Smart Dental Materials Intelligently Responding to Oral pH to Combat Caries: A Literature Review
Abstract
1. Introduction
2. Resins That Can Inhibit Bacterial Acid Production and Raise the pH
3. Suppressing Biofilm Acids and Providing Ions to Increase Enamel Hardness
4. pH-Responsive Antibacterial Resins
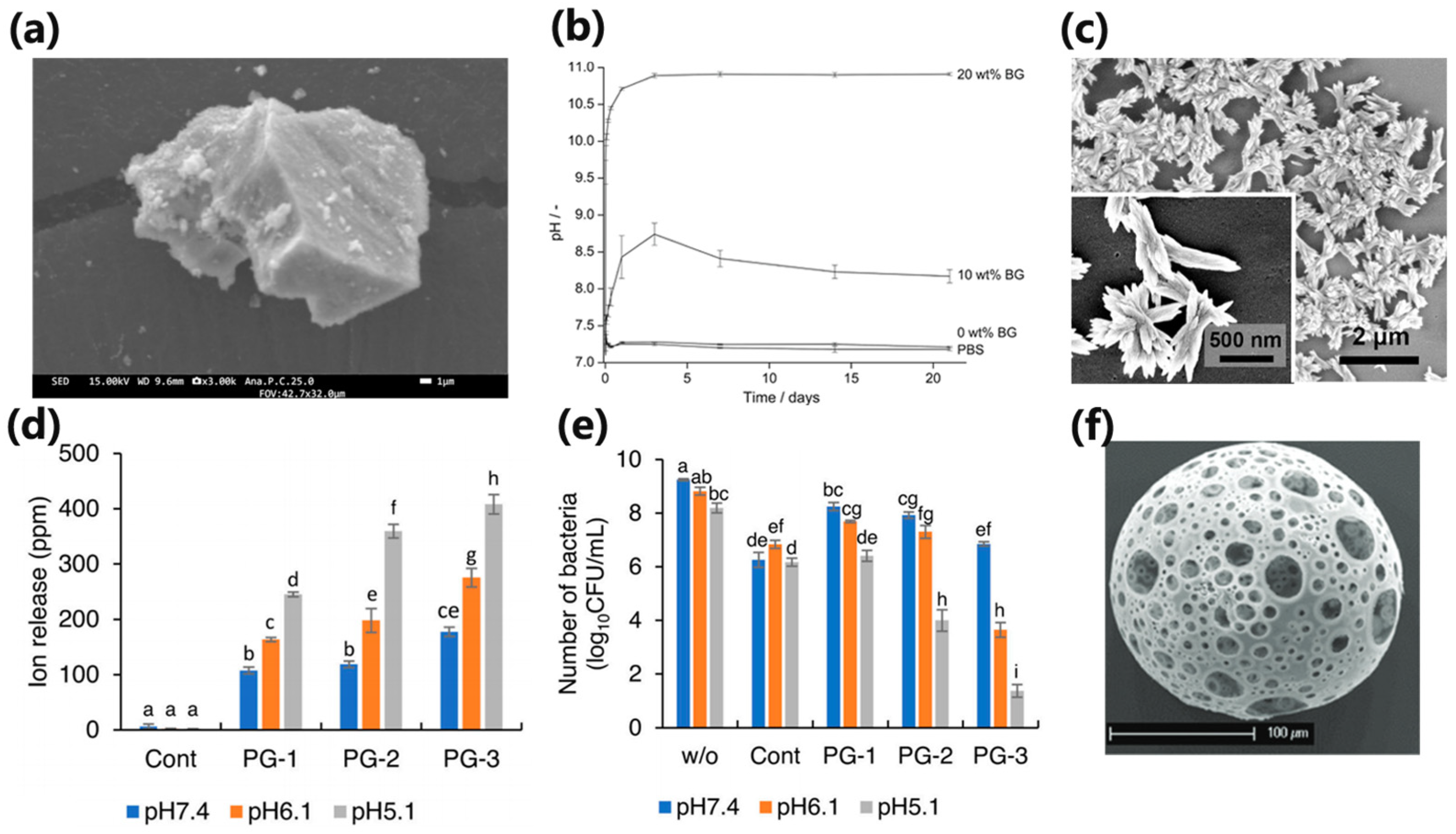
5. Smart pH-Responsive Dental Resins with Bioactive Glass/Bioglass
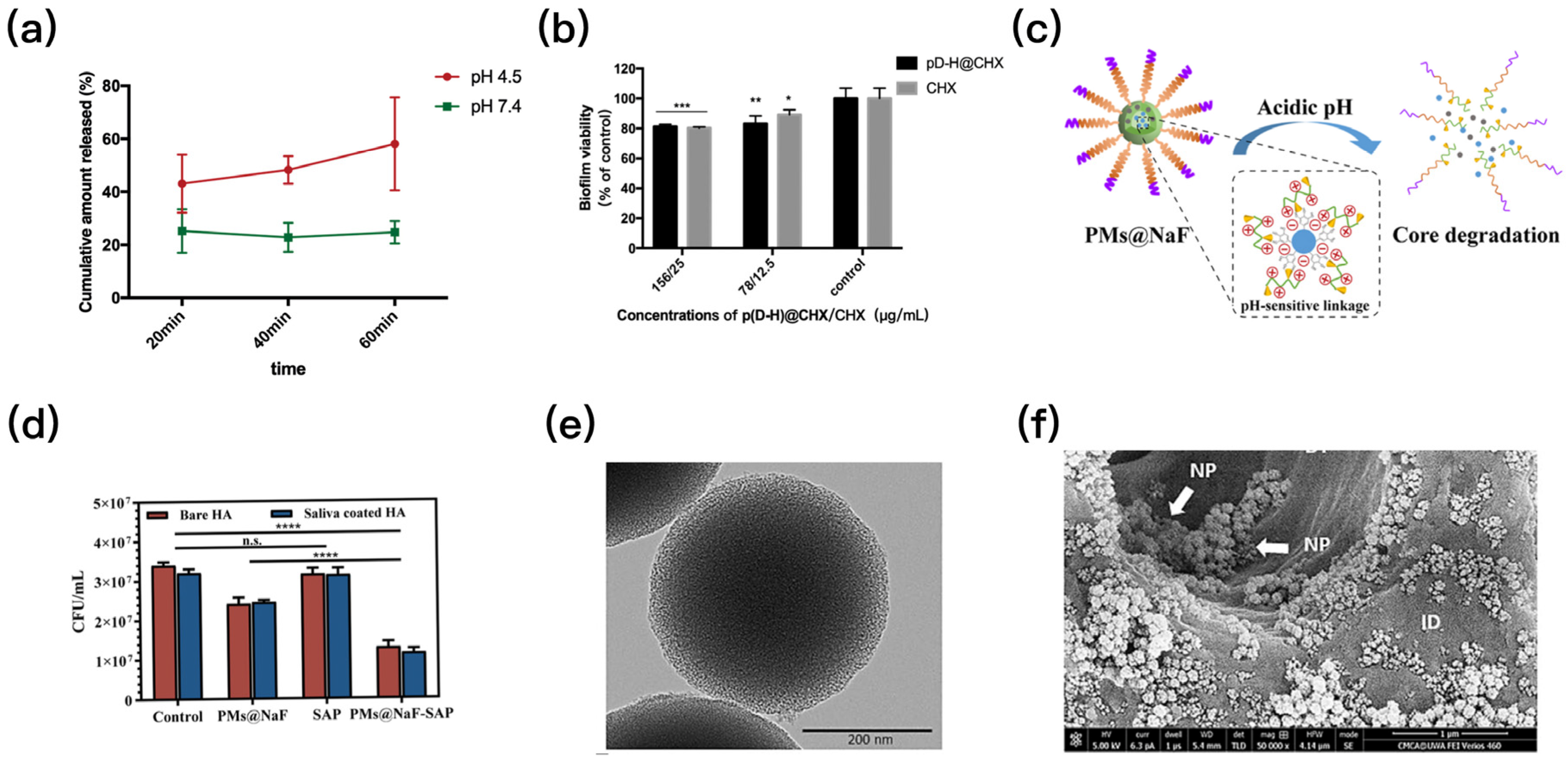
6. Smart Local Drug Delivery System That Can Respond to pH
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-R.; Sun, J.; Du, Y.; Pan, A.; Zeng, L.; Maboudian, R.; Burne, R.A.; Qian, P.-Y.; Zhang, W. Mutanofactin Promotes Adhesion and Biofilm Formation of Cariogenic Streptococcus Mutans. Nat. Chem. Biol. 2021, 17, 576–584. [Google Scholar] [CrossRef]
- Simón-Soro, A.; Mira, A. Solving the Etiology of Dental Caries. Trends Microbiol. 2015, 23, 76–82. [Google Scholar] [CrossRef]
- Namen, F.M.; Galan, J.; De Deus, G.; Cabreira, R.D.; Filho, F.C.e.S. Effect of PH on the Wettability and Fluoride Release of an Ion-Releasing Resin Composite. Oper. Dent. 2008, 33, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart Dental Materials for Antimicrobial Applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef]
- Li, N.; Jiang, L.; Jin, H.; Wu, Y.; Liu, Y.; Huang, W.; Wei, L.; Zhou, Q.; Chen, F.; Gao, Y.; et al. An Enzyme-Responsive Membrane for Antibiotic Drug Release and Local Periodontal Treatment. Colloids Surf. B Biointerfaces 2019, 183, 110454. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Ren, M.; Wang, X.; Li, L.; Liu, F.; Lan, Y.; Yang, S.; Song, J. PH and Lipase-Responsive Nanocarrier-Mediated Dual Drug Delivery System to Treat Periodontitis in Diabetic Rats. Bioact. Mater. 2022, 18, 254–266. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced Smart Biomaterials and Constructs for Hard Tissue Engineering and Regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Maloo, L.M.; Patel, A.; Toshniwal, S.H.; Bagde, A.D. Smart Materials Leading to Restorative Dentistry: An Overview. Cureus 2022, 14, e30789. [Google Scholar] [CrossRef]
- McCabe, J.F.; Yan, Z.; Al Naimi, O.T.; Mahmoud, G.; Rolland, S.L. Smart Materials in Dentistry. Aust. Dent. J. 2011, 56 (Suppl. 1), 3–10. [Google Scholar] [CrossRef]
