How to Develop Drug Delivery System Based on Carbohydrate Nanoparticles Targeted to Brain Tumors
Abstract
1. Introduction
2. Carbohydrates as Carriers for Drug Delivery and Tissue Engineering
2.1. Glucose-Based

2.2. Chitin and Chitosan
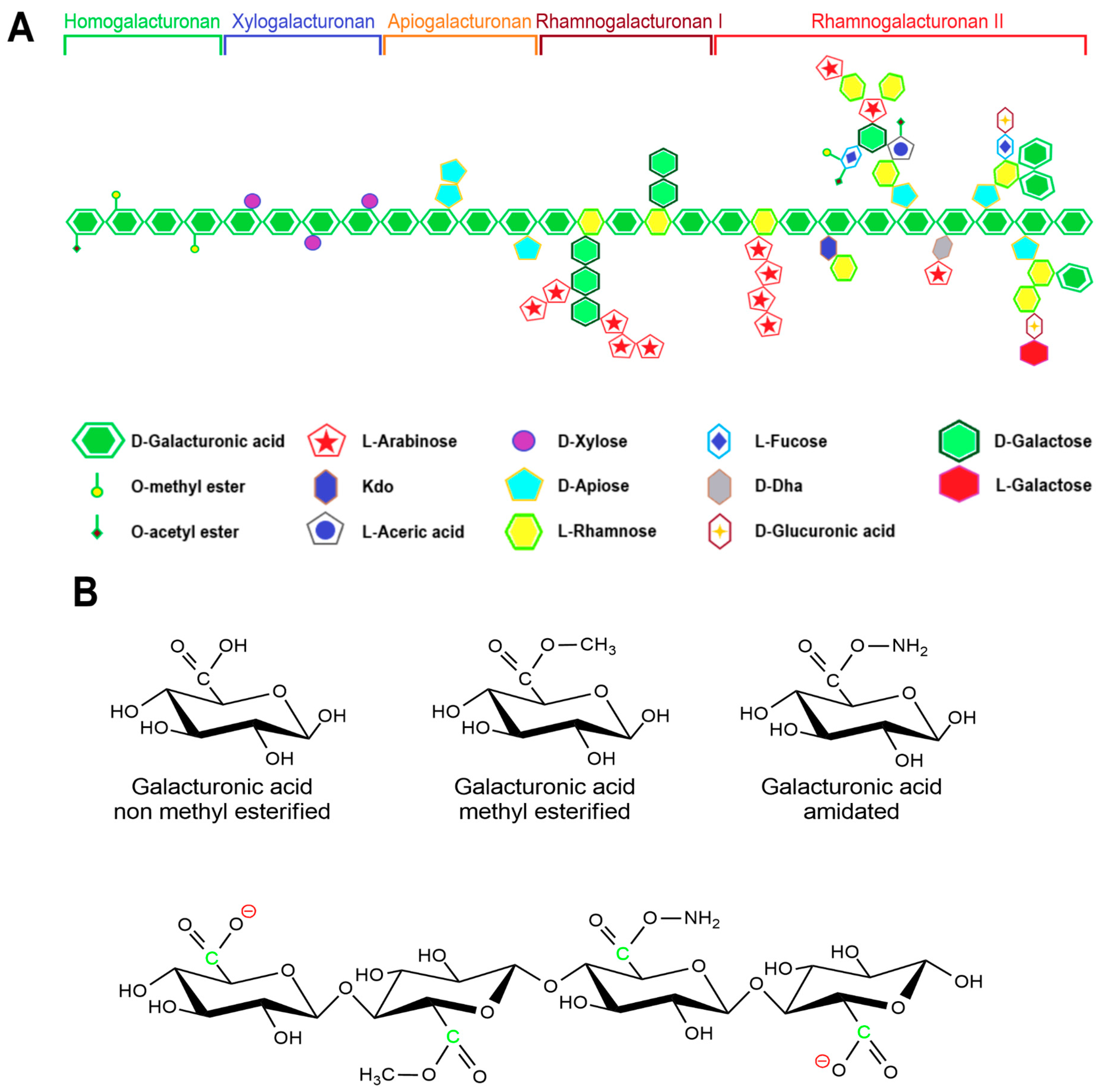
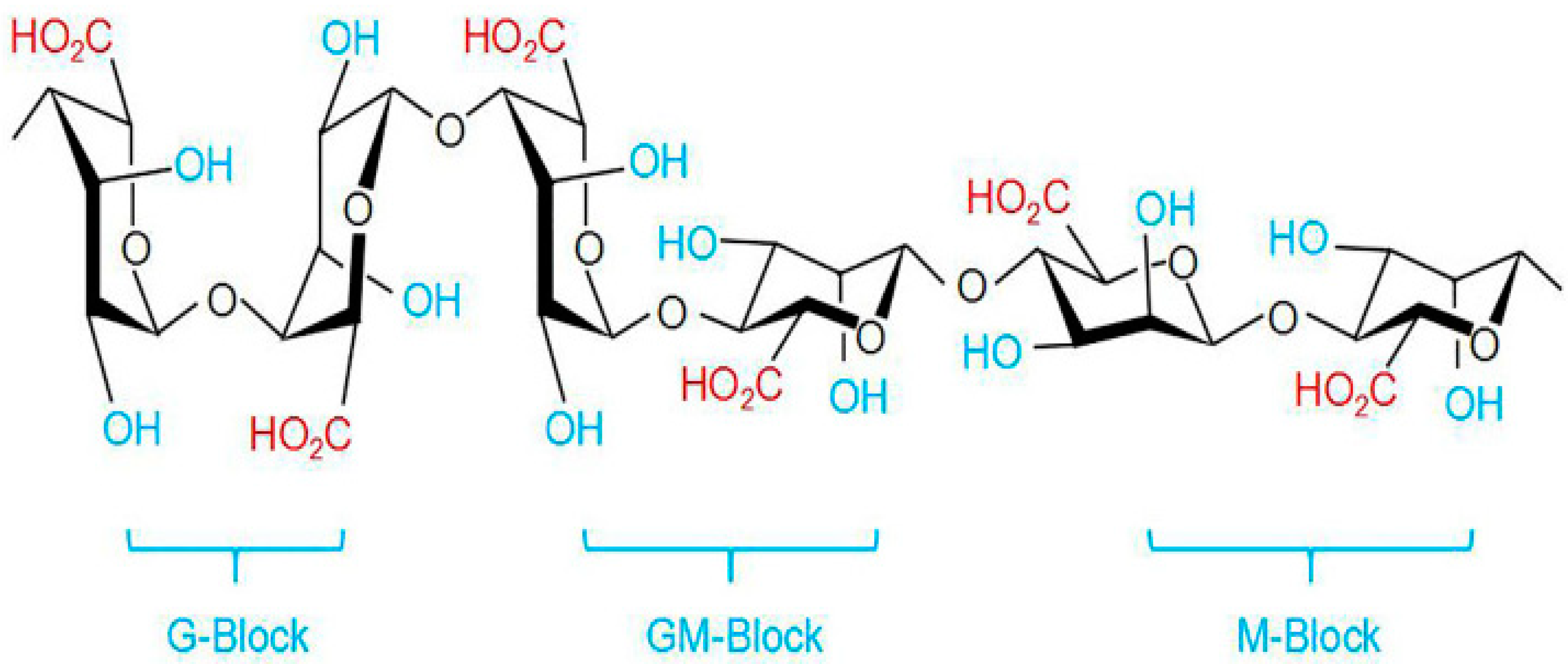
2.3. Pectin
2.4. Hyaluronic Acid
2.5. Alginate
3. Drug Delivery to the Brain
4. Invasive Strategies for Drug Delivery to the Brain
4.1. Intratumoral Drug Delivery
4.2. Blood–Brain Barrier Transient Disruption
5. Noninvasive Strategies for Drug Delivery to the Brain
5.1. Viral Vectors
5.2. Exosomes
5.3. Delivery through Active Transporters in the Blood–Brain Barrier
5.4. Nanoparticles
6. Carbohydrate Nanoparticles for Brain Delivery
6.1. Cellulose-Based Nanoparticles for Brain Delivery
6.2. Cyclodextrin-Based Nanoparticles for Brain Delivery
6.3. Chitosan-Based Nanoparticles for Brain Delivery
6.4. Pectin-Based Nanoparticles for Brain Delivery
6.5. Hyaluronic Acid-Based Nanoparticles for Brain Delivery
6.6. Alginate-Based Nanoparticles for Brain Delivery
6.7. Polysaccharide Nanoparticles Effectiveness and Cytotoxicity
| Nanoparticle Physical Properties | Nanoparticle Biological Impact | References | |||||
|---|---|---|---|---|---|---|---|
| Polysaccharide | Size | Charge | Predominant Synthesis Method | Physical State | Drug Incapsulated | BBB Transmission Showed | |
| Chitosan | 1–200 nm | Positive | Reverse micelle | Hydrogel | DOX, TMZ, lomustine, etc. | Yes | [141,142] |
| Pectin | 25–900 nm | Negative | Ionic gelation | Hydrogel | TMZ, 5-Fluorouracil | No (for non-functionalized) | [184,185] |
