Design and Synthesis of N-Doped Porous Carbons for the Selective Carbon Dioxide Capture under Humid Flue Gas Conditions
Abstract
1. Introduction
2. Materials and Methods
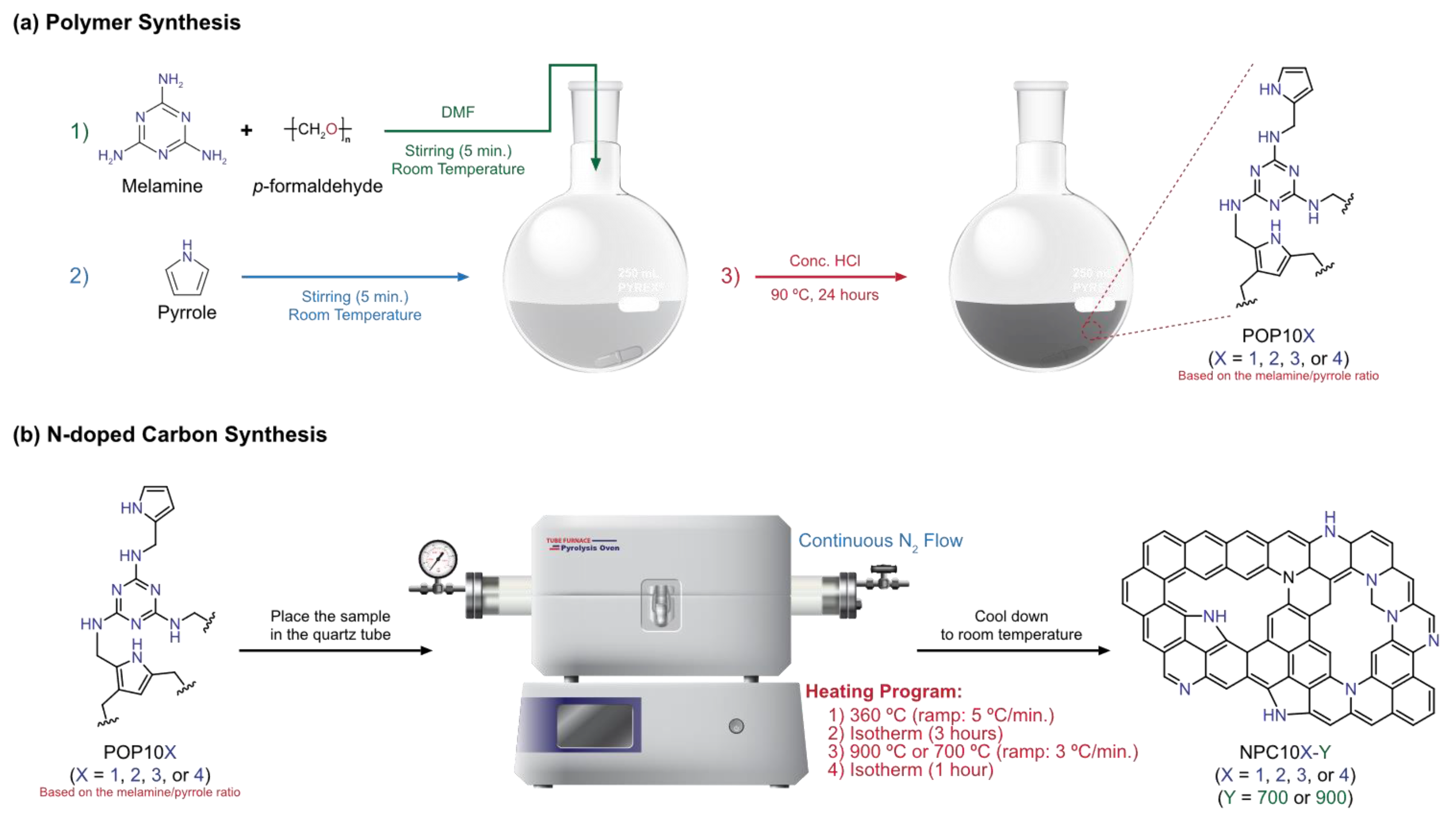
2.1. Polymers Synthesis
2.1.1. POP101
2.1.2. POP102
2.1.3. POP103
2.1.4. POP104
2.2. N-Doped Carbon Synthesis
3. Results and Discussion
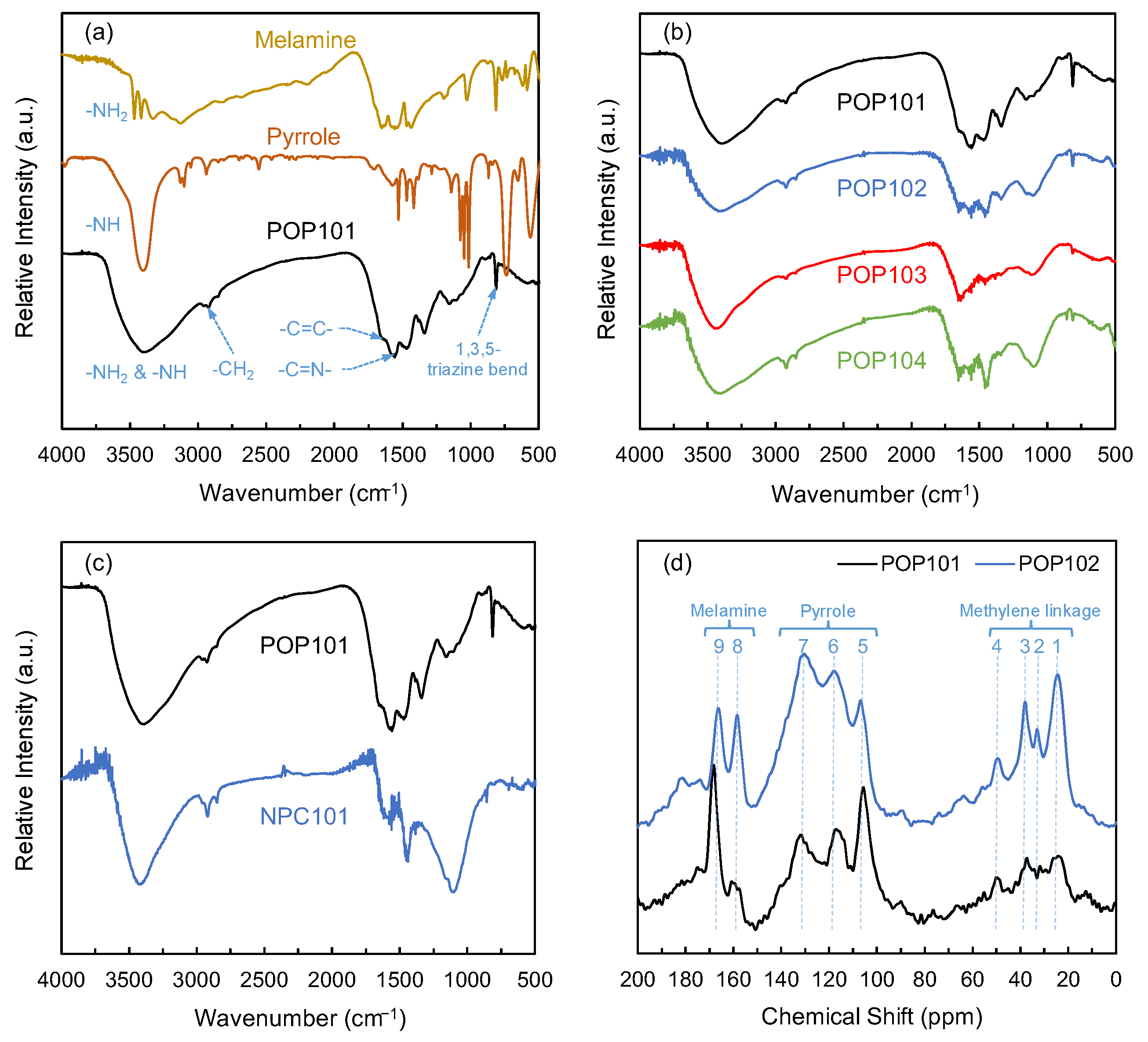
3.1. Structural Characterization & Permanent Porosity
3.2. Thermodynamic Uptake Capacity
3.3. Thermodynamic Analysis of the Adsorption Process
3.4. Dynamic CO2 Separation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Z.; Eden, M.R.; Gani, R. Toward the Development and Deployment of Large-Scale Carbon Dioxide Capture and Conversion Processes. Ind. Eng. Chem. Res. 2016, 55, 3383–3419. [Google Scholar] [CrossRef]
- Bilgen, S. Structure and Environmental Impact of Global Energy Consumption. Renew. Sustain. Energy Rev. 2014, 38, 890–902. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.G.; Quéré, C.L.; Andrew, R.M.; Korsbakken, J.I.; Peters, G.P.; Nakicenovic, N. Reaching Peak Emissions. Nat. Clim. Chang. 2016, 6, 7–10. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, X.; Hu, G.; Bai, R.; Dai, W.; Fan, M.; Luo, M. Enhancement of CO2 Adsorption and Amine Efficiency of Titania Modified by Moderate Loading of Diethylenetriamine. J. Mater. Chem. A 2013, 1, 6208–6215. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 Capture by Solid Adsorbents and Their Applications: Current Status and New Trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent Advances in Solid Sorbents for CO2 Capture and New Development Trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Lee, S.C.; Lin, C.T.; Tsai, M.S. The Pricing of Deposit Insurance in the Presence of Systematic Risk. J. Bank. Financ. 2015, 51, 1–11. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The Chemistry of Metal-Organic Frameworks for CO2 Capture, Regeneration and Conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 Capture in Metal–Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2019, 3, 1800080. [Google Scholar] [CrossRef]
