The Microencapsulation of Tung Oil with a Natural Hydrocolloid Emulsifier for Extrinsic Self-Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microencapsulation Process
2.3. Payload, Yield, and Encapsulation Efficiency
2.4. Characterization process
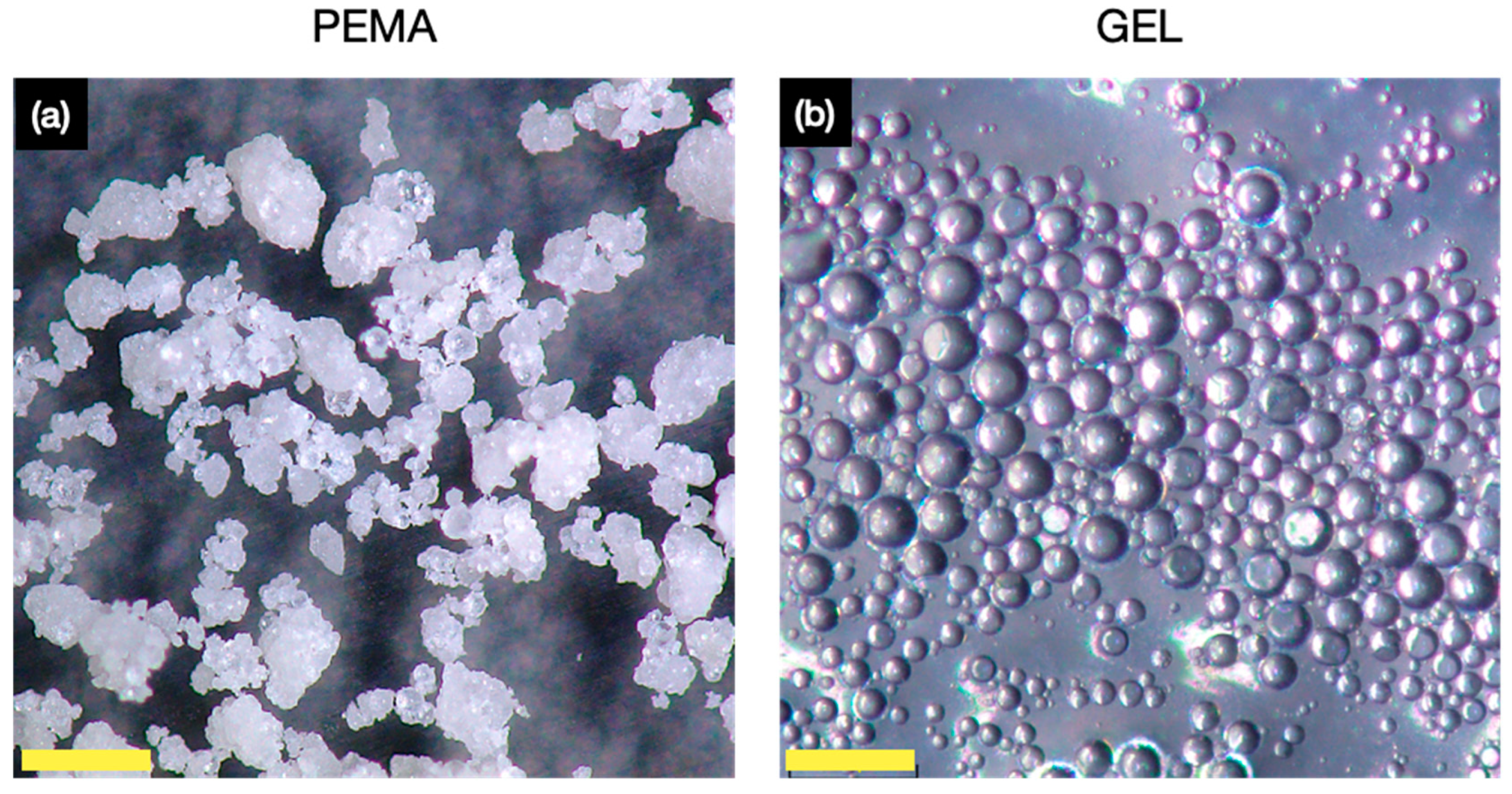
2.4.1. Microscopy of Microcapsules
2.4.2. Particle Size Dstribution
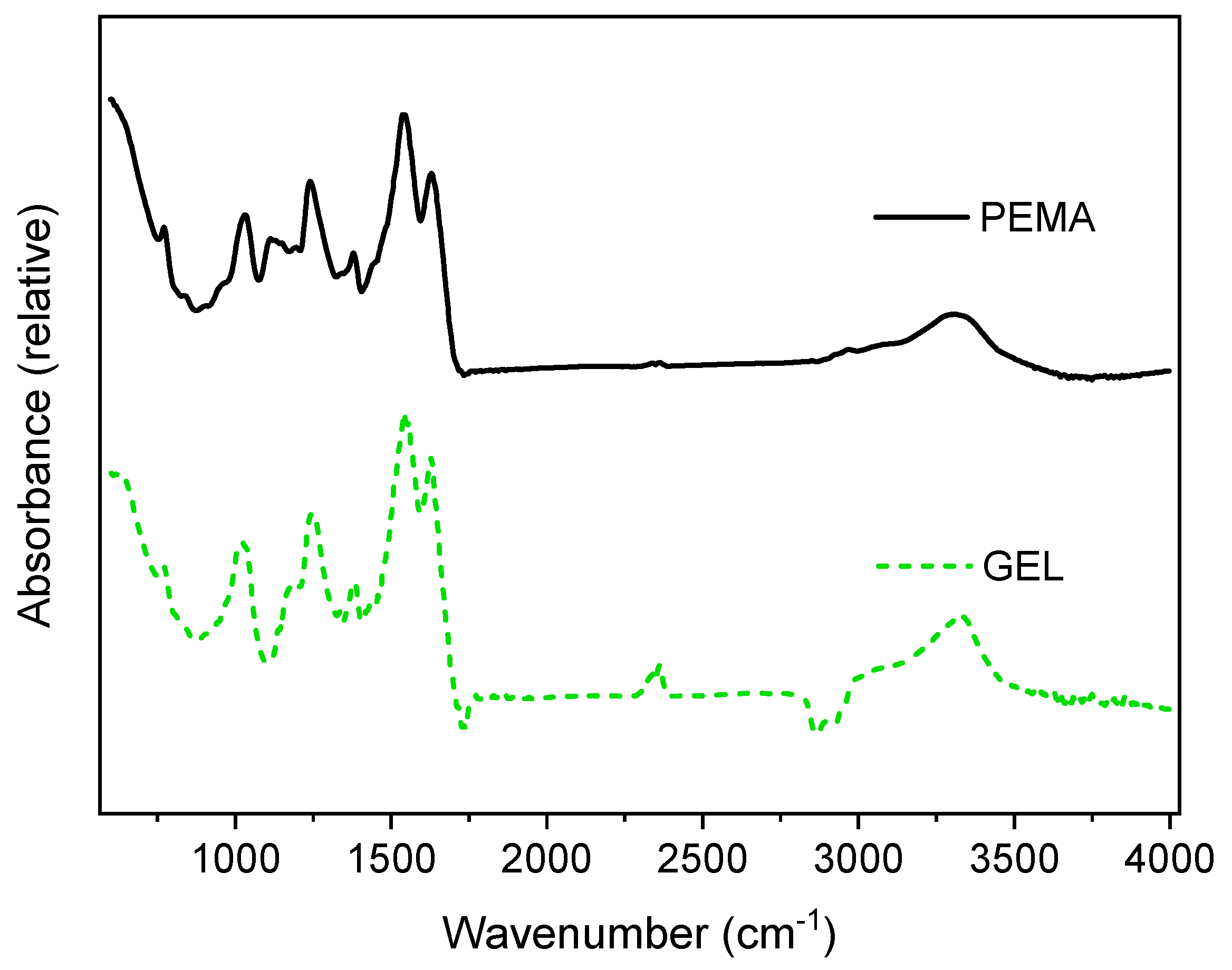
2.4.3. Fourier Transform Infrared Spectroscopy
2.4.4. Thermogravimetric Analysis
2.4.5. X-ray Diffraction
2.4.6. Preparation of the Self-Healing Epoxy Coating
2.4.7. Corrosion Resistance and Self-Healing Performance
2.4.8. Adhesion Testing
2.5. Statistical Analysis
3. Results and Discussion
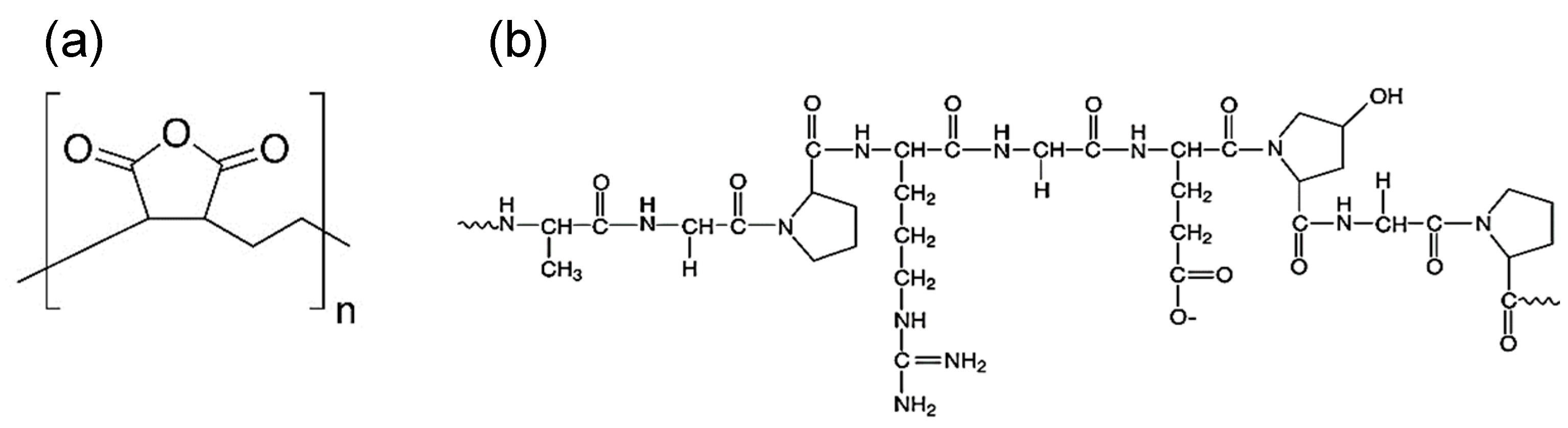
3.1. Gelatin as an Alternative Emulsifier
3.2. Formulation of Microcapsules with PEMA and GEL Emulsifiers
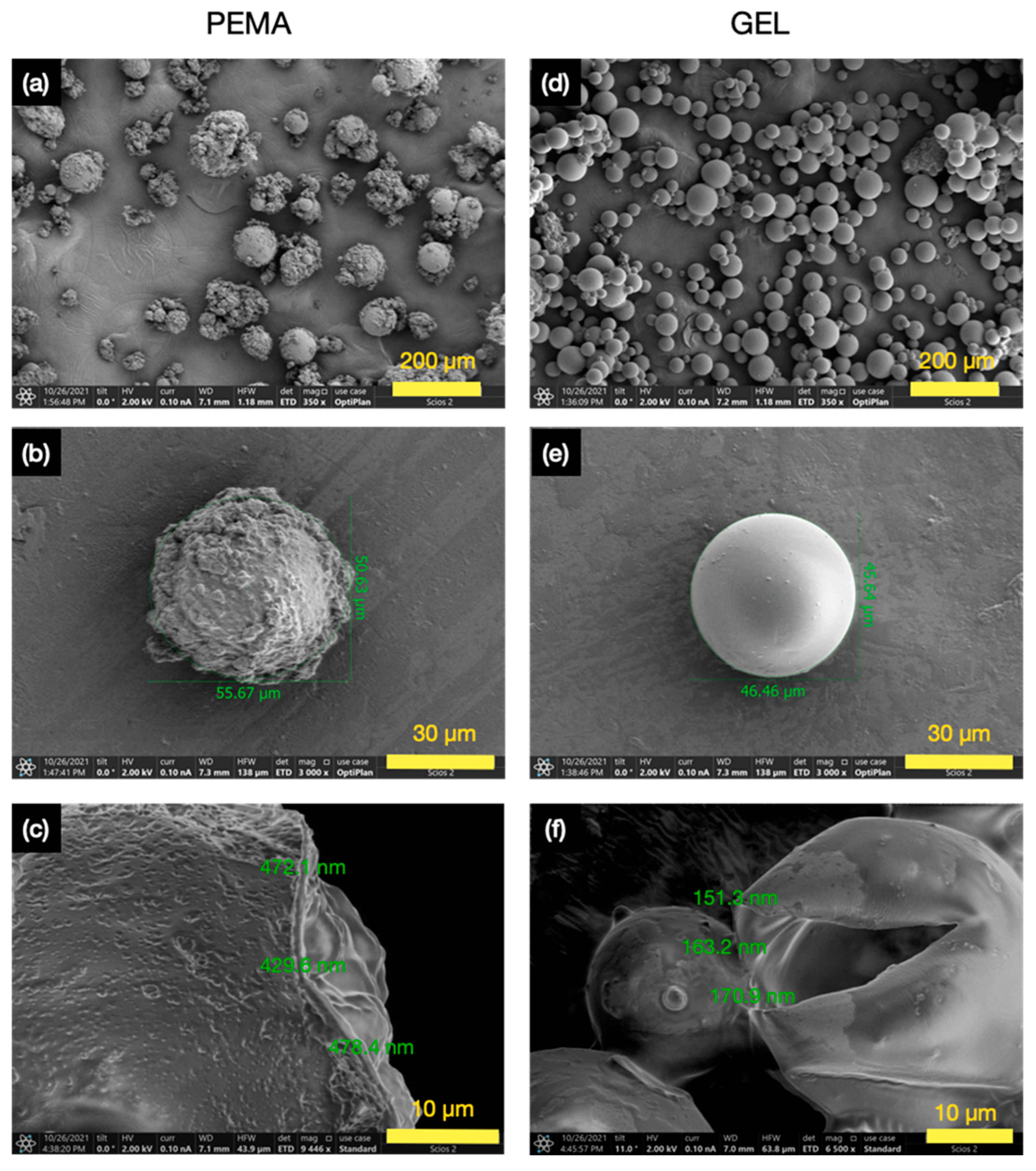
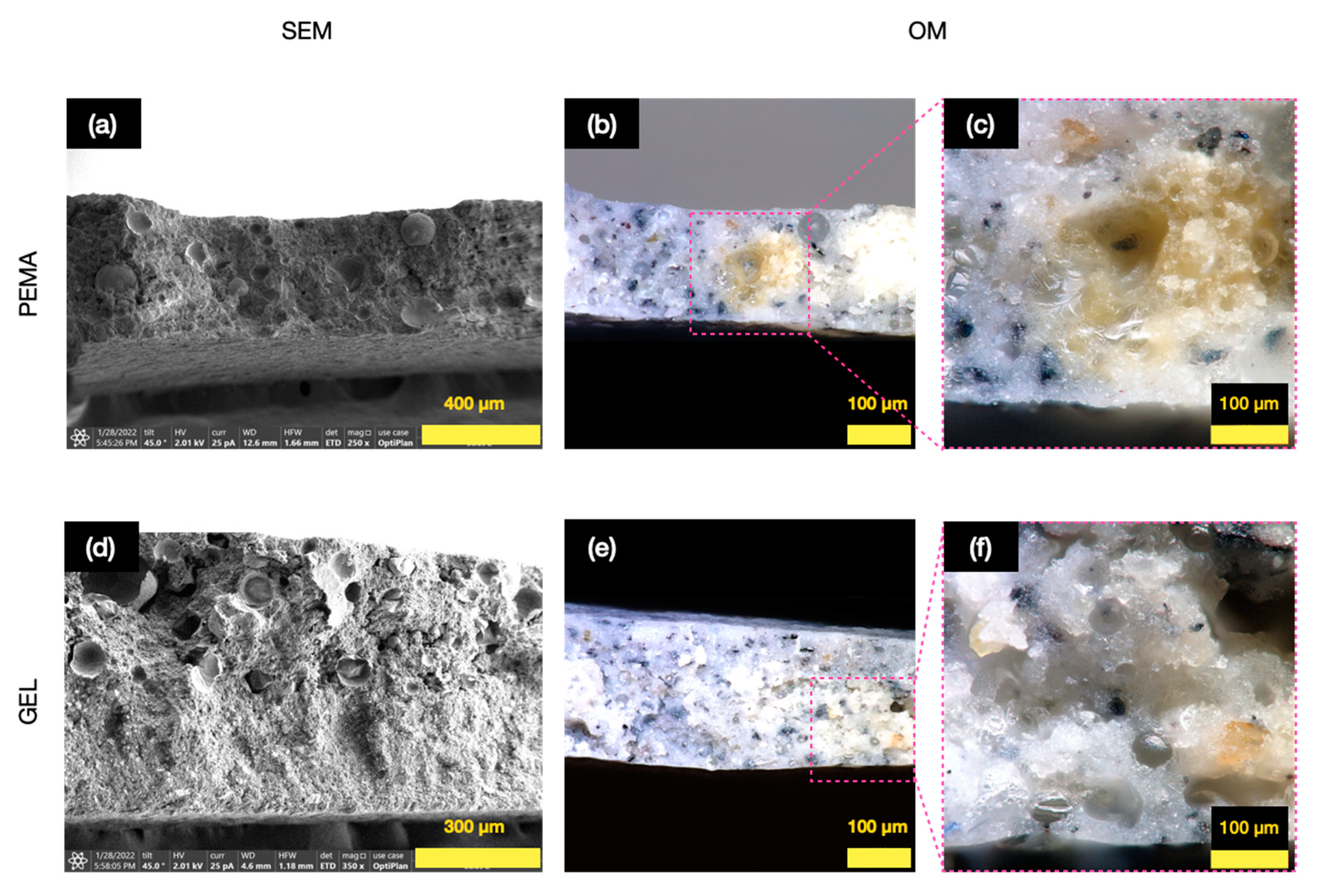
3.3. Morphology and Shell Thickness
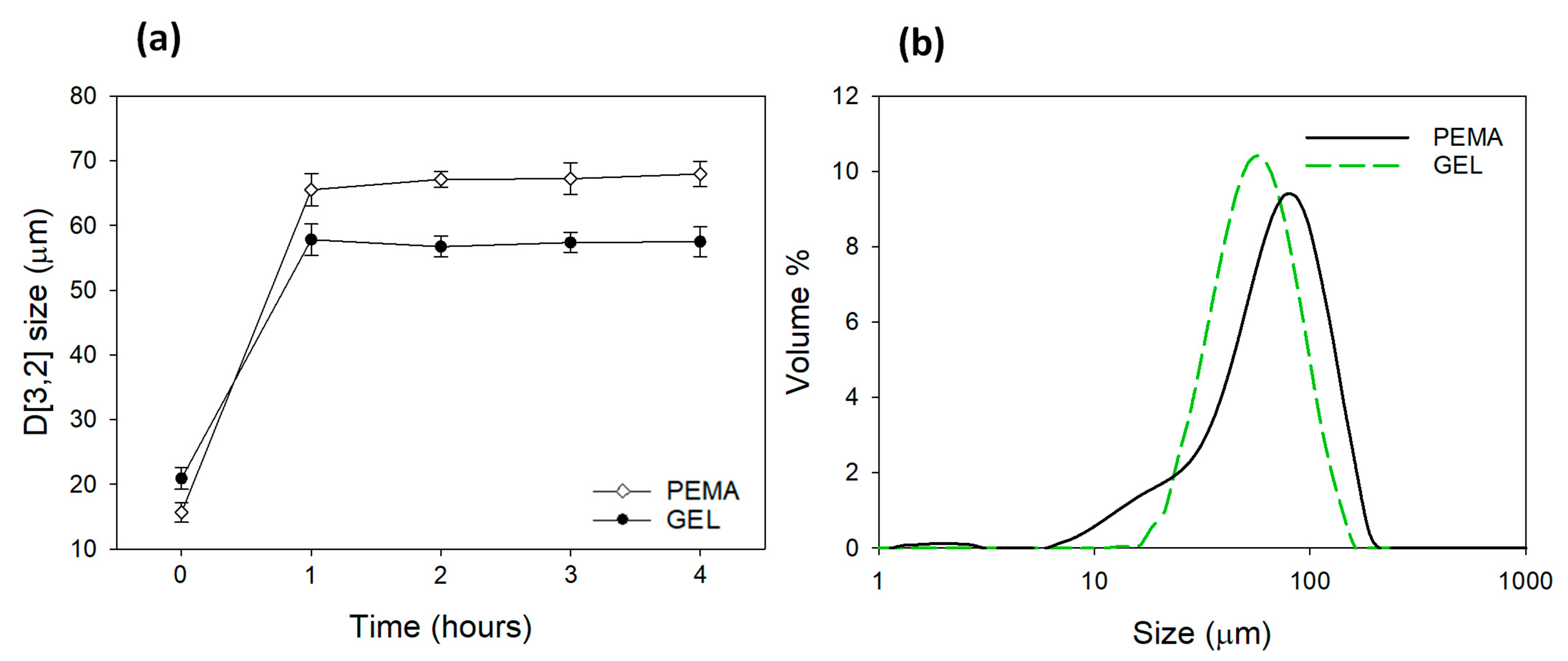
3.4. Particle Size Distribution and Reaction Profile
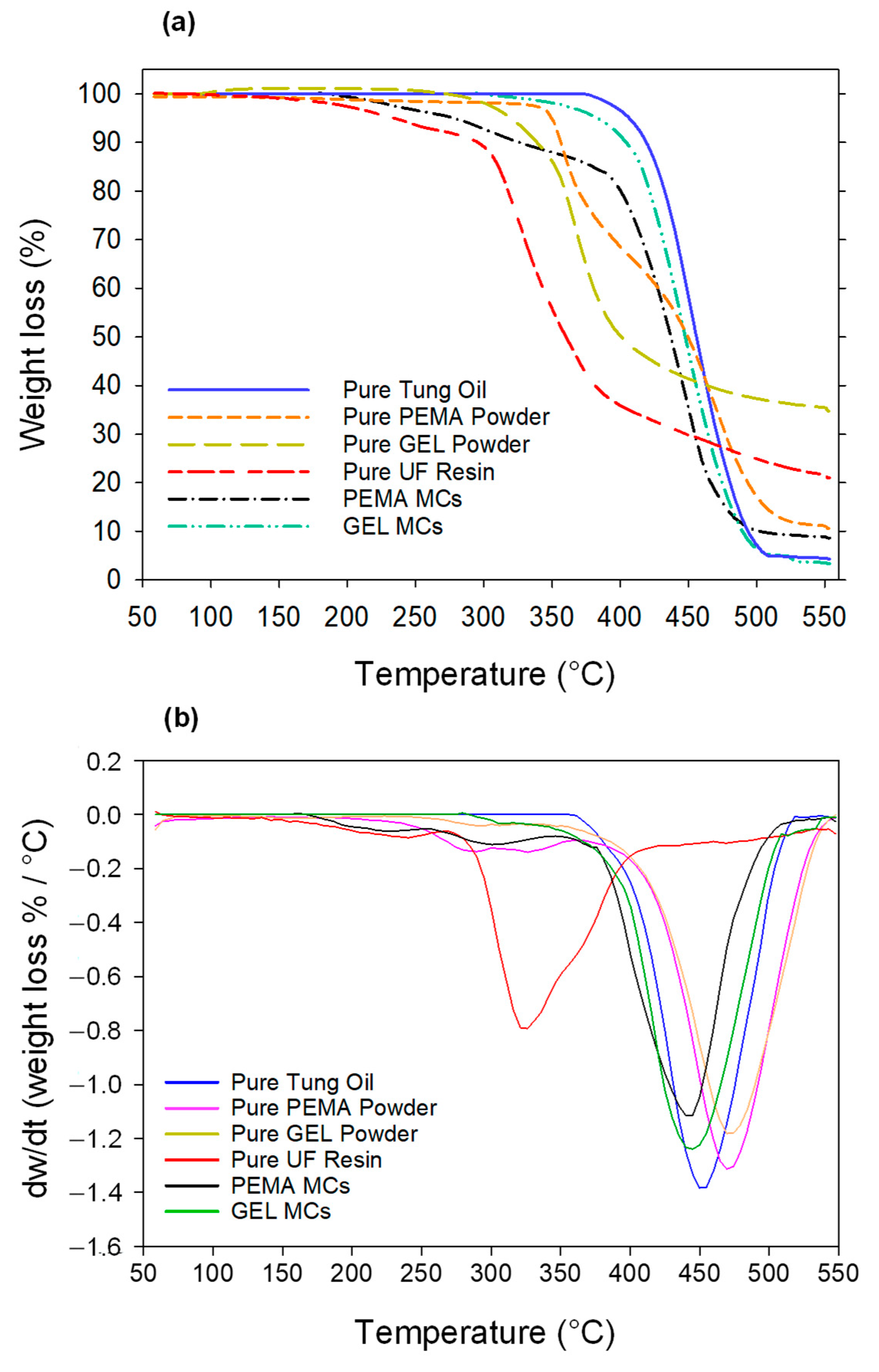
3.5. Thermogravimetric Analysis
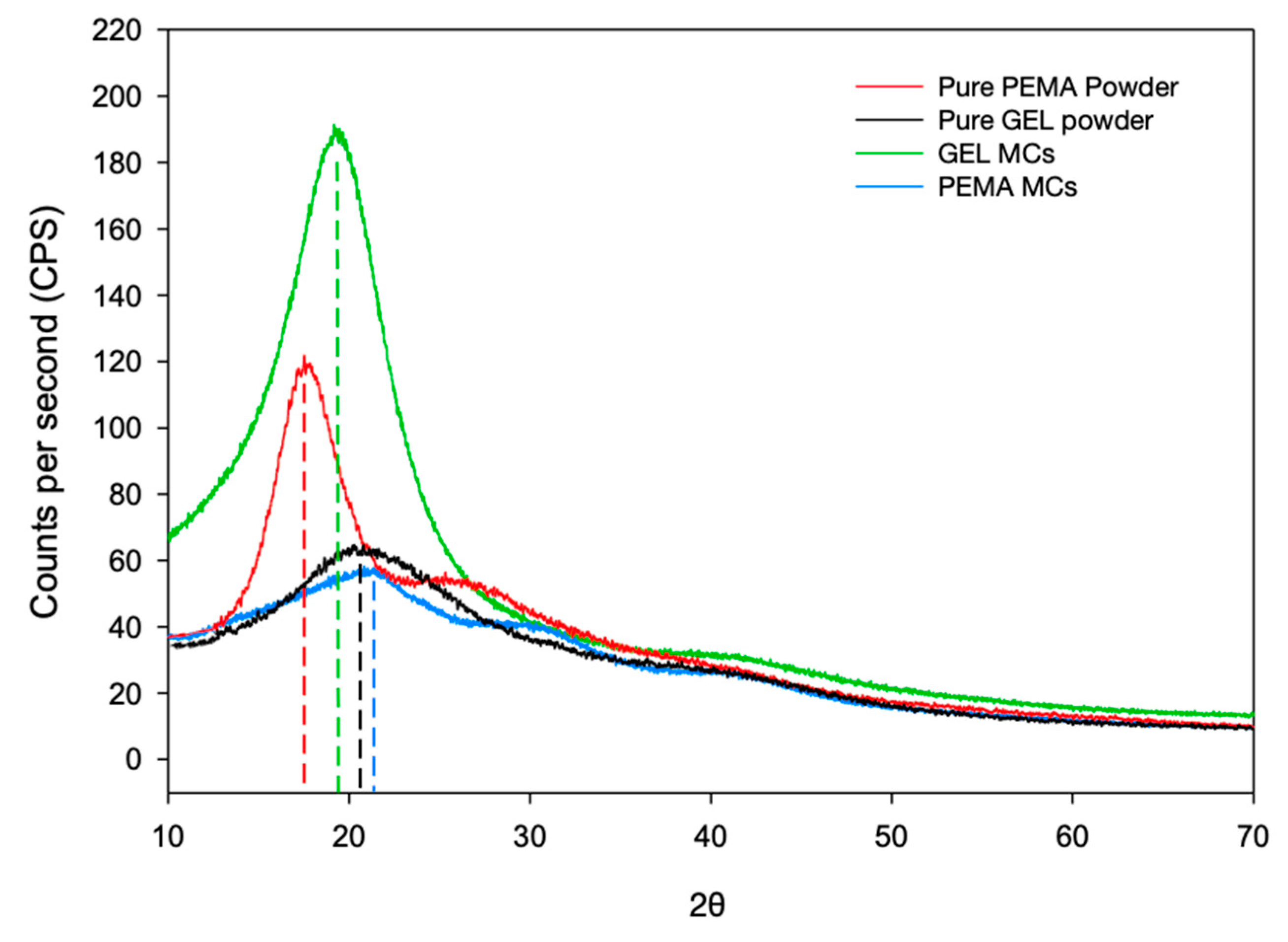
3.6. XRD Investigation
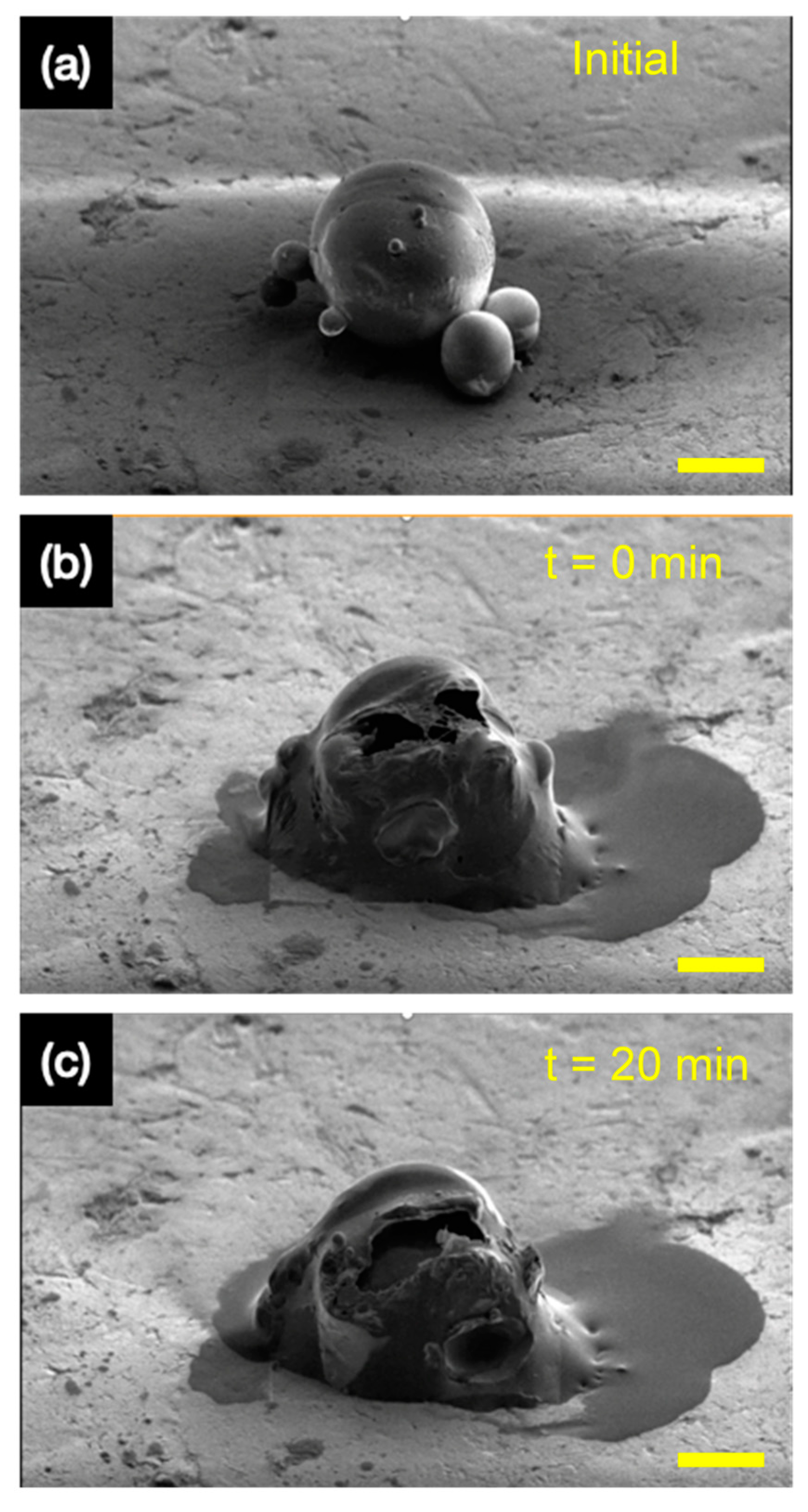
3.7. Self-Healing Microcapsules in Epoxy Primer
3.8. The Release of Tung Oil with GEL Microcapsules
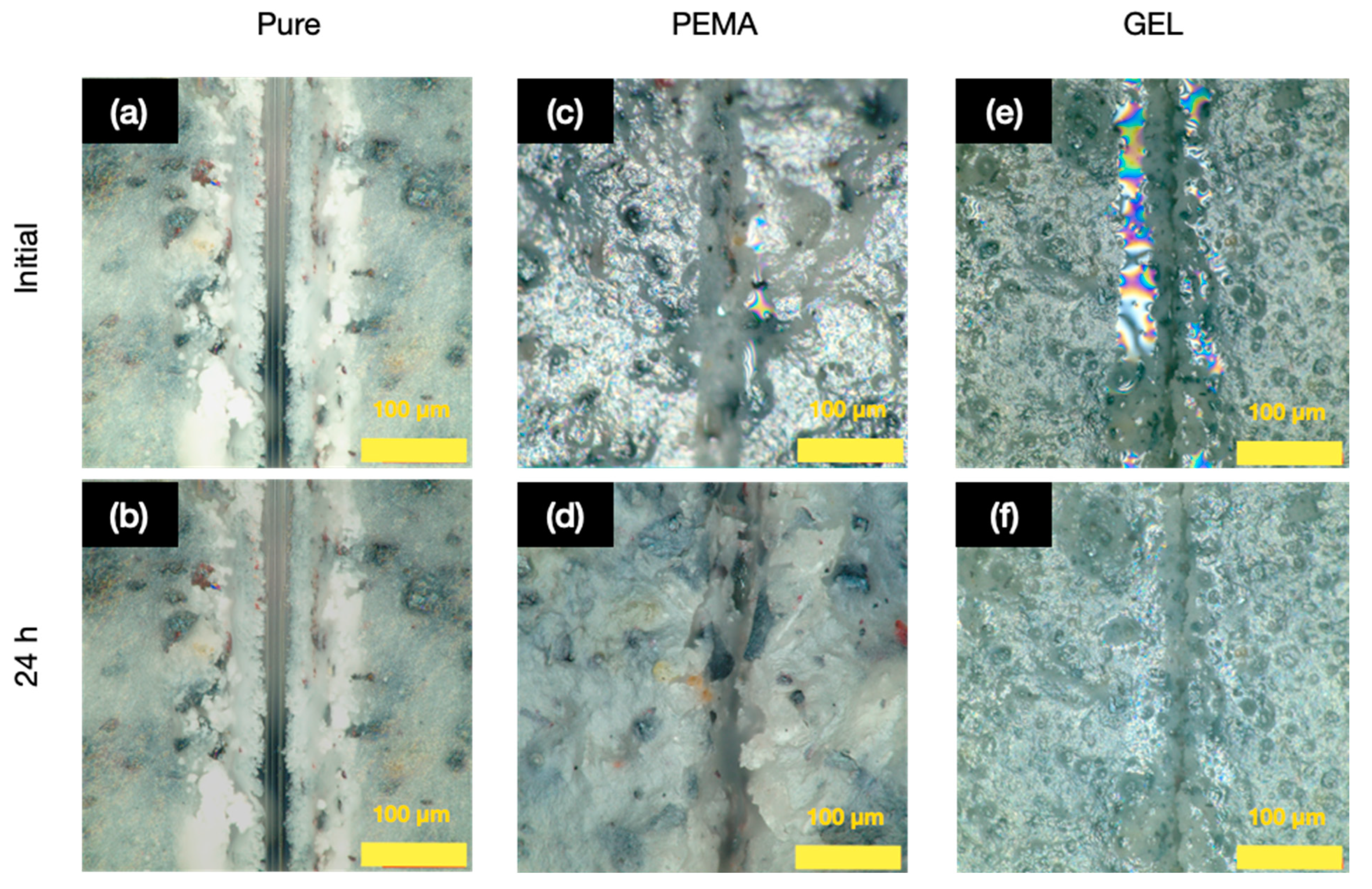
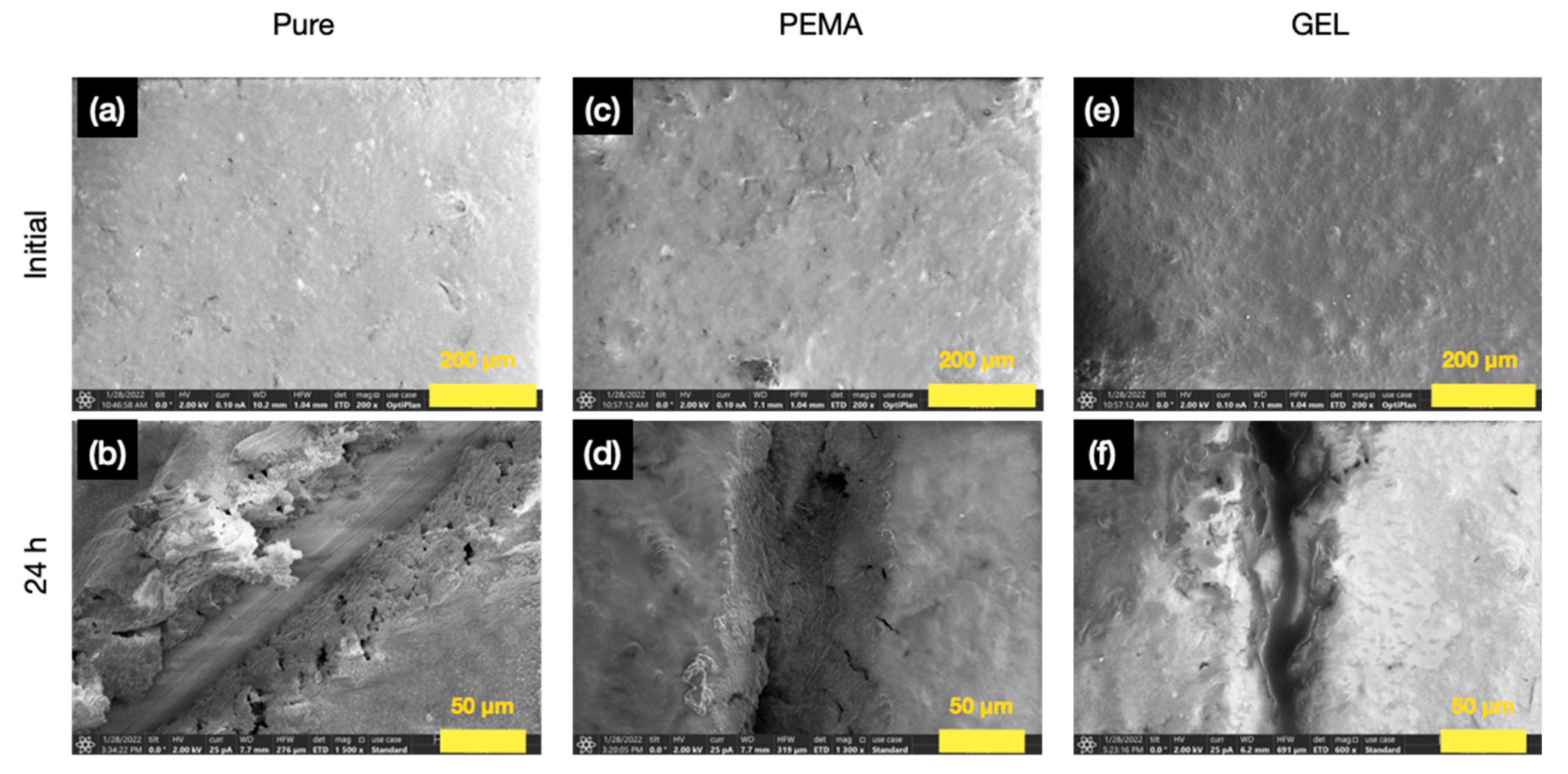
3.9. Self-Healing Performance in Epoxy Resin
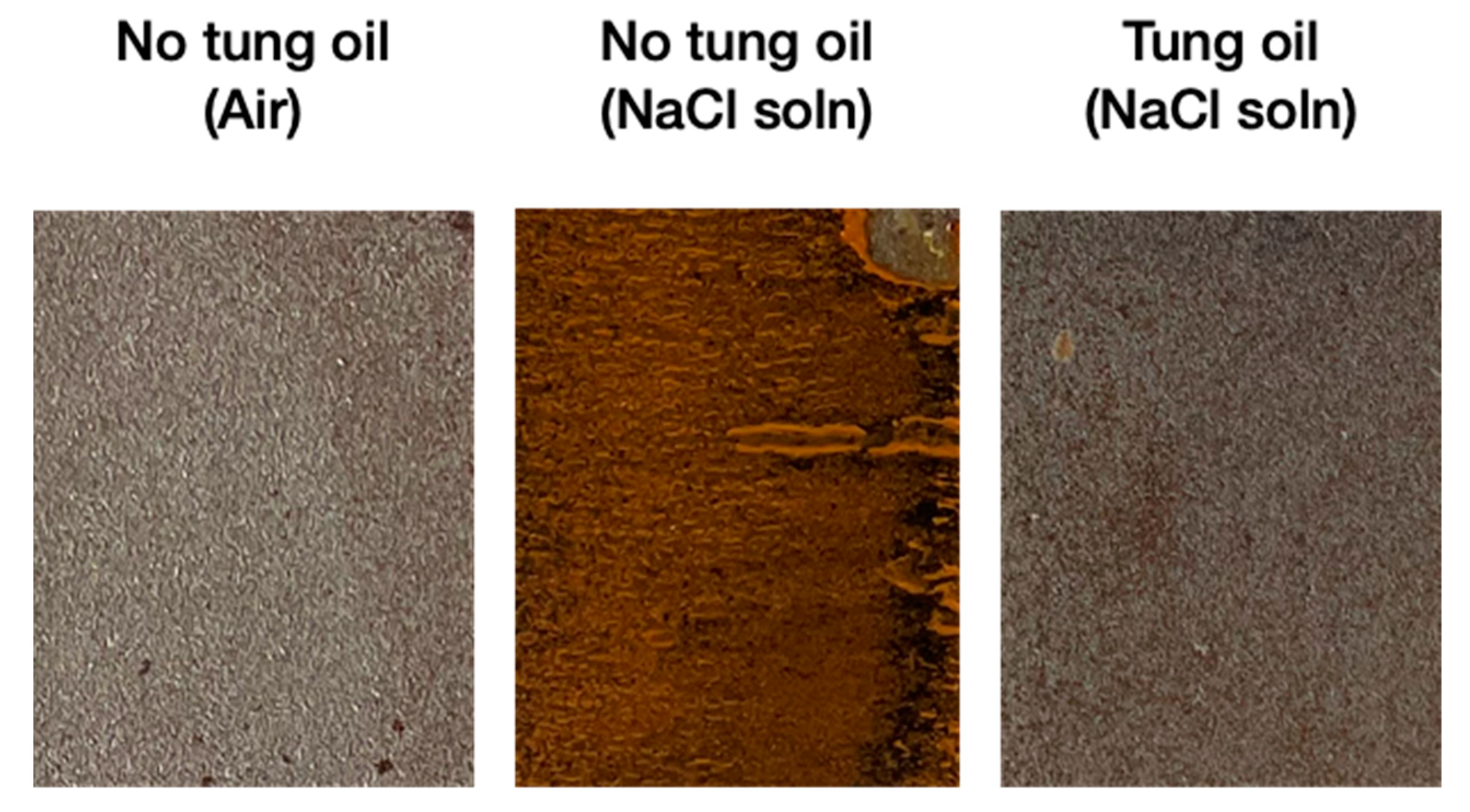
3.10. Corrosion Resistance Performance of the Self-Healing Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thanawala, K.; Khanna, A.; Raman, R. Tung Oil-Urea Formaldehyde Microcapsules for Anti-Corrosive Self-Healing Epoxy Coatings. Mater. Sci. Surf. Eng. 2015, 3, 151–156. [Google Scholar]
