Prospects on Tuning Bioactive and Antimicrobial Denture Base Resin Materials: A Narrative Review
Abstract
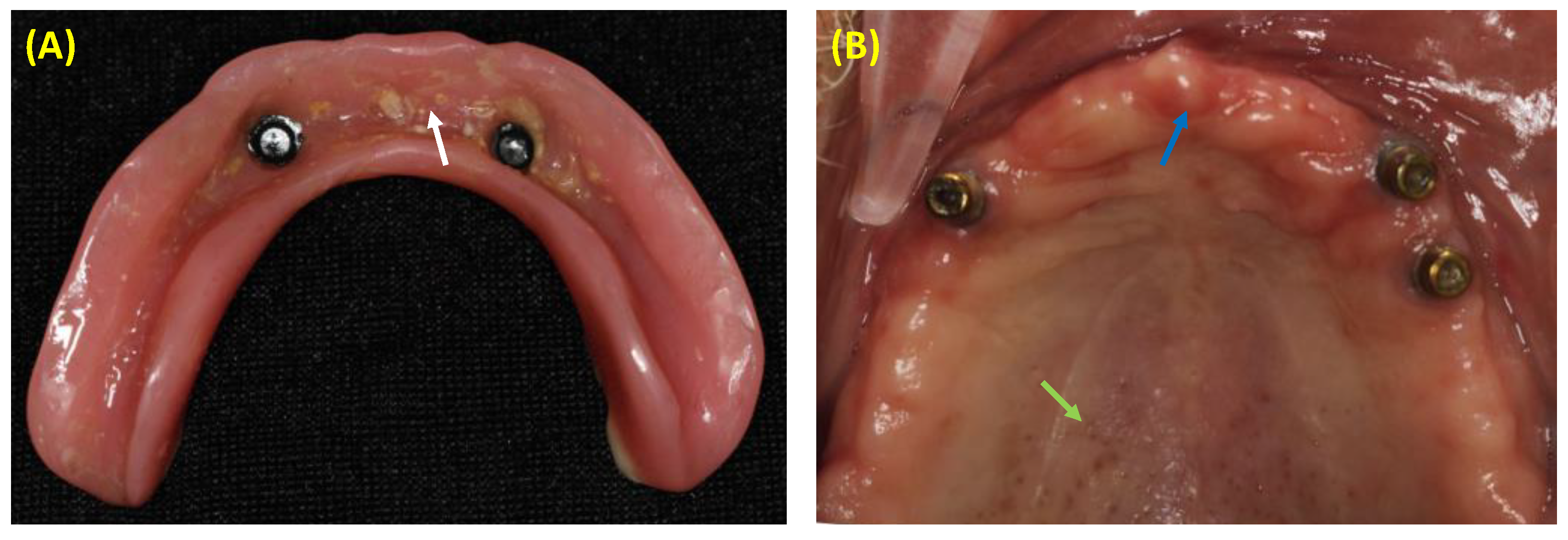
1. Introduction
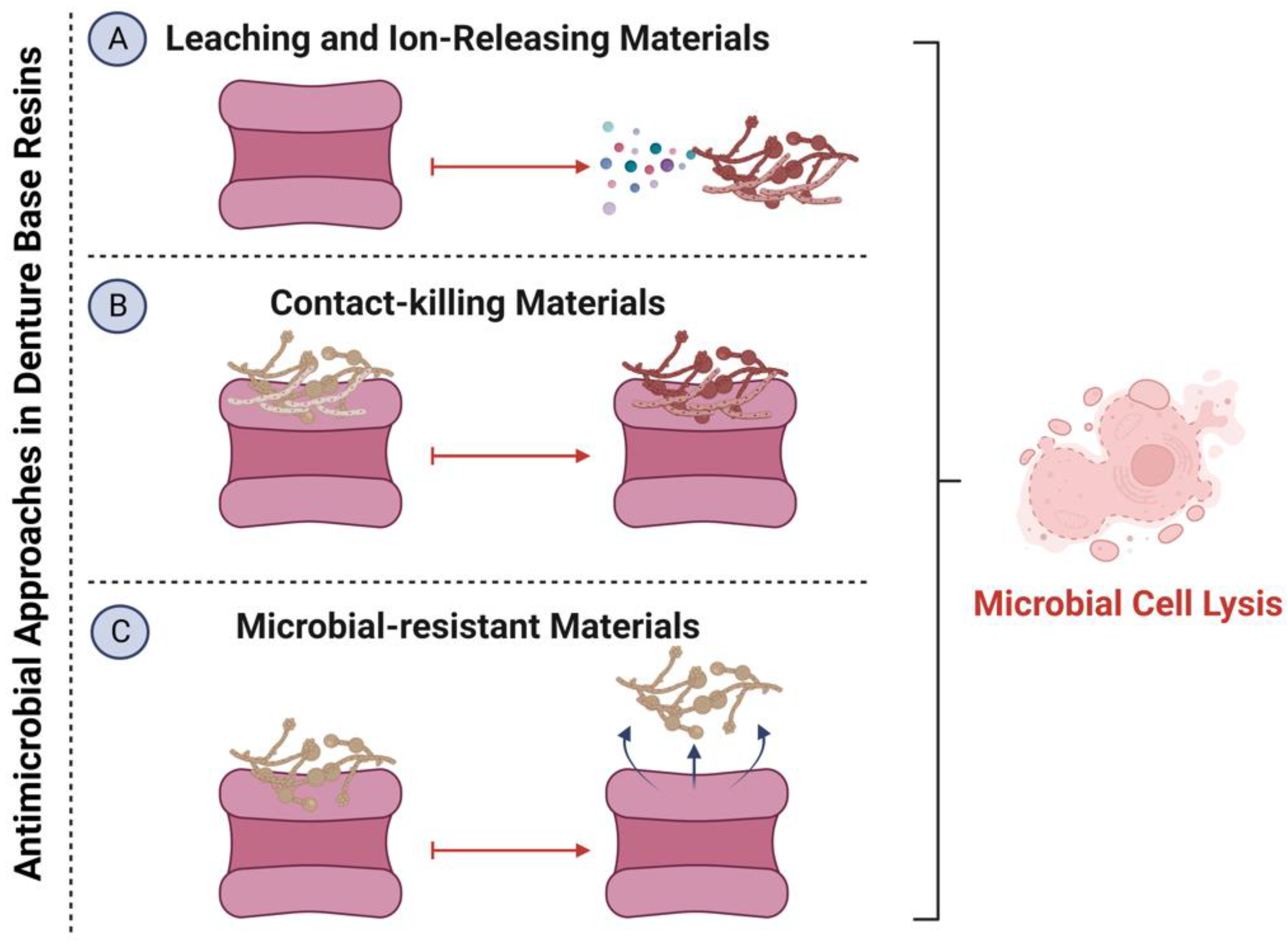
2. Methods Used for Tuning Bioactive and Antimicrobial DBR Materials
2.1. The Incorporation of Organic Compounds into DBRs
2.1.1. Henna
2.1.2. Phytoncide Microcapsules
2.1.3. Neem
2.1.4. 1,4-Diazabicyclo[2.2.2]octane (DABCO) Derivatives
2.2. The Incorporation of Inorganic Particles into DBRs
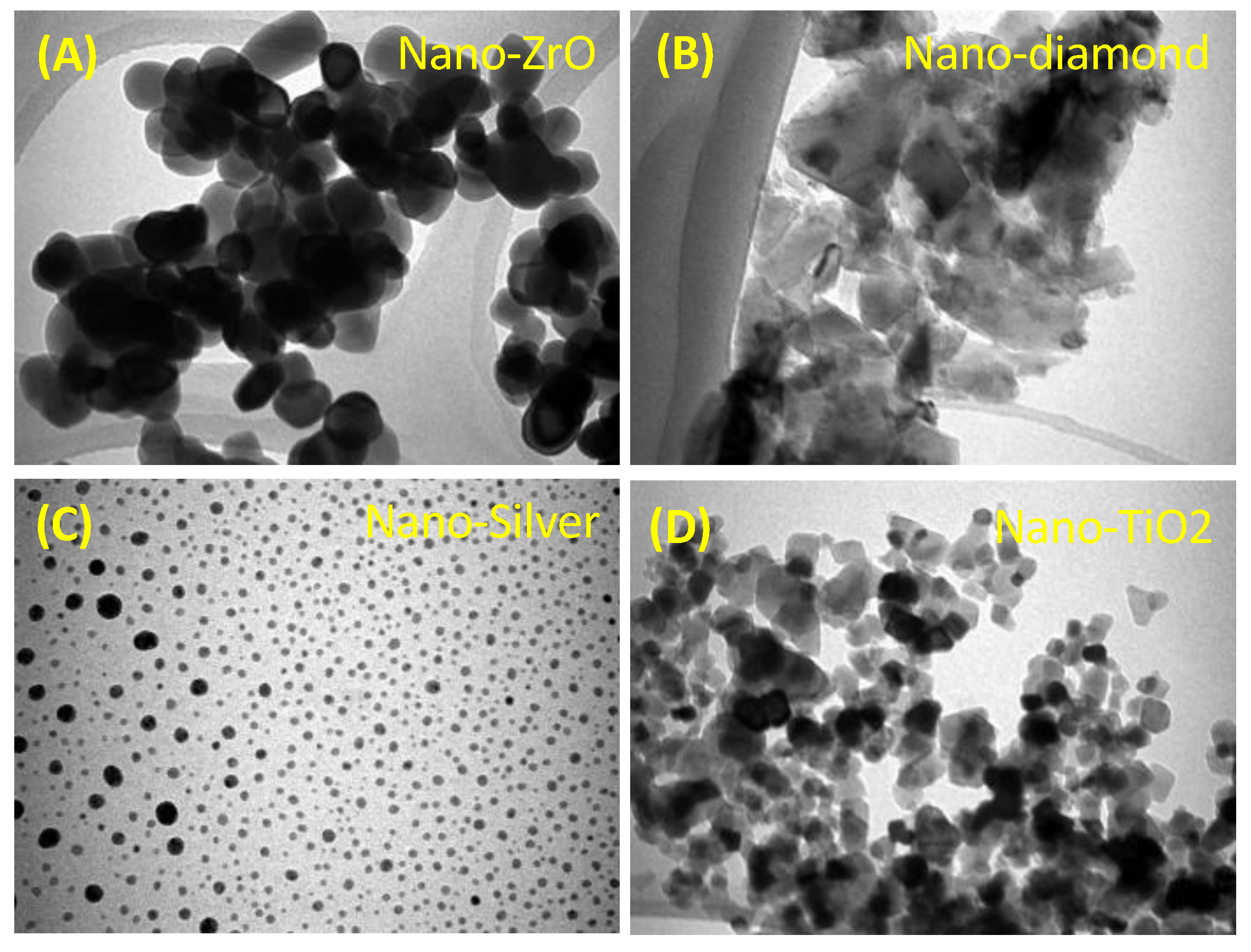
2.2.1. Silver and Silver Zeolites
2.2.2. Pre-Reacted Glass Ionomer Fillers
2.2.3. Zinc Oxide (ZnO)
2.2.4. Zirconium Dioxide (ZrO2)
2.2.5. Silicon Dioxide Nanoparticles (SiO2NPs)
2.2.6. Titanium Dioxide Nanoparticles (TiO2NPs)
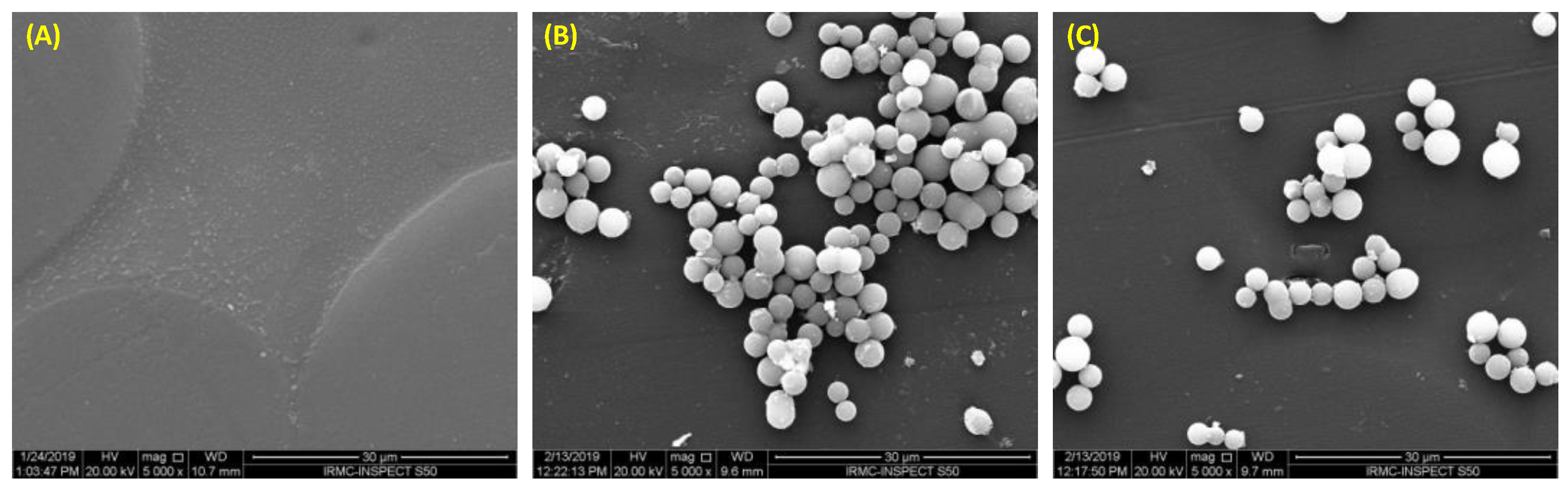
2.2.7. Nanodiamonds (NDs)
2.3. The Incorporation of Polymeric, Quaternary Ammonium Compounds, and Protein-Repelling Agents into DBRs
2.4. Antifungal Medicaments
3. Future Perspectives and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglass, C.W.; Shih, A.; Ostry, L. Will There Be a Need for Complete Dentures in the United States in 2020? J. Prosthet. Dent. 2002, 87, 5–8. [Google Scholar] [CrossRef]
- Polzer, I.; Schimmel, M.; Müller, F.; Biffar, R. Edentulism as Part of the General Health Problems of Elderly Adults. Int. Dent. J. 2010, 60, 143–155. [Google Scholar]
- Kołciuk, L.; Godlewski, T. Oral Health-Related Quality of Life of Patients Using Removable Dentures–Review of Literature. Dent. Med. Probl. 2015, 52, 222–226. [Google Scholar]
- Tandon, R.; Gupta, S.; Agarwal, S.K. Denture Base Materials: From Past to Future. Indian J. Dent. Sci. 2010, 8, 33–39. [Google Scholar]
- Neher, H.T. Acrylic Resins. Ind. Eng. Chem. 1936, 28, 267–271. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials-e-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials; Elsevier Saunders: St. Louis, MO, USA, 2003; Volume 596, pp. 41–43. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine. San Diego Calif. 2004, 31, 162–164. [Google Scholar]
- Palitsch, A.; Hannig, M.; Ferger, P.; Balkenhol, M. Bonding of Acrylic Denture Teeth to MMA/PMMA and Light-Curing Denture Base Materials: The Role of Conditioning Liquids. J. Dent. 2012, 40, 210–221. [Google Scholar] [CrossRef]
- Reichart, P.A. Oral Mucosal Lesions in a Representative Cross-Sectional Study of Aging Germans. Community Dent. Oral Epidemiol. 2000, 28, 390–398. [Google Scholar] [CrossRef]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; López-Ribot, J.L.; Redding, S.W. Denture Stomatitis: A Role for Candida Biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Pachava, K.R.; Nadendla, L.K.; Alluri, L.S.C.; Tahseen, H.; Sajja, N.P. In vitro Antifungal Evaluation of Denture Soft Liner Incorporated with Tea Tree Oil: A New Therapeutic Approach towards Denture Stomatitis. J. Clin. Diagn. Res. 2015, 9, ZC62. [Google Scholar]
- Bajunaid, S.O.; Baras, B.H.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K. Antibiofilm and Protein-Repellent Polymethylmethacrylate Denture Base Acrylic Resin for Treatment of Denture Stomatitis. Materials 2021, 14, 1067. [Google Scholar] [CrossRef]
- Nyquist, G. Denture Sore Mouth. Acta Odontol. Scand. 1952, 10, 1–15. [Google Scholar]
- Budtz-Jörgensen, E.; Bertram, U. Denture Stomatitis I. The Etiology in Relation to Trauma and Infection. Acta Odontol. Scand. 1970, 28, 71–92. [Google Scholar] [CrossRef]
- Vigild, M. Oral Mucosal Lesions among Institutionalized Elderly in Denmark. Community Dent. Oral Epidemiol. 1987, 15, 309–313. [Google Scholar] [CrossRef]
- Jeganathan, S.; Lin, C.C. Denture Stomatitis—A Review of the Aetiology, Diagnosis and Management. Aust. Dent. J. 1992, 37, 107–114. [Google Scholar] [CrossRef]
- Smith, D.; Bains, M. The Detection and Estimation of Residual Monomer in Polymethyl Methacrylate. J. Dent. Res. 1956, 35, 16–24. [Google Scholar] [CrossRef]
