Resistance to Degradation of Silk Fibroin Hydrogels Exposed to Neuroinflammatory Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vivo Pathological Models
2.3. In Vitro Pathological Model
2.4. Preparation of Silk Fibroin Formulations
2.5. Preparation of Biomaterials Formulations
2.6. Proteolytic Activity on Substrate Gels
2.7. Hydrogel-Microglia Interaction Studies
2.8. Mechanical Tests
2.9. Stereotaxic Surgery and Biomaterial Injection
2.10. In Vivo Quantification of Silk Fibroin and Collagen Hydrogels
2.11. Statistical Analysis
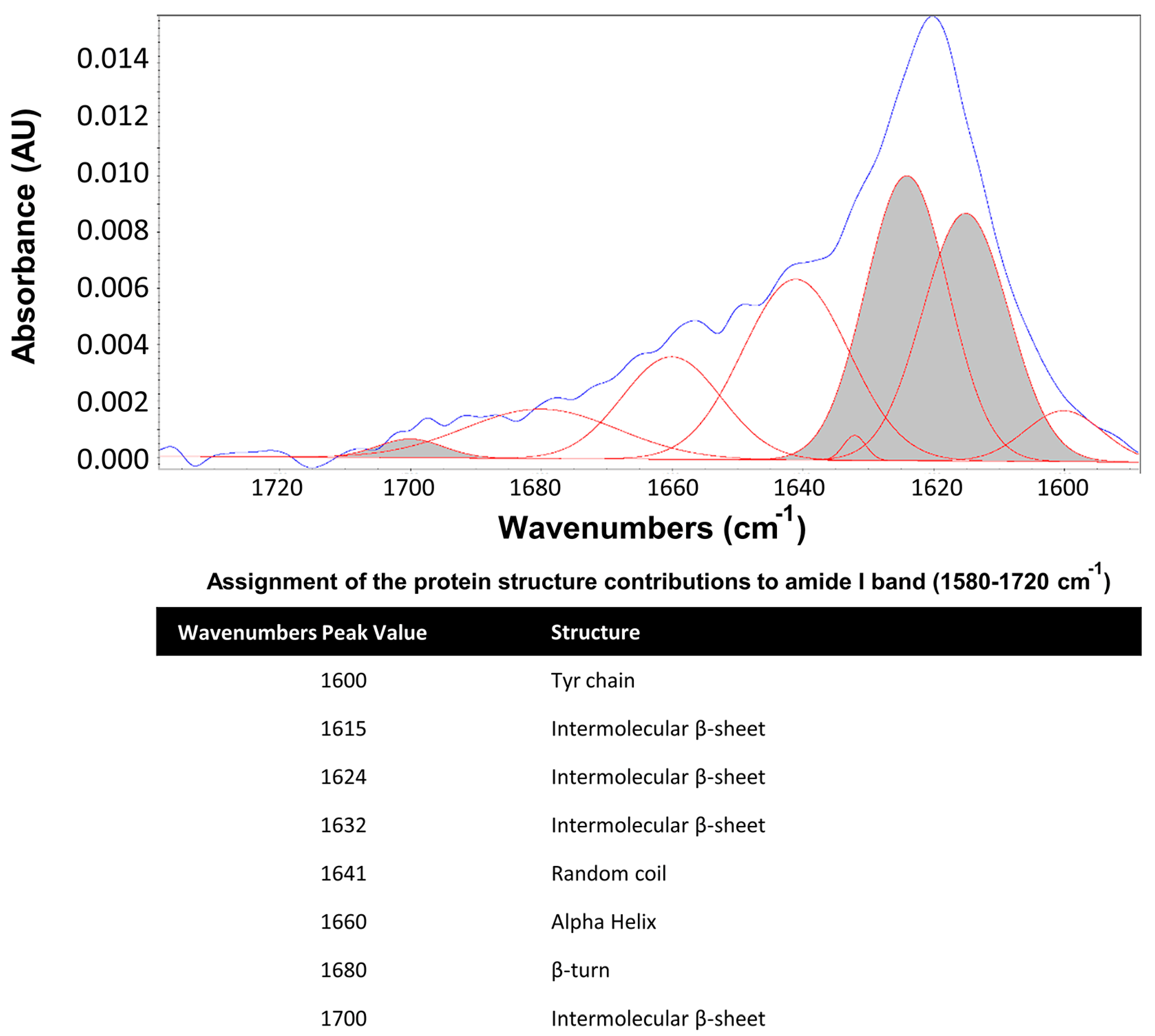
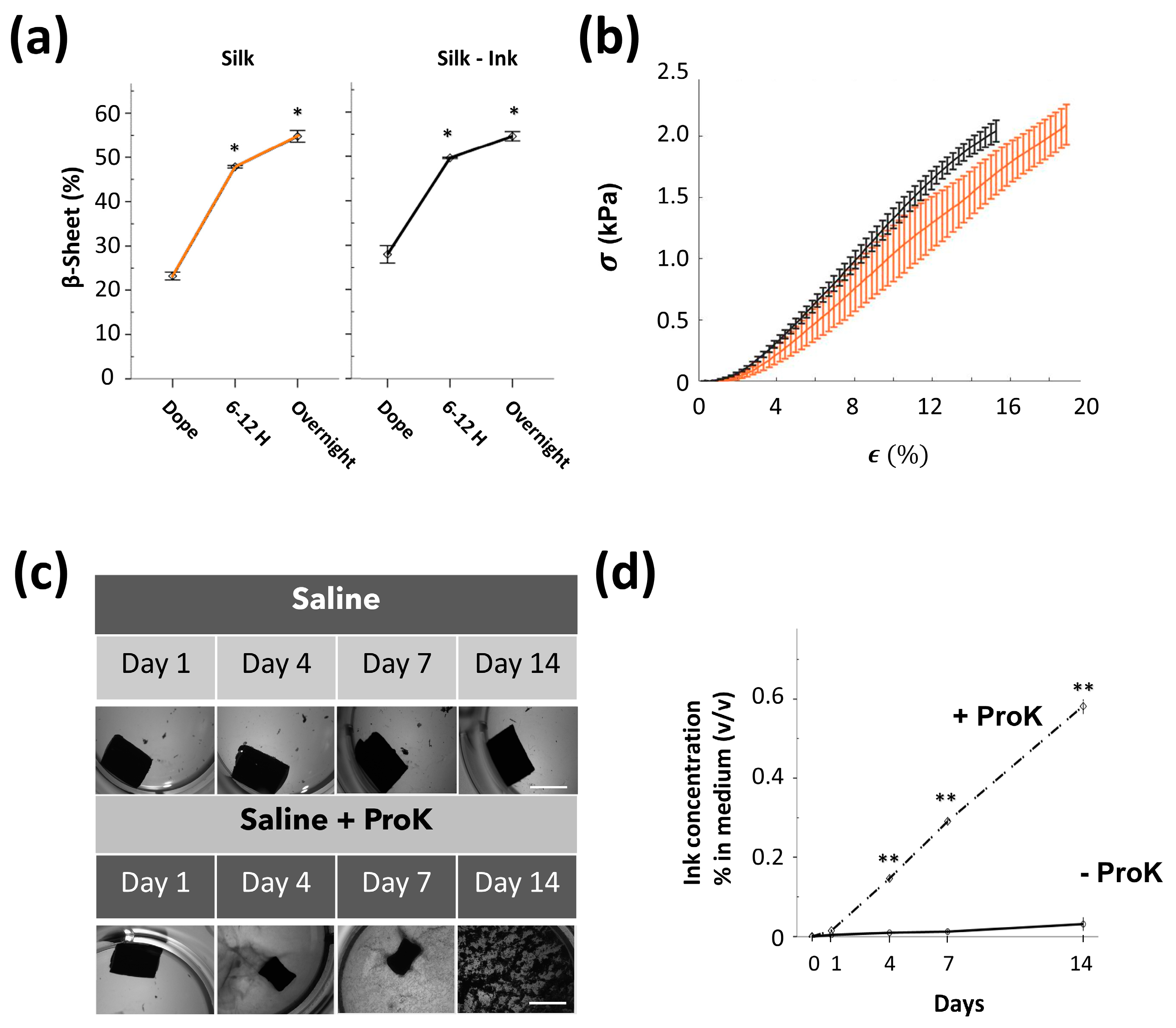
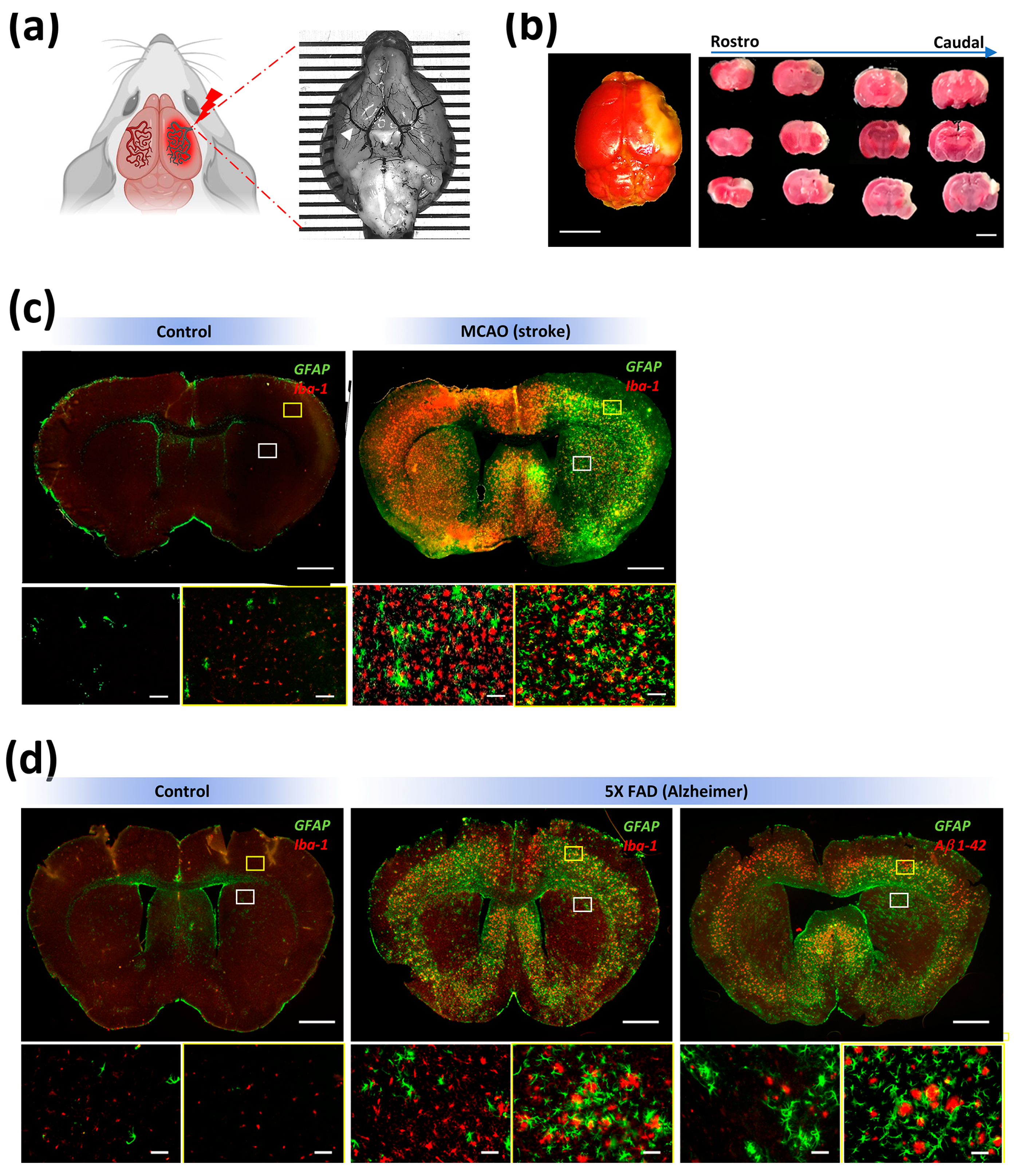
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gernert, M.; Feja, M. Bypassing the Blood-Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies. Pharmaceutics 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Roshani, M.; Kiaie, N.; Aghdam, R.M. Biomaterials and stem cells as drug/gene-delivery vehicles for Parkinson’s treatment: An update. Regen. Med. 2021, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Tang-Schomer, M.D.; Kaplan, D.L.; Whalen, M.J. Film interface for drug testing for delivery to cells in culture and in the brain. Acta Biomater. 2019, 94, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Boisserand, L.S.; Kodama, T.; Papassin, J.; Auzely, R.; Moisan, A.; Rome, C.; Detante, O. Biomaterial Applications in Cell-Based Therapy in Experimental Stroke. Stem Cells Int. 2016, 2016, 6810562. [Google Scholar] [CrossRef]
- Wilson, K.L.; Carmichael, S.T.; Segura, T. Injection of Hydrogel Biomaterial Scaffolds to The Brain After Stroke. J. Vis. Exp. 2020, 164, e61450. [Google Scholar] [CrossRef]
- Modo, M. Bioscaffold-Induced Brain Tissue Regeneration. Front. Neurosci. 2019, 13, 1156. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Conde, G.; de Carvalho, J.R.G.; Dias, P.D.P.; Moranza, H.G.; Montanhim, G.L.; Ribeiro, J.O.; Chinelatto, M.A.; Moraes, P.C.; Taboga, S.R.; Bertolo, P.H.L.; et al. In vivobiocompatibility and biodegradability of poly(lactic acid)/poly(epsilon-caprolactone) blend compatibilized with poly(epsilon-caprolactone-b-tetrahydrofuran) in Wistar rats. Biomed. Phys. Eng. Express 2021, 7, 035005. [Google Scholar] [CrossRef]
- Li, S.; Yu, D.; Ji, H.; Zhao, B.; Ji, L.; Leng, X. In vivo degradation and neovascularization of silk fibroin implants monitored by multiple modes ultrasound for surgical applications. Biomed. Eng. Online 2018, 17, 87. [Google Scholar] [CrossRef]