| Alginate | 10–150 nm | Negative | Ionic gelation | Hydrogel | TMZ, DOX, paclitaxel, etc. | No (for non-functionalized) | [186,187] |
| Cyclodextrin | Any | Uncharged | Ultrasonic emulsification | Hydrogel | 5-fluorouracil, paclitaxel, TMZ | No (for non-functionalized) | [188] |
| Cellulose | Any, different-shaped | Uncharged | Ultrasonic treatment | Solid | DOX, tetracyclines | No (for non-functionalized) | [189,190] |
| Hyaluronic acid | Any | Negative | Ionic gelation | Hydrogel | Doxorubicin, PTX, camptothecin, curcumin, quercetagetin, dexamethasone, chlorhexidine | No (for non-functionalized) | [154,165,166,167,168,169,170,171] |
7. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| BBB | blood–brain barrier |
| CDs | cyclodextrins |
| CED | convection-enhanced delivery |
| CMT | active carriers (carrier-mediated transcytosis) |
| CNCs | nanocrystals |
| CNS | central nervous system |
| DD | degree of deacetylation |
| DE | degree of esterification |
| GBM | glioblastoma multiforme |
| HA | hyaluronic acid |
| NPs | nanoparticles |
| Pgp | P-glycoprotein |
| PTX | paclitaxel |
| RMT | active carrier receptors (receptor-mediated transcytosis) |
| TMZ | temozolomide |
| VLF AG-NPC | venlafaxine-loaded alginate NPs |
References
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Fisher, J.P.; Adamson, D.C. Current FDA-approved therapies for high-grade malignant gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Drugs Approved for Brain Tumors. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/brain (accessed on 5 May 2023).