- Austin, A.; Basker, R.M. The Level of Residual Monomer in Acrylic Denture Base Materials. Br. Dent. J. 1980, 149, 281–286. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and Etiology of Denture Stomatitis. J. Prosthodont. Implant Esthet. Reconstr. Dent. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Bajunaid, S.O. How Effective Are Antimicrobial Agents on Preventing the Adhesion of Candida albicans to Denture Base Acrylic Resin Materials? A Systematic Review. Polymers 2022, 14, 908. [Google Scholar] [CrossRef]
- Jainkittivong, A.; Aneksuk, V.; Langlais, R. Oral Mucosal Conditions in Elderly Dental Patients. Oral Dis. 2002, 8, 218–223. [Google Scholar] [CrossRef]
- Pires, F.; Santos, E.; Bonan, P.; De Almeida, O.; Lopes, M. Denture Stomatitis and Salivary Candida in Brazilian Edentulous Patients. J. Oral Rehabil. 2002, 29, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Collis, J.; Stafford, G. A Survey of Denture Hygiene in Patients Attending Cardiff Dental Hospital. Eur. J. Prosthodont. Restor. Dent. 1994, 3, 67–71. [Google Scholar] [PubMed]
- Barbeau, J.; Séguin, J.; Goulet, J.P.; de Koninck, L.; Avon, S.L.; Lalonde, B.; Rompré, P.; Deslauriers, N. Reassessing the Presence of Candida albicans in Denture-Related Stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 95, 51–59. [Google Scholar] [CrossRef]
- MacEntee, M.I.; Glick, N.; Stolar, E. Age, Gender, Dentures and Oral Mucosal Disorders. Oral Dis. 1998, 4, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Aly, F.; Blackwell, C.; MacKenzie, D.; Weir, D. Identification of Oral Yeast Species Isolated from Individuals with Diabetes Mellitus: Identifizierung Oraler Hefeisolate von Diabetes Mellitus-Patienten. Mycoses 1995, 38, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Rossie, K.; Guggenheimer, J. Oral Candidiasis: Clinical Manifestations, Diagnosis, and Treatment. Pract. Periodontics Aesthetic Dent. 1997, 9, 635–641. [Google Scholar]
- Montes, L.F.; Krumdieck, C.; Cornwell, P.E. Hypovitaminosis A in Patients with Mucocutaneous Candidiasis. J. Infect. Dis. 1973, 128, 227–230. [Google Scholar] [CrossRef]
- Sakki, T.K.; Knuuttila, M.L.; Läärä, E.; Anttila, S.S. The Association of Yeasts and Denture Stomatitis with Behavioral and Biologic Factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1997, 84, 624–629. [Google Scholar] [CrossRef]
- Wilson, J. The Aetiology, Diagnosis and Management of Denture Stomatitis. Br. Dent. J. 1998, 185, 380–384. [Google Scholar] [CrossRef]
- Newton, A. Denture Sore Mouth. Br. Dent. J. 1962, 112, 357–360. [Google Scholar]
- An, S.; Evans, J.L.; Hamlet, S.; Love, R.M. Incorporation of Antimicrobial Agents in Denture Base Resin: A Systematic Review. J. Prosthet. Dent. 2021, 126, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, S.; Dorocka-Bobkowska, B.; Prylinski, M.; Konopka, K.; Duzgunes, N. Miconazole Activity against Candida Biofilms Developed on Acrylic Discs. J. Physiol. Pharmacol. 2014, 65, 593–600. [Google Scholar] [PubMed]
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial Activity, Cytotoxicity and Mechanical Behavior of Nano-Enhanced Denture Base Resin with Different Kinds of Inorganic Antibacterial Agents. Dent. Mater. J. 2017, 36, 693–699. [Google Scholar] [CrossRef]
- Zhang, K.; Ren, B.; Zhou, X.; Xu, H.H.K.; Chen, Y.; Han, Q.; Li, B.; Weir, M.D.; Li, M.; Feng, M.; et al. Effect of Antimicrobial Denture Base Resin on Multi-Species Biofilm Formation. Int. J. Mol. Sci. 2016, 17, 1033. [Google Scholar] [CrossRef] [PubMed]
- Zamperini, C.A.; Machado, A.L.; Vergani, C.E.; Pavarina, A.C.; Giampaolo, E.T.; da Cruz, N.C. Adherence in Vitro of Candida albicans to Plasma Treated Acrylic Resin. Effect of Plasma Parameters, Surface Roughness and Salivary Pellicle. Arch. Oral Biol. 2010, 55, 763–770. [Google Scholar] [CrossRef]