- Wang, Y.; Rudym, D.D.; Walsh, A.; Abrahamsen, L.; Kim, H.J.; Kim, H.S.; Kirker-Head, C.; Kaplan, D.L. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008, 29, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, A.P.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Recent advances in development of functional spider silk-based hybrid materials. Front. Chem. 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.D.; Young, J.H.; Weisman, S.; Hayashi, C.Y.; Merritt, D.J. Insect silk: One name, many materials. Annu. Rev. Entomol. 2010, 55, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Vendrely, C.; Scheibel, T. Biotechnological production of spider-silk proteins enables new applications. Macromol. Biosci 2007, 7, 401–409. [Google Scholar] [CrossRef]
- Banagozar Mohammadi, A.; Sadigh-Eteghad, S.; Torbati, M.; Bagher Fazljou, M.S.; Vatandoust, M.S.; Ej Golzari, S.; Farajdokht, F.; Mahmoudi, J. Identification and applications of neuroactive silk proteins: A narrative review. J. Appl. Biomed. 2019, 17, 147–156. [Google Scholar] [CrossRef]
- Nune, M.; Manchineella, S.; Govindaraju, T.; Narayan, K.S. Melanin incorporated electroactive and antioxidant silk fibroin nanofibrous scaffolds for nerve tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 17–25. [Google Scholar] [CrossRef]
- Wang, F.; Yan, B.; Li, Z.; Wang, P.; Zhou, M.; Yu, Y.; Yuan, J.; Cui, L.; Wang, Q. Rapid antibacterial effects of silk fabric constructed through enzymatic grafting of modified PEI and AgNP deposition. ACS Appl. Mater. Interfaces 2021, 13, 33505–33515. [Google Scholar] [CrossRef]
- Kim, D.W.; Hwang, H.S.; Kim, D.S.; Sheen, S.H.; Heo, D.H.; Hwang, G.; Kang, S.H.; Kweon, H.; Jo, Y.Y.; Kang, S.W.; et al. Effect of silk fibroin peptide derived from silkworm Bombyx mori on the anti-inflammatory effect of Tat-SOD in a mice edema model. BMB Rep. 2011, 44, 787–792. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. [Google Scholar] [CrossRef]
- Montalban, M.G.; Coburn, J.M.; Lozano-Perez, A.A.; Cenis, J.L.; Villora, G.; Kaplan, D.L. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef]
- Patra, C.; Talukdar, S.; Novoyatleva, T.; Velagala, S.R.; Muhlfeld, C.; Kundu, B.; Kundu, S.C.; Engel, F.B. Silk protein fibroin from Antheraea mylitta for cardiac tissue engineering. Biomaterials 2012, 33, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Madurga, R.; Ganan-Calvo, A.M.; Plaza, G.R.; Guinea, G.V.; Elices, M.; Perez-Rigueiro, J. Production of High Performance Bioinspired Silk Fibers by Straining Flow Spinning. Biomacromolecules 2017, 18, 1127–1133. [Google Scholar] [CrossRef]
- Hou, T.-C.; Jeng, S.-C. Application of Bombyx mori Silk Fibroin Films for Liquid-Crystal Devices. ACS Appl. Bio Mater. 2020, 3, 8575–8580. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Wang, Y.; Li, G.; Zheng, Z.; Kaplan, D.L.; Wang, X.; Wang, X. Flexible Water-Absorbing Silk-Fibroin Biomaterial Sponges with Unique Pore Structure for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Yonesi, M.; Garcia-Nieto, M.; Guinea, G.V.; Panetsos, F.; Perez-Rigueiro, J.; Gonzalez-Nieto, D. Silk Fibroin: An Ancient Material for Repairing the Injured Nervous System. Pharmaceutics 2021, 13, 429. [Google Scholar] [CrossRef]