- Jia, J.; Chen, Z.; Jiang, H.; Belmabkhout, Y.; Mouchaham, G.; Aggarwal, H.; Adil, K.; Abou-Hamad, E.; Czaban-Jóźwiak, J.; Tchalala, M.R.; et al. Extremely Hydrophobic POPs to Access Highly Porous Storage Media and Capturing Agent for Organic Vapors. Chem 2019, 5, 180–191. [Google Scholar] [CrossRef]
- Li, L.; Cai, K.; Wang, P.; Ren, H.; Zhu, G. Construction of Sole Benzene Ring Porous Aromatic Frameworks and Their High Adsorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kong, R.; Wang, X.; Xu, Y.; Wang, F.; Ren, W.; Wang, Y.; Su, F.; Jiang, J.X. Porous Carbons Derived from Hypercrosslinked Porous Polymers for Gas Adsorption and Energy Storage. Carbon 2017, 114, 608–618. [Google Scholar] [CrossRef]
- Shen, Z.; Song, Y.; Yin, C.; Luo, X.; Wang, Y.; Li, X. Construction of Hierarchically Porous 3D Graphene-like Carbon Material by B, N Co-Doping for Enhanced CO2 Capture. Microporous Mesoporous Mater. 2021, 322, 111158. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, C.; Yin, C.; Kang, S.; Liu, Y.; Ge, Z.; Xia, Q.; Wang, Y.; Li, X. Facile Large-Scale Synthesis of Macroscopic 3D Porous Graphene-like Carbon Nanosheets Architecture for Efficient CO2 Adsorption. Carbon 2019, 145, 751–756. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Sun, H.; Suvorova, A.; Saunders, M.; Tade, M.; Wang, S. Heteroatom (N or N-S)-Doping Induced Layered and Honeycomb Microstructures of Porous Carbons for CO2 Capture and Energy Applications. Adv. Funct. Mater. 2016, 26, 8651–8661. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Chen, J.; Wei, L.; Modak, A.; Yang, H.; Yang, Q. N-Doped Porous Carbons with Exceptionally High CO2 Selectivity for CO2 Capture. Carbon 2017, 114, 473–481. [Google Scholar] [CrossRef]
- Sevilla, M.; Parra, J.B.; Fuertes, A.B. Assessment of the Role of Micropore Size and N-Doping in CO2 Capture by Porous Carbons. ACS Appl. Mater. Interfaces 2013, 5, 6360–6368. [Google Scholar] [CrossRef]
- Basha, D.B.; Ahmed, S.; Ahmed, A.; Gondal, M.A. Recent Advances on Nitrogen Doped Porous Carbon Micro-Supercapacitors: New Directions for Wearable Electronics. J. Energy Storage 2023, 60, 106581. [Google Scholar] [CrossRef]
