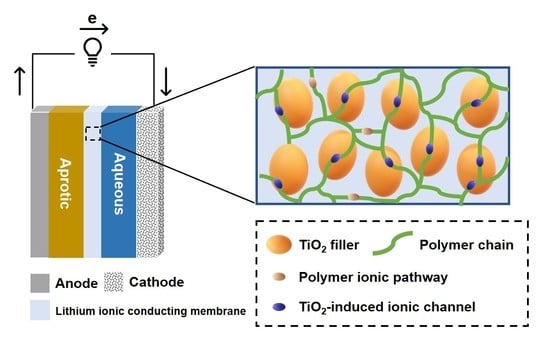
A Flexible Lithium-Ion-Conducting Membrane with Highly Loaded Titanium Oxide Nanoparticles to Promote Charge Transfer for Lithium–Air Battery
Abstract
1. Introduction
2. Experimental Section
2.1. Reagent
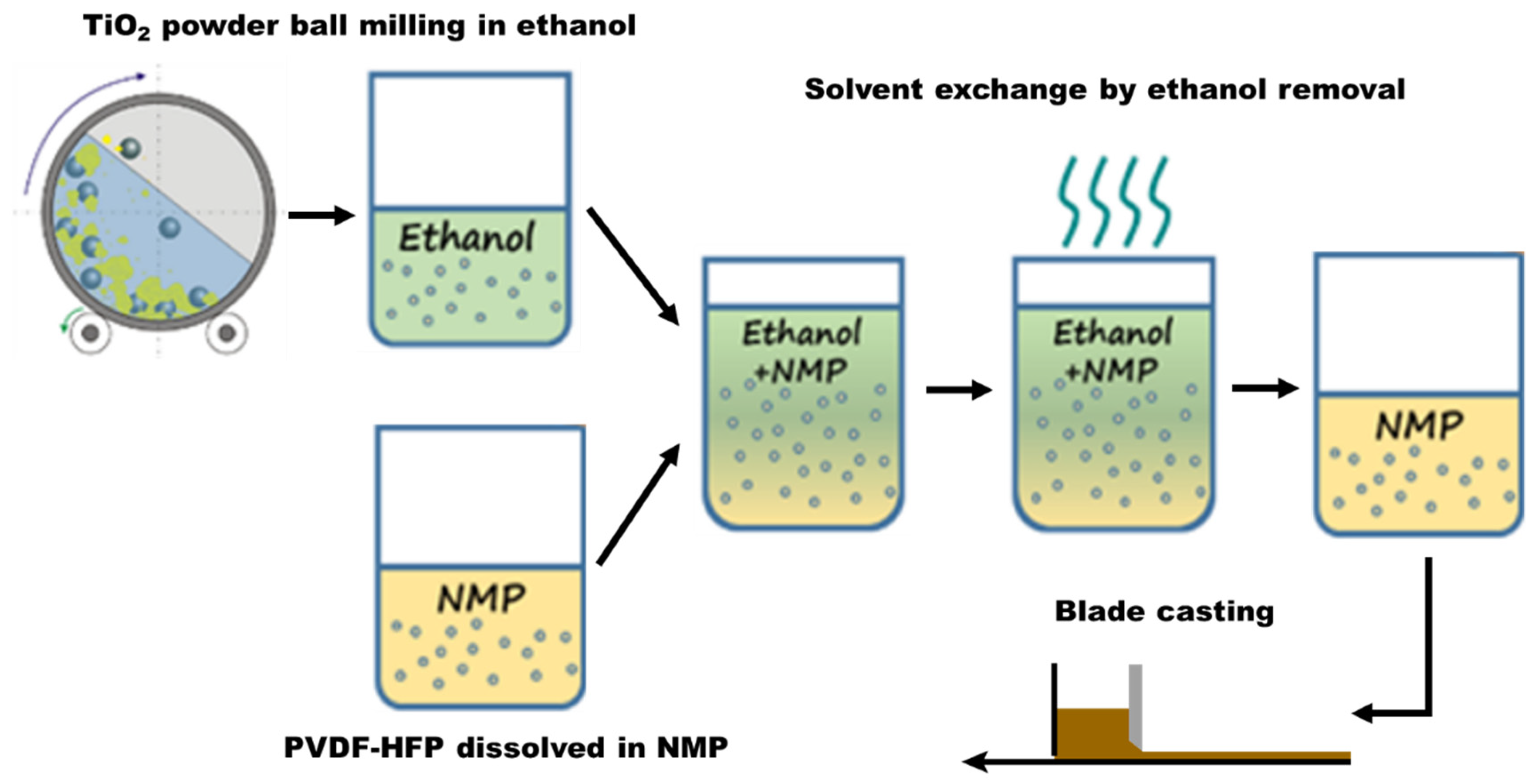
2.2. Pretreatment and Fabrication of the FC-LICM Incorporating TiO2
2.3. Characterization of the Materials
2.4. Electrochemical Measurements and Assembly of the Hybrid Li–Air Battery
3. Results and Discussion
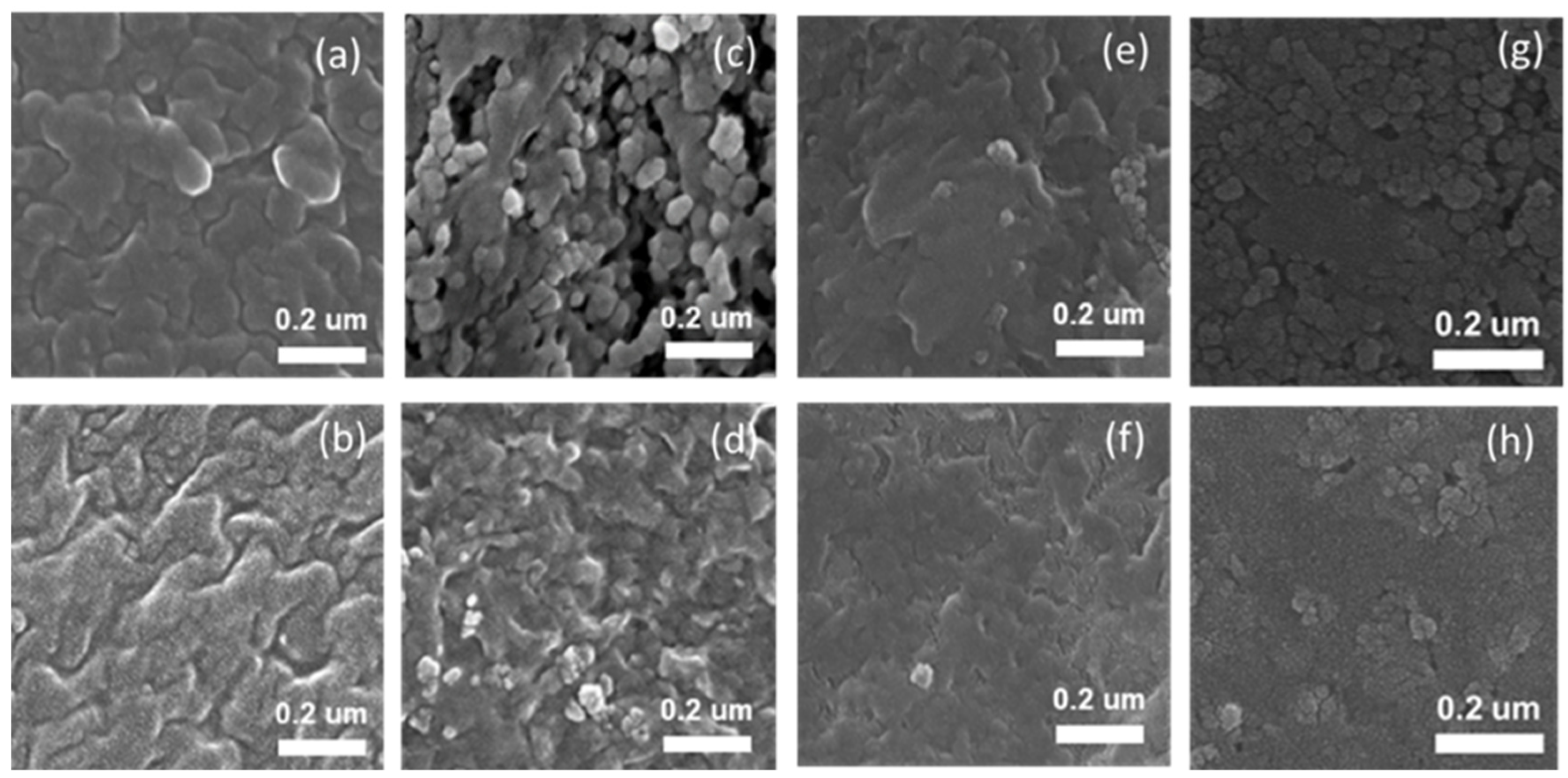
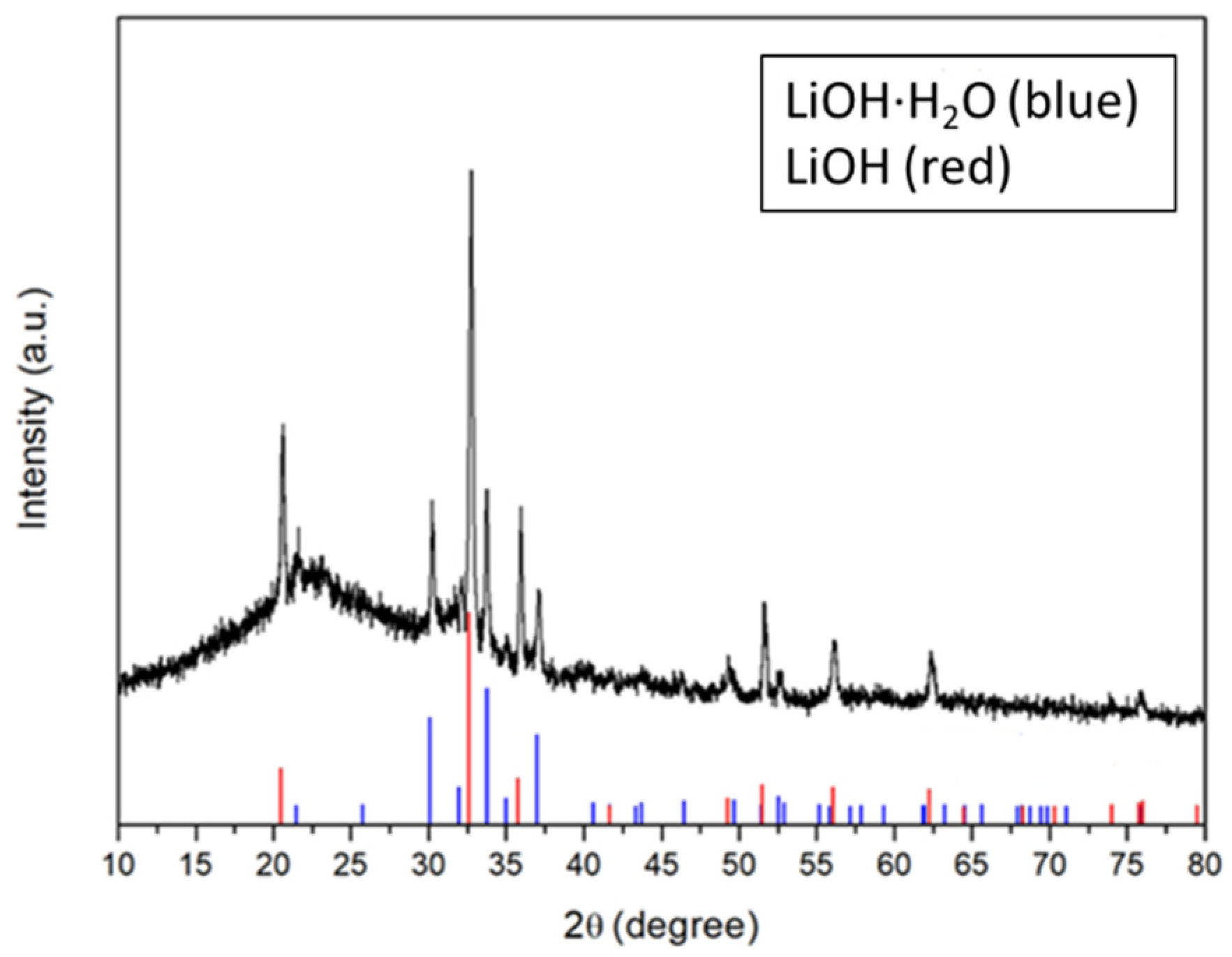
3.1. Characterization of TiO2 Nanoparticle-Filled FC-LICMs
3.2. Swelling Behavior and Permeating Properties of the As-Fabricated TiO2-Filled FC-LICMs
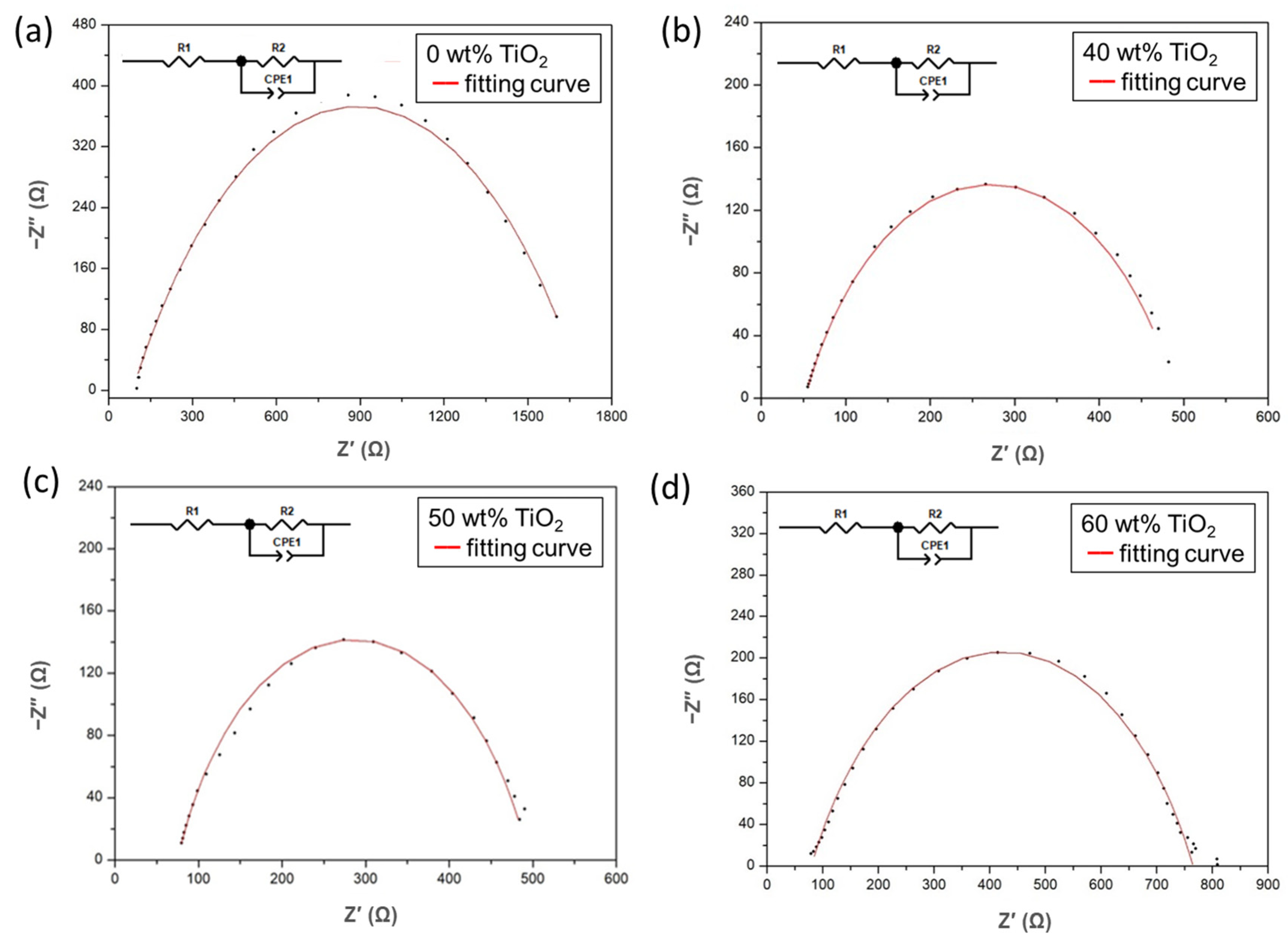
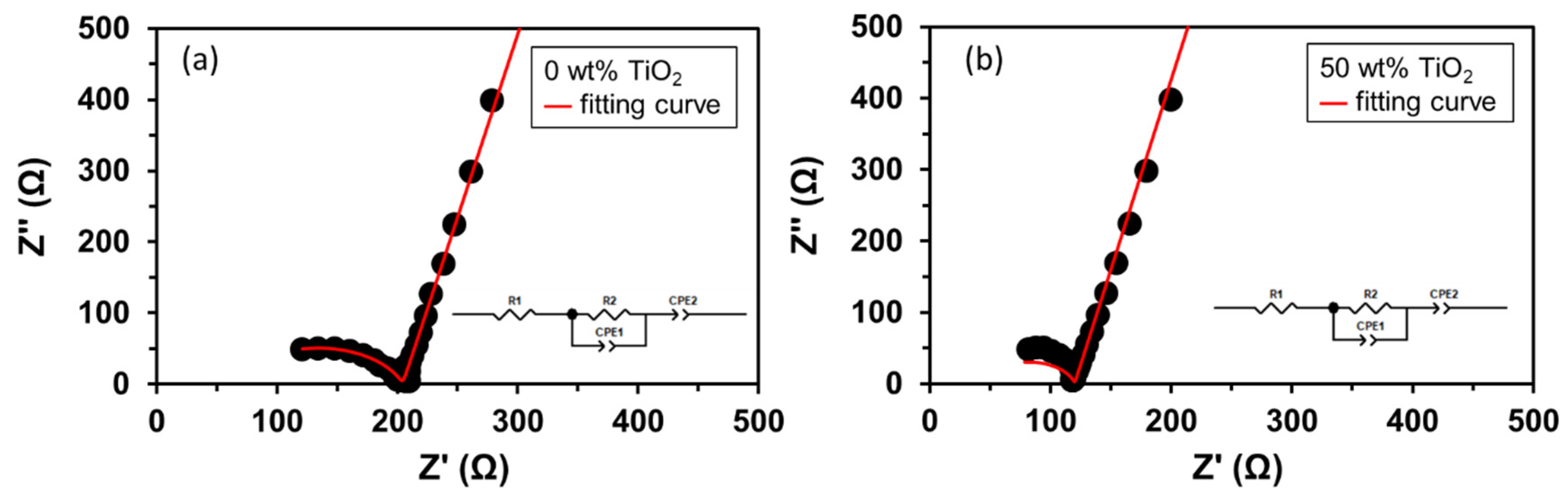
3.3. Electrochemical Impedance Spectroscopy Analyses of FC-LICMs Filled with TiO2
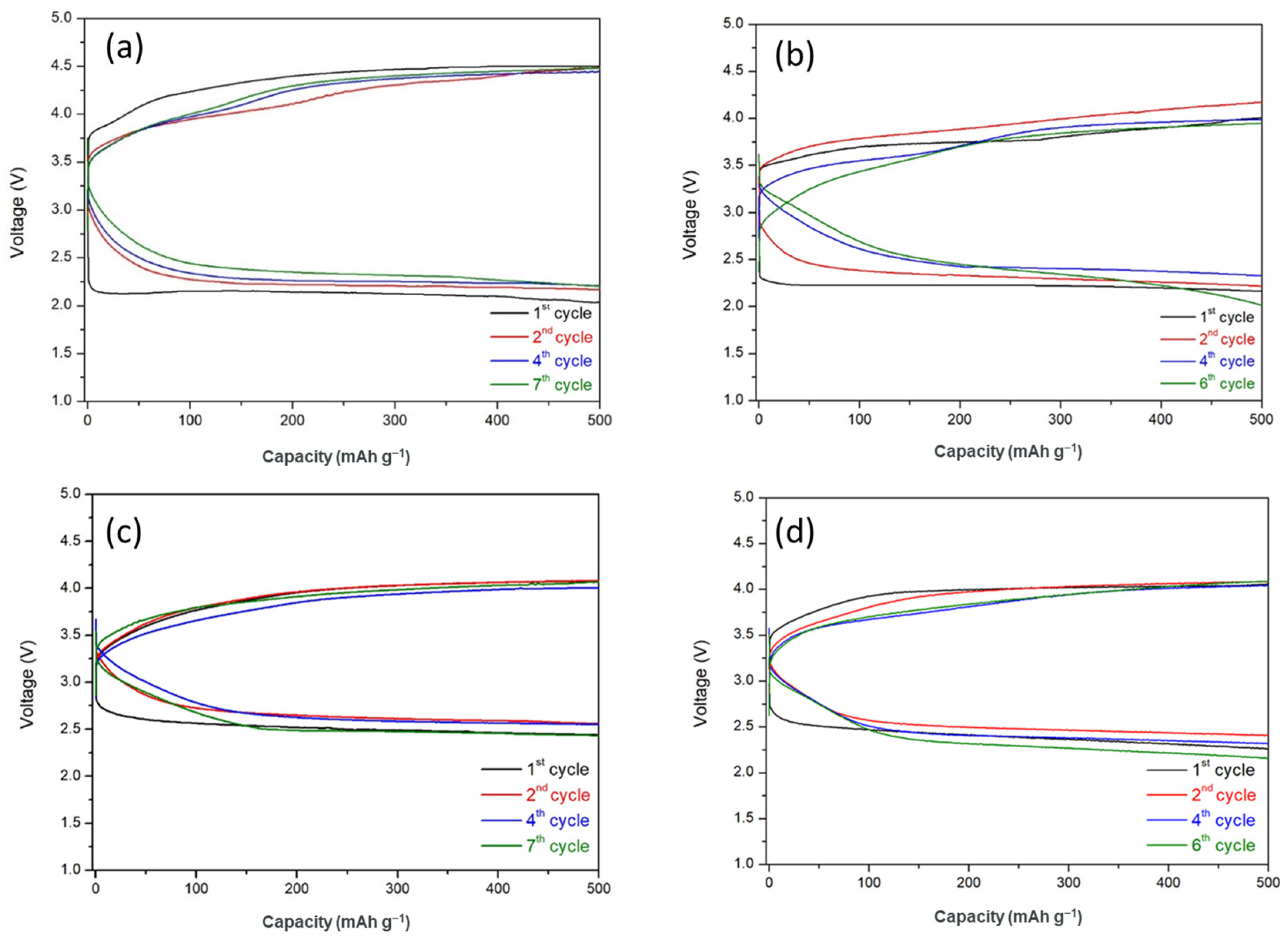
3.4. Cycling Performance of HELABs with Different Ratios of TiO2-Filled FC-LICMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heubner, C.; Nikolowski, K.; Reuber, S.; Schneider, M.; Wolter, M.; Michaelis, A. Recent insights into rate performance limitations of Li-ion batteries. Batter. Supercaps 2021, 4, 268. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review on Li–air batteries—Opportunities, limitations and perspective. J. Power Sources 2011, 196, 886–893. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Yoo, E.; Zhou, H. Li−air rechargeable battery based on metal-free graphene nanosheet catalysts. ACS Nano 2011, 5, 3020–3026. [Google Scholar] [CrossRef]
- Christensen, A.; Albertus, P.; Sanchez-Carrera, R.S.; Lohmann, T.; Kozinsky, B.; Liedtke, R.; Ahmed, J.; Kojic, A. A critical review of Li/air batteries. J. Electrochem. Soc. 2012, 159, R1–R30. [Google Scholar] [CrossRef]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 2016, 1, 16128. [Google Scholar] [CrossRef]