- Nishikawa, R.; Iwata, H.; Sakata, Y.; Muramoto, K.; Matsuoka, T. Safety of gliadel implant for malignant glioma: Report of postmarketing surveillance in Japan. Neurol. Med. Chir. 2021, 61, 536–548. [Google Scholar] [CrossRef]
- Kleinberg, L. Polifeprosan 20, 3.85% carmustine slow release wafer in malignant glioma: Patient selection and perspectives on a low-burden therapy. Patient Prefer. Adherence 2016, 10, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Deepti, R.D.; Archana, D.K.; Madhuri, A.C.; Shilpa, R.G. A review: Brain specific delivery. GSC Biol. Pharm. Sci. 2020, 13, 68–79. [Google Scholar] [CrossRef]
- Raposo, C.D.; Conceição, C.A.; Barros, M.T. Nanoparticles based on novel carbohydrate-functionalized polymers. Molecules 2020, 25, 1744. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Ribovski, L.; Hamelmann, N.M.; Paulusse, J.M.J. Polymeric nanoparticles properties and brain delivery. Pharmaceutics 2021, 13, 2045. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.-X. Role of nanoparticle mechanical properties in cancer drug delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759. [Google Scholar] [CrossRef]
- Silva, A.C.; Oliveira, T.R.; Mamani, J.B.; Malheiros, S.M.F.; Malavolta, L.; Pavon, L.F.; Sibov, T.T.; Amaro, E., Jr.; Tannús, A.; Vidoto, E.L.G.; et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. IJN 2011, 6, 591–603. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano 2019, 13, 386–398. [Google Scholar] [CrossRef]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef]
- Ünal, S.; Aktaş, Y.; Benito, J.M.; Bilensoy, E. Cyclodextrin nanoparticle bound oral camptothecin for colorectal cancer: Formulation development and optimization. Int. J. Pharm. 2020, 584, 119468. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, N.; Wang, Z.; Gao, J.; Zhang, H.; Li, M.; Du, Y.; Gao, X.; Zheng, A. Development and optimization of chitosan nanoparticle-based intranasal vaccine carrier. Molecules 2021, 27, 204. [Google Scholar] [CrossRef] [PubMed]
- Jonassen, H.; Treves, A.; Kjøniksen, A.-L.; Smistad, G.; Hiorth, M. Preparation of ionically cross-linked pectin nanoparticles in the presence of chlorides of divalent and monovalent cations. Biomacromolecules 2013, 14, 3523–3531. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic acid in ocular drug delivery. Carbohydr. Polym. 2021, 264, 118006. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.-S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef]
- Corrêa, L.B.; Pinto, S.R.; Alencar, L.M.R.; Missailidis, S.; Rosas, E.C.; Henriques, M.D.G.M.D.O.; Santos-Oliveira, R. Nanoparticle conjugated with aptamer anti-MUC1/Y for inflammatory arthritis. Colloids Surf. B Biointerfaces 2022, 211, 112280. [Google Scholar] [CrossRef]
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor A2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. IJMS 2019, 20, 5776. [Google Scholar] [CrossRef]
- Cochran, D.B.; Wattamwar, P.P.; Wydra, R.; Hilt, J.Z.; Anderson, K.W.; Eitel, R.E.; Dziubla, T.D. Suppressing iron oxide nanoparticle toxicity by vascular targeted antioxidant polymer nanoparticles. Biomaterials 2013, 34, 9615–9622. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, M.; Zhang, Y.; Ning, L.; Zhao, D.; Ni, Y. Cellulose hollow annular nanoparticles prepared from high-intensity ultrasonic treatment. ACS Nano 2022, 16, 8928–8938. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Lu, S.; Ma, T.; Hu, X.; Zhao, J.; Liao, X.; Song, Y.; Hu, X. Facile extraction and characterization of cellulose nanocrystals from agricultural waste sugarcane straw. J. Sci. Food Agric. 2022, 102, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; An, X.; Seta, F.T.; Ma, M.; Shen, M.; Dai, L.; Liu, H.; Ni, Y. Improving dispersion stability of hydrochloric acid hydrolyzed cellulose nano-crystals. Carbohydr. Polym. 2019, 222, 115037. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W.; Ling, Y.; Liu, B. Facile and rapid one–step extraction of carboxylated cellulose nanocrystals by H2SO4/HNO3 mixed acid hydrolysis. Carbohydr. Polym. 2020, 231, 115701. [Google Scholar] [CrossRef]
- Lugoloobi, I.; Maniriho, H.; Jia, L.; Namulinda, T.; Shi, X.; Zhao, Y. Cellulose nanocrystals in cancer diagnostics and treatment. J. Control. Release 2021, 336, 207–232. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, S.-J. Pectin modifications: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Köse, K.; Tüysüz, M.; Aksüt, D.; Uzun, L. Modification of cyclodextrin and use in environmental applications. Environ. Sci. Pollut. Res. 2022, 29, 182–209. [Google Scholar] [CrossRef]
- Borah, H.J.; Gogoi, M.; Das, D.B.; Hazarika, S. Cyclodextrine-glutaraldehyde cross-linked nanofiltration membrane for recovery of resveratrol from plant extract. J. Environ. Chem. Eng. 2020, 8, 103620. [Google Scholar] [CrossRef]
- Luo, Q.; He, L.; Wang, X.; Huang, H.; Wang, X.; Sang, S.; Huang, X. Cyclodextrin derivatives used for the separation of boron and the removal of organic pollutants. Sci. Total Environ. 2020, 749, 141487. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. IJMS 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in host–guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Ley, C.; Elvers, B. Ullmann’s Encyclopedia of Industrial Chemistry, 1st ed.; Wiley: Weinheim, Germany, 2003; pp. 471–481. ISBN 978-3-527-30385-4. [Google Scholar]
- Kumirska, J.; Weinhold, M.X.; Czerwicka, M.; Kaczyski, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Influence of the chemical structure and physicochemical properties of chitin- and chitosan-based materials on their biomedical activity. In Biomedical Engineering, Trends in Materials Science; Laskovski, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 25–65. ISBN 978-953-307-513-6. [Google Scholar]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Makromol. Chem. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, C.; Liu, Y.; Liu, Z.; Li, J.; Wang, Z.; Han, X. All-in-one bioactive properties of photothermal nanofibers for accelerating diabetic wound healing. Biomaterials 2023, 295, 122029. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Einhorn-Stoll, U.; Archut, A.; Eichhorn, M.; Kastner, H. Pectin—Plant protein systems and their application. Food Hydrocoll. 2021, 118, 106783. [Google Scholar] [CrossRef]
