Fabrication of Woven Jute Fiber Epoxy Bio-Composites through the Epoxy/Thiol-Ene Photopolymerization Technique
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
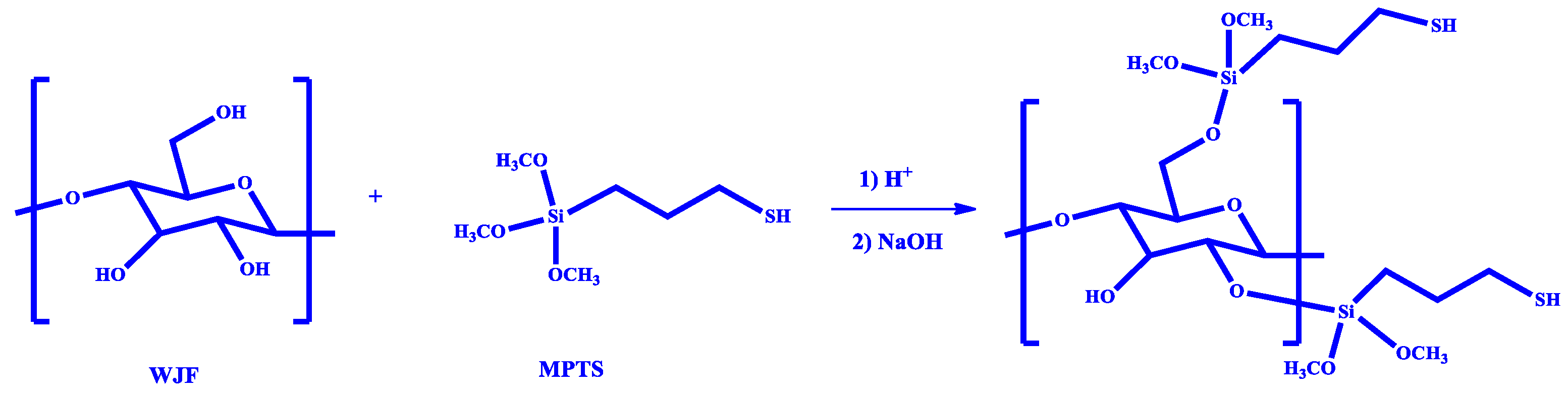
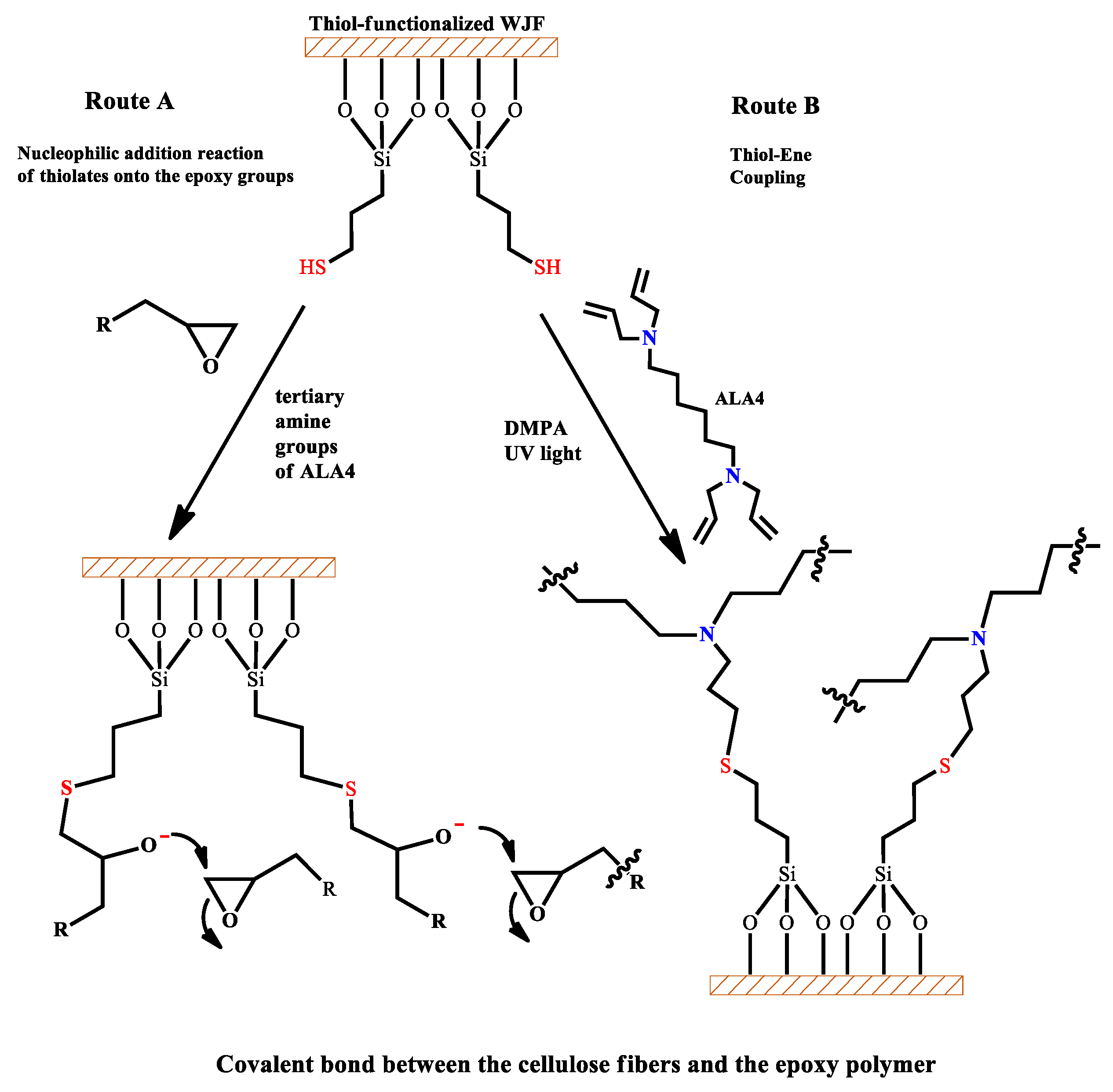
2.2. Thiol-Functionalization of WJF
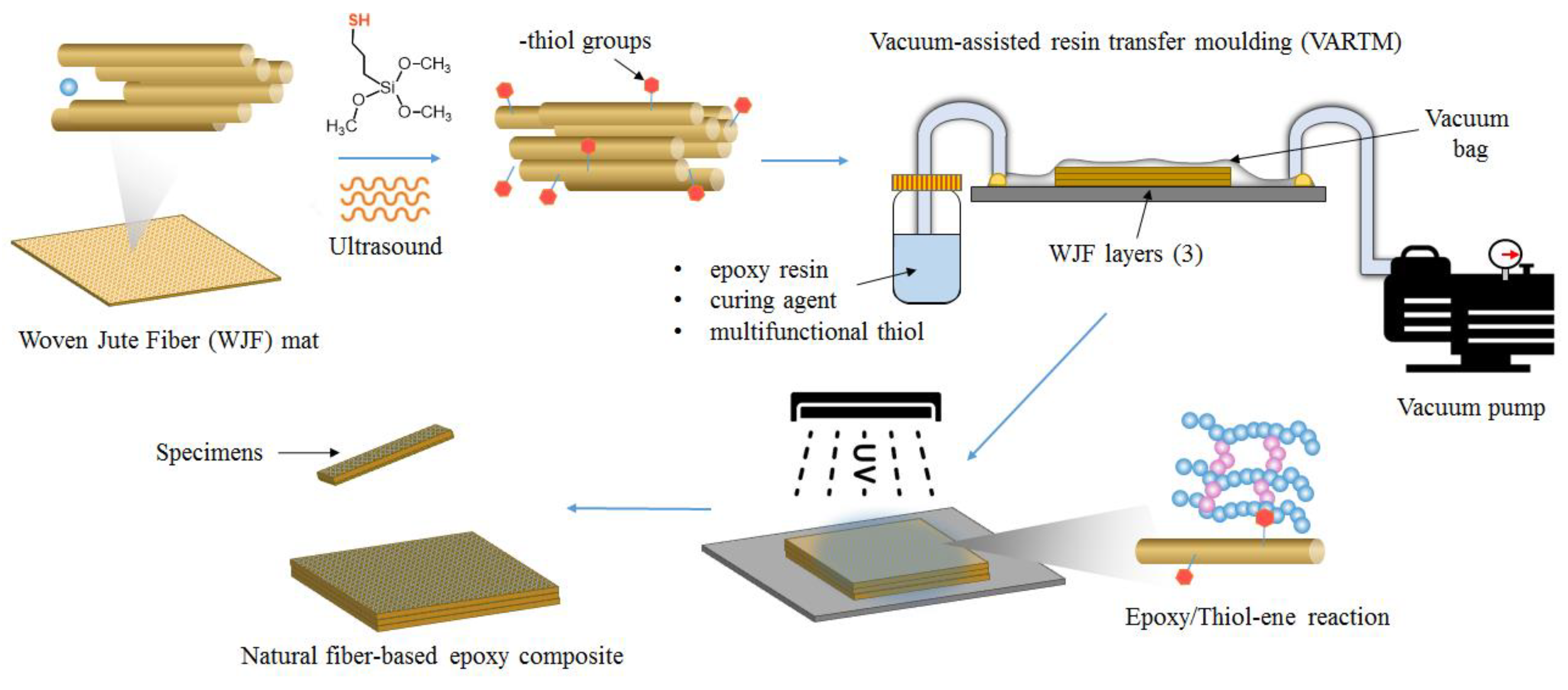
2.3. Photopolymerization of the Photocurable Formulation with the WJF
2.4. Dynamic Mechanical Analysis (DMA) of the Composites
2.5. Flexural Testing of WJF/Epoxy Composites
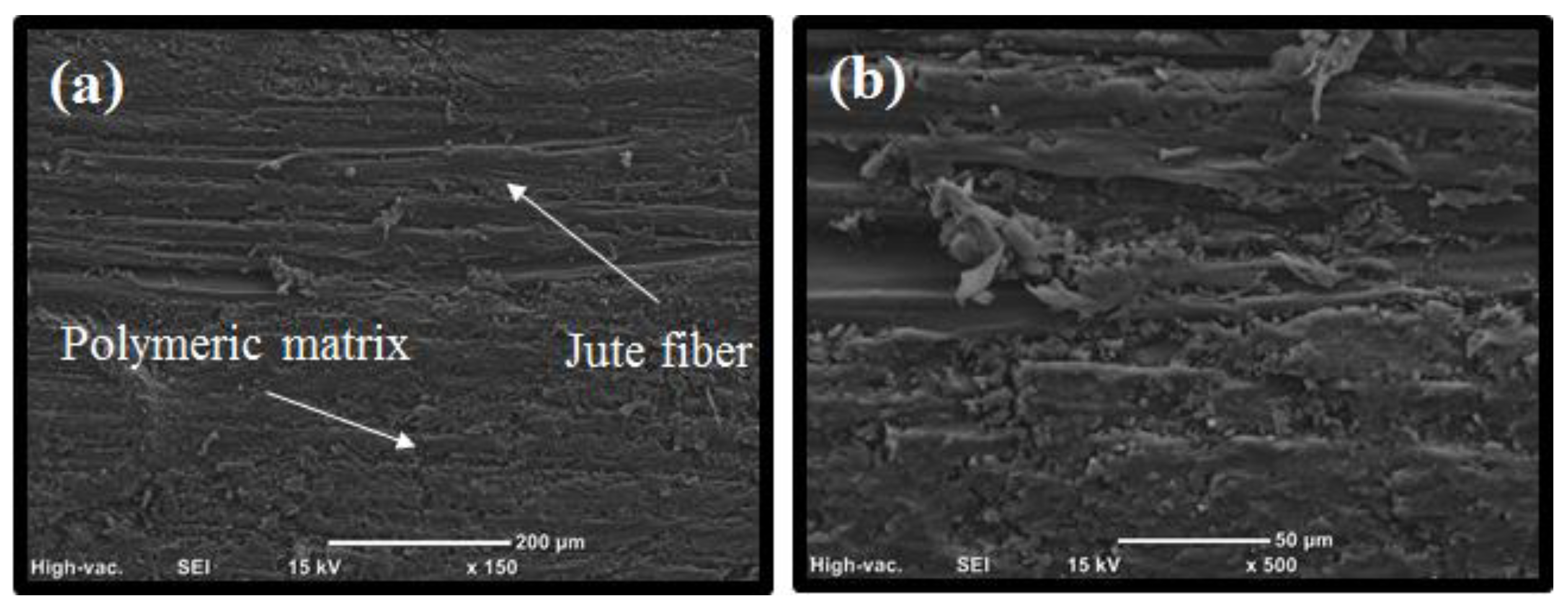
2.6. Scanning Electron Microscopy (SEM)
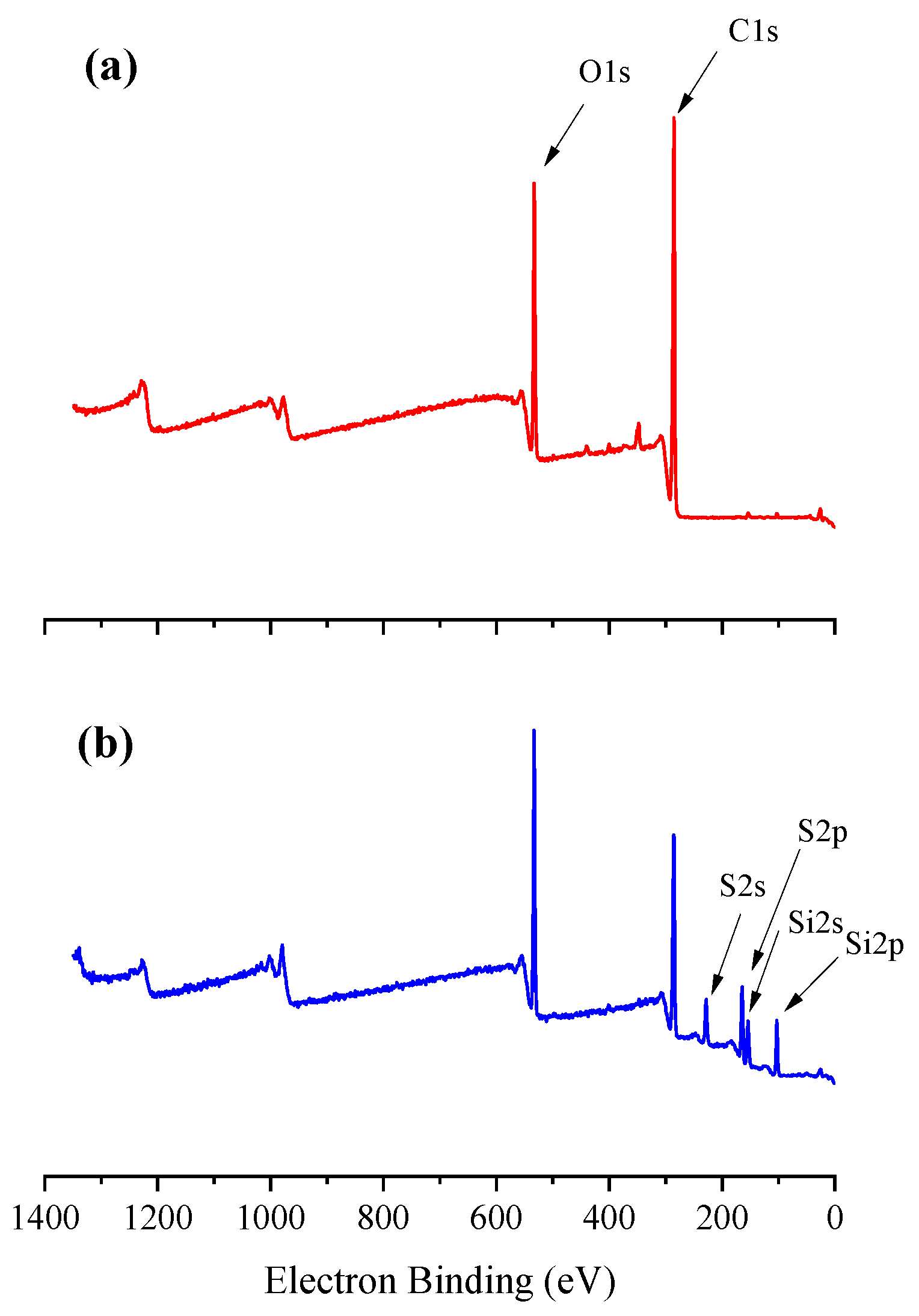
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.8. Thermogravimetric Analysis (TGA)
3. Results and Discussion
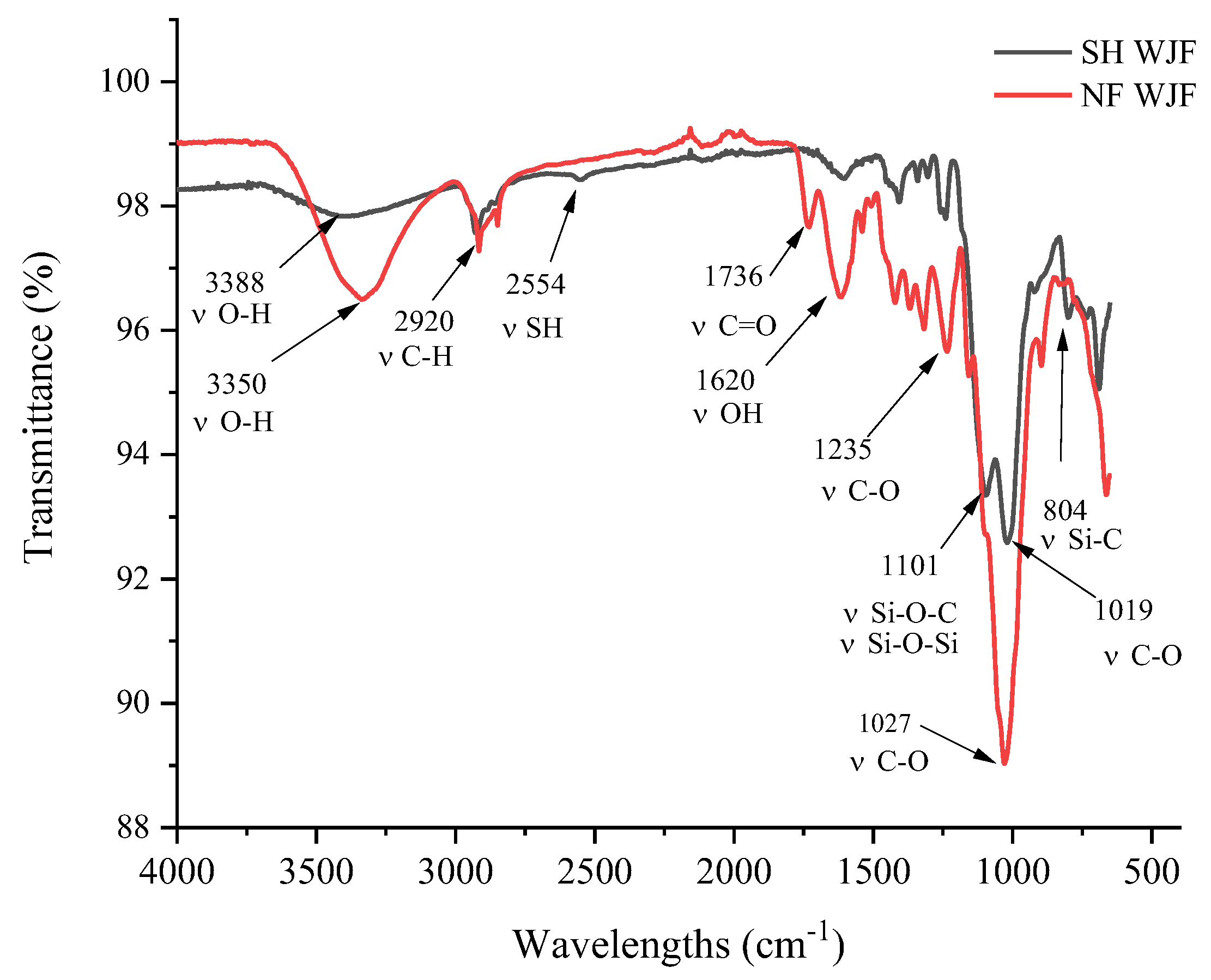
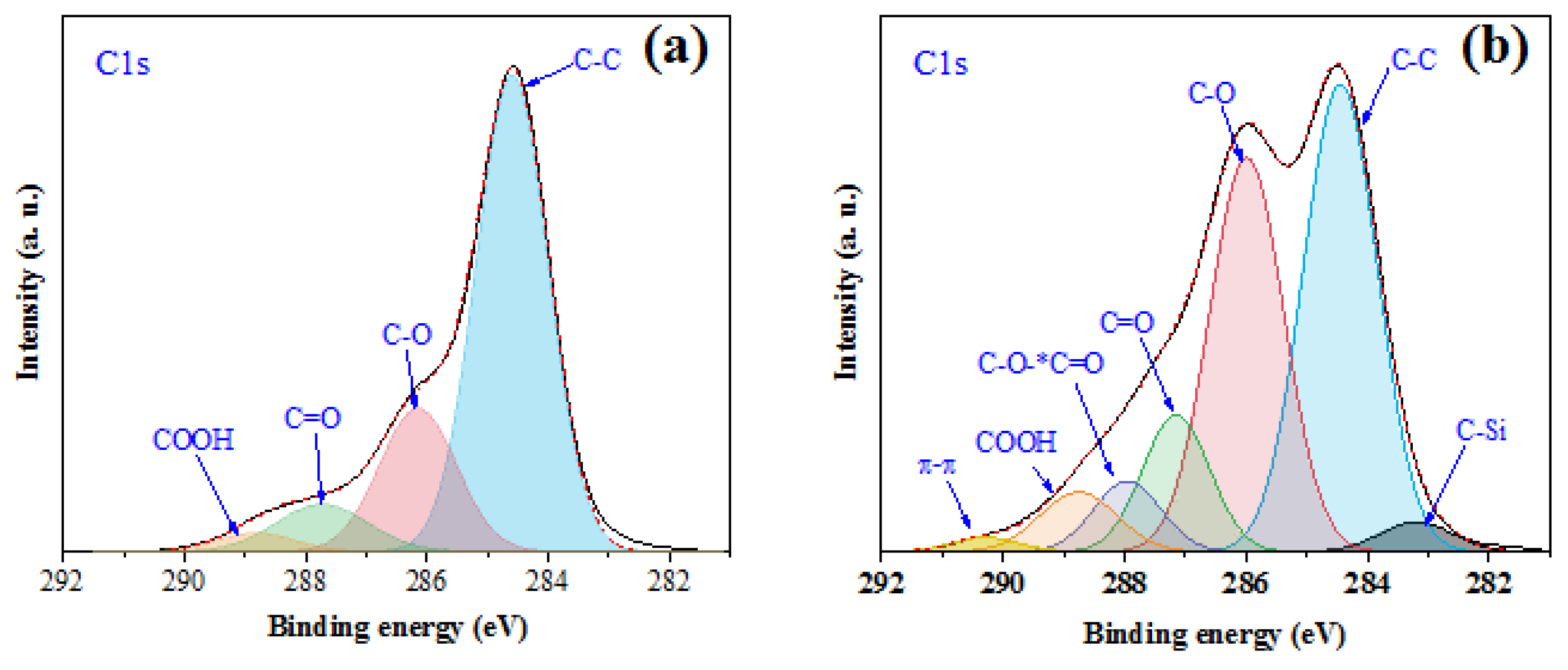
3.1. Characterization of Thiol Functionalized WJF
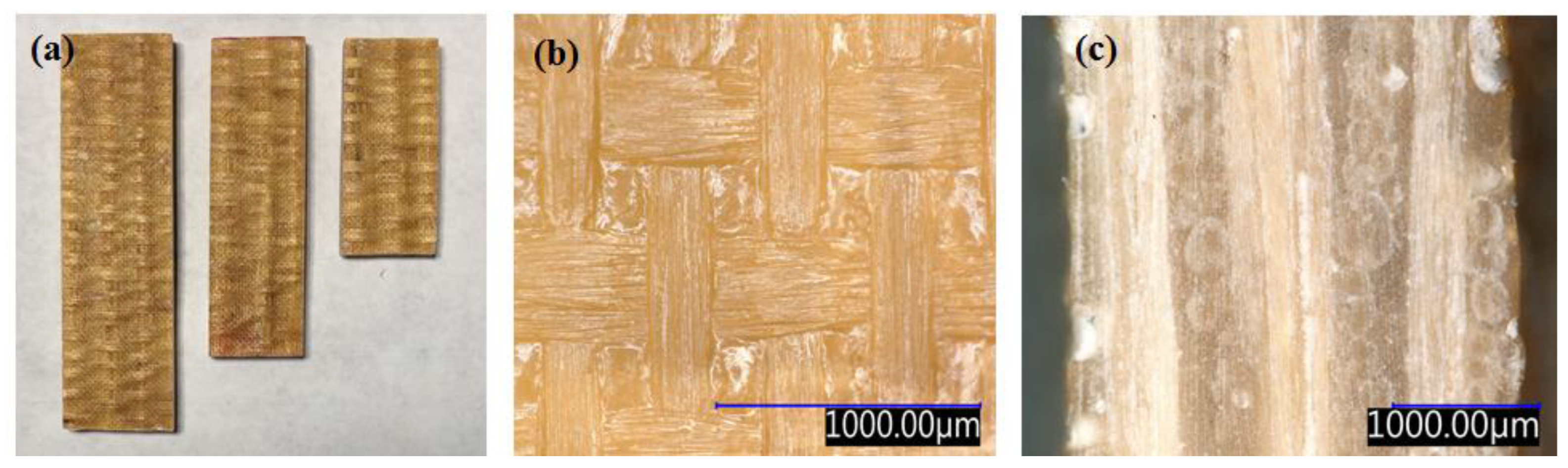
3.2. Preparation of the Photocured Composites
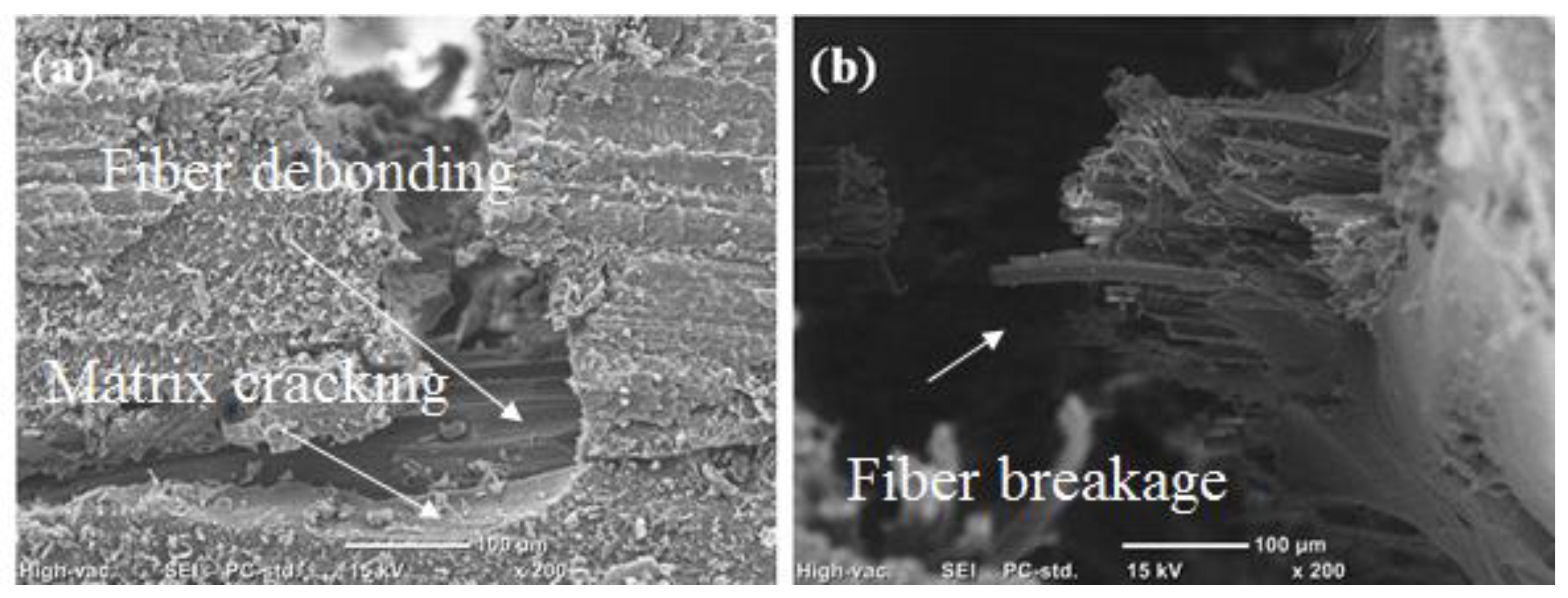
3.3. Mechanical Properties of the WJF/Epoxy Composites
DMA Analysis
3.4. Thermal Stability of the Bio-Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, F.M.; Shah, A.H.; Wang, S.; Mehmood, S.; Wang, J.; Liu, W.; Xu, X. A comprehensive review on epoxy biocomposites base on natural fibers and biofillers, recent developments and applications. Adv. Fibers Mater. 2022, 4, 683–704. [Google Scholar] [CrossRef]
- Gholampour, A.; Ozzbakaloglu, T. A review of natural fiber composites; properties, modification and processing techniques, characterization and applications. J. Mat. Sci. 2020, 55, 829–892. [Google Scholar] [CrossRef]