- Li, M.; Xu, F.; Li, H.; Wang, Y. Nitrogen-Doped Porous Carbon Materials: Promising Catalysts or Catalyst Supports for Heterogeneous Hydrogenation and Oxidation. Catal. Sci. Technol. 2016, 6, 3670–3693. [Google Scholar] [CrossRef]
- Abdelnaby, M.M.; Cordova, K.E.; Abdulazeez, I.; Alloush, A.M.; Al-Maythalony, B.A.; Mankour, Y.; Alhooshani, K.; Saleh, T.A.; Hamouz, O.C.S. Al Novel Porous Organic Polymer for the Concurrent and Selective Removal of Hydrogen Sulfide and Carbon Dioxide from Natural Gas Streams. ACS Appl. Mater. Interfaces 2020, 12, 47984–47992. [Google Scholar] [CrossRef] [PubMed]
- Sethia, G.; Sayari, A. Comprehensive Study of Ultra-Microporous Nitrogen-Doped Activated Carbon for CO2 Capture. Carbon 2015, 93, 68–80. [Google Scholar] [CrossRef]
- Jawaid, S.; Talpur, F.N.; Afridi, H.I.; Nizamani, S.M.; Khaskheli, A.A.; Naz, S. Quick Determination of Melamine in Infant Powder and Liquid Milk by Fourier Transform Infrared Spectroscopy. Anal. Methods 2014, 6, 5269–5273. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Balzer, C.; Braxmeier, S.; Neimark, A.V.; Reichenauer, G. Deformation of Microporous Carbon during Adsorption of Nitrogen, Argon, Carbon Dioxide, and Water Studied by in Situ Dilatometry. Langmuir 2015, 31, 12512–12519. [Google Scholar] [CrossRef]
- Hart, K.E.; Springmeier, J.M.; McKeown, N.B.; Colina, C.M. Simulated Swelling during Low-Temperature N2 Adsorption in Polymers of Intrinsic Microporosity. Phys. Chem. Chem. Phys. 2013, 15, 20161–20169. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Density Functional Theory Model of Adsorption on Amorphous and Microporous Silica Materials. Langmuir 2006, 22, 11171–11179. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Vishnyakov, A.; Russo, R.; Neimark, A.V. Unified Approach to Pore Size Characterization of Microporous Carbonaceous Materials from N2, Ar, and CO2 Adsorption Isotherms†. Langmuir 2000, 16, 2311–2320. [Google Scholar] [CrossRef]
- Blazsó, M. Polyaromatization in Common Synthetic Polymers at Elevated Temperatures. J. Anal. Appl. Pyrolysis 1993, 25, 25–35. [Google Scholar] [CrossRef]