- Zechel, S.; Geitner, R.; Abend, M.; Siegmann, M.; Enke, M.; Kuhl, N.; Klein, M.; Vitz, J.; Gräfe, S.; Dietzek, B.; et al. Intrinsic Self-Healing Polymers with a High E-Modulus Based on Dynamic Reversible Urea Bonds. NPG Asia Mater. 2017, 9, e420. [Google Scholar] [CrossRef] [Green Version]
- Kosarli, M.; Bekas, D.G.; Tsirka, K.; Baltzis, D.; Vaimakis-Tsogkas, D.; Orfanidis, S.; Papavassiliou, G.; Paipetis, A.S. Microcapsule-Based Self-Healing Materials: Healing Efficiency and Toughness Reduction vs. Capsule Size. Compos. Part B Eng. 2019, 171, 78–86. [Google Scholar] [CrossRef]
- Joseph, C.; Jefferson, A.D.; Isaacs, B.; Lark, R.; Gardner, D. Experimental Investigation of Adhesive-Based Self-Healing of Cementitious Materials. Mag. Concr. Res. 2015, 62, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Gu, A.; Liang, G. Preparation and Properties of Poly(Urea-Formaldehyde) Microcapsules Filled with Epoxy Resins. Mater. Chem. Phys. 2008, 110, 417–425. [Google Scholar] [CrossRef]
- Ullah, H.; Azizli, K.; Man, Z.B.; Ismail, M.B.C. Synthesis and Characterization of Urea-Formaldehyde Microcapsules Containing Functionalized Polydimethylsiloxanes. Procedia Eng. 2016, 148, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Fayyad, E.M.; Almaadeed, M.A.; Jones, A. Encapsulation of Tung Oil for Self-Healing Coatings in Corrosion Applications. Sci. Adv. Mater. 2015, 7, 2628–2638. [Google Scholar] [CrossRef]
- Asadi, A.K.; Ebrahimi, M.; Mohseni, M. Preparation and Characterisation of Melamineurea-Formaldehyde Microcapsules Containing Linseed Oil in the Presence of Polyvinylpyrrolidone as Emulsifier. Pigment Resin Technol. 2017, 46, 318–326. [Google Scholar] [CrossRef]