- Hasan, M.; Saifullah, A.; Dhakal, N.H.; Khandaker, S.; Sarker, F. Improved mechanical performances of unidirectional jute fibre composites developed with new fibre architectures. RSC Adv. 2021, 11, 23010–23022. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M. A review on natural fiber reinforced polymer composite and its applications. Int. J. Polym Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Kalali, E.N.; Hu, Y.; Wang, X.; Song, L.; Xing, W. Highly aligned cellulose fibers reinforced epoxy composites derived from bulk natural bamboo. Ind. Crops. Prod. 2019, 129, 434–439. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Wu, H.Y.; Qiu, Y.P. Morphology and properties of hybrid composites based on polypropylene/polylactic acid blend and bamboo fiber. Bioresour. Technol. Rep. 2010, 101, 7944–7950. [Google Scholar]
- Musa, C.; Kervoelen, A.; Danjou, P.E.; Bourmaud, A.; Delattre, F. Bio-based unidirectional composite made of flax fibre and isosorbide-based epoxy resin. Mater. Lett. 2020, 258, 126818. [Google Scholar] [CrossRef]
- Todorovic, A.; Bloessl, Y.; Oreski, G.; Resch-Fauster, K. High Performance composite with 100% bio-based carbon content produced from epoxidized linseed oil, citric acid and flax fiber reinforcement. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106666. [Google Scholar] [CrossRef]
- Li, Y.; Mai, Y.W.; Ye, L. Sisal Fibre and its composites: A review of recent developments. Compos. Sci Technol. 2000, 60, 2037–2060. [Google Scholar] [CrossRef]