- Fernandez-Serra, R.; Gallego, R.; Lozano, P.; Gonzalez-Nieto, D. Hydrogels for neuroprotection and functional rewiring: A new era for brain engineering. Neural Regen. Res. 2020, 15, 783–789. [Google Scholar] [CrossRef]
- Fernandez-Garcia, L.; Perez-Rigueiro, J.; Martinez-Murillo, R.; Panetsos, F.; Ramos, M.; Guinea, G.V.; Gonzalez-Nieto, D. Cortical Reshaping and Functional Recovery Induced by Silk Fibroin Hydrogels-Encapsulated Stem Cells Implanted in Stroke Animals. Front. Cell Neurosci. 2018, 12, 296. [Google Scholar] [CrossRef]
- Florczak, A.; Grzechowiak, I.; Deptuch, T.; Kucharczyk, K.; Kaminska, A.; Dams-Kozlowska, H. Silk Particles as Carriers of Therapeutic Molecules for Cancer Treatment. Materials 2020, 13, 4946. [Google Scholar] [CrossRef]
- Moisenovich, M.M.; Plotnikov, E.Y.; Moysenovich, A.M.; Silachev, D.N.; Danilina, T.I.; Savchenko, E.S.; Bobrova, M.M.; Safonova, L.A.; Tatarskiy, V.V.; Kotliarova, M.S.; et al. Effect of Silk Fibroin on Neuroregeneration After Traumatic Brain Injury. Neurochem. Res. 2019, 44, 2261–2272. [Google Scholar] [CrossRef]
- Fernandez-Garcia, L.; Mari-Buye, N.; Barios, J.A.; Madurga, R.; Elices, M.; Perez-Rigueiro, J.; Ramos, M.; Guinea, G.V.; Gonzalez-Nieto, D. Safety and tolerability of silk fibroin hydrogels implanted into the mouse brain. Acta Biomater. 2016, 45, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Gorenkova, N.; Osama, I.; Seib, F.P.; Carswell, H.V.O. In Vivo Evaluation of Engineered Self-Assembling Silk Fibroin Hydrogels after Intracerebral Injection in a Rat Stroke Model. ACS Biomater. Sci. Eng. 2019, 5, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Age of PISCES: Stem-cell clinical trials in stroke. Lancet 2016, 388, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Preliminary Reports of Stereotaxic Stem Cell Transplants in Chronic Stroke Patients. Mol. Ther. 2016, 24, 1710–1711. [Google Scholar] [CrossRef]
- Gonzalez-Nieto, D.; Fernandez-Garcia, L.; Perez-Rigueiro, J.; Guinea, G.V.; Panetsos, F. Hydrogels-Assisted Cell Engraftment for Repairing the Stroke-Damaged Brain: Chimera or Reality. Polymers 2018, 10, 184. [Google Scholar] [CrossRef]
- Osama, I.; Gorenkova, N.; McKittrick, C.M.; Wongpinyochit, T.; Goudie, A.; Seib, F.P.; Carswell, H.V.O. In vitro studies on space-conforming self-assembling silk hydrogels as a mesenchymal stem cell-support matrix suitable for minimally invasive brain application. Sci. Rep. 2018, 8, 13655. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef]