- Park, S.E.; Periathamby, A.R.; Loza, J.C. Effect of Surface-Charged Poly (Methyl Methacrylate) on the Adhesion of Candida albicans 1. J. Prosthodont. 2003, 12, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Abualsaud, R. Behavior of PMMA Denture Base Materials Containing Titanium Dioxide Nanoparticles: A Literature Review. Int. J. Biomater. 2019, 2019, 6190610. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaijan, Y.A.; Alsulaimi, L.; Alotaibi, R.; Alboainain, A.; Alalawi, H.; Alshehri, S.; Khan, S.Q.; Alsaloum, M.; AlRumaih, H.S.; Alhumaidan, A.A.; et al. Comparative Evaluation of Surface Roughness and Hardness of 3D Printed Resins. Materials 2022, 15, 6822. [Google Scholar] [CrossRef]
- Murakami, N.; Wakabayashi, N.; Matsushima, R.; Kishida, A.; Igarashi, Y. Effect of High-Pressure Polymerization on Mechanical Properties of PMMA Denture Base Resin. J. Mech. Behav. Biomed. Mater. 2013, 20, 98–104. [Google Scholar] [CrossRef]
- Pereira-Cenci, T.; Del Bel Cury, A.A.; Crielaard, W.; Ten Cate, J.M. Development of Candida-Associated Denture Stomatitis: New Insights. J. Appl. Oral Sci. 2008, 16, 86–94. [Google Scholar] [CrossRef]
- Von Fraunhofer, J.; Loewy, Z. Factors Involved in Microbial Colonization of Oral Prostheses. Gen. Dent. 2009, 57, 136–143. [Google Scholar] [PubMed]
- Gad, M.M.; Abualsaud, R.; Khan, S.Q. Hydrophobicity of Denture Base Resins: A Systematic Review and Meta-Analysis. J. Int. Soc. Prev. Community Dent. 2022, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Habimana, O.; Flood, P.; Reynaud, E.G.; Rodriguez, B.J.; Zhang, N.; Casey, E.; Gilchrist, M.D. Material-and Feature-Dependent Effects on Cell Adhesion to Micro Injection Moulded Medical Polymers. Colloids Surf. B Biointerfaces 2016, 145, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ali Sabri, B.; Satgunam, M.; Abreeza, N.; Abed, A.N. A Review on Enhancements of PMMA Denture Base Material with Different Nano-Fillers. Cogent. Eng. 2021, 8, 1875968. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Huang, X.; Chen, B.; Ding, Y.; Zhang, Y.; Yu, D.; Kim, I. Smart L-Borneol-Loaded Hierarchical Hollow Polymer Nanospheres with Antipollution and Antibacterial Capabilities. Mater. Today Chem. 2022, 26, 101252. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly (α-L-Glutamic Acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Mokeem, L.; Balhaddad, A.A.; Garcia, I.M.; Collares, F.M.; Melo, M.A.S. Chapter 18—Nanoparticle-Based Antimicrobial for Dental Restorative Materials. In Emerging Nanomaterials and Nano-Based Drug Delivery Approaches to Combat Antimicrobial Resistance; Saravanan, M., Barabadi, H., Mostafavi, E., Webster, T., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 661–700. [Google Scholar]
- Balhaddad, A.A.; Garcia, I.M.; Mokeem, L.; Alsahafi, R.; Collares, F.M.; Sampaio de Melo, M.A. Metal Oxide Nanoparticles and Nanotubes: Ultrasmall Nanostructures to Engineer Antibacterial and Improved Dental Adhesives and Composites. Bioengineering 2021, 8, 146. [Google Scholar] [CrossRef]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Pagano, S.; Lombardo, G.; Marinucci, L.; Kenny, J.M.; Torre, L.; Cianetti, S. Effect of Nanohydroxyapatite, Antibiotic, and Mucosal Defensive Agent on the Mechanical and Thermal Properties of Glass Ionomer Cements for Special Needs Patients. J. Mater. Res. 2018, 33, 638–649. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Weir, M.D.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Zhang, K.; Xu, H.H. Protein-Repellent Nanocomposite with Rechargeable Calcium and Phosphate for Long-Term Ion Release. Dent. Mater. 2018, 34, 1735–1747. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Cheng, L.; Weir, M.D.; Melo, M.A.S.; Liu, H.; Oates, T.W.; Wang, L.; Xu, H.H. Novel Rechargeable Calcium Phosphate Nanocomposite with Antibacterial Activity to Suppress Biofilm Acids and Dental Caries. J. Dent. 2018, 72, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.; Salloot, Z.; Alshaia, A.; Ibrahim, M.S. The Effect of Bioactive Glass-Enhanced Orthodontic Bonding Resins on Prevention of Demineralization: A Systematic Review. Molecules 2020, 25, 2495. [Google Scholar] [CrossRef] [PubMed]