- Maurya, H.O.; Gupta, M.K.; Srivastava, R.K.; Singh, H. Study on the mechanical properties of epoxy composites using short sisal fibre. Mater. Today Proc. 2015, 2, 1347–1355. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Capela, C.; Manaia, J.; Costa, J.D. Mechanical properties of Woven Mat Jute/Epoxy Composites. Mat. Res. 2016, 19, 702–710. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A. Preparation and mechanical properties of jute fiber reinforced epoxy composites. Ind. Eng. Manage. 2017, 6, 4. [Google Scholar] [CrossRef]
- Wang, D.; Bai, T.; Cheng, W.; Xu, C.; Wang, G.; Cheng, H.; Han, G. Surface modification of bamboo fibers to enhance the interfacial adhesion of epoxy resin-based composites prepared by resin transfer molding. Polymers 2019, 11, 2107. [Google Scholar] [CrossRef]
- Glóriaa, G.O.; Margema, F.M.; Gomes Dias Ribeiroa, C.; Macabu de Moraesa, Y.; Batista da Cruzb, R.; de Andrade Silvac, F.; Neves Monteiro, S. Charpy Impact Tests of Epoxy Composites Reinforced with Giant Bamboo Fibers. Mater. Res. 2015, 18, 178–184. [Google Scholar] [CrossRef]
- Prasad, L.; Kumar, S.; Patel, R.V.; Yadav, A.; Kumar, V.; Winczek, J. Physical and Mechanical Behaviour of Sugarcane Bagasse Fibre-Reinforced Epoxy Bio-Composites. Materials 2020, 13, 5387. [Google Scholar] [CrossRef]
- Anandha Moorthy, A.; Girimurugan, R.; Prakash, E.; Madheswaran, S.; Naveen Sankar, A.; Sriman, P. Investigations on flexural performance of epoxy Matrix composites strengthened with chemically modified and unmodified banana fiber/Used Camellia Sinensis (UCS) particles. Mater. Today Proc. 2021, 45, 8115–8119. [Google Scholar] [CrossRef]