- Kim, M.-S.; Chandika, P.; Jung, W.-K. Recent advances of pectin-based biomedical application: Potential of marine pectin. J. Mar. Biosci. Biotechnol. 2021, 13, 28–47. [Google Scholar] [CrossRef]
- Martínez-Sabando, J.; Coin, F.; Melillo, J.H.; Goyanes, S.; Cerveny, S. A review of pectin-based material for applications in water treatment. Materials 2023, 16, 2207. [Google Scholar] [CrossRef]
- Kedir, W.M.; Deresa, E.M.; Diriba, T.F. Pharmaceutical and drug delivery applications of pectin and its modified nanocomposites. Heliyon 2022, 8, e10654. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Dong, H.; Li, X.; Zhang, J.; Ramaswamy, S.; Xu, F. Pectin in biomedical and drug delivery applications: A review. Int. J. Biol. Macromol. 2021, 185, 49–65. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Cadete, A.; Alonso, M.J. Targeting cancer with hyaluronic acid-based nanocarriers: Recent advances and translational perspectives. Nanomedicine 2016, 11, 2341–2357. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Z. Targeting and delivery of platinum-based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Murphy, E.J.; Montgomery, T.R.; Major, I. Hyaluronic acid: A review of the drug delivery capabilities of this naturally occurring polysaccharide. Polymers 2022, 14, 3442. [Google Scholar] [CrossRef]
- Donelan, W.; Dominguez-Gutierrez, P.R.; Kusmartsev, S. Deregulated hyaluronan metabolism in the tumor microenvironment drives cancer inflammation and tumor-associated immune suppression. Front. Immunol. 2022, 13, 971278. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Nomura, S.; Sato, Y.; Takagi, K.; Ishii, T.; Honma, Y.; Watanabe, K.; Mizukami, Y.; Muto, J. Anti-inflammatory effects of differential molecular weight hyaluronic acids on UVB-induced calprotectin-mediated keratinocyte inflammation. J. Dermatol. Sci. 2022, 107, 24–31. [Google Scholar] [CrossRef]
- Petrey, A.C.; De La Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Hintze, V.; Schnabelrauch, M.; Rother, S. Chemical modification of hyaluronan and their biomedical applications. Front. Chem. 2022, 10, 830671. [Google Scholar] [CrossRef]
- Pandit, A.H.; Mazumdar, N.; Ahmad, S. Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2019, 137, 853–869. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Improvement of hyaluronic acid enzymatic stability by the grafting of amino-acids. Carbohydr. Polym. 2012, 87, 2211–2216. [Google Scholar] [CrossRef]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Lee, W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Verma, M.L.; Dhanya, B.S.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef]
- Chae, T.; Yang, H.; Leung, V.; Ko, F.; Troczynski, T. Novel biomimetic hydroxyapatite/alginate nanocomposite fibrous scaffolds for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 1885–1894. [Google Scholar] [CrossRef]
- Stojkovska, J.; Djurdjevic, Z.; Jancic, I.; Bufan, B.; Milenkovic, M.; Jankovic, R.; Miskovic-Stankovic, V.; Obradovic, B. Comparative in vivo evaluation of novel formulations based on alginate and silver nanoparticles for wound treatments. J. Biomater. Appl. 2018, 32, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Khuller, G. Alginate-based sustained release drug delivery systems for tuberculosis. Expert Opin. Drug Deliv. 2008, 5, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Lo Cascio, C. Developmentally regulated signaling pathways in glioma invasion. Cell. Mol. Life Sci. 2018, 75, 385–402. [Google Scholar] [CrossRef]
- Mathew, E.N.; Berry, B.C.; Yang, H.W.; Carroll, R.S.; Johnson, M.D. Delivering therapeutics to glioblastoma: Overcoming biological constraints. IJMS 2022, 23, 1711. [Google Scholar] [CrossRef]
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; Van Vuurden, D.G.; Hulleman, E. Overview of current drug delivery methods across the blood–brain barrier for the treatment of primary brain tumors. CNS Drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Li, W.; Meng, G.; Wang, P.; Liao, W. Strategies for transporting nanoparticles across the blood–brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the blood–brain barrier for the therapy of malignant brain tumor: Current status and prospects of drug delivery approaches. J. Nanobiotechnol. 2022, 20, 412. [Google Scholar] [CrossRef]
- Ashby, L.S.; Smith, K.A.; Stea, B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: A systematic literature review. World J. Surg. Oncol. 2016, 14, 225. [Google Scholar] [CrossRef]
- Gazaille, C.; Sicot, M.; Saulnier, P.; Eyer, J.; Bastiat, G. Local delivery and glioblastoma: Why not combining sustained release and targeting? Front. Med. Technol. 2021, 3, 791596. [Google Scholar] [CrossRef]
- Iuchi, T.; Inoue, A.; Hirose, Y.; Morioka, M.; Horiguchi, K.; Natsume, A.; Arakawa, Y.; Iwasaki, K.; Fujiki, M.; Kumabe, T.; et al. Long-term effectiveness of gliadel implant for malignant glioma and prognostic factors for survival: 3-year results of a postmarketing surveillance in Japan. Neuro-Oncol. Adv. 2022, 4, vdab189. [Google Scholar] [CrossRef]
- Obermeier, B.; Verma, A.; Ransohoff, R.M. The blood–brain barrier. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 133, pp. 39–59. ISBN 978-0-444-63432-0. [Google Scholar]
- Bellettato, C.M.; Scarpa, M. Possible strategies to cross the blood–brain barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef]