- Sarwar, A.; Ali, M.; Khoja, A.H.; Nawar, A.; Waqas, A.; Liaquat, R.; Naqvi, S.R.; Asjid, M. Synthesis and Characterization of Biomass-Derived Surface-Modified Activated Carbon for Enhanced CO2adsorption. J. CO2 Util. 2021, 46, 101476. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S.; Borhan, A. Isotherm and Thermodynamic Analysis of Carbon Dioxide on Activated Carbon. Procedia Eng. 2016, 148, 630–637. [Google Scholar] [CrossRef]
- Gautam; Sahoo, S. Experimental Investigation on Different Activated Carbons as Adsorbents for CO2 Capture. Therm. Sci. Eng. Prog. 2022, 33, 101339. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Xie, H.; Won, S.W.; Cui, L.; Wu, G. Adsorption of Ag(I) from Aqueous Solution by Waste Yeast: Kinetic, Equilibrium and Mechanism Studies. Bioprocess Biosyst. Eng. 2015, 38, 69–77. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Isotherms, Kinetics and Thermodynamic Studies of Adsorption of Cu2+ from Aqueous Solutions by Mg2+/K+ Type Orange Peel Adsorbents. J. Hazard. Mater. 2010, 174, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Hauchhum, L.; Mahanta, P. Carbon Dioxide Adsorption on Zeolites and Activated Carbon by Pressure Swing Adsorption in a Fixed Bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly Microporous Activated Carbons from Biomass for CO2 Capture and Effective Micropores at Different Conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kiełbasa, K. Advances in Modification of Commercial Activated Carbon for Enhancement of CO2 Capture. Appl. Surf. Sci. 2019, 494, 137–151. [Google Scholar] [CrossRef]
- Singh, G.; Ismail, I.S.; Bilen, C.; Shanbhag, D.; Sathish, C.I.; Ramadass, K.; Vinu, A. A Facile Synthesis of Activated Porous Carbon Spheres from D-Glucose Using a Non-Corrosive Activating Agent for Efficient Carbon Dioxide Capture. Appl. Energy 2019, 255, 113831. [Google Scholar] [CrossRef]
- Nuhnen, A.; Janiak, C. A Practical Guide to Calculate the Isosteric Heat/Enthalpy of Adsorption: Via Adsorption Isotherms in Metal-Organic Frameworks, MOFs. Dalton Trans. 2020, 49, 10295–10307. [Google Scholar] [CrossRef] [PubMed]
