Graphene Oxide Facilitates Transformation of Waste PET into MOF Nanorods in Ionic Liquids
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of NMP Based Ionic Liquid
2.3. Synthesis of Ni-MOF@rGO
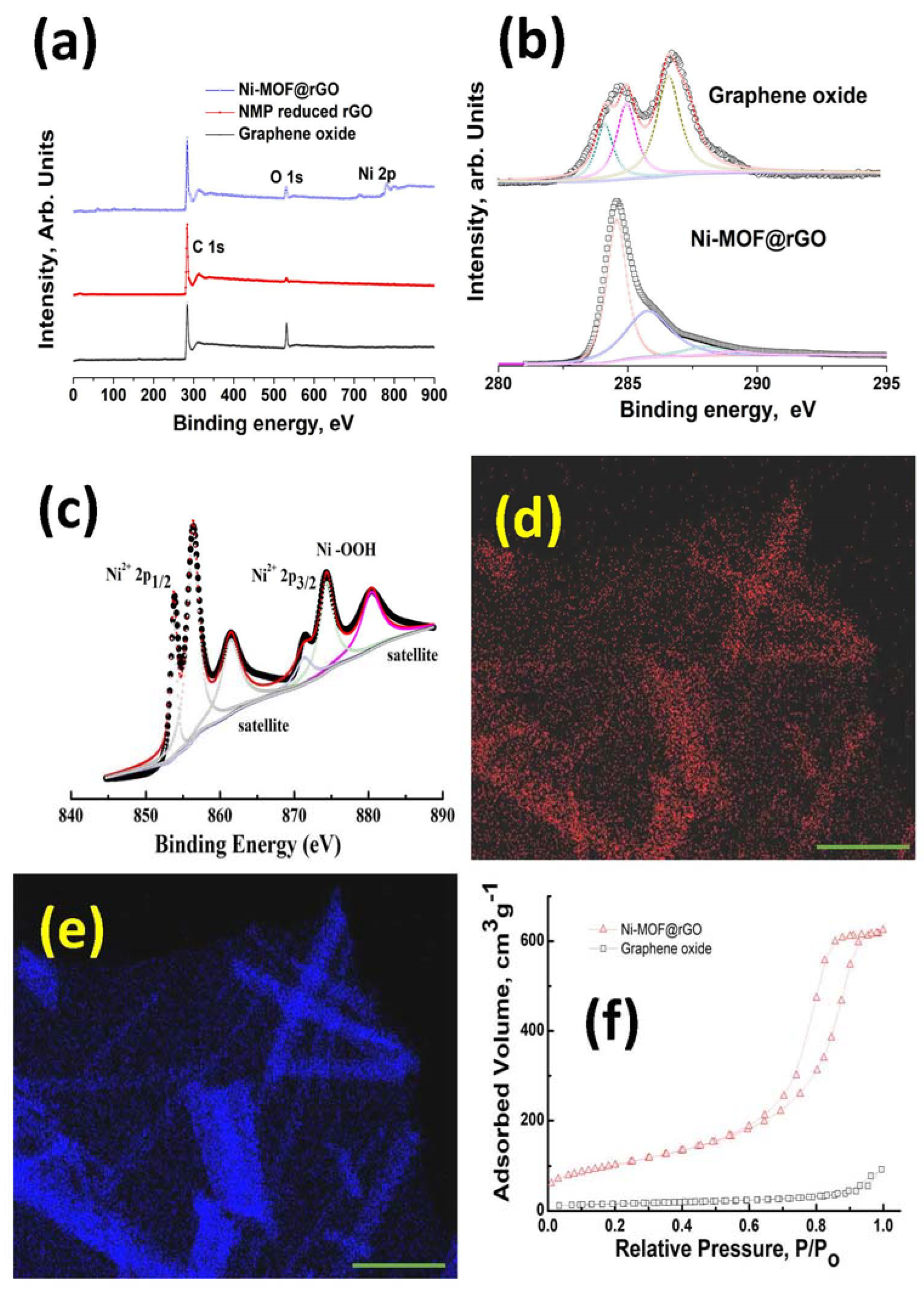
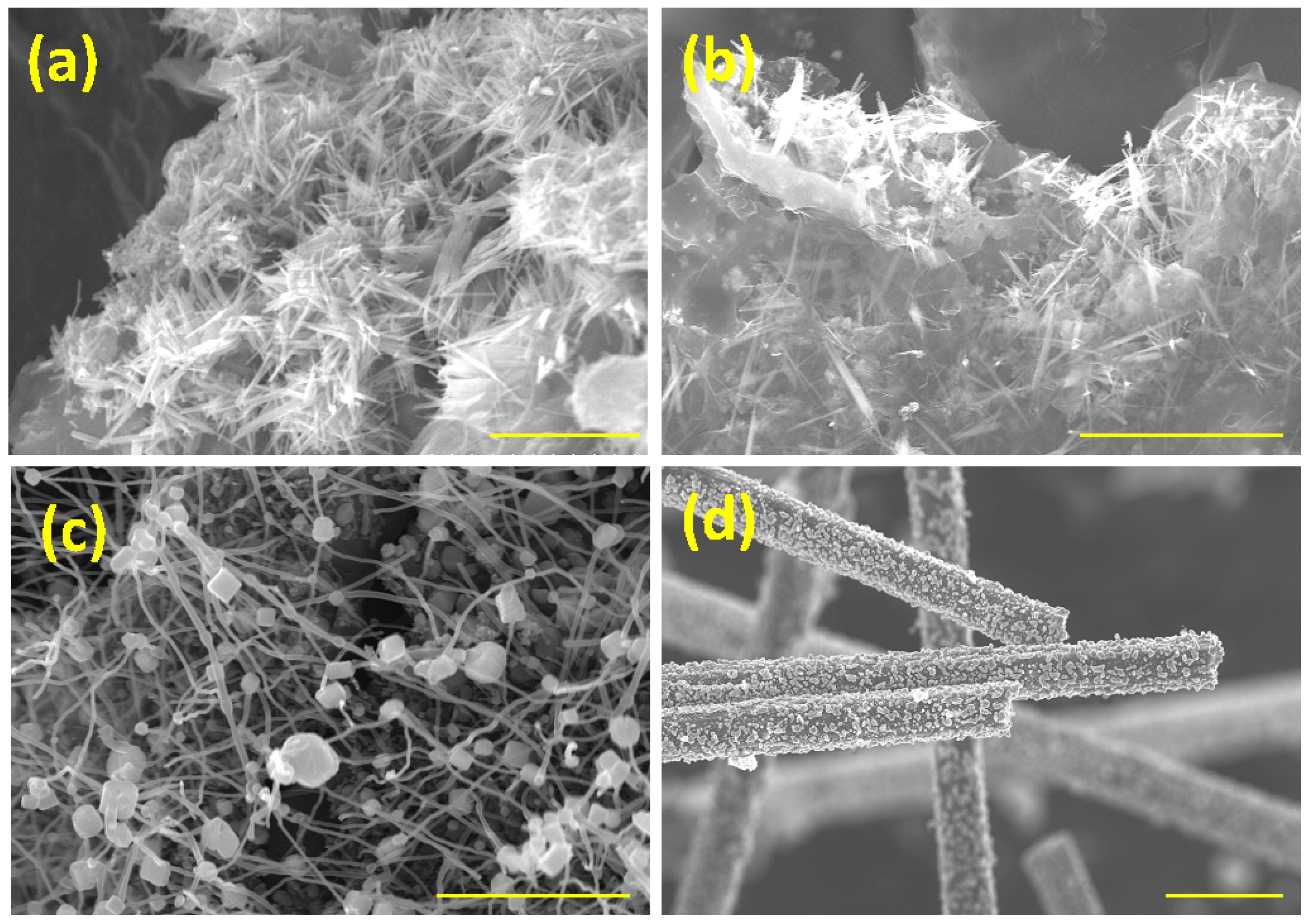
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mandal, S.; Dey, A. PET Chemistry. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. ISBN 978-0-12-811361-5. [Google Scholar]
- New Research Exposes a Crisis in the Global Trade of “Recyclable” Plastics. Available online: https://www.greenpeace.org/international/press-release/21789/new-research-exposes-a-crisis-in-the-global-trade-of-recyclable-plastics/ (accessed on 20 April 2023).