- Iorio-Morin, C.; Gahide, G.; Morin, C.; Vanderweyen, D.; Roy, M.-A.; St-Pierre, I.; Massicotte-Tisluck, K.; Fortin, D. Management of Primary central nervous system lymphoma using intra-arterial chemotherapy with osmotic blood-brain barrier disruption: Retrospective analysis of the sherbrooke cohort. Front. Oncol. 2021, 10, 543648. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Rajendran, S.; Jaquins-Gerstl, A.S.; Hayes, H.J.; Richardson, R.M. Convection-enhanced delivery and principles of extracellular transport in the brain. World Neurosurg. 2021, 151, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Lambride, C.; Vavourakis, V.; Stylianopoulos, T. Convection-enhanced delivery in silico study for brain cancer treatment. Front. Bioeng. Biotechnol. 2022, 10, 867552. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-enhanced delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Bhandari, A.; Jaiswal, K.; Singh, A.; Zhan, W. Convection-enhanced delivery of antiangiogenic drugs and liposomal cytotoxic drugs to heterogeneous brain tumor for combination therapy. Cancers 2022, 14, 4177. [Google Scholar] [CrossRef]
- Zhan, W. Convection enhanced delivery of anti-angiogenic and cytotoxic agents in combination therapy against brain tumour. Eur. J. Pharm. Sci. 2020, 141, 105094. [Google Scholar] [CrossRef]
- Young, J.S.; Aghi, M.K. Chronic convection-enhanced intratumoural delivery of chemotherapy for glioblastoma. Lancet Oncol. 2022, 23, 1347–1348. [Google Scholar] [CrossRef]
- Prabhu, S.S. Convection-enhanced delivery for management of malignant gliomas. In Progress in Neurological Surgery; Chernov, M.F., Muragaki, Y., Kesari, S., McCutcheon, I.E., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 32, pp. 152–158. ISBN 978-3-318-06062-1. [Google Scholar]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neurooncol. 2021, 151, 415–427. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Luo, S.; Tang, P.; Wan, M.; Wu, D.; Gao, W. Ultrasound-assisted brain delivery of nanomedicines for brain tumor therapy: Advance and prospect. J. Nanobiotechnol. 2022, 20, 287. [Google Scholar] [CrossRef]
- Dasgupta, A.; Liu, M.; Ojha, T.; Storm, G.; Kiessling, F.; Lammers, T. Ultrasound-Mediated drug delivery to the brain: Principles, progress and prospects. Drug Discov. Today Technol. 2016, 20, 41–48. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C.; et al. Ultrasound-mediated delivery of paclitaxel for glioma: A comparative study of distribution, toxicity, and efficacy of albumin-bound versus cremophor formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef]
- Meng, Y.; Reilly, R.M.; Pezo, R.C.; Trudeau, M.; Sahgal, A.; Singnurkar, A.; Perry, J.; Myrehaug, S.; Pople, C.B.; Davidson, B.; et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 2021, 13, eabj4011. [Google Scholar] [CrossRef] [PubMed]
- Noroozian, Z.; Xhima, K.; Huang, Y.; Kaspar, B.K.; Kügler, S.; Hynynen, K.; Aubert, I. MRI-guided focused ultrasound for targeted delivery of rAAV to the brain. In Adeno-Associated Virus Vectors; Castle, M.J., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1950, pp. 177–197. ISBN 978-1-4939-9138-9. [Google Scholar]
- Morse, S.V.; Mishra, A.; Chan, T.G.; De Rosales, R.T.M.; Choi, J.J. Liposome delivery to the brain with rapid short-pulses of focused ultrasound and microbubbles. J. Control. Release 2022, 341, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Cruz, E.; Götz, J.; Nisbet, R.M. Ultrasound-mediated delivery of novel tau-specific monoclonal antibody enhances brain uptake but not therapeutic efficacy. J. Control. Release 2022, 349, 634–648. [Google Scholar] [CrossRef]
- Arshadi, S.; Pishevar, A.R. Magnetic drug delivery effects on tumor growth. Inform. Med. Unlocked 2021, 27, 100789. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; McCarty, D.M. Crossing the blood–brain-barrier with viral vectors. Curr. Opin. Virol. 2016, 21, 87–92. [Google Scholar] [CrossRef]
- Chan, K.Y.; Jang, M.J.; Yoo, B.B.; Greenbaum, A.; Ravi, N.; Wu, W.-L.; Sánchez-Guardado, L.; Lois, C.; Mazmanian, S.K.; Deverman, B.E.; et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017, 20, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Schaffer, D.V. Engineered viral vectors for functional interrogation, deconvolution, and manipulation of neural circuits. Curr. Opin. Neurobiol. 2018, 50, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huang, M.; Ma, X.; Chen, H.; Gao, X. Harnessing exosomes for the development of brain drug delivery systems. Bioconjugate Chem. 2019, 30, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Salarpour, S.; Forootanfar, H.; Pournamdari, M.; Ahmadi-Zeidabadi, M.; Esmaeeli, M.; Pardakhty, A. Paclitaxel incorporated exosomes derived from glioblastoma cells: Comparative study of two loading techniques. DARU J. Pharm. Sci. 2019, 27, 533–539. [Google Scholar] [CrossRef]
- Erkan, E.P.; Senfter, D.; Madlener, S.; Jungwirth, G.; Ströbel, T.; Saydam, N.; Saydam, O. Extracellular vesicle-mediated suicide MRNA/protein delivery inhibits glioblastoma tumor growth in vivo. Cancer Gene Ther. 2017, 24, 38–44. [Google Scholar] [CrossRef]
- Hultqvist, G.; Syvänen, S.; Fang, X.T.; Lannfelt, L.; Sehlin, D. Bivalent brain shuttle increases antibody uptake by monovalent binding to the transferrin receptor. Theranostics 2017, 7, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Di, X.; Zhang, S.; Kan, Q.; Liu, H.; Lu, T.; Wang, Y.; Fu, Q.; Sun, J.; He, Z. Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf. B Biointerfaces 2016, 141, 260–267. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [CrossRef]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Med. Chem. 2014, 6, PMC.S13384. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Cupaioli, F.A.; Zucca, F.A.; Boraschi, D.; Zecca, L. Engineered nanoparticles. How brain friendly is this new guest? Prog. Neurobiol. 2014, 119–120, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Mittapalli, R.K.; Liu, X.; Adkins, C.E.; Nounou, M.I.; Bohn, K.A.; Terrell, T.B.; Qhattal, H.S.; Geldenhuys, W.J.; Palmieri, D.; Steeg, P.S.; et al. Paclitaxel–hyaluronic nanoconjugates prolong overall survival in a preclinical brain metastases of breast cancer model. Mol. Cancer Ther. 2013, 12, 2389–2399. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Md, S.; Sahni, J.K.; Ali, J.; Baboota, S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J. Psychiatr. Res. 2014, 48, 1–12. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, W.I.; Kim, Y.H.; Tae, G. Brain-targeted delivery of protein using chitosan- and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials 2013, 34, 1170–1178. [Google Scholar] [CrossRef]