- Wang, L.; Pan, J.; Zhang, Y.; Cheng, X.; Liu, L.; Peng, H. A Li–air battery with ultralong cycle life in ambient air. Adv. Mater. 2018, 30, 1704378. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, J.; Peng, Z. Achilles Heel of lithium–air batteries: Lithium carbonate. Angew. Chem. Int. Ed. 2018, 57, 3874–3886. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, J.; Li, F.; Wang, Z.L.; Sun, C. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- He, H.; Niu, W.; Asl, N.M.; Salim, J.; Chen, R.R.; Kim, Y. Effects of aqueous electrolytes on the voltage behaviors of rechargeable Li-air batteries. Electrochim. Acta 2012, 67, 87–94. [Google Scholar] [CrossRef]
- Sun, C.; Li, F.; Ma, C.; Wang, Y.; Ren, Y.; Yang, W.; Ma, Z.; Li, J.; Chen, Y.; Kim, Y.; et al. Graphene–Co3O4 nanocomposite as an efficient bifunctional catalyst for lithium–air batteries. J. Mater. Chem. A 2014, 2, 7188. [Google Scholar] [CrossRef]
- Manthiram, A.; Li, L. Hybrid and Aqueous Lithium-Air Batteries. Adv. Energy Mater. 2015, 5, 1401302. [Google Scholar] [CrossRef]
- Peng, S.-H.; Chen, T.-H.; Li, C.-H.; Lu, H.-C.; Lue, S.J. Optimal cobalt oxide (Co3O4): Graphene (GR) ratio in Co3O4/GR as air cathode catalyst for air-breathing hybrid electrolyte lithium-air battery. J. Power Sources 2020, 471, 228373. [Google Scholar] [CrossRef]
- Peng, S.-H.; Lu, H.-C.; Lue, S.J. Nanocrystal cobalt oxide (Co3O4) decorated graphene as efficient cathode catalyst for air-breathing hybrid electrolyte lithium-air battery. Nanomaterials 2020, 10, 1122. [Google Scholar] [CrossRef]