- Al-Qarni, F.; Weir, M.; Melo, M.A.; Al-Dulaijan, Y.; Almulhim, K.S.; Xu, H.H. Novel Calcium Phosphate Ion-Rechargeable and Antibacterial Adhesive to Inhibit Dental Caries. Clin. Oral Investig. 2022, 26, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Carli, E.; Pasini, M.; Lardani, L.; Giuca, G.; Miceli, M. Impact of Self-Ligating Orthodontic Brackets on Dental Biofilm and Periodontal Pathogens in Adolescents. J. Biol. Regul. Homeost. Agents 2021, 35, 107–115. [Google Scholar] [PubMed]
- AlSahafi, R.; Balhaddad, A.A.; Mitwalli, H.; Ibrahim, M.S.; Melo, M.A.S.; Oates, T.W.; Xu, H.H.K.; Weir, M.D. Novel Crown Cement Containing Antibacterial Monomer and Calcium Phosphate Nanoparticles. Nanomaterials 2020, 10, 2001. [Google Scholar] [CrossRef]
- AlShahrani, S.S.; AlAbbas, M.S.; Garcia, I.M.; AlGhannam, M.I.; AlRuwaili, M.A.; Collares, F.M.; Ibrahim, M.S. The Antibacterial Effects of Resin-Based Dental Sealants: A Systematic Review of In Vitro Studies. Materials 2021, 14, 413. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Abd Rahman, N.A.; Ming, L.C.; Dhaliwal, S.K.S.; Knights, J.; Albuquerque Junior, R.F. Microbial Biofilm Decontamination on Dental Implant Surfaces: A Mini Review. Front. Cell. Infect. Microbiol. 2021, 11, 736186. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Thumbigere-Math, V.; Tay, F.R.; Fouad, A.F.; Oates, T.W.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Novel Bioactive and Therapeutic Root Canal Sealers with Antibacterial and Remineralization Properties. Materials 2020, 13, 1096. [Google Scholar] [CrossRef]
- Nawasrah, A.; AlNimr, A.; Ali, A.A. Antifungal Effect of Henna against Candida albicans Adhered to Acrylic Resin as a Possible Method for Prevention of Denture Stomatitis. Int. J. Environ. Res. Public Health 2016, 13, E520. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Sunni, M.; Al-Shayeb, A.; Al-Namsy, R.; Al-Naser, Z.; Khan, S.Q. The In-Vitro Effects of White Henna Addition on the Candida albicans Adhesion and Physical Properties of Denture Base Resin. Eur. Oral Res. 2021, 55, 86–93. [Google Scholar] [CrossRef]
- Lee, M.-J.; Kim, M.-J.; Oh, S.-H.; Kwon, J.-S. Novel Dental Poly (Methyl Methacrylate) Containing Phytoncide for Antifungal Effect and Inhibition of Oral Multispecies Biofilm. Materials 2020, 13, 371. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Jo, Y.-H.; Yoon, H.-I.; Han, J.-S. Antifungal Effect, Surface Roughness, and Cytotoxicity of Three-Dimensionally Printed Denture Base with Phytoncide-Filled Microcapsules: An In-Vitro Study. J. Dent. 2022, 120, 104098. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Jo, Y.-H.; Yoon, H.-I.; Han, J.-S. Effect of Phytochemical-Filled Microcapsules with Antifungal Activity on Material Properties and Dimensional Accuracy of Denture Base Resin for Three-Dimensional Printing. BMC Oral Health 2022, 22, 178. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.-K.; Al-Dubayan, A.-H.; Al-Awami, H.; Khan, S.-Q.; Gad, M.-M. In Vitro Assessment of the Antifungal Effects of Neem Powder Added to Polymethyl Methacrylate Denture Base Material. J. Clin. Exp. Dent. 2019, 11, e170–e178. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.L.; Wang, Y.; Lilly, E.A.; Lallier, T.E.; Peters, B.M.; Hamdan, S.; Xu, X.; Fidel, P.L.; Noverr, M.C. Synthesis, Antifungal Activity, and Biocompatibility of Novel 1,4-Diazabicyclo [2.2.2]Octane (DABCO) Compounds and DABCO-Containing Denture Base Resins. Antimicrob. Agents Chemother. 2017, 61, e02575-16. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.-Y.; Huang, L.; Chai, Z.-G.; Shen, L.-J.; Xiao, Y.-H. The Antifungal Effects and Mechanical Properties of Silver Bromide/Cationic Polymer Nano-Composite-Modified Poly-Methyl Methacrylate-Based Dental Resin. Sci. Rep. 2017, 7, 1547. [Google Scholar] [CrossRef]