- Paolini, N.A.; Cordeiro Neto, A.G.; Pellanda, A.C.; de Carvalho Jorge, A.R.; de Barros Soares, B.; Floriano, J.B.; Berton, M.A.C.; Vijayan P, P.; Thomas, S. Evaluation of Corrosion Protection of Self-Healing Coatings Containing Tung and Copaiba Oil Microcapsules. Int. J. Polym. Sci. 2021, 2021, 6650499. [Google Scholar] [CrossRef]
- Li, H.; Cui, Y.; Li, Z.; Zhu, Y.; Wang, H. Fabrication of Microcapsules Containing Dual-Functional Tung Oil and Properties Suitable for Self-Healing and Self-Lubricating Coatings. Prog. Org. Coat. 2018, 115, 164–171. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Morales-Hernández, N.; García-Márquez, E.; Rodríguez-Rodríguez, R. Development of Fish Oil Microcapsules by Spray Drying Using Mesquite Gum and Chitosan as Wall Materials: Physicochemical Properties, Microstructure, and Lipid Hydroperoxide Concentration. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–10. [Google Scholar] [CrossRef]
- Liu, M.; Thirumalai, R.V.K.G.; Wu, Y.; Wan, H. Characterization of the Crystalline Regions of Cured Urea Formaldehyde Resin. RSC Adv. 2017, 7, 49536–49541. [Google Scholar] [CrossRef] [Green Version]
- Nguon, O.; Lagugné-Labarthet, F.; Brandys, F.A.; Li, J.; Gillies, E.R. Microencapsulation by in Situ Polymerization of Amino Resins. Polym. Rev. 2017, 58, 1–50. [Google Scholar] [CrossRef] [Green Version]
- Antunes, A.; Duarte, M.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Barros-Timmons, A.; Magalhães, F.D. Partial Replacement of Melamine by Benzoguanamine in MUF Resins towards Improved Flexibility of Agglomerated Cork Panels. Int. J. Adhes. Adhes. 2018, 87, 142–150. [Google Scholar] [CrossRef]
- Antunes, A.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Barros-Timmons, A.; Magalhães, F.D. Highly Flexible Glycol-Urea-Formaldehyde Resins. Eur. Polym. J. 2018, 105, 167–176. [Google Scholar] [CrossRef]