- Francois, P.; Fouquet, V.; Attal, J.-P.; Dursun, E. Commercially Available Fluoride-Releasing Restorative Materials: A Review and a Proposal for Classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus Mutans. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Lingström, P.; van Ruyven, F.O.; van Houte, J.; Kent, R. The PH of Dental Plaque in Its Relation to Early Enamel Caries and Dental Plaque Flora in Humans. J. Dent. Res. 2000, 79, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Gelli, R.; Ridi, F.; Baglioni, P. The Importance of Being Amorphous: Calcium and Magnesium Phosphates in the Human Body. Adv. Colloid Interface Sci. 2019, 269, 219–235. [Google Scholar] [CrossRef]
- Regnault, W.F.; Icenogle, T.B.; Antonucci, J.M.; Skrtic, D. Amorphous Calcium Phosphate/Urethane Methacrylate Resin Composites. I. Physicochemical Characterization. J. Mater. Sci. Mater. Med. 2008, 19, 507–515. [Google Scholar] [CrossRef]
- Xie, X.; Wang, L.; Xing, D.; Zhang, K.; Weir, M.D.; Liu, H.; Bai, Y.; Xu, H.H.K. Novel Dental Adhesive with Triple Benefits of Calcium Phosphate Recharge, Protein-Repellent and Antibacterial Functions. Dent. Mater. 2017, 33, 553–563. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite Containing Amorphous Calcium Phosphate Nanoparticles for Caries Inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, X.; Darvell, B.W.; Lu, W.W. Apatite-Formation Ability—Predictor of “Bioactivity”? Acta Biomater 2010, 6, 4181–4188. [Google Scholar] [CrossRef]
- Dickens, S.H.; Flaim, G.M.; Takagi, S. Mechanical Properties and Biochemical Activity of Remineralizing Resin-Based Ca-PO4 Cements. Dent. Mater. 2003, 19, 558–566. [Google Scholar] [CrossRef]
- Moreau, J.L.; Sun, L.; Chow, L.C.; Xu, H.H.K. Mechanical and Acid Neutralizing Properties and Bacteria Inhibition of Amorphous Calcium Phosphate Dental Nanocomposite. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 80–88. [Google Scholar] [CrossRef]
- Zhang, L.; Weir, M.D.; Hack, G.; Fouad, A.F.; Xu, H.H.K. Rechargeable Dental Adhesive with Calcium Phosphate Nanoparticles for Long-Term Ion Release. J. Dent. 2015, 43, 1587–1595. [Google Scholar] [CrossRef]
- Liang, K.; Wang, S.; Tao, S.; Xiao, S.; Zhou, H.; Wang, P.; Cheng, L.; Zhou, X.; Weir, M.D.; Oates, T.W.; et al. Dental Remineralization via Poly(Amido Amine) and Restorative Materials Containing Calcium Phosphate Nanoparticles. Int. J. Oral. Sci. 2019, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.; Bienek, D.R. Use of Protein Repellents to Enhance the Antimicrobial Functionality of Quaternary Ammonium Containing Dental Materials. J. Funct. Biomater. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaijan, Y.A.; Weir, M.D.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Zhang, K.; Xu, H.H.K. Protein-Repellent Nanocomposite with Rechargeable Calcium and Phosphate for Long-Term Ion Release. Dent. Mater. 2018, 34, 1735–1747. [Google Scholar] [CrossRef]