- Sahin, A.; Yoyen-Ermis, D.; Caban-Toktas, S.; Horzum, U.; Aktas, Y.; Couvreur, P.; Esendagli, G.; Capan, Y. Evaluation of brain-targeted chitosan nanoparticles through blood–brain barrier cerebral microvessel endothelial cells. J. Microencapsul. 2017, 34, 659–666. [Google Scholar] [CrossRef]
- Yemisci, M.; Caban, S.; Fernandez-Megia, E.; Capan, Y.; Couvreur, P.; Dalkara, T. Preparation and characterization of biocompatible chitosan nanoparticles for targeted brain delivery of peptides. In Neurotrophic Factors; Skaper, S.D., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1727, pp. 443–454. ISBN 978-1-4939-7570-9. [Google Scholar]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T.; et al. Development of SiRNA-loaded chitosan nanoparticles targeting galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef]
- Ansari, R.; Sadati, S.M.; Mozafari, N.; Ashrafi, H.; Azadi, A. Carbohydrate polymer-based nanoparticle application in drug delivery for CNS-related disorders. Eur. Polym. J. 2020, 128, 109607. [Google Scholar] [CrossRef]
- Romruen, O.; Kaewprachu, P.; Karbowiak, T.; Rawdkuen, S. Isolation and characterization cellulose nanosphere from different agricultural by-products. Polymers 2022, 14, 2534. [Google Scholar] [CrossRef]
- Qian, H.; Wang, X.; Yuan, K.; Xie, C.; Wu, W.; Jiang, X.; Hu, L. Delivery of doxorubicin in vitro and in vivo using bio-reductive cellulose nanogels. Biomater. Sci. 2014, 2, 220–232. [Google Scholar] [CrossRef]
- Dong, S.; Cho, H.J.; Lee, Y.W.; Roman, M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules 2014, 15, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; Taharabaru, T.; Nishida, T.; Ohno, Y.; Maeda, Y.; Sato, M.; Ishikura, K.; Yanagihara, K.; Takagi, H.; Nakamura, T.; et al. Lactose-appended β-cyclodextrin as an effective nanocarrier for brain delivery. J. Control. Release 2020, 328, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Gill, E.S.; Wu, L.; Xu, L.; Lowe, T.L. β-cyclodextrin-poly(β-amino ester) nanoparticles for sustained drug delivery across the blood–brain barrier. Biomacromolecules 2012, 13, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 2012, 209, 2501–2513. [Google Scholar] [CrossRef]
- Wong, K.H.; Xie, Y.; Huang, X.; Kadota, K.; Yao, X.-S.; Yu, Y.; Chen, X.; Lu, A.; Yang, Z. Delivering crocetin across the blood-brain barrier by using γ-cyclodextrin to treat Alzheimer’s disease. Sci. Rep. 2020, 10, 3654. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Postnova, I.V. Water-soluble polyelectrolyte complexes of oppositely charged polysaccharides. Compos. Interfaces 2009, 16, 251–279. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, W.; Wang, W.; Xiao, Y.; Chen, Y.; Wang, X. Antibacterial electrospun nanofibrous materials for wound healing. Adv. Fiber Mater. 2023, 5, 107–129. [Google Scholar] [CrossRef]
- Singh, D.; Rashid, M.; Hallan, S.S.; Mehra, N.K.; Prakash, A.; Mishra, N. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif. Cells Nanomed. Biotechnol. 2015, 44, 865–877. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Khadrawy, Y.A.; Abd-El Daim, T.M.; Elfeky, A.S.; Abd Rabo, A.A.; Mustafa, A.B.; Mostafa, I.T. Thymoquinone-encapsulated chitosan nanoparticles coated with polysorbate 80 as a novel treatment agent in a reserpine-induced depression animal model. Physiol. Behav. 2020, 222, 112934. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Vaidya, B.; Prakash, A.; Rath, G.; Goyal, A.K. Brain delivery of intranasal in situ gel of nanoparticulated polymeric carriers containing antidepressant drug: Behavioral and biochemical assessment. J. Drug Target. 2015, 23, 275–286. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Eslahi, M.; Dana, P.M.; Asemi, Z.; Hallajzadeh, J.; Mansournia, M.A.; Yousefi, B. The effects of chitosan-based materials on glioma: Recent advances in its applications for diagnosis and treatment. Int. J. Biol. Macromol. 2021, 168, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Bigdeli, M.; Aligholi, H.; Rasoulian, B.; Khaksarian, M. Sustained release of silibinin-loaded chitosan nanoparticle induced apoptosis in glioma cells. J. Biomed. Mater. Res. 2020, 108, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Md, S.; Alhakamy, N.A.; Kesharwani, P. Recent development of aptamer conjugated chitosan nanoparticles as cancer therapeutics. Int. J. Pharm. 2022, 620, 121751. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Bao, X.; Du, Y.-Z.; You, J.; Hu, F.-Q. Preparation and evaluation of SiO2-deposited stearic acid-g-chitosan nanoparticles for doxorubicin delivery. IJN 2012, 7, 5119–5128. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef]
- Opanasopit, P.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Ruktanonchai, U. Development and characterization of pectinate micro/nanoparticles for gene delivery. AAPS PharmSciTech 2008, 9, 67–74. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fishman, M.L.; Hicks, K.B. Pectin in controlled drug delivery—A review. Cellulose 2006, 14, 15–24. [Google Scholar] [CrossRef]
- Wong, T.W.; Colombo, G.; Sonvico, F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech 2011, 12, 201–214. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Nan-Shan, C.; Boackle, R.J. Hyaluronic acid-complement interactions—II. Role of divalent cations and gelatin. Mol. Immunol. 1985, 22, 843–848. [Google Scholar] [CrossRef]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef]