- Venkateshwaran, N.; Elaya Perumal, E.; Arunsundaranayagam, D. Fiber Surface treatment and its effect on mechanical and visco-elastic behavior of banana composite. Mat. Desig. 2013, 47, 151–159. [Google Scholar] [CrossRef]
- Andre, N.G.; Ariawan, D.; Mohd Ishak, Z.A. Mechanical properties and micromechanical analysis of nonwoven kenaf fibre/epoxy composites produced by resin transfer moulding. J. Compos. Mat. 2017, 51, 1875–1885. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Hassan, A.; Alothman, O.Y.; Paridah, M.T. Recent Advances in epoxy resins, natural fiber-reinforced epoxy composites and their applicactions. J. Reinf. Plast. Compos. 2016, 35, 447–470. [Google Scholar] [CrossRef]
- Babu, T.N.; Shyam, S.; Kaul, S.; Prabha, D.R. Natural Fiber composites an alternative to plastics in the automotive industry: A review. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2022, 236, 237–266. [Google Scholar] [CrossRef]
- MordorIntelligence. Composite Material Market—Growth, Trends, COVID-19 Impact, and Forecasts (2022–2027). Available online: https://www.mordorintelligence.com/industry-reports/composite-material-market (accessed on 14 November 2022).
- CompositesWorld. Natural Fiber Composites: What’s Holding Them Back? Available online: https://www.compositesworld.com/articles/natural-fiber-composites-whats-holding-them-back (accessed on 14 November 2022).
- Ahmad, F.; Choi, H.S.; Park, M.K. A review: Natural fiber composites selection in view of mechanical, light weight and economic properties. Macromol. Mater. Eng. 2015, 300, 10–24. [Google Scholar] [CrossRef]