- Szybala, C.; Pritchard, E.M.; Lusardi, T.A.; Li, T.; Wilz, A.; Kaplan, D.L.; Boison, D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp. Neurol. 2009, 219, 126–135. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Heinz, R.; Brandenburg, S.; Nieminen-Kelha, M.; Kremenetskaia, I.; Boehm-Sturm, P.; Vajkoczy, P.; Schneider, U.C. Microglia as target for anti-inflammatory approaches to prevent secondary brain injury after subarachnoid hemorrhage (SAH). J. Neuroinflamm. 2021, 18, 36. [Google Scholar] [CrossRef]
- Hemonnot, A.L.; Hua, J.; Ulmann, L.; Hirbec, H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front. Aging Neurosci. 2019, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nieto, D.; Fernandez-Serra, R.; Perez-Rigueiro, J.; Panetsos, F.; Martinez-Murillo, R.; Guinea, G.V. Biomaterials to Neuroprotect the Stroke Brain: A Large Opportunity for Narrow Time Windows. Cells 2020, 9, 1074. [Google Scholar] [CrossRef]
- Barios, J.A.; Pisarchyk, L.; Fernandez-Garcia, L.; Barrio, L.C.; Ramos, M.; Martinez-Murillo, R.; Gonzalez-Nieto, D. Long-term dynamics of somatosensory activity in a stroke model of distal middle cerebral artery oclussion. J. Cereb. Blood Flow Metab. 2016, 36, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Overk, C.R.; Cartier, A.; Shaked, G.; Rockenstein, E.; Ubhi, K.; Spencer, B.; Price, D.L.; Patrick, C.; Desplats, P.; Masliah, E. Hippocampal neuronal cells that accumulate alpha-synuclein fragments are more vulnerable to Abeta oligomer toxicity via mGluR5—Implications for dementia with Lewy bodies. Mol. Neurodegener. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Nam, J.H.; Yoon, G.; Lee, J.Y.; Nam, Y.; Kang, H.J.; Cho, H.J.; Kim, J.; Hoe, H.S. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflamm. 2018, 15, 271. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Rajan, N.; Habermehl, J.; Cote, M.F.; Doillon, C.J.; Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protoc. 2006, 1, 2753–2758. [Google Scholar] [CrossRef]
- Atamer, Z.; Post, A.E.; Schubert, T.; Holder, A.; Boom, R.M.; Hinrichs, J. Bovine β-casein: Isolation, properties and functionality. A review. Int. Dairy J. 2017, 66, 115–125. [Google Scholar] [CrossRef]
- Toth, M.; Fridman, R. Assessment of Gelatinases (MMP-2 and MMP-9 by Gelatin Zymography. Methods Mol. Med. 2001, 57, 163–174. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Common artifacts and mistakes made in electrophoresis. Methods Mol. Biol. 2012, 869, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Madurga, R.; Gañán-Calvo, A.M.; Plaza, G.R.; Guinea, G.V.; Elices, M.; Pérez-Rigueiro, J. Straining flow spinning: Production of regenerated silk fibers under a wide range of mild coagulating chemistries. Green Chem. 2017, 19, 3380–3389. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K. The Mouse Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Ren, Z.; Chen, J.; Khalil, R.A. Zymography as a Research Tool in the Study of Matrix Metalloproteinase Inhibitors. Methods Mol. Biol. 2017, 1626, 79–102. [Google Scholar] [CrossRef]