- De La Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2016. [Google Scholar] [CrossRef]
- Matha, K.; Lollo, G.; Taurino, G.; Respaud, R.; Marigo, I.; Shariati, M.; Bussolati, O.; Vermeulen, A.; Remaut, K.; Benoit, J.-P. Bioinspired hyaluronic acid and polyarginine nanoparticles for DACHPt delivery. Eur. J. Pharm. Biopharm. 2020, 150, 1–13. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Koksal, A.F.; Kilic Iyilik, E. Complex coacervation of hyaluronic acid and chitosan: Effects of PH, ionic strength, charge density, chain length and the charge ratio. Soft Matter 2015, 11, 8605–8612. [Google Scholar] [CrossRef]
- Du, X.; Dubin, P.L.; Hoagland, D.A.; Sun, L. Protein-selective coacervation with hyaluronic acid. Biomacromolecules 2014, 15, 726–734. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.; Park, H. Design and investigation of nanoemulsified carrier based on amphiphile-modified hyaluronic acid. Carbohydr. Polym. 2011, 83, 462–469. [Google Scholar] [CrossRef]
- Jha, A.K.; Hule, R.A.; Jiao, T.; Teller, S.S.; Clifton, R.J.; Duncan, R.L.; Pochan, D.J.; Jia, X. Structural analysis and mechanical characterization of hyaluronic acid-based doubly cross-linked networks. Macromolecules 2009, 42, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, C.; Wei, S.; Yang, T.; Lan, Y.; Cao, A.; Yang, J.; Hou, Y. Paclitaxel delivered by CD44 receptor-targeting and endosomal PH sensitive dual functionalized hyaluronic acid micelles for multidrug resistance reversion. Colloids Surf. B Biointerfaces 2018, 170, 330–340. [Google Scholar] [CrossRef]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-targeted nanocarrier for cancer therapy. Front. Pharmacol. 2022, 12, 800481. [Google Scholar] [CrossRef]
- Avenoso, A.; D’Ascola, A.; Scuruchi, M.; Mandraffino, G.; Calatroni, A.; Saitta, A.; Campo, S.; Campo, G.M. Hyaluronan in the experimental injury of the cartilage: Biochemical action and protective effects. Inflamm. Res. 2018, 67, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Qiu, B.; Deng, R.; Li, H.; Xu, X.; Shang, X. Chondroprotective effects of hyaluronic acid-chitosan nanoparticles containing plasmid DNA encoding cytokine response modifier A in a rat knee osteoarthritis model. Cell Physiol. Biochem. 2018, 47, 1207–1216. [Google Scholar] [CrossRef]
- Zhang, M.; Asghar, S.; Tian, C.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. Lactoferrin/phenylboronic acid-functionalized hyaluronic acid nanogels loading doxorubicin hydrochloride for targeting glioma. Carbohydr. Polym. 2021, 253, 117194. [Google Scholar] [CrossRef]
- Li, K.; Liu, H.; Gao, W.; Chen, M.; Zeng, Y.; Liu, J.; Xu, L.; Wu, D. Mulberry-like dual-drug complicated nanocarriers assembled with apogossypolone amphiphilic starch micelles and doxorubicin hyaluronic acid nanoparticles for tumor combination and targeted therapy. Biomaterials 2015, 39, 131–144. [Google Scholar] [CrossRef]
- Thomas, R.G.; Moon, M.; Lee, S.; Jeong, Y.Y. Paclitaxel loaded hyaluronic acid nanoparticles for targeted cancer therapy: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2015, 72, 510–518. [Google Scholar] [CrossRef]
- Sun, W.; Du, Y.; Liang, X.; Yu, C.; Fang, J.; Lu, W.; Guo, X.; Tian, J.; Jin, Y.; Zheng, J. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials 2019, 217, 119264. [Google Scholar] [CrossRef]
- Pepe, G.; Calce, E.; Verdoliva, V.; Saviano, M.; Maglione, V.; Di Pardo, A.; De Luca, S. Curcumin-loaded nanoparticles based on amphiphilic hyaluronan-conjugate explored as targeting delivery system for neurodegenerative disorders. IJMS 2020, 21, 8846. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Han, Y.; Huang, J.; Dai, L.; Du, J.; McClements, D.J.; Mao, L.; Liu, J.; Gao, Y. Fabrication and characterization of layer-by-layer composite nanoparticles based on zein and hyaluronic acid for codelivery of curcumin and quercetagetin. ACS Appl. Mater. Interfaces 2019, 11, 16922–16933. [Google Scholar] [CrossRef] [PubMed]
- Abuelella, K.E.; Abd-Allah, H.; Soliman, S.M.; Abdel-Mottaleb, M.M.A. Skin targeting by chitosan/hyaluronate hybrid nanoparticles for the management of irritant contact dermatitis: In vivo therapeutic efficiency in mouse-ear dermatitis model. Int. J. Biol. Macromol. 2023, 232, 123458. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Yu, X.; Liu, J.; Wu, J.; Wan, Y. Chitosan-based hydrogels embedded with hyaluronic acid complex nanoparticles for controlled delivery of bone morphogenetic protein-2. Pharmaceutics 2019, 11, 214. [Google Scholar] [CrossRef]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef]
- Mamo, J.C.; Lam, V.; Al-Salami, H.; Brook, E.; Mooranian, A.; Nesbit, M.; Graneri, L.; D’Alonzo, Z.; Fimognari, N.; Stephenson, A.; et al. Sodium alginate capsulation increased brain delivery of probucol and suppressed neuroinflammation and neurodegeneration. Ther. Deliv. 2018, 9, 703–709. [Google Scholar] [CrossRef]
- Spadari, C.C.; Lanser, D.M.; Araújo, M.V.; De Jesus, D.F.F.; Lopes, L.B.; Gelli, A.; Ishida, K. Oral delivery of brain-targeted miltefosine-loaded alginate nanoparticles functionalized with polysorbate 80 for the treatment of cryptococcal meningitis. J. Antimicrob. Chemother. 2023, 78, 1092–1101. [Google Scholar] [CrossRef]
- Sorrentino, A.; Cataldo, A.; Curatolo, R.; Tagliatesta, P.; Mosca, L.; Bellucci, S. Novel optimized biopolymer-based nanoparticles for nose-to-brain delivery in the treatment of depressive diseases. RSC Adv. 2020, 10, 28941–28949. [Google Scholar] [CrossRef]
- Chavanpatil, M.D.; Khdair, A.; Gerard, B.; Bachmeier, C.; Miller, D.W.; Shekhar, M.P.V.; Panyam, J. Surfactant–polymer nanoparticles overcome P-glycoprotein-mediated drug efflux. Mol. Pharm. 2007, 4, 730–738. [Google Scholar] [CrossRef]