- Yuan, L.; Liang, G.; Xie, J.Q.; Li, L.; Guo, J. Preparation and Characterization of Poly(Urea-Formaldehyde) Microcapsules Filled with Epoxy Resins. Polymer 2006, 47, 5338–5349. [Google Scholar] [CrossRef]
- Sharma, S.; Choudhary, V. Parametric Study for Epoxy Loaded PMMA Microcapsules Using Taguchi and ANOVA Methods. Express Polym. Lett. 2017, 11, 1023–1036. [Google Scholar] [CrossRef]
- Bolimowski, P.A.; Kozera, R.; Boczkowska, A. Poly(Urea-Formaldehyde) Microcapsules—Synthesis and Influence of Stirring Speed on Capsules Size. Polimery 2018, 63, 339–346. [Google Scholar] [CrossRef]
- Zhang, Y.; Baiocco, D.; Mustapha, A.N.; Zhang, X.; Yu, Q.; Wellio, G.; Zhang, Z.; Li, Y. Hydrocolloids: Nova Materials Assisting Encapsulation of Volatile Phase Change Materials for Cryogenic Energy Transport and Storage. Chem. Eng. J. 2020, 382, 123028. [Google Scholar] [CrossRef]
- Mustapha, A.N.; Zhang, Y.; Zhang, Z.; Ding, Y.; Yuan, Q.; Li, Y. Taguchi and ANOVA Analysis for the Optimization of the Microencapsulation of a Volatile Phase Change Material. J. Mater. Res. Technol. 2021, 11, 667–680. [Google Scholar] [CrossRef]
- Yoshizawa, H.; Kamio, E.; Kobayashi, E.; Jacobson, J.; Kitamura, Y. Investigation of Alternative Compounds to Poly(E-MA) as a Polymeric Surfactant for Preparation of Microcapsules by Phase Separation Method. J. Microencapsul. 2007, 24, 249–357. [Google Scholar] [CrossRef] [PubMed]
- Fashandi, M.; Leung, S.N. Preparation and Characterization of 100% Bio-Based Polylactic Acid/Palmitic Acid Microcapsules for Thermal Energy Storage. Mater. Renew. Sustain. Energy 2017, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Farzi, G.; Davoodi, A.; Ahmadi, A.; Neisiany, R.E.; Anwer, M.K.; Aboudzadeh, M.A. Encapsulation of Cerium Nitrate within Poly(Urea-Formaldehyde)Microcapsules for the Development of Self-Healing Epoxy-Based Coating. ACS Omega 2021, 6, 31147. