Thiol-Surface-Engineered Cellulose Nanocrystals in Favor of Copper Ion Uptake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Thioglycolic Acid-Esterified Cellulose Nanocrystals
2.3. Characterization of Thioglycolic Acid-Esterified Cellulose Nanocrystals
2.4. The Uptake of Divalent Copper Ions on Thioglycolic Acid-Esterified Cellulose Nanocrystals
3. Results and Discussion
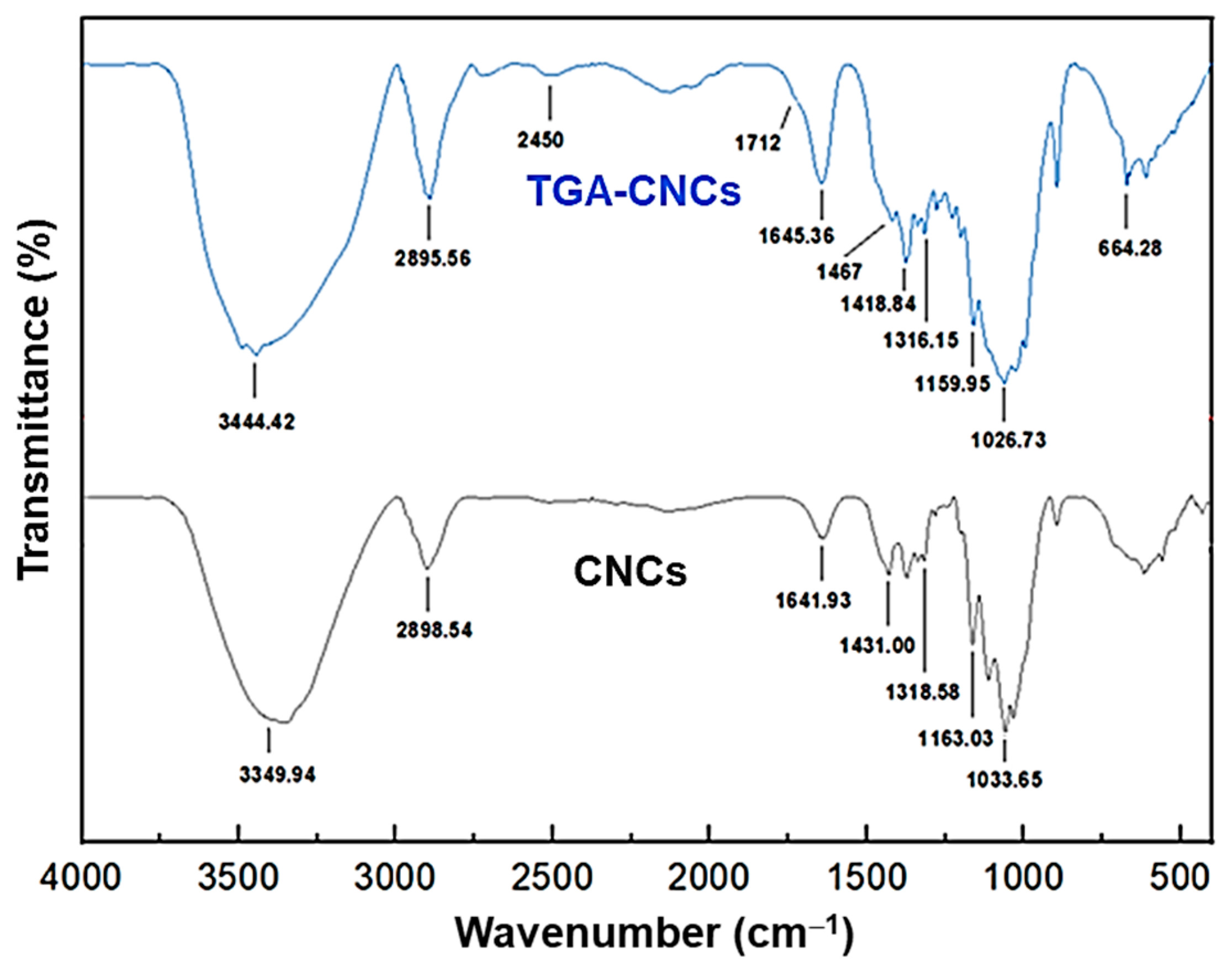
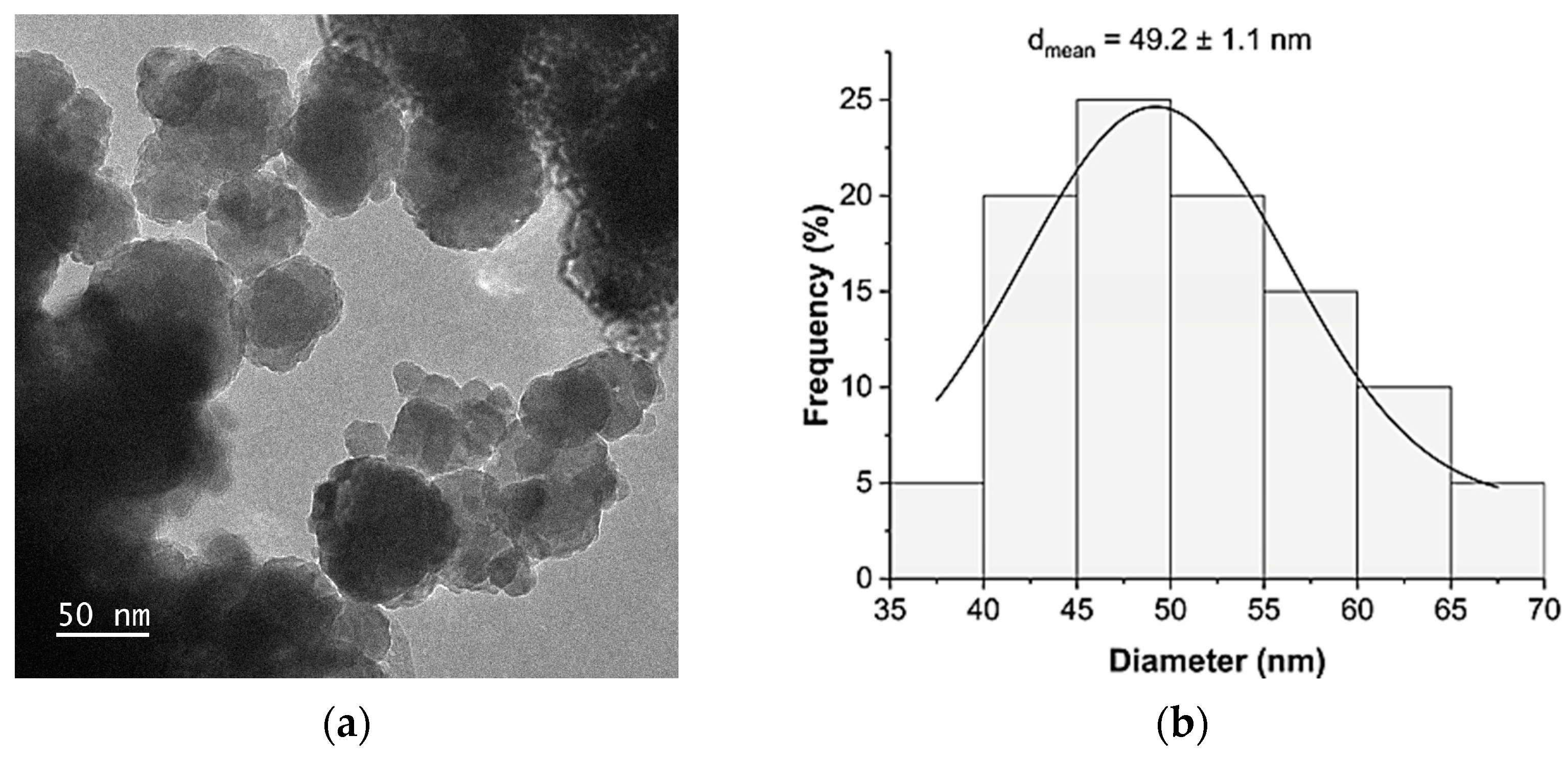
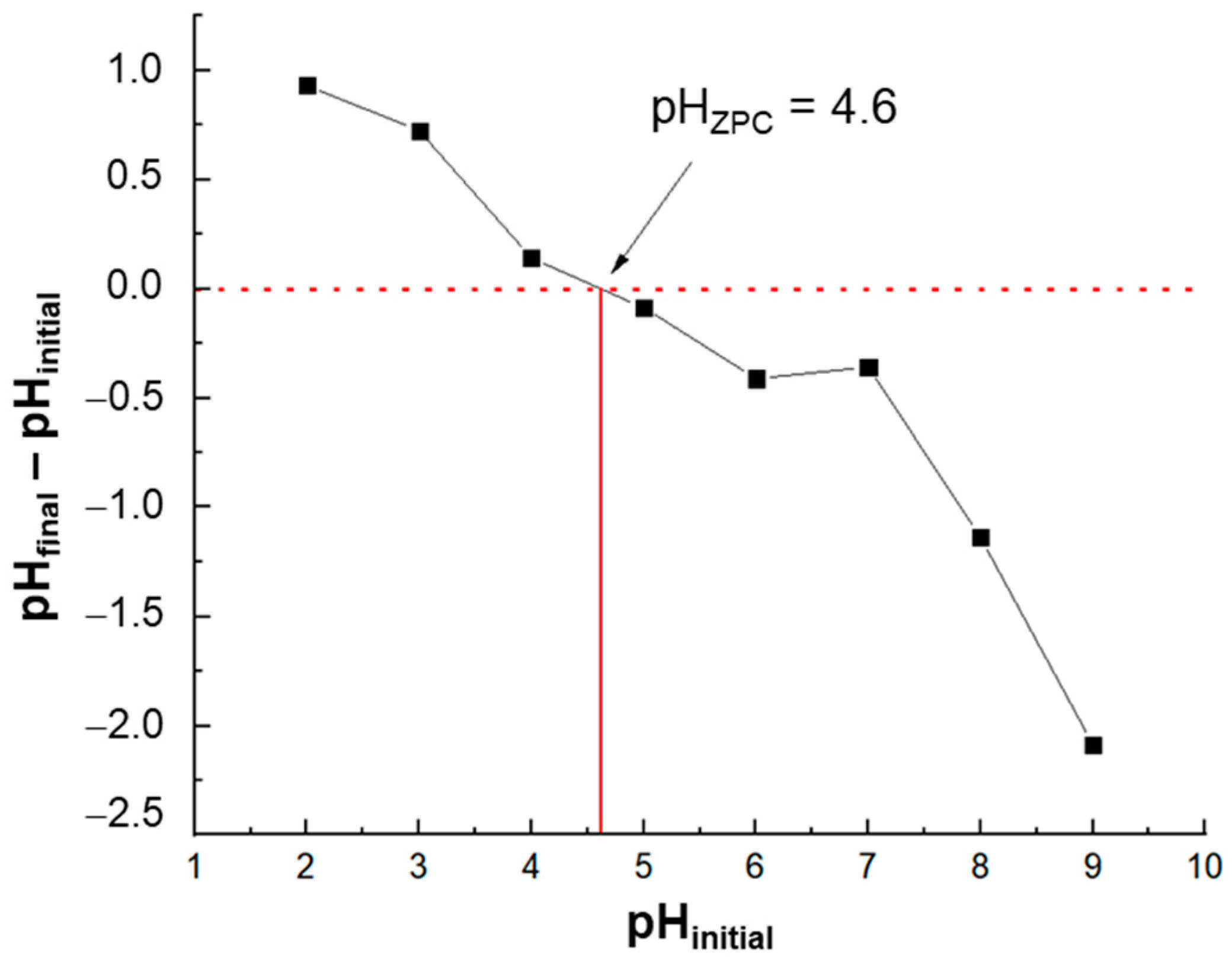
3.1. Characteristics of Thioglycolic Acid-Esterified Cellulose Nanocrystals
3.2. Processing Parameters for the Uptake of Divalent Copper Ions on Thioglycolic Acid-Esterified Cellulose Nanocrystals: Isotherm and Kinetic Studies
- (i)
- The ion exchange between copper ions and protons from –OH or –SH groups;
- (ii)
- The surface metal chelation between copper ions and thiol groups;
- (iii)
- The electrostatic forces between positively charged copper ions and negatively charged groups (hydroxyl, carbonyl, thiolate).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L., Jr.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Long, R.; Wang, L.; Liu, C.; Bai, Z.; Liu, X. A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Sep. Purif. Technol. 2022, 284, 120099. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, S.; Xi, C.; Zhang, F. A Review: Adsorption and Removal of Heavy Metals Based on Polyamide-amines Composites. Front. Chem. 2022, 10, 814643. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of copper (II) on mesoporous silica: The effect of nano-scale confinement. Geochem. Trans. 2018, 19, 13. [Google Scholar] [CrossRef] [Green Version]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. Adsorption of copper ions from polluted water using biochar derived from waste renewable resources: Static and dynamic analysis. Int. J. Environ. Anal. Chem. 2022, 102, 4067–4088. [Google Scholar] [CrossRef]
- Demiral, H.; Güngör, C. Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J. Clean. Prod. 2016, 124, 103–113. [Google Scholar] [CrossRef]
- Xie, R.; Jin, Y.; Chen, Y.; Jiang, W. The importance of surface functional groups in the adsorption of copper onto walnut shell derived activated carbon. Water Sci. Technol. 2017, 76, 3022–3034. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Wang, Y.; Zhang, Y.; Niu, Z.; Li, X. Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater. Open Chem. 2020, 18, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A review on heavy metal ions adsorption from water by graphene oxide and its composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Bhargavi, R.J.; Maheshwari, U.; Gupta, S. Synthesis and use of alumina nanoparticles as an adsorbent for the removal of Zn(II) and CBG dye from wastewater. Int. J. Ind. Chem. 2015, 6, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Gossuin, Y.; Vuong, Q.L. NMR relaxometry for adsorption studies: Proof of concept with copper adsorption on activated alumina. Sep. Purif. Technol. 2018, 202, 138–143. [Google Scholar] [CrossRef]