- De Castro, D.T.; Valente, M.L.C.; da Silva, C.H.L.; Watanabe, E.; Siqueira, R.L.; Schiavon, M.A.; Alves, O.L.; Dos Reis, A.C. Evaluation of Antibiofilm and Mechanical Properties of New Nanocomposites Based on Acrylic Resins and Silver Vanadate Nanoparticles. Arch. Oral Biol. 2016, 67, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, J.; Lan, J.; Qi, Q. Effect of a Denture Base Acrylic Resin Containing Silver Nanoparticles on Candida albicans Adhesion and Biofilm Formation. Gerodontology 2016, 33, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, C.; Takakuda, K.; Wakabayashi, N. Reduction of Candida Biofilm Adhesion by Incorporation of Prereacted Glass Ionomer Filler in Denture Base Resin. J. Dent. 2016, 44, 37–43. [Google Scholar] [CrossRef]
- Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Antifungal, Optical, and Mechanical Properties of Polymethylmethacrylate Material Incorporated with Silanized Zinc Oxide Nanoparticles. Int. J. Nanomed. 2017, 12, 2353–2360. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Akhtar, S.; Fouda, S.M. Double-Layered Acrylic Resin Denture Base with Nanoparticle Additions: An in Vitro Study. J. Prosthet. Dent. 2022, 127, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.K.; Alghamdi, L.A.; Alshahrani, F.A.; Khan, S.Q.; Matin, A.; Gad, M.M. In Vitro Assessment of Artificial Aging on the Antifungal Activity of PMMA Denture Base Material Modified with ZrO2 Nanoparticles. Int. J. Dent. 2021, 2021, 5560443. [Google Scholar] [CrossRef] [PubMed]
- Alzayyat, S.T.; Almutiri, G.A.; Aljandan, J.K.; Algarzai, R.M.; Khan, S.Q.; Akhtar, S.; Matin, A.; Gad, M.M. Antifungal Efficacy and Physical Properties of Poly(Methylmethacrylate) Denture Base Material Reinforced with SiO2 Nanoparticles. J. Prosthodont. 2021, 30, 500–508. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, G.M.; AlSuhail, H.S.; Alqater, N.K.; AlTaisan, S.A.; Akhtar, S.; Khan, S.Q.; Gad, M.M. Polymethylmethacrylate Denture Base Layering as a New Approach for the Addition of Antifungal Agents. J. Prosthodont. 2022. early view. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Ali, M.S.; Al-Thobity, A.M.; Al-Dulaijan, Y.A.; El Zayat, M.; Emam, A.-N.M.; Akhtar, S.; Khan, S.Q.; Al-Harbi, F.A.; Fouda, S.M. Polymethylmethacrylate Incorporating Nanodiamonds for Denture Repair: In Vitro Study on the Mechanical Properties. Eur. J. Dent. 2022, 16, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; Al Ghamdi, M.A.; Khan, S.Q.; Akhtar, S.; Al Eraky, D.M.; Al-Harbi, F.A. Effect of Low Nanodiamond Concentrations and Polymerization Techniques on Physical Properties and Antifungal Activities of Denture Base Resin. Polymers 2021, 13, 4331. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Zhong, Z.; Lin, L. Evaluation of Chitosan Quaternary Ammonium Salt-Modified Resin Denture Base Material. Int. J. Biol. Macromol. 2016, 85, 102–110. [Google Scholar] [CrossRef]
- Hayashi, R.; Kubota, T.; Mega, J. Application of Fluoroalkyl Acrylate Monomer for a Denture Base Material. Int. J. Oral Med. Sci. 2003, 1, 124–129. [Google Scholar] [CrossRef]
- Karkosh, Z.S.A.; Hussein, B.M.A.; AL-Wattar, W.M.A. Effect of Phosphoric Containing and Varnish-Coated Groups on Candida albicans Adhesion and Porosity of Heat Cure Acrylic Denture Base Material. Biomed. Pharmacol. J. 2018, 11, 179–185. [Google Scholar] [CrossRef]
- Da Silva Barboza, A.; Fang, L.K.; Ribeiro, J.S.; Cuevas-Suárez, C.E.; Moraes, R.R.; Lund, R.G. Physicomechanical, Optical, and Antifungal Properties of Polymethyl Methacrylate Modified with Metal Methacrylate Monomers. J. Prosthet. Dent. 2021, 125, e1–e6. [Google Scholar] [CrossRef]