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Zhang, Z.; Ding, Y.; Yu, Q.; Li, Y. Polysaccharide Assisted Microencapsulation for Volatile Phase Change Materials with a Fluorescent Retention Indicator. Chem. Eng. J. 2019, 359, 1234–1243. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Li, Z.; Wang, W. Thermochromic Microcapsules with Highly Transparent Shells Obtained through In-Situ Polymerization of Urea Formaldehyde around Thermochromic Cores for Smart Wood Coatings. Sci. Rep. 2018, 8, 4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.N.; Kessler, M.R.; Sottos, N.R.; White, S.R. In Situ Poly(Urea-Formaldehyde) Microencapsulation of Dicyclopentadiene. J. Microencapsul. 2003, 20, 719–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, D.K. What Is the Proper Way to Apply the Multiple Comparison Test? Korean J. Anesthesiol. 2018, 71, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, W. Tukey’s Test. Encycl. Syst. Biol. 2013, 2013, 2303–2304. [Google Scholar] [CrossRef]
- Fan, C.; Zhou, X. Effect of Emulsifier on Poly(Urea-Formaldehyde) Microencapsulation of Tetrachloroethylene. Polym. Bull. 2011, 67, 15–27. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Almaadeed, M.A.; Jones, A. Preparation and Characterization of Urea–Formaldehyde Microcapsules Filled with Paraffin Oil. Polym. Bull. 2016, 73, 631–646. [Google Scholar] [CrossRef]
- Katoueizadeh, E.; Zebarjad, S.M.; Janghorban, K. Investigating the Effect of Synthesis Conditions on the Formation of Urea-Formaldehyde Microcapsules. J. Mater. Res. Technol. 2019, 8, 541–552. [Google Scholar] [CrossRef]
- Pizzi, A.; Garcia, R.; Wang, S. On the Networking Mechanisms of Additives-Accelerated Phenol-Formaldehyde Polycondensates. J. Appl. Polym. Sci. 1997, 66, 255–266. [Google Scholar] [CrossRef]