- Al-Qarni, F.D.; Tay, F.; Weir, M.D.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Xie, X.; Xu, H.H.K. Protein-Repelling Adhesive Resin Containing Calcium Phosphate Nanoparticles with Repeated Ion-Recharge and Re-Releases. J. Dent. 2018, 78, 91–99. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial Quaternary Ammonium Compounds in Dental Materials: A Systematic Review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.-X.; Xu, H.H.K. Effects of Quaternary Ammonium Chain Length on the Antibacterial and Remineralizing Effects of a Calcium Phosphate Nanocomposite. Int. J. Oral. Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef]
- Moreau, J.L.; Weir, M.D.; Giuseppetti, A.A.; Chow, L.C.; Antonucci, J.M.; Xu, H.H.K. Long-Term Mechanical Durability of Dental Nanocomposites Containing Amorphous Calcium Phosphate Nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1264–1273. [Google Scholar] [CrossRef]
- Weir, M.D.; Moreau, J.L.; Levine, E.D.; Strassler, H.E.; Chow, L.C.; Xu, H.H.K. Nanocomposite Containing CaF2 Nanoparticles: Thermal Cycling, Wear and Long-Term Water-Aging. Dent. Mater. 2012, 28, 642–652. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Weir, M.D.; Sun, L. Calcium and Phosphate Ion Releasing Composite: Effect of PH on Release and Mechanical Properties. Dent. Mater. 2009, 25, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Geraldeli, S.; Soares, E.F.; Alvarez, A.J.; Farivar, T.; Shields, R.C.; Sinhoreti, M.A.C.; Nascimento, M.M. A New Arginine-Based Dental Adhesive System: Formulation, Mechanical and Anti-Caries Properties. J. Dent. 2017, 63, 72–80. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, X.; Zhou, X.; Bonavente, A.; Weir, M.D.; Melo, M.A.S.; Imazato, S.; Oates, T.W.; Cheng, L.; Xu, H.H.K. Novel Nanocomposite Inhibiting Caries at the Enamel Restoration Margins in an In Vitro Saliva-Derived Biofilm Secondary Caries Model. IJMS 2020, 21, 6369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, X.; Huang, X.; Zhu, C.; Weir, M.D.; Melo, M.A.S.; Bonavente, A.; Lynch, C.D.; Imazato, S.; Oates, T.W.; et al. Antibacterial and Remineralizing Nanocomposite Inhibit Root Caries Biofilms and Protect Root Dentin Hardness at the Margins. J. Dent. 2020, 97, 103344. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, X.; Zhou, X.; Weir, M.D.; Melo, M.A.S.; Tay, F.R.; Imazato, S.; Oates, T.W.; Cheng, L.; Xu, H.H.K. In Vitro Evaluation of Composite Containing DMAHDM and Calcium Phosphate Nanoparticles on Recurrent Caries Inhibition at Bovine Enamel-Restoration Margins. Dent. Mater. 