- Kumar, B.; Roy, S.; Agumba, D.O.; Pham, D.H.; Kim, J. Effect of bio-based derived epoxy resin on interfacial adhesion of cellulose film and applicability towards natural jute fiber-reinforced composites. Int. J. Biol. Mol. 2022, 222, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Torres-Arellano, M.; Renteria-Rodriguez, V.; Franco-Urquiza, E. Mechanical Properties of Natural-Fiber-Reinforced Biobased Epoxy Resins Manufactured by Resin Infusion Process. Polymers 2020, 12, 2841. [Google Scholar] [CrossRef] [PubMed]
- Fouassier, J.P. Photoinitiation, Photopolymerization and Photocuring: Fundamentals and Applications; HANSER: Cincinnati, OH, USA, 1995. [Google Scholar]
- Peiffer, R.W. Applications of Photopolymer Technology. In Photopolymerization: Fundamentals and Applications; Scranton, A.B., Bowman, C.N., Peiffer, R.W., Eds.; ACS Symp Series 673; American Chemical Society: New Orleans, LA, USA, 1996. [Google Scholar]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaiford, S.; Goyanes, A.; Basit, A.W.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Pagac, M.; Hajnys, J.; Ma, Q.P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J.W. A Review of Vat Photopolymerization Technology: Materials, Applications, Challenges, and Future Trends of 3D Printing. Polymers 2021, 13, 958. [Google Scholar] [CrossRef]
- Baroli, B. Photopolymerization of biomaterials: Issues and potentialities in drug delivery, tissue engineering, and cell encapsulation applications. J. Chem. Technol. Biotechnol. 2006, 81, 491–499. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Miller, K.A.; Zhu, H.; Egap, E. Lead Halide Perovskite Nanocrystals as Photocatalysts for PET-RAFT Polymerization under Visible and Near-Infrared Irradiation. ACS Macro Lett. 2020, 9, 725–730. [Google Scholar] [CrossRef]