- Saikia, B.J.; Dolui, S.K. Preparation and Characterization of an Azide–Alkyne Cycloaddition Based Self-Healing System via a Semiencapsulation Method. RSC Adv. 2015, 5, 92480–92489. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Chi, Y.; Yan, J. TG-FTIR Study on Urea-Formaldehyde Resin Residue during Pyrolysis and Combustion. undefined 2010, 173, 205–210. [Google Scholar] [CrossRef]
- Ismail, N.A.; Khan, A.; Fayyad, E.; Kahraman, R.; Abdullah, A.M.; Shakoor, R.A. Self-Healing Performance of Smart Polymeric Coatings Modified with Tung Oil and Linalyl Acetate. Polymers 2021, 13, 1609. [Google Scholar] [CrossRef]
- Park, B.D.; Jeong, H.W. Hydrolytic Stability and Crystallinity of Cured Ureaformaldehyde Resin Adhesives with Different Formaldehyde/Urea Mole Ratios. Int. J. Adhes. Adhes. 2011, 31, 524–529. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, H.; Ma, J.; Huang, L.; Huang, L.; Chen, X.; Zeng, H.; Ma, S. Preparation and Properties of Corrosion-Resistant Coatings From Waterborne Polyurethane Modified Epoxy Emulsion. Front. Mater. 2019, 6, 185. [Google Scholar] [CrossRef] [Green Version]
- Boumezgane, O.; Suriano, R.; Fedel, M.; Tonelli, C.; Deflorian, F.; Turri, S. Self-Healing Epoxy Coatings with Microencapsulated Ionic PDMS Oligomers for Corrosion Protection Based on Supramolecular Acid-Base Interactions. Prog. Org. Coat. 2022, 162, 106558. [Google Scholar] [CrossRef]
- Cordeiro Neto, A.G.; Pellanda, A.C.; de Carvalho Jorge, A.R.; Floriano, J.B.; Coelho Berton, M.A. Preparation and Evaluation of Corrosion Resistance of a Self-Healing Alkyd Coating Based on Microcapsules Containing Tung Oil. Prog. Org. Coat. 2020, 147, 105874. [Google Scholar] [CrossRef]