- PET Recycling. Available online: https://www.plasteurope.com/news/PET_RECYCLING_t236441/ (accessed on 20 April 2023).
- Fulgencio-Medrano, L.; García-Fernández, S.; Asueta, A.; Lopez-Urionabarrenechea, A.; Perez-Martinez, B.B.; Arandes, J.M. Oil Production by Pyrolysis of Real Plastic Waste. Polymers 2022, 14, 553. [Google Scholar] [CrossRef] [PubMed]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- El Essawy, N.A.; Ali, S.M.; Farag, H.A.; Konsowa, A.H.; Elnouby, M.; Hamad, H.A. Green Synthesis of Graphene from Recycled PET Bottle Wastes for Use in the Adsorption of Dyes in Aqueous Solution. Ecotoxicol. Environ. Saf. 2017, 145, 57–68. [Google Scholar] [CrossRef]
- Liu, X.; Xie, W.; Widenmeyer, M.; Ding, H.; Chen, G.; De Carolis, D.M.; Lakus-Wollny, K.; Molina-Luna, L.; Riedel, R.; Weidenkaff, A. Upcycling Waste Plastics into Multi-Walled Carbon Nanotube Composites via NiCo2O4 Catalytic Pyrolysis. Catalysts 2021, 11, 1353. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Transforming Waste Poly(Ethylene Terephthalate) into Nitrogen Doped Carbon Nanotubes and Its Utility in Oxygen Reduction Reaction and Bisphenol-A Removal from Contaminated Water. Materials 2020, 13, 4144. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Fisse, J.; Vogt, D. Optimization and Kinetic Evaluation for Glycolytic Depolymerization of Post-Consumer PET Waste with Sodium Methoxide. Polymers 2023, 15, 687. [Google Scholar] [CrossRef]
- Otaibi, A.A.A.; Alsukaibi, A.K.D.; Rahman, M.A.; Mushtaque, M.; Haque, A. From Waste to Schiff Base: Upcycling of Aminolysed Poly(ethylene terephthalate) Product. Polymers 2022, 14, 1861. [Google Scholar] [CrossRef]
- Deleu, W.P.; Stassen, I.; Jonckheere, D.; Ameloot, R.; De Vos, D.E. Waste PET (bottles) as a resource or substrate for MOF synthesis. J. Mater. Chem. A 2016, 4, 9519–9525. [Google Scholar] [CrossRef]
- Araujo, D.; Azevedo, J.; Cardoso, P.; Lazarus, B.; Morreira, M.; Silva, L.; Barbosa, J. Polymeric Composite Reinforced with PET Fiber Waste for Application in Civil Construction as a Cladding Element. Polymers 2022, 14, 1293. [Google Scholar] [CrossRef]
- Fernandes, P.D.; Magalhães, F.D.; Pereira, R.F.; Pinto, A.M. Metal-Organic Frameworks Applications in Synergistic Cancer Photo-Immunotherapy. Polymers 2023, 15, 1490. [Google Scholar] [CrossRef]
- Borzehandani, M.Y.; Jorabchi, M.N.; Abdulmalek, E.; Abdul Rahman, M.B.; Mohammad Latif, M.A. Exploring the Potential of a Highly Scalable Metal-Organic Framework CALF-20 for Selective Gas Adsorption at Low Pressure. Polymers 2023, 15, 760. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Selyutin, A.; Ermakov, S.; Penkova, A. Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions. Polymers 2023, 15, 1341. [Google Scholar] [CrossRef]
- Lou, R.; Cao, Q.; Niu, T.; Zhang, Y.; Zhang, Y.; Wang, Z.; Zhang, X. Metal–Organic-Framework-Mediated Fast Self-Assembly 3D Interconnected Lignin-Based Cryogels in Deep Eutectic Solvent for Supercapacitor Applications. Polymers 2023, 15, 1824. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lis, M.J. Barrier Effects of Cellulosic Fibers with Hybrid Coating Based on Zirconium Metal-Organic Framework. Polymers 2022, 14, 3071. [Google Scholar] [CrossRef] [PubMed]