- Zhu, Y.; Egap, E. PET-RAFT polymerization catalyzed by cadmium selenide quantum dots (QDs): Grafting-from QDs photocatalyst to make polymer nanocomposites. Polym. Chem. 2020, 11, 1018–1024. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Garcia Valdez, A.E.; Navarro Tovar, A.G.; Hilario de la Cruz, A.A.; Gonzalez Sanchez, L.F.; Trejo Garcia, J.H.; Espinoza Muñoz, J.F.; Sangermano, M. Development of an hybrid epoxy-amine/thiol-ene photocurable system. J. Polym. Res. 2014, 132, 504. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Acosta Ortiz, R.; Garcia Valdez, A.E.; Navarro, A.G.; Berlanga Duarte, M.L. Interpenetrated hybrid thiol-ene/epoxy UV-cured network with enhanced impact resistance. Prog. Org. Coat. 2015, 78, 244–248. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Garcıa Valdez, A.E.; Sangermano, M.; Hilario de la Cruz, A.A.; Aguirre Flores, R.; Espinoza Munoz, J.F. Comparison of the Performance of Two Bifunctional Curing Agents for the Photopolymerization of Epoxy Resins and the Study of the Mechanical Properties of the Obtained Polymers. Macromol. Symp. 2015, 358, 35–40. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Garcıa Valdez, A.E.; Garcia Padilla, E.E.; Aguirre Flores, R.; Espinoza Munoz, J.F. Development of a photocurable glass-fiber reinforced epoxy-amine/thiol-ene composite. J. Polym. Res. 2016, 23, 30. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Ku Herrera, J.J.; García Santos, A.O.; García Valdez, A.E.; Soria Arguello, G. Tensile Strength and Fracture Mode I Toughness of Photocurable Carbon Fiber/Polyether–polythioether Composites. J. Polym. Res. 2021, 28, 46. [Google Scholar] [CrossRef]