- Vorokhta, M.; Morávková, J.; Dopita, M.; Zhigunov, A.; Šlouf, M.; Pilař, R.; Sazama, P. Effect of Micropores on CO2 Capture in Ordered Mesoporous CMK-3 Carbon at Atmospheric Pressure. Adsorption 2021, 27, 1221–1236. [Google Scholar] [CrossRef]
- Burri, H.; Anjum, R.; Gurram, R.B.; Mitta, H.; Mutyala, S.; Jonnalagadda, M. Mesoporous Carbon Supported MgO for CO2 Capture and Separation of CO2/N2. Korean J. Chem. Eng. 2019, 36, 1482–1488. [Google Scholar] [CrossRef]
- Tao, D.J.; Mao, F.F.; Luo, J.J.; Zhou, Y.; Li, Z.M.; Zhang, L. Mesoporous N-Doped Carbon Derived from Tea Waste for High-Performance CO2 Capture and Conversion. Mater. Today Commun. 2020, 22, 100849. [Google Scholar] [CrossRef]
- Singh, M.G.; Lakhi, K.S.; Park, D.H.; Srivastava, P.; Naidu, R.; Vinu, A. Facile One-Pot Synthesis of Activated Porous Biocarbons with a High Nitrogen Content for CO2 Capture. ChemNanoMat 2018, 4, 281–290. [Google Scholar] [CrossRef]
- Abdelnaby, M.M.; Qasem, N.A.A.; Al-Maythalony, B.A.; Cordova, K.E.; Al Hamouz, O.C.S. A Microporous Organic Copolymer for Selective CO2 Capture under Humid Conditions. ACS Sustain. Chem. Eng. 2019, 7, 13941–13948. [Google Scholar] [CrossRef]
- Xu, C.; Ruan, C.Q.; Li, Y.; Lindh, J.; Strømme, M. High-Performance Activated Carbons Synthesized from Nanocellulose for CO2 Capture and Extremely Selective Removal of Volatile Organic Compounds. Adv. Sustain. Syst. 2018, 2, 1700147. [Google Scholar] [CrossRef]
- Lu, T.; Ma, C.; Demir, M.; Yu, Q.; Aghamohammadi, P.; Wang, L.; Hu, X. One-Pot Synthesis of Potassium Benzoate-Derived Porous Carbon for CO2 Capture and Supercapacitor Application. Sep. Purif. Technol. 2022, 301, 122053. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Wang, Z.; Wang, S.; Fu, J. Tunable-Quaternary (N, S, O, P)-Doped Porous Carbon Microspheres with Ultramicropores for CO2 Capture. Appl. Surf. Sci. 2020, 507, 145130. [Google Scholar] [CrossRef]
- Prasankumar, T.; Salpekar, D.; Bhattacharyya, S.; Manoharan, K.; Yadav, R.M.; Campos Mata, M.A.; Miller, K.A.; Vajtai, R.; Jose, S.; Roy, S.; et al. Biomass Derived Hierarchical Porous Carbon for Supercapacitor Application and Dilute Stream CO2 Capture. Carbon 2022, 199, 249–257. [Google Scholar] [CrossRef]