- Hoang Huy, V.P.; So, S.; Hur, J. Inorganic fillers in composite gel polymer electrolytes for high-performance lithium and non-lithium polymer batteries. Nanomaterials 2021, 11, 614. [Google Scholar] [CrossRef]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.C.; Li, Y.; Lee, H.W.; Cui, Y. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Liu, J.; Sun, L.; Cong, L.; Fu, F.; Mauger, A.; Julien, C.M.; Xie, H.; Pan, X. “Polymer-in-ceramic” based poly(ε-caprolactone)/ceramic composite electrolyte for all-solid-state batteries. J. Energy Chem. 2021, 52, 318–325. [Google Scholar] [CrossRef]
- Hu, T.; Shen, X.; Peng, L.; Liu, Y.; Wang, X.; Ma, H.; Zhang, P.; Zhao, J. Preparation of single-ion conductor solid polymer electrolyte by multi-nozzle electrospinning process for lithium-ion batteries. J. Phys. Chem. Solids. 2021, 158, 110229. [Google Scholar] [CrossRef]
- Ge, Z.H.; Wei, K.; Lewis, H.; Martin, J.; Nolas, G.S. Bottom-up processing and low temperature transport properties of polycrystalline SnSe. J. Solid State Chem. 2015, 225, 354–358. [Google Scholar] [CrossRef]
- Pignanelli, F.; Romero, M.; Castiglioni, J.; Faccio, R.; Mombrú, A.W. Novel synergistic in situ synthesis of lithium-ion poly (ethylene citrate)-TiO2 nanocomposites as promising fluorine-free solid polymer electrolytes for lithium batteries. J. Phys. Chem. Solids 2019, 135, 109082. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, X.; Liu, L.; Zhang, Z.; Teng, Y.; Yu, D.; Sui, J.; Wang, X. SiO2/C composite derived from rice husks with enhanced capacity as anodes for lithium-ion batteries. Chem. Select. 2018, 3, 10338–10344. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.P.; Fan, L.Z.; Nan, C.W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Lu, S.-H.; Lu, H.-C. Pouch-type hybrid Li-air battery enabled by flexible composite lithium-ion conducting membrane. J. Power Sources 2021, 489, 229431. [Google Scholar] [CrossRef]