- Beaumont, M.; Bacher, M.; Opietnik, M.; Gindl-Altmutter, W.; Potthast, A.; Rosenau, T. A general aqueous silanization protocol to introduce vinyl, mercapto or azido functionalities onto cellulose fibers and nanocelluloses. Molecules 2018, 23, 1427. [Google Scholar] [CrossRef]
- Prasad, L.; Saini, A.; Kumar, V. Mechanical Performance of Jute and Basalt Fiber Geo-grid-Reinforced Epoxy Hibrid Composite Material. J. Nat. Fibers 2021, 18, 694–704. [Google Scholar] [CrossRef]
- Oladele, I.O.; Ogunwande, G.S.; Taiwo, A.S.; Lephuthing, S.S. Development and characterization of Moringa Oleifera fuit waste pod derived particulate cellulosic reinforced epoxy bio-composites for structural applications. Heliyon 2022, 8, e09755. [Google Scholar] [CrossRef]
- Acosta Ortiz, R.; Sánchez Huerta, R.S.; Ledezma Pérez, A.S.; García Valdez, A.E. Synthesis of a Curing Agent Derived from Limonene and the Study of its Performance to Polymerize a Biobased Epoxy Resin using the Epoxy/Thiol-Ene Photopolymerization Technique. Polymers 2022, 14, 2192. [Google Scholar] [CrossRef]





| Sample SH WJF 30 | Equivalents | mmol | grams |
|---|---|---|---|
| Greenpoxy 28 | 1 | 250.0 | 86.0 |
| ALA4 | 0.3 | 76.0 | 37.0 |
| PTKMP | 0.3 | 76.0 | 20.9 |
| DMPA | 0.002 | 1.5 | 0.38 |
| Sample SH WJF 40 | Equivalents | mmol | grams |
| Greenpoxy 28 | 1 | 220.0 | 75.0 |
| ALA4 | 0.4 | 88.0 | 43.1 |
| PTKMP | 0.4 | 88.0 | 24.3 |
| DMPA | 0.002 | 2.0 | 0.45 |
| Element | NF WJF | SH WJF |
|---|---|---|