- Du, J.; Li, W.C.; Ren, Z.X.; Guo, L.P.; Lu, A.H. Synthesis of Mechanically Robust Porous Carbon Monoliths for CO2 Adsorption and Separation. J. Energy Chem. 2020, 42, 56–61. [Google Scholar] [CrossRef]







| Entry | Material | Precursors | Method | Temperature |
|---|---|---|---|---|
| 1 | POP101 | Melamine/Pyrrole Molar ratio: 1:1 | Solvothermal (DMF) | 90 °C |
| 2 | POP102 | Melamine/Pyrrole Molar ratio: 1:2 | Solvothermal (DMF) | 90 °C |
| 3 | POP103 | Melamine/Pyrrole Molar ratio: 1:3 | Solvothermal (DMF) | 90 °C |
| 4 | POP104 | Melamine/Pyrrole Molar ratio: 1:4 | Solvothermal (DMF) | 90 °C |
| 5 | NPC101-700 | POP101 | Pyrolysis | 700 °C |
| 6 | NPC102-700 | POP102 | Pyrolysis | 700 °C |
| 7 | NPC103-700 | POP103 | Pyrolysis | 700 °C |
| 8 | NPC104-700 | POP104 | Pyrolysis | 700 °C |
| 9 | NPC101-900 | POP101 | Pyrolysis | 900 °C |
| 10 | NPC102-900 | POP102 | Pyrolysis | 900 °C |
| Material | BET Area (m2 g−1) | Langmuir Area (m2 g−1) | Pore Volume 1 (cm3 g−1) | Micropore Volume 2 (cm3 g−1) | DFT Pore Radius (nm) |
|---|---|---|---|---|---|
| POP101 | 205 | 324 | 0.59 | 0.000 | 0.25 |
| POP102 | 263 | 441 | 0.62 | 0.000 | 0.62 |
| POP103 | 333 | 532 | 0.50 | 0.000 | 0.59 |
| POP104 | 436 | 695 | 0.55 | 0.010 | 0.63 |
| NPC101-700 | 570 | 792 | 0.60 | 0.201 | 0.39 |
| NPC102-700 | 369 | 552 | 0.42 | 0.094 | 0.39 |
| NPC103-700 | 387 | 542 | 0.34 | 0.080 | 0.54 |
| NPC104-700 | 348 | 495 | 0.30 | 0.065 | 0.54 |
| NPC101-900 | 537 | 807 | 0.78 | 0.141 | 0.39 |
| NPC102-900 | 659 | 1067 | 0.83 | 0.120 | 0.50 |
| Samples | CO2 Uptake (cm3 g−1) | CO2 Uptake (cm3 g−1) |
|---|---|---|
| 273 K | 298 K | |
| POP101 | 30.8 | 20.7 |
| POP102 | 29.3 | 19.2 |
| POP103 | 28.9 | 19.3 |
| POP104 | 30.8 | 19.6 |
| NPC101-900 | 59.4 | 44.1 |
| NPC102-900 | 57.5 | 46.5 |
| Material | ΔS° (kJ/mol K) | ΔH° (kJ/mol) | ΔG° (kJ/mol) | |
|---|---|---|---|---|
| 273 K | 298 K | |||
| NPC101-900 | −0.074 | −23.620 | −3.35 | −1.50 |
| NPC102-900 | −0.044 | −14.827 | −2.73 | −1.62 |
| Material | SABET (m2 g−1) | CO2 Uptake (mmol g−1) | Temp. (K) | CO2/N2 Selectivity | Ref. |