- Sowińska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Task-Specific Ionic Liquids with Lactate Anion Applied to Improve ZnO Dispersibility in the Ethylene-Propylene-Diene Elastomer. Polymers 2021, 13, 774. [Google Scholar] [CrossRef]
- Domingo Huguet, D.; Gual, A.; Garcia-Valls, R.; Nogalska, A. Supported Imidazolium-Based Ionic Liquids on a Polysulfone Matrix for Enhanced CO2 Capture. Polymers 2022, 14, 4865. [Google Scholar] [CrossRef]
- Kerche, E.F.; Kairytė, A.; Członka, S.; da Silva, V.D.; Salles, N.A.; Schrekker, H.S.; Amico, S.C. Imidazolium Ionic Liquids as Compatibilizer Agents for Microcrystalline Cellulose/Epoxy Composites. Polymers 2023, 15, 333. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, B.; Yu, H.; Chen, G.Z. Synergistic Effects of 1-Octyl-3-Methylimidazolium Hexafluorophosphate and Cellulose Nanocrystals on Improving Polyacrylate Waterborne Anti-Corrosion Coatings. Polymers 2023, 15, 810. [Google Scholar] [CrossRef]
- Volmajer Valh, J.; Stopar, D.; Selaya Berodia, I.; Erjavec, A.; Šauperl, O.; Fras Zemljič, L. Economical Chemical Recycling of Complex PET Waste in the Form of Active Packaging Material. Polymers 2022, 14, 3244. [Google Scholar] [CrossRef]
- Orduna, L.; Otaegi, I.; Aranburu, N.; Guerrica-Echevarría, G. Effect of the Simultaneous Addition of Polycaprolactone and Carbon Nanotubes on the Mechanical, Electrical, and Adhesive Properties of Epoxy Resins Cured with Ionic Liquids. Polymers 2023, 15, 1607. [Google Scholar] [CrossRef]
- Wang, A.; Tu, Y.; Wang, S.; Zhang, H.; Yu, F.; Chen, Y.; Li, D. A PEGylated Chitosan as Gel Polymer Electrolyte for Lithium-Ion Batteries. Polymers 2022, 14, 4552. [Google Scholar] [CrossRef]
- Khan, S.; Rauber, D.; Shanmugam, S.; Kay, C.W.M.; Konist, A.; Kikas, T. Efficient Lignin Fractionation from Scots Pine (Pinus sylvestris) Using Ammonium-Based Protic Ionic Liquid: Process Optimization and Characterization of Recovered Lignin. Polymers 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, N.; Nguyen, T.T.; Qian, E.W. Catalytic Depolymerization of Lignin in Ionic Liquid Using a Continuous Flow Fixed-Bed Reaction System. Ind. Eng. Chem. Res. 2018, 57, 16995–17002. [Google Scholar] [CrossRef]
- Kamimura, A.; Yamamoto, S. An Efficient Method To Depolymerize Polyamide Plastics: A New Use of Ionic Liquids. Org. Lett. 2007, 9, 2533–2535. [Google Scholar] [CrossRef] [PubMed]
- Marullo, S.; Rizzo, C.; Dintcheva, N.T.; D’Anna, F. Amino Acid-Based Cholinium Ionic Liquids as Sustainable Catalysts for PET Depolymerization. ACS Sustain. Chem. Eng. 2021, 9, 15157–15165. [Google Scholar] [CrossRef]
- Mouawia, A.; Nourry, A.; Gaumont, A.-C.; Pilard, J.-F.; Dez, I. Controlled Metathetic Depolymerization of Natural Rubber in Ionic Liquids: From Waste Tires to Telechelic Polyisoprene Oligomers. ACS Sustain. Chem. Eng. 2017, 5, 696–700. [Google Scholar] [CrossRef]
- Liu, M.; Guo, J.; Gu, Y.; Gao, J.; Liu, F.; Yu, S. Pushing the Limits in Alcoholysis of Waste Polycarbonate with DBU-Based Ionic Liquids under Metal- and Solvent-Free Conditions. ACS Sustain. Chem. Eng. 2018, 6, 13114–13121. [Google Scholar] [CrossRef]