| Mass% | Mass% | |
| C | 58.86 | 51.64 |
| O | 12.76 | 12.49 |
| Al | 0.67 | 1.78 |
| Si | 0.53 | 2.39 |
| S | ------- | 3.20 |
| Ca | 2.39 | 1.12 |
| Pd | 10.44 | 11.08 |
| Au | 14.35 | 16.3 |
| Total | 100% | 100% |
| Sample | Storage Modulus (MPa) | Tg (°C) | Flexural Strength (σmax) MPa | Flexural Modulus (Ef) GPa |
|---|---|---|---|---|
| NF WJF30 | 3399 | 76 | 37.7 | 1.21 |
| NF WJF 40 | 2796 | 70 | 39.4 | 1.04 |
| SH WJF30 | 3356 | 81 | 57.2 | 1.70 |
| SH WJF40 | 2736 | 77 | 42.1 | 1.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta Ortiz, R.; Yañez Macías, R.; Ku Herrera, J.d.J.; García Valdez, A.E. Fabrication of Woven Jute Fiber Epoxy Bio-Composites through the Epoxy/Thiol-Ene Photopolymerization Technique. Polymers 2023, 15, 60. https://doi.org/10.3390/polym15010060
Acosta Ortiz R, Yañez Macías R, Ku Herrera JdJ, García Valdez AE. Fabrication of Woven Jute Fiber Epoxy Bio-Composites through the Epoxy/Thiol-Ene Photopolymerization Technique. Polymers. 2023; 15(1):60. https://doi.org/10.3390/polym15010060
Chicago/Turabian StyleAcosta Ortiz, Ricardo, Roberto Yañez Macías, José de Jesús Ku Herrera, and Aida Esmeralda García Valdez. 2023. "Fabrication of Woven Jute Fiber Epoxy Bio-Composites through the Epoxy/Thiol-Ene Photopolymerization Technique" Polymers 15, no. 1: 60. https://doi.org/10.3390/polym15010060
APA StyleAcosta Ortiz, R., Yañez Macías, R., Ku Herrera, J. d. J., & García Valdez, A. E. (2023). Fabrication of Woven Jute Fiber Epoxy Bio-Composites through the Epoxy/Thiol-Ene Photopolymerization Technique. Polymers, 15(1), 60. https://doi.org/10.3390/polym15010060