- Qiao, A.; Cui, M.; Huang, R.; Ding, G.; Qi, W.; He, Z.; Klemeš, J.J.; Su, R. Advances in nanocellulose-based materials as adsorbents of heavy metals and dyes. Carbohydr. Polym. 2021, 272, 118471. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Salama, A.; Abouzeid, R.; Leong, W.S.; Jeevanandam, J.; Samyn, P.; Dufresne, A.; Bechelany, M.; Barhoum, A. Nanocellulose-Based Materials for Water Treatment: Adsorption, Photocatalytic Degradation, Disinfection, Antifouling, and Nanofiltration. Nanomaterials 2021, 11, 3008. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Potenza, M.; Bergamonti, L.; Lottici, P.P.; Righi, L.; Lazzarini, L.; Graiff, C. Green Extraction of Cellulose Nanocrystals of Polymorph II from Cynara scolymus L.: Challenge for a “Zero Waste” Economy. Crystals 2022, 12, 672. [Google Scholar] [CrossRef]
- Gröndahl, J.; Karisalmi, K.; Vapaavuori, J. Micro- and nanocelluloses from non-wood waste sources; processes and use in industrial applications. Soft Matter 2021, 17, 9842–9858. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef] [Green Version]
- Thach-Nguyen, R.; Dang-Bao, T. Noble metal nanoparticles dispersed on nanocellulose: A green platform for catalytic organic transformations. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1258, 012014. [Google Scholar] [CrossRef]
- Thach-Nguyen, R.; Lam, H.-H.; Phan, H.-P.; Dang-Bao, T. Cellulose nanocrystals isolated from corn leaf: Straightforward immobilization of silver nanoparticles as a reduction catalyst. RSC Adv. 2022, 12, 35436–35444. [Google Scholar] [CrossRef] [PubMed]