- Lang, S.; Zhou, Q. Synthesis and Characterization of Poly(Urea-Formaldehyde) Microcapsules Containing Linseed Oil for Self-Healing Coating Development. Prog. Org. Coat. 2017, 105, 99–110. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Ashrafi, A.; Kasiriha, M. Tung Oil: An Autonomous Repairing Agent for Self-Healing Epoxy Coatings. Prog. Org. Coat. 2010, 70, 383–387. [Google Scholar] [CrossRef]
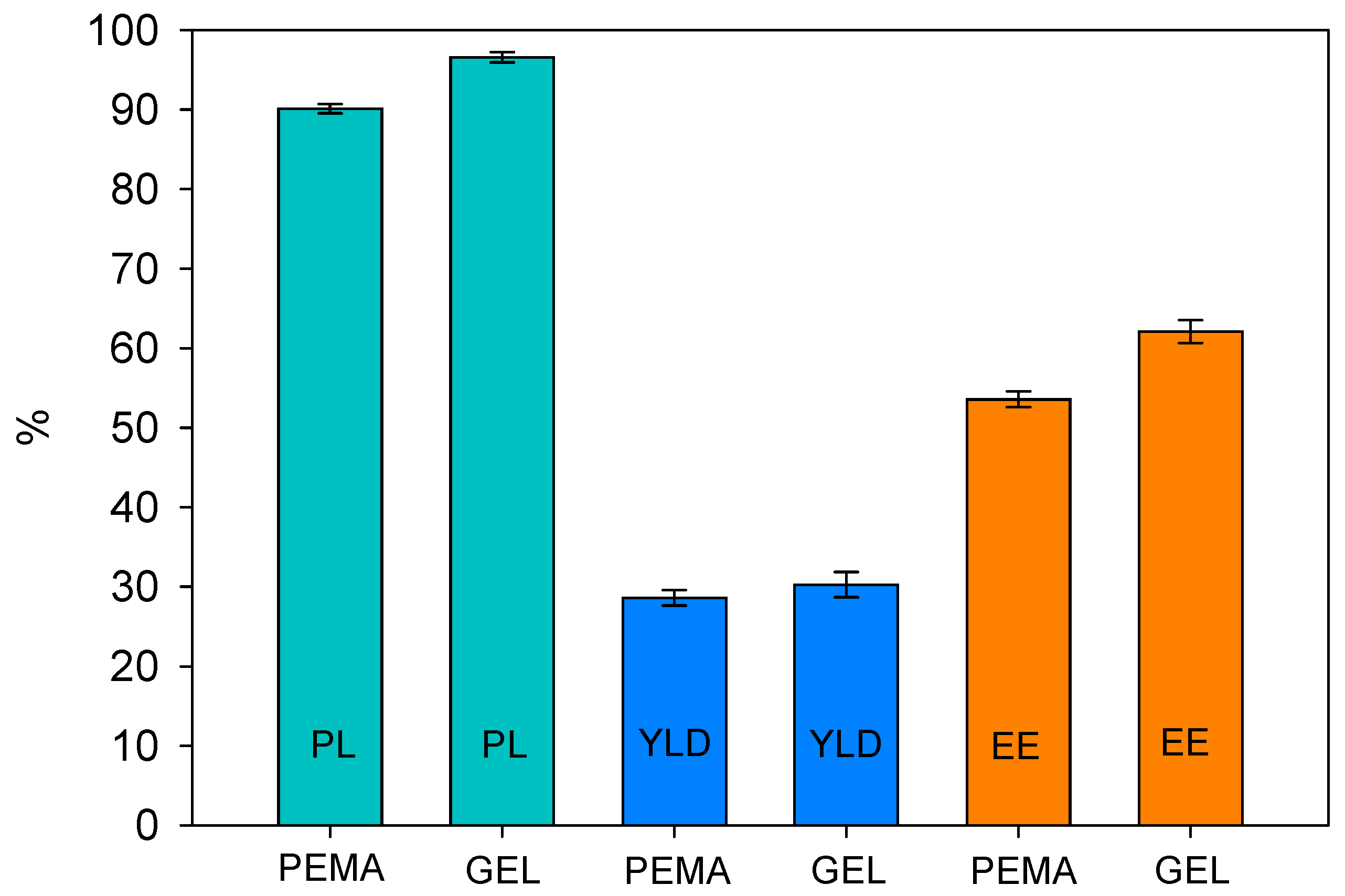
| Pair | Tukey’s HSD Q Statistic | Tukey’s HSD p-Value | Tukey’s HSD Inference |
|---|---|---|---|
| PEMA PL v. GEL PL | 18.0 | 1.00 × 10−3 | p < 0.05 |
| PEMA YLD v. GEL YLD | 2.20 | 0.20 | Insignificant |
| PEMA EE v. GEL EE | 11.90 | 1.10 × 10−3 | p < 0.05 |
| Adhesion Strength (MPa) | Time until Failure (s) | |
|---|---|---|
| Pure | 12.76 0.3 | 13.8 0.2 |
| 5 wt.% PEMA | 12.36 0.2 | 14.0 0.6 |
| 10 wt.% PEMA | 12.19 0.1 | 13.5 0.3 |
| 5 wt.% GEL | 12.66 0.3 | 13.9 0.4 |
| 10 wt.% GEL | 12.27 0.5 | 13.1 0.5 |
| Pair | Tukey’s HSD Q Statistic | Tukey’s HSD p-Value | Tukey’s HSD Inference |
|---|---|---|---|
| Pure v. 5 wt.% PEMA | 2.35 | 0.49 | Insignificant |
| Pure v.10 wt.% PEMA | 3.35 | 0.20 | Insignificant |
| Pure v. 5 wt.% GEL | 0.59 | 0.90 | Insignificant |
| Pure v. 10 wt.% GEL | 2.68 | 0.38 | Insignificant |
| 5 wt.% PEMA v. 10 wt.% PEMA | 1.00 | 0.90 | Insignificant |
| 5 wt.% PEMA v. 5 wt.% GEL | 1.76 | 0.71 | Insignificant |
| 5 wt.% PEMA v. 10 wt.% GEL | 0.33 | 0.90 | Insignificant |
| 10 wt.% PEMA v. 5 wt.% GEL | 2.77 | 0.35 | Insignificant |
| 10 wt.% PEMA v. 10 wt.% GEL | 0.67 | 0.89 | Insignificant |
| 5 wt.% GEL v. 10 wt.% GEL | 2.10 | 0.57 | Insignificant |
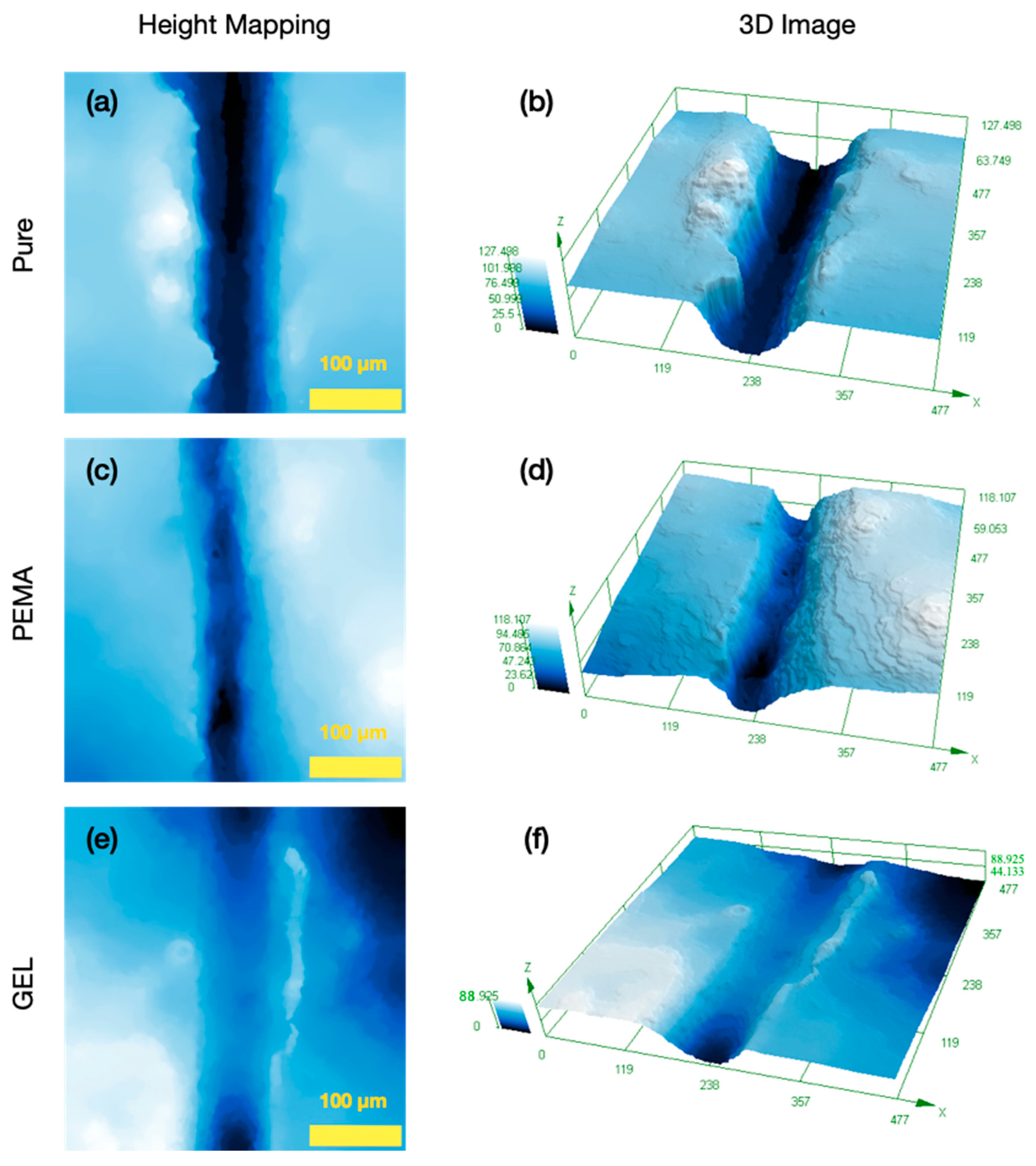
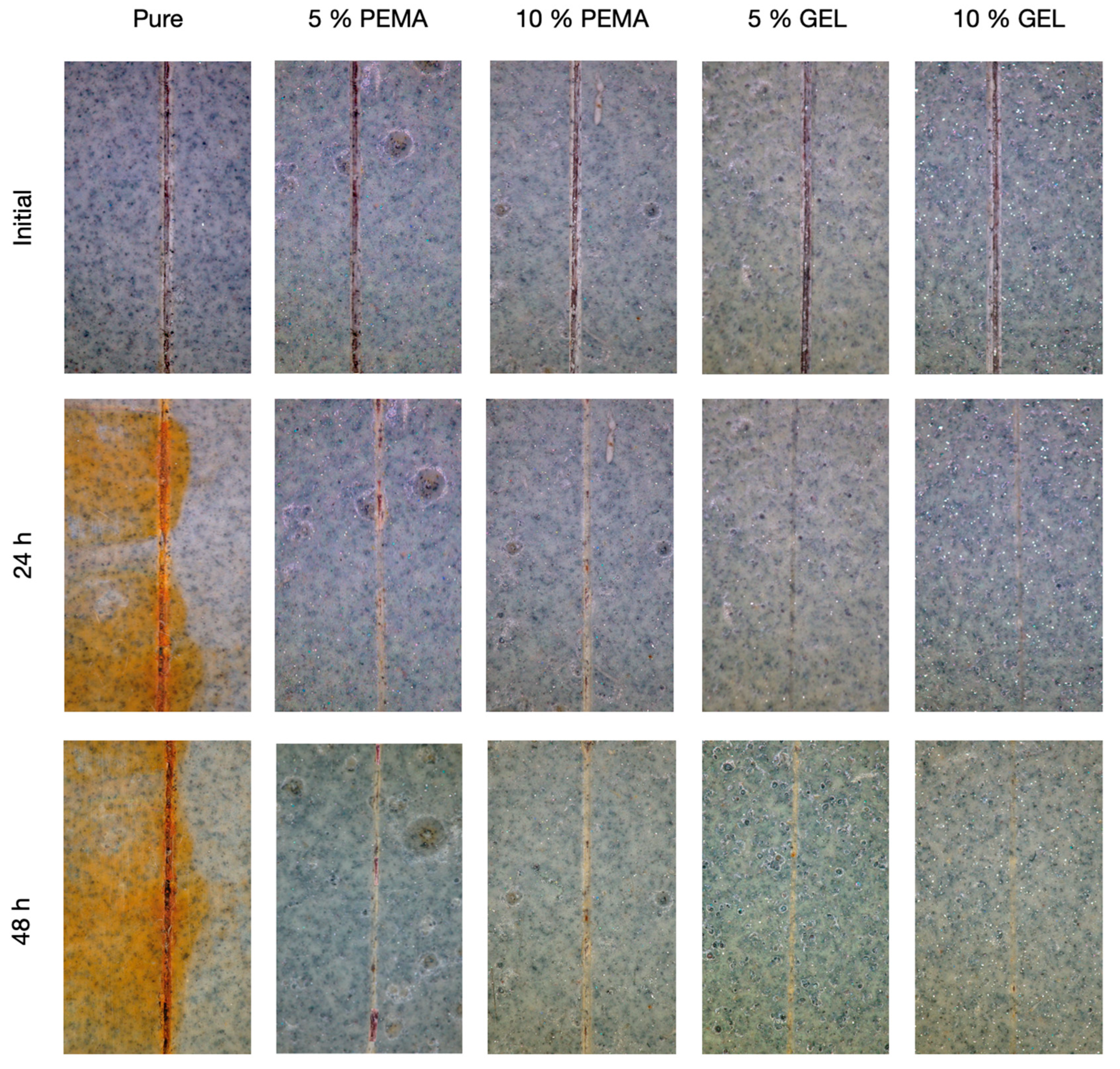
| Initial Scratch Depth (µm) | 24 h Scratch Depth (µm) | Healing Depth Difference (µm) | |
|---|---|---|---|