2020, 36, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Bhadila, G.; Filemban, H.; Wang, X.; Melo, M.A.S.; Arola, D.D.; Tay, F.R.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Bioactive Low-Shrinkage-Stress Nanocomposite Suppresses S. Mutans Biofilm and Preserves Tooth Dentin Hardness. Acta Biomater. 2020, 114, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bhadila, G.; Wang, X.; Weir, M.D.; Melo, M.A.S.; Martinho, F.; Fay, G.G.; Oates, T.W.; Sun, J.; Xu, H.H.K. Low-Shrinkage-Stress Nanocomposite: An Insight into Shrinkage Stress, Antibacterial, and Ion Release Properties. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1124–1134. [Google Scholar] [CrossRef]
- Bhadila, G.; Wang, X.; Zhou, W.; Menon, D.; Melo, M.A.S.; Montaner, S.; Oates, T.W.; Weir, M.D.; Sun, J.; Xu, H.H.K. Novel Low-Shrinkage-Stress Nanocomposite with Remineralization and Antibacterial Abilities to Protect Marginal Enamel under Biofilm. J. Dent. 2020, 99, 103406. [Google Scholar] [CrossRef]
- AlSahafi, R.; Balhaddad, A.A.; Mitwalli, H.; Ibrahim, M.S.; Melo, M.A.S.; Oates, T.W.; Xu, H.H.K.; Weir, M.D. Novel Crown Cement Containing Antibacterial Monomer and Calcium Phosphate Nanoparticles. Nanomaterials 2020, 10, 2001. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Xia, Y.; Ji, Y.; Ruan, J.; Weir, M.D.; Lin, X.; Nie, Z.; Gu, N.; Masri, R.; et al. Novel Magnetic Nanoparticle-Containing Adhesive with Greater Dentin Bond Strength and Antibacterial and Remineralizing Capabilities. Dent. Mater. 2018, 34, 1310–1322. [Google Scholar] [CrossRef]
- Staben, L.R.; Koenig, S.G.; Lehar, S.M.; Vandlen, R.; Zhang, D.; Chuh, J.; Yu, S.-F.; Ng, C.; Guo, J.; Liu, Y.; et al. Targeted Drug Delivery through the Traceless Release of Tertiary and Heteroaryl Amines from Antibody-Drug Conjugates. Nat. Chem. 2016, 8, 1112–1119. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. PH-Activated Nanoparticles for Controlled Topical Delivery of Farnesol to Disrupt Oral Biofilm Virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef]
- Liang, J.; Liu, F.; Zou, J.; Xu, H.H.K.; Han, Q.; Wang, Z.; Li, B.; Yang, B.; Ren, B.; Li, M.; et al. PH-Responsive Antibacterial Resin Adhesives for Secondary Caries Inhibition. J. Dent. Res. 2020, 99, 1368–1376. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, J.; Zhou, X.; Ren, B.; Wang, H.; Han, Q.; Li, H.; Cheng, L. Effects of a Novel, Intelligent, PH-Responsive Resin Adhesive on Cariogenic Biofilms In Vitro. Pathogens 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Y.; Zhou, X.; Zhu, C.; Han, Q.; Wang, H.; Xu, H.H.K.; Ren, B.; Cheng, L. Intelligent PH-Responsive Dental Sealants to Prevent Long-Term Microleakage. Dent. Mater. 2021, 37, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, J.; Zhou, W.; Ma, T.; Weir, M.D.; Hack, G.D.; Fay, G.G.; Oates, T.W.; Cheng, L.; Xu, H.H.K. Novel Dental Resin Infiltrant Containing Smart Monomer Dodecylmethylaminoethyl Methacrylate. Front. Cell. Infect. Microbiol. 2022, 12, 1063143. [Google Scholar] [CrossRef]