- Compagnoni, M.A.; Pero, A.C.; Ramos, S.M.M.; Marra, J.; Paleari, A.G.; Rodriguez, L.S. Antimicrobial Activity and Surface Properties of an Acrylic Resin Containing a Biocide Polymer. Gerodontology 2014, 31, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.A.; Dentino, A.R. New Phosphated Poly(Methyl Methacrylate) Polymers for the Prevention of Denture-Induced Microbial Infection: An In Vitro Study. Clin. Cosmet. Investig. Dent. 2011, 3, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bajunaid, S.O.; Baras, B.H.; Weir, M.D.; Xu, H.H.K. Denture Acrylic Resin Material with Antibacterial and Protein-Repelling Properties for the Prevention of Denture Stomatitis. Polymers 2022, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Q.; Zhou, X.; Zhang, K.; Wang, S.; Xu, H.H.K.; Weir, M.D.; Feng, M.; Li, M.; Peng, X.; et al. Heat-Polymerized Resin Containing Dimethylaminododecyl Methacrylate Inhibits Candida albicans Biofilm. Materials 2017, 10, 431. [Google Scholar] [CrossRef]
- Mirizadeh, A.; Atai, M.; Ebrahimi, S. Fabrication of Denture Base Materials with Antimicrobial Properties. J. Prosthet. Dent. 2018, 119, 292–298. [Google Scholar] [CrossRef]
- Al-Haddad, A.; Vahid Roudsari, R.; Satterthwaite, J.D. Fracture Toughness of Heat Cured Denture Base Acrylic Resin Modified with Chlorhexidine and Fluconazole as Bioactive Compounds. J. Dent. 2014, 42, 180–184. [Google Scholar] [CrossRef]
- Ryalat, S.; Darwish, R.; Amin, W. New Form of Administering Chlorhexidine for Treatment of Denture-Induced Stomatitis. Ther. Clin. Risk Manag. 2011, 7, 219–225. [Google Scholar] [CrossRef]
- Maluf, C.V.; Peroni, L.V.; Menezes, L.R.; Coutinho, W.; Lourenço, E.J.V.; Telles, D.d.M. Evaluation of the Physical and Antifungal Effects of Chlorhexidine Diacetate Incorporated into Polymethyl Methacrylate. J. Appl. Oral Sci. Rev. FOB 2020, 28, e20190039. [Google Scholar] [CrossRef]
- Ottaviani, A.; Iacovelli, F.; Fiorani, P.; Desideri, A. Natural Compounds as Therapeutic Agents: The Case of Human Topoisomerase IB. Int. J. Mol. Sci. 2021, 22, 4138. [Google Scholar] [CrossRef]
- Al-Rubiay, K.K.; Jaber, N.N.; Al-Mhaawe, B.H.; Alrubaiy, L.K. Antimicrobial Efficacy of Henna Extracts. Oman Med. J. 2008, 23, 253. [Google Scholar]
- Gozubuyuk, G.S.; Aktas, E.; Yigit, N. An Ancient Plant Lawsonia Inermis (Henna): Determination of In Vitro Antifungal Activity against Dermatophytes Species. J. Mycol. Med. 2014, 24, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of Phytoncide from Trees on Human Natural Killer Cell Function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Judge, R.B.; Wong, R.H.; Arzmi, M.H.; Palamara, J.E.; Dashper, S.G. Incorporation of the Microencapsulated Antimicrobial Agent Phytoncide into Denture Base Resin. Aust. Dent. J. 2018, 63, 302–311. [Google Scholar] [CrossRef]
- Bharitkar, Y.P.; Bathini, S.; Ojha, D.; Ghosh, S.; Mukherjee, H.; Kuotsu, K.; Chattopadhyay, D.; Mondal, N.B. Antibacterial and Antiviral Evaluation of Sulfonoquinovosyldiacylglyceride: A Glycolipid Isolated from Azadirachta Indica Leaves. Lett. Appl. Microbiol. 2014, 58, 184–189. [Google Scholar] [CrossRef]
- Bansal, V.; Gupta, M.; Bhaduri, T.; Shaikh, S.A.; Sayed, F.R.; Bansal, V.; Agrawal, A. Assessment of Antimicrobial Effectiveness of Neem and Clove Extract Against Streptococcus Mutans and Candida albicans: An In Vitro Study. Niger. Med. J. 2019, 60, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Barua, D.R.; Basavanna, J.M.; Varghese, R.K. Efficacy of Neem Extract and Three Antimicrobial Agents Incorporated into Tissue Conditioner in Inhibiting the Growth of C. albicans and S. mutans. J. Clin. Diagn. Res. 2017, 11, ZC97–ZC101. [Google Scholar] [CrossRef]
