M.; Bourauel, C.; ElBanna, A.; Salah Eldin, T. Micro versus Macro Shear Bond Strength Testing of Dentin-Composite Interface Using Chisel and Wireloop Loading Techniques. Dent. J. 2021, 9, 140. [Google Scholar] [CrossRef]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles-An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, L.B.T.; Oliveira, M.T.; Saraceni, C.H.C.; Lima, A.F. The influence of nanofillers on the properties of ethanol-solvated and non-solvated dental adhesives. Restor. Dent. Endod. 2019, 44, e28. [Google Scholar] [CrossRef] [PubMed]
- Sirisha, K.; Rambabu, T.; Ravishankar, Y.; Ravikumar, P. Validity of bond strength tests: A critical review-Part II. J. Conserv. Dent. 2014, 17, 420. [Google Scholar] [CrossRef] [PubMed]
- Jarahi, N.; Borouziniat, A.; Jarahi, L.; Nejat, A.H. Effect of different storage solutions and autoclaving on shear bond strength of composite to dentin. J. Res. Med. Dent. Sci. 2018, 6, 50–53. [Google Scholar]
- Kim, J.-S.; Cho, B.-H.; Lee, I.-B.; Um, C.-M.; Lim, B.-S.; Oh, M.-H.; Chang, C.-G.; Son, H.-H. Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72B, 284–291. [Google Scholar] [CrossRef]
- Cadenaro, M.; Breschi, L.; Rueggeberg, F.A.; Suchko, M.; Grodin, E.; Agee, K.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent. Mater. 2009, 25, 621–628. [Google Scholar] [CrossRef]
- Ikeda, T.; De Munck, J.; Shirai, K.; Hikita, K.; Inoue, S.; Sano, H.; Lambrechts, P.; Van Meerbeek, B. Effect of evaporation of primer components on ultimate tensile strengths of primer–adhesive mixture. Dent. Mater. 2005, 21, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.I.; Feilzer, A.J. Four-year water degradation of a total-etch and two self-etching adhesives bonded to dentin. J. Dent. 2008, 36, 611–617. [Google Scholar] [CrossRef]
- Daood, U.; Omar, H.; Qasim, S.; Nogueira, L.P.; Pichika, M.R.; Mak, K.-K.; Steier, L.; Cky, Y.; Lin, S.L.; Fawzy, A.S. New antimicrobial and collagen crosslinking formulated dentin adhesive with improved bond durability. J. Mech. Behav. Biomed. Mater. 2020, 110, 103927. [Google Scholar] [CrossRef]
- Daood, U.; Yiu, C.; Burrow, M.F.; Niu, L.-N.; Tay, F.R. Effect of a novel quaternary ammonium silane on dentin protease activities. J. Dent. 2017, 58, 19–27. [Google Scholar] [CrossRef]
- Lung, Y.; Matinlinna, J.P. Silanes for adhesion promotion and surface modification. In Silane Chemistry, Applications and Performance; Moriguchi, K., Utagawa, S., Eds.; Nova Publishers: Hauppauge, NY, USA, 2013; pp. 87–109. [Google Scholar]
- Sabatini, C.; Campillo, M.; Aref, J. Color Stability of Ten Resin-Based Restorative Materials. J. Esthet. Restor. Dent. 2012, 24, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Mar Perez, M.d. Color Difference Thresholds in Dentistry. J. Esthet. Restor. Dent. 2015, 27, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Štruncová, M.; Toma, S.H.; Araki, K.; Bresciani, E.; Rodrigues, F.P.; Medeiros, I.S.; Dutra-Correa, M. Silver nanoparticles added to a commercial adhesive primer: Colour change and resin colour stability with ageing. Int. J. Adhes. Adhes. 2020, 102, 102694. [Google Scholar] [CrossRef]