- Hasegawa, S.; Imanishi, N.; Zhang, T.; Xie, J.; Hirano, A.; Takeda, Y.; Yamamoto, O. Study on lithium/air secondary batteries—Stability of NASICON-type lithium ion conducting glass–ceramics with water. J. Power Sources 2009, 189, 371–377. [Google Scholar] [CrossRef]
- Zhang, T.; Imanishi, N.; Shimonishi, Y.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. A novel high energy density rechargeable lithium/air battery. Chem. Commun. 2010, 46, 1661–1663. [Google Scholar] [CrossRef]
- Imanishi, N.; Hasegawa, S.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Lithium anode for lithium-air secondary batteries. J. Power Sources 2008, 185, 1392–1397. [Google Scholar] [CrossRef]
- Kumar, J.; Kumar, B. Development of membranes and a study of their interfaces for rechargeable lithium–air battery. J. Power Sources 2009, 194, 1113–1119. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Wu, L.; Duan, Z.; Jiang, J.; Zhao, L. Synthesis of a thin-layer MnO2 nanosheet-coated Fe3O4 nanocomposite as a magnetically separable photocatalyst. Langmuir 2014, 30, 7006–7013. [Google Scholar] [CrossRef]
- Tseng, Y.; Ramdhani, F.; Hsiang, S.; Lee, T.; Teng, H.; Jan, J.S. Lithium battery enhanced by the combination of in-situ generated poly (ionic liquid) systems and TiO2 nanoparticles. J. Membr. Sci. 2022, 641, 119891. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Garcia-Belmonte, G.; Bisquert, J.; Zaban, A.; Salvador, P. Decoupling of transport, charge storage, and interfacial charge transfer in the nanocrystalline TiO2/electrolyte system by impedance methods. J. Phys. Chem. B. 2002, 106, 334–339. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, J.Y.; Hong, L. Morphology, crystallinity, and electrochemical properties of in situ formed poly(ethylene oxide)/TiO2 nanocomposite polymer electrolytes. J. Appl. Polym. Sci. 2003, 89, 2815–2822. [Google Scholar] [CrossRef]