- Eleftheriadou, D.; Evans, R.E.; Atkinson, E.; Abdalla, A.; Gavins, F.K.H.; Boyd, A.S.; Williams, G.R.; Knowles, J.C.; Roberton, V.H.; Phillips, J.B. An alginate-based encapsulation system for delivery of therapeutic cells to the CNS. RSC Adv. 2022, 12, 4005–4015. [Google Scholar] [CrossRef]
- Kutlu, C.; Çakmak, A.S.; Gümüşderelioğlu, M. Double-effective chitosan scaffold-PLGA nanoparticle system for brain tumour therapy: In vitro study. J. Microencapsul. 2014, 31, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-I.; Jin, S.-G.; Kim, I.-Y.; Pei, J.; Wen, M.; Jung, T.-Y.; Moon, K.-S.; Jung, S. Doxorubicin-incorporated nanoparticles composed of poly(ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro. Colloids Surf. B Biointerfaces 2010, 79, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gherardini, L.; Vetri Buratti, V.; Maturi, M.; Inzalaco, G.; Locatelli, E.; Sambri, L.; Gargiulo, S.; Barone, V.; Bonente, D.; Bertelli, E.; et al. Loco-regional treatment with temozolomide-loaded thermogels prevents glioblastoma recurrences in orthotopic human xenograft models. Sci. Rep. 2023, 13, 4630. [Google Scholar] [CrossRef]
- Schwinn, S.; Mokhtari, Z.; Thusek, S.; Schneider, T.; Sirén, A.-L.; Tiemeyer, N.; Caruana, I.; Miele, E.; Schlegel, P.G.; Beilhack, A.; et al. Cytotoxic effects and tolerability of gemcitabine and axitinib in a xenograft model for C-Myc amplified medulloblastoma. Sci. Rep. 2021, 11, 14062. [Google Scholar] [CrossRef]
- K, S.K.; Choppala, A.D. Development and optimization of osimertinib-loaded biodegradable polymeric nanoparticles enhance in-vitro cytotoxicity in mutant EGFR NSCLC cell models and in-vivo tumor reduction in H1975 xenograft mice models. AAPS PharmSciTech 2022, 23, 159. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Wang, Y.-M.; Li, N.-M.; Liu, G.-S.; Yang, S.; Tang, G.-T.; He, D.-X.; Tan, X.-W.; Wei, H. In vitro and in vivo evaluation of pectin-based nanoparticles for hepatocellular carcinoma drug chemotherapy. Mol. Pharm. 2014, 11, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Pavelkova, A.; Maciulyte, S.; Budriene, S.; Sedlarik, V. Polysaccharide-based nanocomplexes for co-encapsulation and controlled release of 5-fluorouracil and temozolomide. Eur. J. Pharm. Sci. 2016, 92, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yao, L.; Nie, S.; Xu, Y. Low-viscosity sodium alginate combined with TiO2 nanoparticles for improving neuroblastoma treatment. Int. J. Biol. Macromol. 2021, 167, 921–933. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Liu, T.; Wu, Y.; Guo, H.; Wang, P.; Tian, Q.; Wang, Y.; Yuan, Z. Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials 2012, 33, 2187–2196. [Google Scholar] [CrossRef]
- Prabha, G.; Raj, V. Formation and characterization of β-cyclodextrin (β-CD)–polyethyleneglycol (PEG)–polyethyleneimine (PEI) coated Fe 3 O 4 nanoparticles for loading and releasing 5-fluorouracil drug. Biomed. Pharmacother. 2016, 80, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr. Polym. 2017, 175, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Letchford, K.; Jackson, J.K.; Wasserman, B.; Ye, L.; Hamad, W.Y.; Burt, H.M. The Use of nanocrystalline cellulose for the binding and controlled release of drugs. IJN 2011, 6, 321–330. [Google Scholar] [CrossRef] [PubMed]


| Method | Main Principle(s) | Advantage(s) | Drawback(s) |
|---|---|---|---|
| Emulsification and crosslinking | Covalent crosslinking | Simple process steps | Use of harmful chemicals |
| Reversed micelles | Covalent crosslinking | Ultrafine NPs below 100 nm | Time-consuming process Complex application Use of harmful chemicals |
| Phase inversion precipitation | Precipitation | High encapsulation capacity for specific compounds | Requires high shear force Use of harmful chemicals |
| Emulsion-droplet coalescence | Precipitation | Requires high shear force Use of harmful chemicals | |
| Ionic gelation | Ionic crosslinking | Use of mild chemicals. Simple process. Ease of adjusting NP size | |
| Ionic gelation with radical polymerization | Polymerization and crosslinking | Time-consuming process Complex application | |
| Self-assembly | Electrostatic and/or hydrophobic interaction | Highly stable NPs. Use of mild chemicals Adjustable procedure | Hard to control when carried out on a large scale |
| Top-down | Acid hydrolysis and deacetylation | Time-consuming process Complex application Need an extra step for drug loading | |
| Spray drying | Atomization | Simple and fast process. Does not require another separation or drying steps | Large particle size Not suitable for use with temperature-sensitive substances |
| SCASA | Atomization | Acid- or harmful solvent-free method. Does not require another separation or drying steps | Time-consuming process Requires a specially designed system Large particle size |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silant’ev, V.E.; Shmelev, M.E.; Belousov, A.S.; Patlay, A.A.; Shatilov, R.A.; Farniev, V.M.; Kumeiko, V.V. How to Develop Drug Delivery System Based on Carbohydrate Nanoparticles Targeted to Brain Tumors. Polymers 2023, 15, 2516. https://doi.org/10.3390/polym15112516
Silant’ev VE, Shmelev ME, Belousov AS, Patlay AA, Shatilov RA, Farniev VM, Kumeiko VV. How to Develop Drug Delivery System Based on Carbohydrate Nanoparticles Targeted to Brain Tumors. Polymers. 2023; 15(11):2516. https://doi.org/10.3390/polym15112516
Chicago/Turabian StyleSilant’ev, Vladimir E., Mikhail E. Shmelev, Andrei S. Belousov, Aleksandra A. Patlay, Roman A. Shatilov, Vladislav M. Farniev, and Vadim V. Kumeiko. 2023. "How to Develop Drug Delivery System Based on Carbohydrate Nanoparticles Targeted to Brain Tumors" Polymers 15, no. 11: 2516. https://doi.org/10.3390/polym15112516
APA StyleSilant’ev, V. E., Shmelev, M. E., Belousov, A. S., Patlay, A. A., Shatilov, R. A., Farniev, V. M., & Kumeiko, V. V. (2023). How to Develop Drug Delivery System Based on Carbohydrate Nanoparticles Targeted to Brain Tumors. Polymers, 15(11), 2516. https://doi.org/10.3390/polym15112516