- Ritter, D.D.; Rocha, R.O.; Soares, F.Z.M.; Lenzi, T.L. Do Adhesive Systems Influence the Color Match of Resin Composites? J. Appl. Biomater. Funct. Mater. 2016, 14, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, F.; Imazato, S.; Cheng, L.; Liu, H.; Arola, D.D.; Bai, Y.; Xu, H.H.K. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J. Biomed. Mater. Res. Part B. Appl. Biomater. 2013, 101B, 929–938. [Google Scholar] [CrossRef]
- Dutra-Correa, M.; Leite, A.A.B.V.; de Cara, S.P.H.M.; Diniz, I.M.A.; Marques, M.M.; Suffredini, I.B.; Fernandes, M.S.; Toma, S.H.; Araki, K.; Medeiros, I.S. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018, 77, 66–71. [Google Scholar] [CrossRef]




| Wavenumber (cm−1) | Assignned | Reference |
|---|---|---|
| 3650–3000 | Stretching O-H | [32] |
| 3310 | Stretching N-H | [33] |
| 2968 | Asymmetric stretching C-H | [34] |
| 2880 | Symmetric stretching C-H | [33,35] |
| 1720 | C=O | [34] |
| 1640 | C=C Aliphatic | [33,35] |
| 1611 | C-C Aromatic | [33,35] |
| 1542 | Bending N-H | [35] |
| 1320 | Bending C-H | [35] |
| 1245 | Symmetric stretching C-O | [34] |
| 930 | Asymmetric stretching C-O-C | [34] |
| DC (%) Peak Height/Peak Area | Micro-Hardness (KHN) | Flexural Strength (MPa) | Flexural Modulus (GPa) | |
|---|---|---|---|---|
| Com | 73.68 a (2.14)/76.46 (2.54) | 17.21 a (0.82) | 105.58 a (4.70) | 1.40 a (0.19) |
| Exp-0 | 55.73 a (1.50)/56.32 (2.05) | 24.30 (1.30) | 174 (10.66) | 2.74 (0.62) |
| Exp-0.15 | 54.71 a (2.50)/56.15 (2.90) | 27.35 (2.24) | 184.37 (7.59) | 3.26 (0.16) |
| Exp-0.25 | 66.66 (1.80)/70.76 (2.50) | 30.50 (2.30) | 188.55 (7.20) | 4.24 (0.46) |
| Exp-0.5 | 64.44 (3.65)/69.04 (3.05) | 39.11 a (4.01) | 187 (8.51) | 3.45 (0.40) |
| Com | Exp-0 | Exp-0.15 | Exp-0.25 | Exp-0.5 | |
|---|---|---|---|---|---|
| Day 1 vs. Day 30 | 3.27 (0.44) | 2.66 (0.63) | 2.70 (2.25) | 1.33 a (0.40) | 1.06 a (0.09) |
| Day 1 vs. Day 60 | 2.97 (0.82) | 2.58 (0.01) | 1.833 (0.42) | 2.16 (0.94) | 2.83 b (1.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.S.; Alhamdan, Y.; Alibrahim, H.; Almulhim, K.S.; Nawaz, M.; Ahmed, S.Z.; Aljuaid, K.; Ateeq, I.S.; Akhtar, S.; Ansari, M.A.; et al. Analyses of Experimental Dental Adhesives Based on Zirconia/Silver Phosphate Nanoparticles. Polymers 2023, 15, 2614. https://doi.org/10.3390/polym15122614
Khan AS, Alhamdan Y, Alibrahim H, Almulhim KS, Nawaz M, Ahmed SZ, Aljuaid K, Ateeq IS, Akhtar S, Ansari MA, et al. Analyses of Experimental Dental Adhesives Based on Zirconia/Silver Phosphate Nanoparticles. Polymers. 2023; 15(12):2614. https://doi.org/10.3390/polym15122614
Chicago/Turabian StyleKhan, Abdul Samad, Yasmin Alhamdan, Hala Alibrahim, Khalid S. Almulhim, Muhammad Nawaz, Syed Zubairuddin Ahmed, Khalid Aljuaid, Ijlal Shahrukh Ateeq, Sultan Akhtar, Mohammad Azam Ansari, and et al. 2023. "Analyses of Experimental Dental Adhesives Based on Zirconia/Silver Phosphate Nanoparticles" Polymers 15, no. 12: 2614. https://doi.org/10.3390/polym15122614
APA StyleKhan, A. S., Alhamdan, Y., Alibrahim, H., Almulhim, K. S., Nawaz, M., Ahmed, S. Z., Aljuaid, K., Ateeq, I. S., Akhtar, S., Ansari, M. A., & Siddiqui, I. A. (2023). Analyses of Experimental Dental Adhesives Based on Zirconia/Silver Phosphate Nanoparticles. Polymers, 15(12), 2614. https://doi.org/10.3390/polym15122614