- Jayanthi, S.; Kulasekarapandian, K.; Arulsankar, A.; Sankaranarayanan, K.; Sundaresan, B. Influence of nano-sized TiO2 on the structural, electrical, and morphological properties of polymer-blend electrolytes PEO–PVC–LiClO4. J. Compos. Mater. 2014, 49, 1035–1045. [Google Scholar] [CrossRef]
- Lin, C.W.; Hung, C.L.; Venkateswarlu, M.; Hwang, B.J. Influence of TiO2 nano-particles on the transport properties of composite polymer electrolyte for lithium-ion batteries. J. Power Sources 2005, 146, 397–401. [Google Scholar] [CrossRef]
- Sasikumar, M.; Hari Krishna, R.; Raja, M.; Therese, H.A.; Balakrishnan, N.T.M.; Raghavan, P.; Sivakumar, P. Titanium dioxide nano-ceramic filler in solid polymer electrolytes: Strategy towards suppressed dendrite formation and enhanced electrochemical performance for safe lithium ion batteries. J. Alloy. Compd. 2021, 882, 16070. [Google Scholar] [CrossRef]
- Huo, H.; Zhao, N.; Sun, J.; Du, F.; Li, Y.; Guo, X. Composite electrolytes of polyethylene oxides/garnets interfacially wetted by ionic liquid for room-temperature solid-state lithium battery. J. Power Sources 2017, 372, 1–7. [Google Scholar] [CrossRef]
- Kumar, B.; Scanlon, L.G. Polymer-ceramic composite electrolytes. J. Power Sources 1994, 52, 261–268. [Google Scholar] [CrossRef]
- Yang, T.; Shu, C.; Zheng, R.; Li, M.; Hou, Z.; Hei, P. Dendrite-free solid-state Li–O2 batteries enabled by organic–inorganic interaction reinforced gel polymer electrolyte. ACS Sustain. Chem. Eng. 2019, 7, 17362–17371. [Google Scholar] [CrossRef]
- Song, D.Y.; Xu, C.; Chen, Y.F.; He, J.R.; Zhao, Y.; Li, P.J.; Lin, W.; Fu, F. Enhanced thermal and electrochemical properties of PVDF-HFP/PMMA polymer electrolyte by TiO2 nanoparticles. Solid State Ion. 2015, 282, 31–36. [Google Scholar] [CrossRef]
- Liang, X.; Han, D.; Wang, Y.; Lan, L.; Mao, J. Preparation and performance study of a PVDF–LATP ceramic composite polymer electrolyte membrane for solid-state batteries. RSC Adv. 2018, 8, 40498–40504. [Google Scholar] [CrossRef]