- Hasan, M.R.; Herz, J.; Hermann, D.M.; Doeppner, T.R. Intravascular perfusion of carbon black ink allows reliable visualization of cerebral vessels. J. Vis. Exp. 2013, 71, e4374. [Google Scholar] [CrossRef]
- Frankola, K.A.; Greig, N.H.; Luo, W.; Tweedie, D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2011, 10, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef]
- Liu, X.; Quan, N. Microglia and CNS Interleukin-1: Beyond Immunological Concepts. Front. Neurol. 2018, 9, 8. [Google Scholar] [CrossRef]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef]
- Nuttall, R.K.; Silva, C.; Hader, W.; Bar-Or, A.; Patel, K.D.; Edwards, D.R.; Yong, V.W. Metalloproteinases are enriched in microglia compared with leukocytes and they regulate cytokine levels in activated microglia. Glia 2007, 55, 516–526. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Crapser, J.D.; Arreola, M.A.; Tsourmas, K.I.; Green, K.N. Microglia as hackers of the matrix: Sculpting synapses and the extracellular space. Cell Mol. Immunol. 2021, 18, 2472–2488. [Google Scholar] [CrossRef]
- Konnecke, H.; Bechmann, I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin. Dev. Immunol. 2013, 2013, 914104. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef]
- Sharon, A.; Jankowski, M.M.; Shmoel, N.; Erez, H.; Spira, M.E. Inflammatory foreign body response induced by neuro-implants in rat cortices depleted of resident microglia by a CSF1R inhibitor and its implications. Front. Neurosci. 2021, 15, 646914. [Google Scholar] [CrossRef]
- He, Y.; Taylor, N.; Yao, X.; Bhattacharya, A. Mouse primary microglia respond differently to LPS and poly(I:C) in vitro. Sci. Rep. 2021, 11, 10447. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, H.S. The anti-inflammatory role of tissue inhibitor of metalloproteinase-2 in lipopolysaccharide-stimulated microglia. J. Neuroinflamm. 2014, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Crocker, S.J.; Frausto, R.F.; Whitton, J.L.; Milner, R. A novel method to establish microglia-free astrocyte cultures: Comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia 2008, 56, 1187–1198. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Prinz, M.; Tay, T.L.; Wolf, Y.; Jung, S. Microglia: Unique and common features with other tissue macrophages. Acta Neuropathol. 2014, 128, 319–331. [Google Scholar] [CrossRef]