- Singhania, A.; Sathe, S.; Ranka, R.; Godbole, S. Individual and Synergistic Effects of Tea Tree Oil and Neem Extract on Candida albicans Adhesion to Denture Soft Liner. Cureus 2022, 14, e27869. [Google Scholar] [CrossRef]
- Pappas, H.C.; Sylejmani, R.; Graus, M.S.; Donabedian, P.L.; Whitten, D.G.; Neumann, A.K. Antifungal Properties of Cationic Phenylene Ethynylenes and Their Impact on β-Glucan Exposure. Antimicrob. Agents Chemother. 2016, 60, 4519–4529. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward Dental Caries: Exploring Nanoparticle-Based Platforms and Calcium Phosphate Compounds for Dental Restorative Materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Köroğlu, A.; Şahin, O.; Kürkçüoğlu, I.; Dede, D.Ö.; Özdemir, T.; Hazer, B. Silver Nanoparticle Incorporation Effect on Mechanical and Thermal Properties of Denture Base Acrylic Resins. J. Appl. Oral Sci. 2016, 24, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Hamedi-Rad, F.; Ghaffari, T.; Rezaii, F.; Ramazani, A. Effect of Nanosilver on Thermal and Mechanical Properties of Acrylic Base Complete Dentures. J. Dent. Tehran Iran 2014, 11, 495–505. [Google Scholar]
- Ito, S.; Iijima, M.; Hashimoto, M.; Tsukamoto, N.; Mizoguchi, I.; Saito, T. Effects of Surface Pre-Reacted Glass-Ionomer Fillers on Mineral Induction by Phosphoprotein. J. Dent. 2011, 39, 72–79. [Google Scholar] [CrossRef]
- Saku, S.; Kotake, H.; Scougall-Vilchis, R.J.; Ohashi, S.; Hotta, M.; Horiuchi, S.; Hamada, K.; Asaoka, K.; Tanaka, E.; Yamamoto, K. Antibacterial Activity of Composite Resin with Glass-Ionomer Filler Particles. Dent. Mater. J. 2010, 29, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, K.; Mukai, Y.; Tominaga, T.; Iwaya, I.; Fujino, F.; Hirata, Y.; Teranaka, T. Fluoride Release and Recharge Characteristics of Denture Base Resins Containing Surface Pre-Reacted Glass-Ionomer Filler. Dent. Mater. J. 2009, 28, 227–233. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal Activity of ZnO Nanoparticles—The Role of ROS Mediated Cell Injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef]
- Anwander, M.; Rosentritt, M.; Schneider-Feyrer, S.; Hahnel, S. Biofilm Formation on Denture Base Resin Including ZnO, CaO, and TiO2 Nanoparticles. J. Adv. Prosthodont. 2017, 9, 482–485. [Google Scholar] [CrossRef]
- Gowri, S.; Rajiv Gandhi, R.; Sundrarajan, M. Structural, Optical, Antibacterial and Antifungal Properties of Zirconia Nanoparticles by Biobased Protocol. J. Mater. Sci. Technol. 2014, 30, 782–790. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A.S. Influence of Incorporation of ZrO2 Nanoparticles on the Repair Strength of Polymethyl Methacrylate Denture Bases. Int. J. Nanomed. 2016, 11, 5633–5643. [Google Scholar] [CrossRef]
- Gomes, D.G.; Pieretti, J.C.; Rolim, W.R.; Seabra, A.B.; Oliveira, H.C. 5—Advances in nano-based delivery systems of micronutrients for a greener agriculture. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2021; pp. 111–143. [Google Scholar]
- Lührs, A.-K.; Geurtsen, W. The Application of Silicon and Silicates in Dentistry: A Review. Prog. Mol. Subcell. Biol. 2009, 47, 359–380. [Google Scholar] [CrossRef]
- Lee, K.-I.; Su, C.-C.; Fang, K.-M.; Wu, C.-C.; Wu, C.-T.; Chen, Y.-W. Ultrafine Silicon Dioxide Nanoparticles Cause Lung Epithelial Cells Apoptosis via Oxidative Stress-Activated PI3K/Akt-Mediated Mitochondria- and Endoplasmic Reticulum Stress-Dependent Signaling Pathways. Sci. Rep. 2020, 10, 9928. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Seshan, C.A.; Chelliah, R. Toxicity Mechanism of Titanium Dioxide and Zinc Oxide Nanoparticles against Food Pathogens. Colloids Surf. B Biointerfaces 2016, 148, 600–606. [Google Scholar] [CrossRef]
- Szunerits, S.; Barras, A.; Boukherroub, R. Antibacterial Applications of Nanodiamonds. Int. J. Environ. Res. Public Health 2016, 13, 413. [Google Scholar] [CrossRef]