- Xie, X.-J.; Xing, D.; Wang, L.; Zhou, H.; Weir, M.D.; Bai, Y.-X.; Xu, H.H. Novel Rechargeable Calcium Phosphate Nanoparticle-Containing Orthodontic Cement. Int. J. Oral. Sci. 2017, 9, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Sui, B.; Ilyas, K.; Boccaccini, A.R. Porous Bioactive Glass Micro- and Nanospheres with Controlled Morphology: Developments, Properties and Emerging Biomedical Applications. Mater. Horiz. 2021, 8, 300–335. [Google Scholar] [CrossRef]
- Tauböck, T.T.; Zehnder, M.; Schweizer, T.; Stark, W.J.; Attin, T.; Mohn, D. Functionalizing a Dentin Bonding Resin to Become Bioactive. Dent. Mater. 2014, 30, 868–875. [Google Scholar] [CrossRef]
- Deng, F.; Sakai, H.; Kitagawa, H.; Kohno, T.; Thongthai, P.; Liu, Y.; Kitagawa, R.; Abe, G.L.; Sasaki, J.-I.; Imazato, S. Fabrication of PH-Responsive Zn2+-Releasing Glass Particles for Smart Antibacterial Restoratives. Molecules 2022, 27, 7202. [Google Scholar] [CrossRef]
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of Bioactive Glasses in Various Dental Fields. Biomater. Res. 2022, 26, 31. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Tauböck, T.T. Ion Release and Hydroxyapatite Precipitation of Resin Composites Functionalized with Two Types of Bioactive Glass. J. Dent. 2022, 118, 103950. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Ahmed, M.H.; Li, X.; Nedeljkovic, I.; Vandooren, J.; Mercelis, B.; Zhang, F.; Van Landuyt, K.L.; Huang, C.; Van Meerbeek, B. Zinc-Calcium-Fluoride Bioglass-Based Innovative Multifunctional Dental Adhesive with Thick Adhesive Resin Film Thickness. ACS Appl. Mater. Interfaces 2020, 12, 30120–30135. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Aati, S.; Ngo, H.; Fawzy, A. PH-Dependent Delivery of Chlorhexidine from PGA Grafted Mesoporous Silica Nanoparticles at Resin-Dentin Interface. J. Nanobiotechnol. 2021, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Kuthati, Y.; Kankala, R.K.; Lin, S.-X.; Weng, C.-F.; Lee, C.-H. PH-Triggered Controllable Release of Silver-Indole-3 Acetic Acid Complexes from Mesoporous Silica Nanoparticles (IBN-4) for Effectively Killing Malignant Bacteria. Mol. Pharm. 2015, 12, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, Z.; Lo, E.C.M. A New PH-Responsive Nano Micelle for Enhancing the Effect of a Hydrophobic Bactericidal Agent on Mature Streptococcus Mutans Biofilm. Front. Microbiol. 2021, 12, 761583. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, H.; Cheng, L.; Wang, Y.; Liu, F.; Wang, S. The Application of Mesoporous Silica Nanoparticles as a Drug Delivery Vehicle in Oral Disease Treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1124411. [Google Scholar] [CrossRef]