- Zeplin, P.H.; Maksimovikj, N.C.; Jordan, M.C.; Nickel, J.; Lang, G.; Leimer, A.H.; Römer, L.; Scheibel, T. Spider silk coatings as a bioshield to reduce periprosthetic fibrous capsule formation. Adv. Funct. Mater. 2014, 24, 2658–2666. [Google Scholar] [CrossRef]
- Ghuman, H.; Mauney, C.; Donnelly, J.; Massensini, A.R.; Badylak, S.F.; Modo, M. Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke. Acta Biomater. 2018, 80, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- d’Errico, P.; Ziegler-Waldkirch, S.; Aires, V.; Hoffmann, P.; Mezo, C.; Erny, D.; Monasor, L.S.; Liebscher, S.; Ravi, V.M.; Joseph, K.; et al. Microglia contribute to the propagation of Abeta into unaffected brain tissue. Nat. Neurosci. 2022, 25, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.R.; Li, Y.M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Schall, K.; Smith, D.; Pandya, R.; Persidsky, Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J. Neurochem 2007, 101, 566–576. [Google Scholar] [CrossRef]
- Wang, X.X.; Tan, M.S.; Yu, J.T.; Tan, L. Matrix metalloproteinases and their multiple roles in Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 908636. [Google Scholar] [CrossRef]
- Li, M.; Ogiso, M.; Minoura, N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 2003, 24, 357–365. [Google Scholar] [CrossRef]
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation of Bombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2004, 91, 2383–2390. [Google Scholar] [CrossRef]
- Brown, J.; Lu, C.L.; Coburn, J.; Kaplan, D.L. Impact of silk biomaterial structure on proteolysis. Acta Biomater. 2015, 11, 212–221. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Kweon, H.; Kim, D.W.; Kim, M.K.; Kim, S.G.; Kim, J.Y.; Chae, W.S.; Hong, S.P.; Park, Y.H.; Lee, S.Y.; et al. Accelerated biodegradation of silk sutures through matrix metalloproteinase activation by incorporating 4-hexylresorcinol. Sci. Rep. 2017, 7, 42441. [Google Scholar] [CrossRef] [PubMed]
- Fine, N.A.; Lehfeldt, M.; Gross, J.E.; Downey, S.; Kind, G.M.; Duda, G.; Kulber, D.; Horan, R.; Ippolito, J.; Jewell, M. SERI surgical scaffold, prospective clinical trial of a silk-derived biological scaffold in two-stage breast reconstruction: 1-year data. Plast Reconstr. Surg 2015, 135, 339–351. [Google Scholar] [CrossRef]
- Siavashani, A.Z.; Mohammadi, J.; Rottmar, M.; Senturk, B.; Nourmohammadi, J.; Sadeghi, B.; Huber, L.; Maniura-Weber, K. Silk fibroin/sericin 3D sponges: The effect of sericin on structural and biological properties of fibroin. Int. J. Biol. Macromol. 2020, 153, 317–326. [Google Scholar] [CrossRef]
- Massensini, A.R.; Ghuman, H.; Saldin, L.T.; Medberry, C.J.; Keane, T.J.; Nicholls, F.J.; Velankar, S.S.; Badylak, S.F.; Modo, M. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 2015, 27, 116–130. [Google Scholar] [CrossRef]