- Tyagi, U.; Anand, N. Facile Depolymerization of Microcrystalline Cellulose in Ionic Liquid Medium Catalyzed by Carbon Materials as Catalysts. Curr. Opin. Green Sustain. Chem. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R. Ionic Liquids Based upon Metal Halide/Substituted Quaternary Ammonium Salt Mixtures. Inorg. Chem. 2004, 43, 3447–3452. [Google Scholar] [CrossRef]
- Neve, F.; Francescangeli, O.; Crispini, A.; Charmant, J. A2[MX4] Copper(II) Pyridinium Salts. From Ionic Liquids to Layered Solids to Liquid Crystals. Chem. Mater. 2001, 13, 2032–2041. [Google Scholar] [CrossRef]
- Lin, I.J.B.; Vasam, C.S. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety. J. Organomet. Chem. 2005, 690, 3498–3512. [Google Scholar] [CrossRef]
- Dutra, G.V.S.; Teixeira, T.S.; Medeiros, G.A.; Abdelnur, P.V.; Hermes de Araújo, P.H.; Sayer, C.; Neto, B.A.D.; Machado, F. On the Role of Metal-Containing Imidazolium-Based Ionic Liquid Catalysts in the Formation of Tailored Polystyrene. Ind. Eng. Chem. Res. 2020, 59, 21685–21699. [Google Scholar] [CrossRef]
- Amde, M.; Liu, J.-F.; Pang, L. Environmental Application, Fate, Effects, and Concerns of Ionic Liquids: A Review. Environ. Sci. Technol. 2015, 49, 12611–12627. [Google Scholar] [CrossRef] [PubMed]
- Poulakis, J.G.; Papaspyrides, C.D. Dissolution/reprecipitation: A model process for PET bottle recycling. J. Appl. Polym. Sci. 2001, 81, 91–95. [Google Scholar] [CrossRef]
- Li, F.-T.; Wu, B.; Liu, R.-H.; Wang, X.-J.; Chen, L.-J.; Zhao, D.-S. An inexpensive N-methyl-2-pyrrolidone-based ionic liquid as efficient extractant and catalyst for desulfurization of dibenzothiophene. Chem. Eng. J. 2015, 274, 192–199. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Chowdhury, M.R.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic liquids with N-methyl-2-pyrrolidonium cation as an enhancer for topical drug delivery: Synthesis, characterization, and skin-penetration evaluation. J. Mol. Liq. 2020, 299, 112166. [Google Scholar] [CrossRef]
- Fang, L.; Shen, Z.; Shen, X.; Kang, S.; Song, H.; Liang, T. A study on thiophene removals from model oils with different molecular compositions using an inexpensive N-methylpyrrolidone-FeCl3 ionic liquid. J. Mol. Liq. 2021, 333, 115913. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Y.; Chai, L.; Lai, Z.; Fang, X.; Liu, B.; Zhang, W.; Lu, M.; Xu, Y.; Xu, H. Nmp-based ionic liquids: Recyclable catalysts for both hetero-Michael addition and Knoevenagel condensation in water. Synth. Commun. 2018, 48, 1060–1067. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Chen, L.; Chu, M.; Dong, Y.; Liu, D.; Liu, P.; Qu, D.; Duan, J.; Li, X. MOF(Ni)/CNT composites with layer structure for high capacitive performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128802. [Google Scholar] [CrossRef]
- Yan, L.; Jiang, H.; Xing, Y.; Wang, Y.; Liu, D.; Gu, X.; Dai, P.; Li, L.; Zhao, X. Nickel metal–organic framework implanted on graphene and incubated to be ultrasmall nickel phosphide nanocrystals acts as a highly efficient water splitting electrocatalyst. J. Mater. Chem. A 2018, 6, 1682–1691. [Google Scholar] [CrossRef]