|---|---|---|---|---|---|
| POP-derived NPCs | 570–659 | 1.9–2.1 | 298 | 50–53 | This work |
| CMK-3 carbon | 624 | 1.7 | 293 | 35–38 | [42] |
| Mesoporous carbon-MgO | 0.9 | 298 | — | [43] | |
| Mesoporous N-doped carbon tea waste (TW-900) | 354 | 1.7 | 298 | 79 | [44] |
| Activated porous biocarbons (NEPB-3UK) | 982 | 2.2 | 298 | — | [45] |
| BPL Carbon | 1210 | 2.1 | 298 | — | [46] |
| Cellulose-based carbons -AC-N2 | 500 | 2.6 | — | [47] | |
| benzoate-derived porous carbon | 777 | 3.6 | 298 | — | [48] |
| (N, S, O, P)-doped porous carbon microspheres (PCMS—750) | 342 | 1.8 | 298 | 10.6 | [49] |
| PCMSs—800 | 481 | 2.8 | 12.7 | [49] | |
| Hierarchical porous carbon | 4.5 | 273 | — | [50] | |
| Carbon monoliths | 595–621 | 2.3–3.0 | 298 | — | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelnaby, M.M.; Aliyu, M.; Nemitallah, M.A.; Alloush, A.M.; Mahmoud, E.-H.M.; Ossoss, K.M.; Zeama, M.; Dowaidar, M. Design and Synthesis of N-Doped Porous Carbons for the Selective Carbon Dioxide Capture under Humid Flue Gas Conditions. Polymers 2023, 15, 2475. https://doi.org/10.3390/polym15112475
Abdelnaby MM, Aliyu M, Nemitallah MA, Alloush AM, Mahmoud E-HM, Ossoss KM, Zeama M, Dowaidar M. Design and Synthesis of N-Doped Porous Carbons for the Selective Carbon Dioxide Capture under Humid Flue Gas Conditions. Polymers. 2023; 15(11):2475. https://doi.org/10.3390/polym15112475
Chicago/Turabian StyleAbdelnaby, Mahmoud M., Mansur Aliyu, Medhat A. Nemitallah, Ahmed M. Alloush, El-Hassan M. Mahmoud, Khaled M. Ossoss, Mostafa Zeama, and Moataz Dowaidar. 2023. "Design and Synthesis of N-Doped Porous Carbons for the Selective Carbon Dioxide Capture under Humid Flue Gas Conditions" Polymers 15, no. 11: 2475. https://doi.org/10.3390/polym15112475
APA StyleAbdelnaby, M. M., Aliyu, M., Nemitallah, M. A., Alloush, A. M., Mahmoud, E.-H. M., Ossoss, K. M., Zeama, M., & Dowaidar, M. (2023). Design and Synthesis of N-Doped Porous Carbons for the Selective Carbon Dioxide Capture under Humid Flue Gas Conditions. Polymers, 15(11), 2475. https://doi.org/10.3390/polym15112475