| Pure | 82.64 ± 6.2 | 81.22 ± 4.1 | 1.42 |
| PEMA | 78.4 ± 5.9 | 28.72 ± 5.3 | 49.58 |
| GEL | 67.68 ± 4.8 | 5.8 ± 2.6 | 61.88 |
| Pair | Tukey’s HSD Q Statistic | Tukey’s HSD p-Value | Tukey’s HSD Inference |
|---|---|---|---|
| Pure v. PEMA | 1.30 | 0.65 | Insignificant |
| Pure v. GEL | 4.57 | 0.04 | p < 0.05 |
| PEMA v. GEL | 3.28 | 0.13 | Insignificant |
| Pair | Tukey’s HSD Q Statistic | Tukey’s HSD p-Value | Tukey’s HSD Inference |
|---|---|---|---|
| Pure v. PEMA | 21.50 | 1.01 × 10−3 | p < 0.05 |
| Pure v. GEL | 30.90 | 1.01 × 10−3 | p < 0.05 |
| PEMA v. GEL | 9.37 | 1.37 × 10−3 | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapha, A.N.; AlMheiri, M.; AlShehhi, N.; Rajput, N.; Joshi, S.; Antunes, A.; AlTeneiji, M. The Microencapsulation of Tung Oil with a Natural Hydrocolloid Emulsifier for Extrinsic Self-Healing Applications. Polymers 2022, 14, 1907. https://doi.org/10.3390/polym14091907
Mustapha AN, AlMheiri M, AlShehhi N, Rajput N, Joshi S, Antunes A, AlTeneiji M. The Microencapsulation of Tung Oil with a Natural Hydrocolloid Emulsifier for Extrinsic Self-Healing Applications. Polymers. 2022; 14(9):1907. https://doi.org/10.3390/polym14091907
Chicago/Turabian StyleMustapha, Abdullah Naseer, Maitha AlMheiri, Nujood AlShehhi, Nitul Rajput, Sachin Joshi, Ana Antunes, and Mohamed AlTeneiji. 2022. "The Microencapsulation of Tung Oil with a Natural Hydrocolloid Emulsifier for Extrinsic Self-Healing Applications" Polymers 14, no. 9: 1907. https://doi.org/10.3390/polym14091907
APA StyleMustapha, A. N., AlMheiri, M., AlShehhi, N., Rajput, N., Joshi, S., Antunes, A., & AlTeneiji, M. (2022). The Microencapsulation of Tung Oil with a Natural Hydrocolloid Emulsifier for Extrinsic Self-Healing Applications. Polymers, 14(9), 1907. https://doi.org/10.3390/polym14091907