- Han, X.; Lai, J.H.C.; Huang, J.; Park, S.W.; Liu, Y.; Chan, K.W.Y. Imaging Self-Healing Hydrogels and Chemotherapeutics Using CEST MRI at 3 T. ACS Appl. Bio Mater. 2021, 4, 5605–5616. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Feng, L.; Chen, Z.; Lan, Y.; Liu, Y.; Li, D.; Yan, C.; Xu, Y. Ultrasmall Superparamagnetic Iron Oxide Labeled Silk Fibroin/Hydroxyapatite Multifunctional Scaffold Loaded With Bone Marrow-Derived Mesenchymal Stem Cells for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.; Rezin, G.T.; Zanoni, E.T.; de Souza Notoya, F.; Leffa, D.D.; Damiani, A.P.; Daumann, F.; Rodriguez, J.C.; Benavides, R.; da Silva, L.; et al. Acute and chronic administration of gold nanoparticles cause DNA damage in the cerebral cortex of adult rats. Mutat Res. 2014, 766–767, 25–30. [Google Scholar] [CrossRef]
- Enea, M.; Peixoto de Almeida, M.; Eaton, P.; Dias da Silva, D.; Pereira, E.; Soares, M.E.; Bastos, M.L.; Carmo, H. A multiparametric study of gold nanoparticles cytotoxicity, internalization and permeability using an in vitro model of blood-brain barrier. Influence of size, shape and capping agent. Nanotoxicology 2019, 13, 990–1004. [Google Scholar] [CrossRef]
- Vales, G.; Suhonen, S.; Siivola, K.M.; Savolainen, K.M.; Catalan, J.; Norppa, H. Size, Surface Functionalization, and Genotoxicity of Gold Nanoparticles In Vitro. Nanomaterials 2020, 10, 271. [Google Scholar] [CrossRef]
- Long, Y.; Cheng, X.; Tang, Q.; Chen, L. The antigenicity of silk-based biomaterials: Sources, influential factors and applications. J. Mater. Chem B 2021, 9, 8365–8377. [Google Scholar] [CrossRef]
- Etienne, O.; Schneider, A.; Kluge, J.A.; Bellemin-Laponnaz, C.; Polidori, C.; Leisk, G.G.; Kaplan, D.L.; Garlick, J.A.; Egles, C. Soft tissue augmentation using silk gels: An in vitro and in vivo study. J. Periodontol. 2009, 80, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yang, D.; Sun, C.; Huang, J.; Chen, K.; Zhang, C.; Chen, H.; Yao, Q. A quicker degradation rate is yielded by a novel kind of transgenic silk fibroin consisting of shortened silk fibroin heavy chains fused with matrix metalloproteinase cleavage sites. J. Mater. Sci. Mater. Med. 2014, 25, 1833–1842. [Google Scholar] [CrossRef]
- Lyons, F.G.; Al-Munajjed, A.A.; Kieran, S.M.; Toner, M.E.; Murphy, C.M.; Duffy, G.P.; O’Brien, F.J. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials 2010, 31, 9232–9243. [Google Scholar] [CrossRef] [PubMed]
- Flaig, I.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M. In vivo analysis of the biocompatibility and immune response of jellyfish collagen scaffolds and its suitability for bone regeneration. Int. J. Mol. Sci. 2020, 21, 4518. [Google Scholar] [CrossRef]