- Kou, Z.; Miao, C.; Wang, Z.; Mei, P.; Zhang, Y.; Yan, X.; Jiang, Y.; Xiao, W. Enhanced ionic conductivity of novel composite polymer electrolytes with Li1.3Al0.3Ti1.7(PO4)3 NASICON-type fast ion conductor powders. Solid State Ionics 2019, 338, 138–143. [Google Scholar] [CrossRef]
- Hsia, T.; Lu, H.; Hsueh, Y.; Kumar, S.; Yen, C.; Yang, C.; Lue, S.J. Superdry poly (vinylidene fluoride-co-hexafluoropropylene) coating on a lithium anode as a protective layer and separator for a high-performance lithium-oxygen battery. J. Colloid Interface Sci. 2022, 626, 524–534. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, K.; Zhang, P.; Li, H.; Mi, H. PVDF-HFP based polymer electrolytes with high Li+ transference number enhancing the cycling performance and rate capability of lithium metal batteries. Appl. Surf. Sci. 2022, 574, 151593. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Zhu, X.; Kong, X.Z. Preparation of hollow TiO2 nanoparticles through TiO2 deposition on polystyrene latex particles and characterizations of their structure and photocatalytic activity. Nanoscale Res. Lett. 2012, 7, 646. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR surface characterization of TiO2 particles used in polymer encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- Subianto, S.; Choudhury, N.; Dutta, N. Composite electrolyte membranes from partially fluorinated polymer and hyperbranched, sulfonated polysulfone. Nanomaterials 2013, 4, 1–18. [Google Scholar] [CrossRef]
- Hinckley, A.C.; Wang, C.; Pfattner, R.; Kong, D.; Zhou, Y.; Ecker, B.; Bao, Z. Investigation of a solution-processable, nonspecific surface modifier for low cost, high work function electrodes. ACS Appl. Mater. Interfaces. 2016, 8, 19658–19664. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Knápek, A.; Burda, D.; Orudzhev, F.; Hadaš, Z. Characterization of polyvinylidene fluoride (Pvdf) electrospun fibers doped by carbon flakes. Polymers 2020, 12, 2766. [Google Scholar] [CrossRef]