- Peng, X.; Han, Q.; Zhou, X.; Chen, Y.; Huang, X.; Guo, X.; Peng, R.; Wang, H.; Peng, X.; Cheng, L. Effect of PH-Sensitive Nanoparticles on Inhibiting Oral Biofilms. Drug Deliv. 2022, 29, 561–573. [Google Scholar] [CrossRef]
- Zhao, Z.; Ding, C.; Wang, Y.; Tan, H.; Li, J. PH-Responsive Polymeric Nanocarriers for Efficient Killing of Cariogenic Bacteria in Biofilms. Biomater. Sci. 2019, 7, 1643–1651. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, L.; Chen, L.; Lin, Y.; Luo, Z.; Chen, Z.; Li, T.; Wu, J.; Zhong, Z. Farnesal-Loaded PH-Sensitive Polymeric Micelles Provided Effective Prevention and Treatment on Dental Caries. J. Nanobiotechnol. 2020, 18, 89. [Google Scholar] [CrossRef]
- Zhou, J.; Horev, B.; Hwang, G.; Klein, M.I.; Koo, H.; Benoit, D.S.W. Characterization and Optimization of PH-Responsive Polymer Nanoparticles for Drug Delivery to Oral Biofilms. J. Mater. Chem. B 2016, 4, 3075–3085. [Google Scholar] [CrossRef]
- Zhu, Y.; Marin, L.M.; Xiao, Y.; Gillies, E.R.; Siqueira, W.L. PH-Sensitive Chitosan Nanoparticles for Salivary Protein Delivery. Nanomaterials 2021, 11, 1028. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, F.; Hu, S.; Kong, M.; Feng, C.; Liu, Y.; Cheng, X.; Ji, Q.; Chen, X. PH-Activated Nanoparticles with Targeting for the Treatment of Oral Plaque Biofilm. J. Mater. Chem. B 2018, 6, 586–592. [Google Scholar] [CrossRef]
- Xu, Y.; You, Y.; Yi, L.; Wu, X.; Zhao, Y.; Yu, J.; Liu, H.; Shen, Y.; Guo, J.; Huang, C. Dental Plaque-Inspired Versatile Nanosystem for Caries Prevention and Tooth Restoration. Bioact. Mater. 2023, 20, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-M.; Ge, Y.; Qiu, J.; Shao, D.; Zhang, Y.; Bai, J.; Zheng, X.; Chang, Z.-M.; Wang, Z.; Dong, W.-F.; et al. Redox/PH Dual-Controlled Release of Chlorhexidine and Silver Ions from Biodegradable Mesoporous Silica Nanoparticles against Oral Biofilms. Int. J. Nanomed. 2018, 13, 7697–7709. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, S.; Li, J.; Bu, X.; Dong, X.; Chen, N.; Li, F.; Zhu, J.; Sang, L.; Zeng, Y.; et al. Dual-Sensitive Antibacterial Peptide Nanoparticles Prevent Dental Caries. Theranostics 2022, 12, 4818–4833. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Hong, J.H.; Hatton, B.D.; Finer, Y. Responsive Antimicrobial Dental Adhesive Based on Drug-Silica Co-Assembled Particles. Acta Biomater. 2018, 76, 283–294. [Google Scholar] [CrossRef] [PubMed]