- Fouda, S.M.; Gad, M.M.; Ellakany, P.; Al-Thobity, A.M.; Al-Harbi, F.A.; Virtanen, J.I.; Raustia, A. The Effect of Nanodiamonds on Candida albicans Adhesion and Surface Characteristics of PMMA Denture Base Material—An in Vitro Study. J. Appl. Oral Sci. Rev. FOB 2019, 27, e20180779. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Garcia, I.M.; Ibrahim, M.S.; Rolim, J.P.M.L.; Gomes, E.A.B.; Martinho, F.C.; Collares, F.M.; Xu, H.; Melo, M.A.S. Prospects on Nano-Based Platforms for Antimicrobial Photodynamic Therapy Against Oral Biofilms. Photobiomodulation Photomed. Laser Surg. 2020, 38, 481–496. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Garcia, I.M.; Kensara, A.; Balhaddad, A.A.; Collares, F.M.; Williams, M.A.; Ibrahim, A.S.; Lin, N.J.; Weir, M.D.; Xu, H.H.K.; et al. How We Are Assessing the Developing Antibacterial Resin-Based Dental Materials? A Scoping Review. J. Dent. 2020, 99, 103369. [Google Scholar] [CrossRef]
- Schipper, N.G.; Olsson, S.; Hoogstraate, J.A.; deBoer, A.G.; Vårum, K.M.; Artursson, P. Chitosans as Absorption Enhancers for Poorly Absorbable Drugs 2: Mechanism of Absorption Enhancement. Pharm. Res. 1997, 14, 923–929. [Google Scholar] [CrossRef]
- Regis, R.R.; Della Vecchia, M.P.; Pizzolitto, A.C.; Compagnoni, M.A.; Souza, P.P.C.; de Souza, R.F. Antimicrobial Properties and Cytotoxicity of an Antimicrobial Monomer for Application in Prosthodontics. J. Prosthodont. 2012, 21, 283–290. [Google Scholar] [CrossRef]
- Kurt, A.; Erkose-Genc, G.; Uzun, M.; Sarı, T.; Isik-Ozkol, G. The Effect of Cleaning Solutions on a Denture Base Material: Elimination of Candida albicans and Alteration of Physical Properties. J. Prosthodont. 2018, 27, 577–583. [Google Scholar] [CrossRef]
- De Melo, M.A.S. Designing Bioactive Polymeric Materials for Restorative Dentistry; de Melo, M.A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]

| Type of the Material | Main Findings | |
|---|---|---|
| Organic Compounds | Henna | |
| Phytoncide Microcapsules |
| |
| Neem |
| |
| 1,4-diazabicyclo[2.2.2]octane (DABCO) derivatives |
| |
| Inorganic Particles | Silver |
|
| Pre-reacted Glass Ionomer Fillers |
| |
| Zinc oxide (ZnO) |
| |
| Zirconium dioxide (ZrO2) | ||
| Silicon dioxide (SiO2NPs) |
| |
| Titanium dioxide nanoparticles (TiO2NPs) |
| |
| Nanodiamonds (NDs) | ||
| Polymeric Compounds | Chitosan |
|
| Fluoroalkyl acrylate |
| |
| 2-hydroxyethyl methacrylate ester |
| |
| Zirconium methacrylate (ZM) |
| |
| Tin methacrylate (TM) | ||
| Di-n-butyldimethacrylate-tin (DNBMT) | ||
| Poly (2-tert-butylaminoethyl) methacrylate (PTBAEMA) |
| |
| Phosphated poly(methyl methacrylate) |
| |
| Quaternary Ammonium Compounds | Dimethylaminohexadecyl methacrylate (DMAHDM) |
|
| Dimethylaminododecyl methacrylate (DMADDM) |
| |
| N,N-dimethylaminoethyl methacrylate (DMAEMA) |
| |
| Protein-repelling Agents | 2-methacryloyloxyethyl phosphorylcholine (MPC) |
|
| Antifungal medicaments | Chlorohexidine |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Dulaijan, Y.A.; Balhaddad, A.A. Prospects on Tuning Bioactive and Antimicrobial Denture Base Resin Materials: A Narrative Review. Polymers 2023, 15, 54. https://doi.org/10.3390/polym15010054
Al-Dulaijan YA, Balhaddad AA. Prospects on Tuning Bioactive and Antimicrobial Denture Base Resin Materials: A Narrative Review. Polymers. 2023; 15(1):54. https://doi.org/10.3390/polym15010054
Chicago/Turabian StyleAl-Dulaijan, Yousif A., and Abdulrahman A. Balhaddad. 2023. "Prospects on Tuning Bioactive and Antimicrobial Denture Base Resin Materials: A Narrative Review" Polymers 15, no. 1: 54. https://doi.org/10.3390/polym15010054
APA StyleAl-Dulaijan, Y. A., & Balhaddad, A. A. (2023). Prospects on Tuning Bioactive and Antimicrobial Denture Base Resin Materials: A Narrative Review. Polymers, 15(1), 54. https://doi.org/10.3390/polym15010054