- Fahmi, E.M.; Ahmad, A.; Rahman, M.Y.A.; Hamzah, H. Effect of NiO nanofiller concentration on the properties of PEO-NiO-LiClO4 composite polymer electrolyte. J. Solid State Electrochem. 2012, 16, 2487–2491. [Google Scholar] [CrossRef]
- Sasikumar, M.; Jagadeesan, A.; Raja, M.; Hari Krishna, R.; Sivakumar, P. The effects of PVAc on surface morphological and electrochemical performance of P(VdF-HFP)-based blend solid polymer electrolytes for lithium ion-battery applications. Ionics 2019, 25, 2171–2181. [Google Scholar] [CrossRef]
- Kim, K.M.; Ko, J.M.; Park, N.G.; Ryu, K.S.; Chang, S.H. Characterization of poly (vinylidenefluoride-co-hexafluoropropylene)-based polymer electrolyte filled with rutile TiO2 nanoparticles. Solid State Ion. 2003, 161, 121–131. [Google Scholar] [CrossRef]
- Kim, H.S.; Mora-Sero, I.; Gonzalez-Pedro, V.; Fabregat-Santiago, F.; Juarez-Perez, E.; Park, N.G.; Bisquert, J. Mechanism of Carrier Accumulation in Perovskite Thin-absorber Solar Cells. Nat. Commun. 2013, 4, 2242. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Shang, M.; Liu, J.; Lu, G.; Wang, X.; Bi, Y. The charge transfer mechanism of Bi modified TiO2 nanotube arrays: TiO2 serving as a “charge-transfer-bridge”. Nano Energy 2017, 31, 96–104. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Sassmann, P.B.; Weichold, O. Preparation and characterisation of ion-conductive unsaturated polyester resins for the on-site production of resistivity sensors. Ionics 2019, 25, 3971–3978. [Google Scholar] [CrossRef]
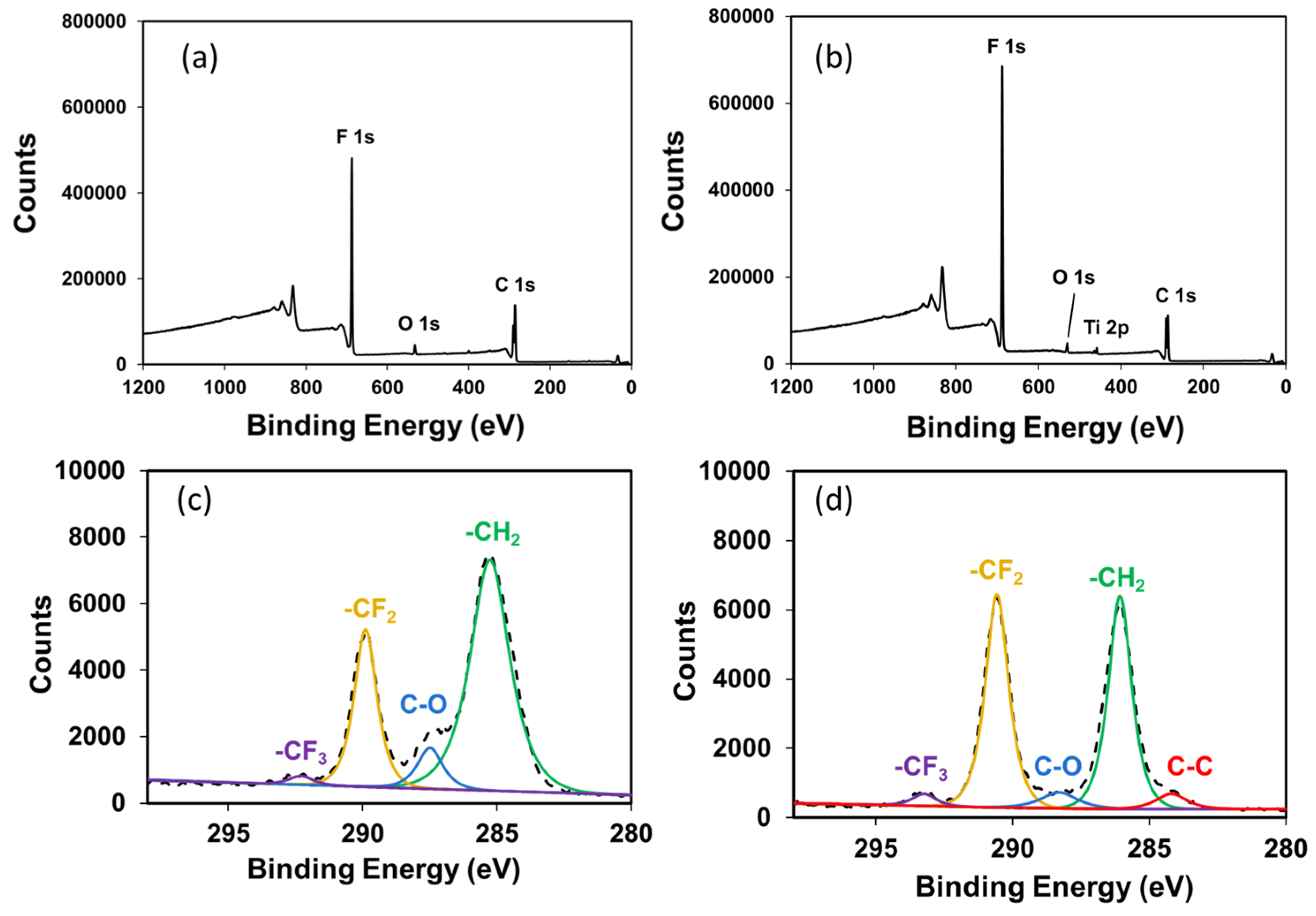
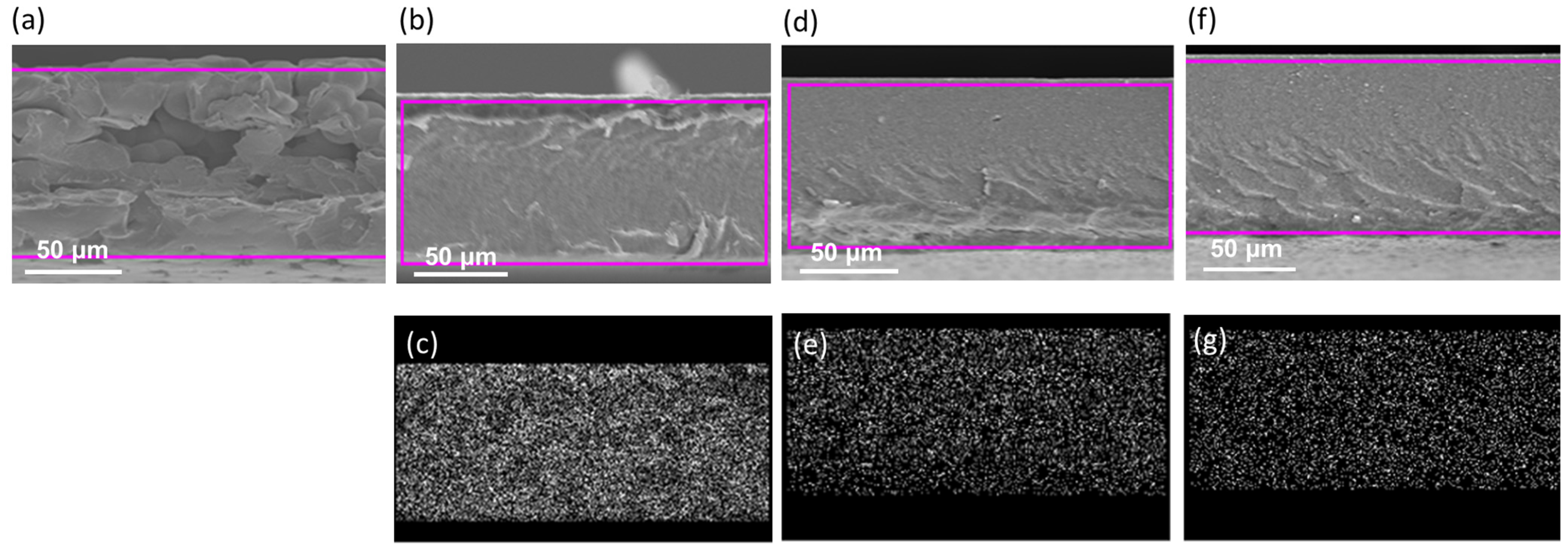