| Type | Component | Features |
|---|---|---|
| Composites | 32% BT(BisGMA+TEGDMA) + 35% Glass particles + 3% DMAHDM+30% NACP [33,34] | Inhibit bacteria; inhibit enamel demineralization; increase Ca and P ion release at low pH |
| 35-38% UV (UDMA+TEG-DVBE) + 2%–5% DMAHDM + 20% NACP + 43% glass [36,37,38] | Inhibit bacteria; remineralize; lower shrinkage-stress | |
| 25% (BisGMA+TEGDMA) + 75% TTCP [31] | Increase Ca and P ion release at low pH | |
| Adhesive | PEHB&PM primer, containing 5% MPC + 5% DMAHDM + 30% NACP [17] | Reduce protein adsorption and bacterial adhesion; inhibit bacteria; increase Ca and P ion release at low pH |
| SBMP adhesive containing 5% DMADDM + 0.1% NAg + 20% NACP; SBMP primer containing 5% DMADDM + 0.1% NAg [30] | Inhibit bacteria; increase Ca and P ion release at low pH | |
| Clearfil SE Bond containing 5% TAs (DMAEM, HMAEM) [43,44] | Inhibit bacteria; increase Ca and P ion release at low pH | |
| Scotchbond™ bond (3M ESPE) + 5% CHX-loaded/MSN-PGA [54] | Inhibit bacteria; pH-response | |
| Adper Scotchbond Multi-Purpose Adhesive (SBMP) + 10% OCT-DMSNs (octenidine dihydrochloride, OCT) [66] | Inhibit bacteria; pH-response | |
| Resin infiltrant | BisGMA + TEGDMA + 5% DMAEM [46] | Inhibit bacteria; pH-response |
| Resin sealant | ClinproTM Sealant (3 MTM ESPETM) + 2.5–10% DMAEM [45] | Inhibit bacteria; pH-response; reduce microleakage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Zhang, Q.; Dai, Z.; Zhu, M.; Xiao, L.; Zhao, Z.; Bai, Y.; Zhang, K. Smart Dental Materials Intelligently Responding to Oral pH to Combat Caries: A Literature Review. Polymers 2023, 15, 2611. https://doi.org/10.3390/polym15122611
Yu K, Zhang Q, Dai Z, Zhu M, Xiao L, Zhao Z, Bai Y, Zhang K. Smart Dental Materials Intelligently Responding to Oral pH to Combat Caries: A Literature Review. Polymers. 2023; 15(12):2611. https://doi.org/10.3390/polym15122611
Chicago/Turabian StyleYu, Kan, Qinrou Zhang, Zixiang Dai, Minjia Zhu, Le Xiao, Zeqing Zhao, Yuxing Bai, and Ke Zhang. 2023. "Smart Dental Materials Intelligently Responding to Oral pH to Combat Caries: A Literature Review" Polymers 15, no. 12: 2611. https://doi.org/10.3390/polym15122611
APA StyleYu, K., Zhang, Q., Dai, Z., Zhu, M., Xiao, L., Zhao, Z., Bai, Y., & Zhang, K. (2023). Smart Dental Materials Intelligently Responding to Oral pH to Combat Caries: A Literature Review. Polymers, 15(12), 2611. https://doi.org/10.3390/polym15122611