- Gao, J.; He, P.; Yang, T.; Wang, X.; Zhou, L.; He, Q.; Jia, L.; Deng, H.; Hui, Z.; Bin, J.; et al. Short rod-like Ni-MOF anchored on graphene oxide nanosheets: A promising voltammetric platform for highly sensitive determination of p-chloronitrobenzene. J. Electroanal. Chem. 2020, 861, 113954. [Google Scholar] [CrossRef]
- Yoshioka, T.; Ota, M.; Okuwaki, A. Conversion of a Used Poly(ethylene terephthalate) Bottle into Oxalic Acid and Terephthalic Acid by Oxygen Oxidation in Alkaline Solutions at Elevated Temperatures. Ind. Eng. Chem. Res. 2003, 42, 675–679. [Google Scholar] [CrossRef]
- Canossa, S.; Gonzalez-Nelson, A.; Shupletsov, L.; Carmen Martin, M.; Van der Veen, M.A. Overcoming Crystallinity Limitations of Aluminium Metal-Organic Frameworks by Oxalic Acid Modulated Synthesis. Chem.-Eur. J. 2020, 26, 3564–3570. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.J.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Harandizadeh, A.H.; Aghamiri, S.; Hojjat, M.; Ranjbar-Mohammadi, M.; Talaie, M.R. Adsorption of Carbon Dioxide with Ni-MOF-74 and MWCNT Incorporated Poly Acrylonitrile Nanofibers. Nanomaterials 2022, 12, 412. [Google Scholar] [CrossRef]
- Manyani, N.; Sharma, K.; Siwatch, P.; Triapthi, S.K. Study of Electrochemical Performance of Ni-BTC MOF as a Supercapacitor Electrode. AIP Conf. Proc. 2021, 2352, 020035. [Google Scholar]
- Graphene Number of Layers Calculator from ID/IG Ratio and I2D/IG Ratio via Raman Spectroscopy. Available online: https://instanano.com/characterization/raman/graphene-layers/ (accessed on 20 April 2023).
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Zhang, K.; Xia, X.; Deng, S.; Zhong, Y.; Xie, D.; Pan, G.; Wu, J.; Liu, Q.; Wang, X.; Tu, J. Nitrogen-Doped Sponge Ni Fibers as Highly Efficient Electrocatalysts for Oxygen Evolution Reaction. Nano-Micro Lett. 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Gangaraju, D.; Shanmugharaj, A.M.; Sridhar, V.; Park, H. Fabrication of Nanometer-Sized Nickel-Based Metal Organic Frameworks on Carbon Nanotubes for Electro-Catalytic Oxidation of Urea and Arsenic Removal. ACS Appl. Nano Mater. 2022, 5, 19035–19042. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Coordination Polymer Framework-Derived Ni-N-Doped Carbon Nanotubes for Electro-Oxidation of Urea. Materials 2022, 15, 2048. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangaraju, D.; Shanmugharaj, A.M.; Sridhar, V. Graphene Oxide Facilitates Transformation of Waste PET into MOF Nanorods in Ionic Liquids. Polymers 2023, 15, 2479. https://doi.org/10.3390/polym15112479
Gangaraju D, Shanmugharaj AM, Sridhar V. Graphene Oxide Facilitates Transformation of Waste PET into MOF Nanorods in Ionic Liquids. Polymers. 2023; 15(11):2479. https://doi.org/10.3390/polym15112479
Chicago/Turabian StyleGangaraju, Deepa, Andikkadu Masilamani Shanmugharaj, and Vadahanambi Sridhar. 2023. "Graphene Oxide Facilitates Transformation of Waste PET into MOF Nanorods in Ionic Liquids" Polymers 15, no. 11: 2479. https://doi.org/10.3390/polym15112479
APA StyleGangaraju, D., Shanmugharaj, A. M., & Sridhar, V. (2023). Graphene Oxide Facilitates Transformation of Waste PET into MOF Nanorods in Ionic Liquids. Polymers, 15(11), 2479. https://doi.org/10.3390/polym15112479