- Gao, J.; Ma, Y.; Guo, Z.; Zhang, Y.; Xing, F.; Zhang, T.; Kong, Y.; Luo, X.; Xu, L.; Zhang, G. Evaluating the Degradation Process of Collagen Sponge and Acellular Matrix Implants In Vivo Using the Standardized HPLC-MS/MS Method. Separations 2023, 10, 47. [Google Scholar] [CrossRef]
- Choy, S.; Lam, D.V.; Lee, S.-M.; Hwang, D.S. Prolonged biodegradation and improved mechanical stability of collagen via vapor-phase Ti stitching for long-term tissue regeneration. ACS Appl. Mater. Interfaces 2019, 11, 38440–38447. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; Ahn, J.H.; Lee, J.W.; Hong, J.; Lee, T.K.; Kim, B.; Kim, S.S.; Won, M.H. Brain Factor-7(R) improves learning and memory deficits and attenuates ischemic brain damage by reduction of ROS generation in stroke in vivo and in vitro. Lab Anim. Res. 2020, 36, 24. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bucci, L.R. Effects of Silk Fibroin Enzyme Hydrolysates on Memory and Learning: A Review. Molecules 2022, 27, 5407. [Google Scholar] [CrossRef]
- Chen, S.; Yang, W.; Yan, S.; Han, G.; Zhang, Q. In vitro degradation of silk fibroin/hyaluronic acid composite hydrogels. AATCC J. Res. 2021, 8, 51–54. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, H.; Gil, E.S.; Mandal, B.B.; Min, B.H.; Kaplan, D.L. Silk-fibrin/hyaluronic acid composite gels for nucleus pulposus tissue regeneration. Tissue Eng. 2011, 17, 2999–3009. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonesi, M.; Ramos, M.; Ramirez-Castillejo, C.; Fernández-Serra, R.; Panetsos, F.; Belarra, A.; Chevalier, M.; Rojo, F.J.; Pérez-Rigueiro, J.; Guinea, G.V.; et al. Resistance to Degradation of Silk Fibroin Hydrogels Exposed to Neuroinflammatory Environments. Polymers 2023, 15, 2491. https://doi.org/10.3390/polym15112491
Yonesi M, Ramos M, Ramirez-Castillejo C, Fernández-Serra R, Panetsos F, Belarra A, Chevalier M, Rojo FJ, Pérez-Rigueiro J, Guinea GV, et al. Resistance to Degradation of Silk Fibroin Hydrogels Exposed to Neuroinflammatory Environments. Polymers. 2023; 15(11):2491. https://doi.org/10.3390/polym15112491
Chicago/Turabian StyleYonesi, Mahdi, Milagros Ramos, Carmen Ramirez-Castillejo, Rocío Fernández-Serra, Fivos Panetsos, Adrián Belarra, Margarita Chevalier, Francisco J. Rojo, José Pérez-Rigueiro, Gustavo V. Guinea, and et al. 2023. "Resistance to Degradation of Silk Fibroin Hydrogels Exposed to Neuroinflammatory Environments" Polymers 15, no. 11: 2491. https://doi.org/10.3390/polym15112491
APA StyleYonesi, M., Ramos, M., Ramirez-Castillejo, C., Fernández-Serra, R., Panetsos, F., Belarra, A., Chevalier, M., Rojo, F. J., Pérez-Rigueiro, J., Guinea, G. V., & González-Nieto, D. (2023). Resistance to Degradation of Silk Fibroin Hydrogels Exposed to Neuroinflammatory Environments. Polymers, 15(11), 2491. https://doi.org/10.3390/polym15112491