| Sample Name | B.E. (-CH2-) | B.E. (-CF2-) | Peak Area Ratio (-CH2- to -CF2-) |
|---|---|---|---|
| 0 wt% of TiO2 in FC-LICM | 285.2 eV | 289.8 eV | 2.54 |
| 50 wt% of TiO2 in FC-LICM | 286 eV | 290.5 eV | 1 |
| TiO2 Content in FC-LICM | Thickness (μm) | Permeability (cm2 s−1) | Swelling Ratio (%) 1 | Aprotic Electrolyte Uptake (%) 2 |
|---|---|---|---|---|
| 0 wt% | 94 | 1.23 × 10−9 | 8.5% | 13.2% |
| 40 wt% | 84 | 5.12 × 10−7 | 7.1% | 11.8% |
| 50 wt% | 86 | 3.91 × 10−7 | 4.7% | 5.7% |
| 60 wt% | 90 | 1.13 × 10−6 | 3.3% | 2.7% |
| TiO2 Content in FC-LICM | Rb (Ω) | Rct (Ω) | Conductivity (S cm−1) |
|---|---|---|---|
| 0 wt% | 85 | 1609 | 4.31 × 10−5 |
| 40 wt% | 48 | 476 | 6.86 × 10−5 |
| 50 wt% | 74 | 420 | 4.54 × 10−5 |
| 60 wt% | 79 | 685 | 4.43 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.-H.; Yu, Y.-H.; Lu, H.-C.; Lue, S.J. A Flexible Lithium-Ion-Conducting Membrane with Highly Loaded Titanium Oxide Nanoparticles to Promote Charge Transfer for Lithium–Air Battery. Polymers 2023, 15, 2409. https://doi.org/10.3390/polym15102409
Peng S-H, Yu Y-H, Lu H-C, Lue SJ. A Flexible Lithium-Ion-Conducting Membrane with Highly Loaded Titanium Oxide Nanoparticles to Promote Charge Transfer for Lithium–Air Battery. Polymers. 2023; 15(10):2409. https://doi.org/10.3390/polym15102409
Chicago/Turabian StylePeng, Si-Han, Yen-Hsiang Yu, Hsin-Chun Lu, and Shingjiang Jessie Lue. 2023. "A Flexible Lithium-Ion-Conducting Membrane with Highly Loaded Titanium Oxide Nanoparticles to Promote Charge Transfer for Lithium–Air Battery" Polymers 15, no. 10: 2409. https://doi.org/10.3390/polym15102409
APA StylePeng, S.-H., Yu, Y.-H., Lu, H.-C., & Lue, S. J. (2023). A Flexible Lithium-Ion-Conducting Membrane with Highly Loaded Titanium Oxide Nanoparticles to Promote Charge Transfer for Lithium–Air Battery. Polymers, 15(10), 2409. https://doi.org/10.3390/polym15102409