- Dang-Bao, T.; Nguyen, L.-N.; Lam, H.-H. Corncob-derived nanocellulose-supported palladium nanoparticles towards catalytic reduction of 4-nitrophenol. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Afewerki, S.; Alimohammadzadeh, R.; Osong, S.H.; Tai, C.-W.; Engstrand, P.; Córdova, A. Sustainable Design for the Direct Fabrication and Highly Versatile Functionalization of Nanocelluloses. Glob. Chall. 2017, 1, 1700045. [Google Scholar] [CrossRef]
- Kanoth, B.P.; Claudino, M.; Johansson, M.; Berglund, L.A.; Zhou, Q. Biocomposites from Natural Rubber: Synergistic Effects of Functionalized Cellulose Nanocrystals as Both Reinforcing and Cross-Linking Agents via Free-Radical Thiol–ene Chemistry. ACS Appl. Mater. Interfaces 2015, 7, 16303–16310. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadzadeh, R.; Rafi, A.A.; Goclik, L.; Tai, C.-W.; Cordova, A. Direct organocatalytic thioglycolic acid esterification of cellulose nanocrystals: A simple entry to click chemistry on the surface of nanocellulose. Carbohydr. Polym. Technol. Appl. 2022, 3, 100205. [Google Scholar] [CrossRef]
- Xu, Q.; Ke, X.; Zhang, Y.; Fu, F.; Liu, X. Facile Fabrication of Durable Antibacterial Cotton Fabric Realized by Thioglycolic Acid and Silver Nanoparticles. Fibers Polym. 2018, 19, 2307–2316. [Google Scholar] [CrossRef]
- Ram, B.; Chauhan, G.S. New spherical nanocellulose and thiol-based adsorbent for rapid and selective removal of mercuric ions. Chem. Eng. J. 2018, 331, 587–596. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xiao, N.; Wang, H.; Liu, C.; Pan, X. Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate. Polymers 2018, 10, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariano, M.; Kissi, N.E.; Dufresne, A. Cellulose nanomaterials: Size and surface influence on the thermal and rheological behavior. Polimeros 2018, 28, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Somseemee, O.; Saeoui, P.; Schevenels, F.T.; Siriwong, C. Enhanced interfacial interaction between modified cellulose nanocrystals and epoxidized natural rubber via ultraviolet irradiation. Sci. Rep. 2022, 12, 6682. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Sauvage, F.; Fraire, J.C.; Huang, C.; Smedt, S.C.D.; Braeckmans, K. Photothermal Nanomaterial-Mediated Photoporation. Acc. Chem. Res. 2023, 56, 631–643. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Y.; Lu, H.; Fang, Q.; Rong, H. Efficient Adsorption of Tetracycline from Aqueous Solutions by Modified Alginate Beads after the Removal of Cu(II) Ions. ACS Omega 2021, 6, 6240–6251. [Google Scholar] [CrossRef]
- Dang-Bao, T.; Lam, H.-H.; Dang, T.-H.-L. Defluoridation of water by Ce-Ti hybrid oxide nanoparticles. IOP Conf. Ser. Earth Environ. Sci. 2021, 947, 012026. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]



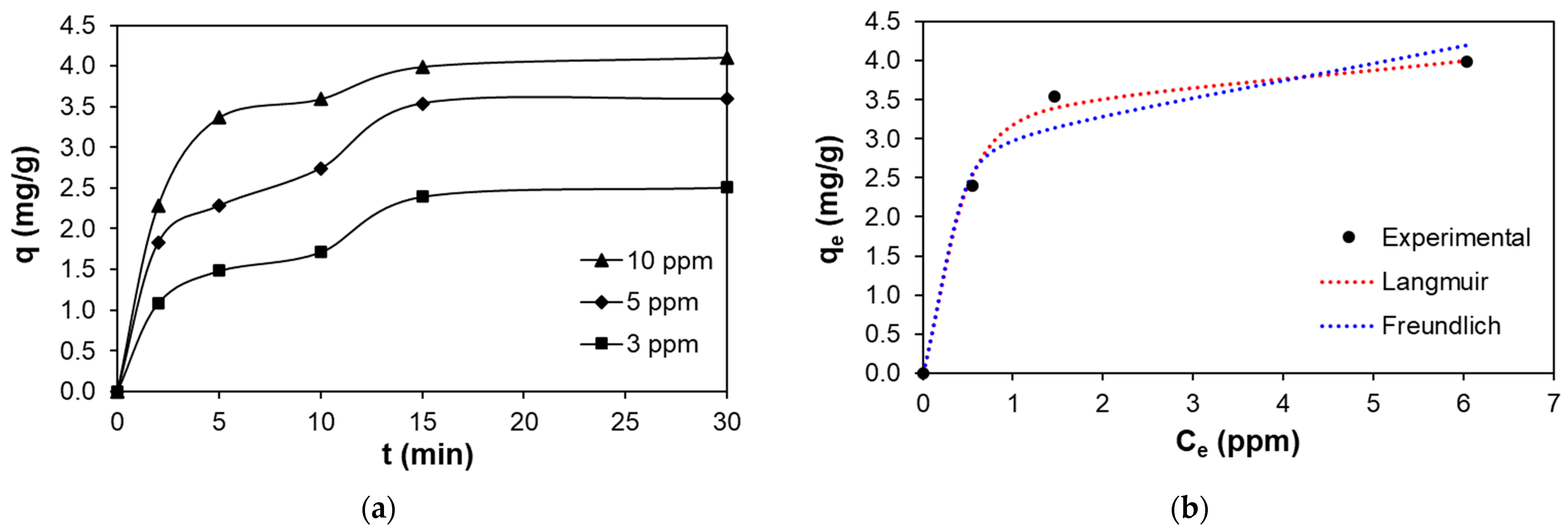

| Isotherm Models | Parameters | R2 | ||
|---|---|---|---|---|
| Langmuir | qm (mg g−1) | 4.244 | 0.9995 | |
| KL (L mg−1) | 2.733 | |||
| RL | 0.035–0.109 | |||
| Freundlich | n | 4.885 | 0.8483 | |
| KF (mg g−1)(L mg−1)1/n | 2.903 | |||
| Kinetic Models | Parameters | Initial Copper Concentrations | |||
|---|---|---|---|---|---|
| 3 ppm | 5 ppm | 10 ppm | |||
| Pseudo-first order | qe (mg g−1) | 1.937 | 2.794 | 2.967 | |
| k (min−1) | 0.1158 | 0.1357 | 0.2242 | ||
| R2 | 0.8696 | 0.8899 | 0.8930 | ||
| Pseudo-second order | qe (mg g−1) | 2.880 | 3.992 | 4.352 | |
| k (g mg−1 min−1) | 0.0778 | 0.0786 | 0.1352 | ||
| R2 | 0.9817 | 0.9908 | 0.9992 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang-Bao, T.; Nguyen, T.-M.-C.; Hoang, G.-H.; Lam, H.-H.; Phan, H.-P.; Tran, T.-K.-A. Thiol-Surface-Engineered Cellulose Nanocrystals in Favor of Copper Ion Uptake. Polymers 2023, 15, 2562. https://doi.org/10.3390/polym15112562
Dang-Bao T, Nguyen T-M-C, Hoang G-H, Lam H-H, Phan H-P, Tran T-K-A. Thiol-Surface-Engineered Cellulose Nanocrystals in Favor of Copper Ion Uptake. Polymers. 2023; 15(11):2562. https://doi.org/10.3390/polym15112562
Chicago/Turabian StyleDang-Bao, Trung, Thi-My-Chau Nguyen, Gia-Han Hoang, Hoa-Hung Lam, Hong-Phuong Phan, and Thi-Kieu-Anh Tran. 2023. "Thiol-Surface-Engineered Cellulose Nanocrystals in Favor of Copper Ion Uptake" Polymers 15, no. 11: 2562. https://doi.org/10.3390/polym15112562
APA StyleDang-Bao, T., Nguyen, T.-M.-C., Hoang, G.-H., Lam, H.-H., Phan, H.-P., & Tran, T.-K.-A. (2023). Thiol-Surface-Engineered Cellulose Nanocrystals in Favor of Copper Ion Uptake. Polymers, 15(11), 2562. https://doi.org/10.3390/polym15112562






