Fabrication of a Nickel Ferrite/Nanocellulose-Based Nanocomposite as an Active Sensing Material for the Detection of Chlorine Gas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
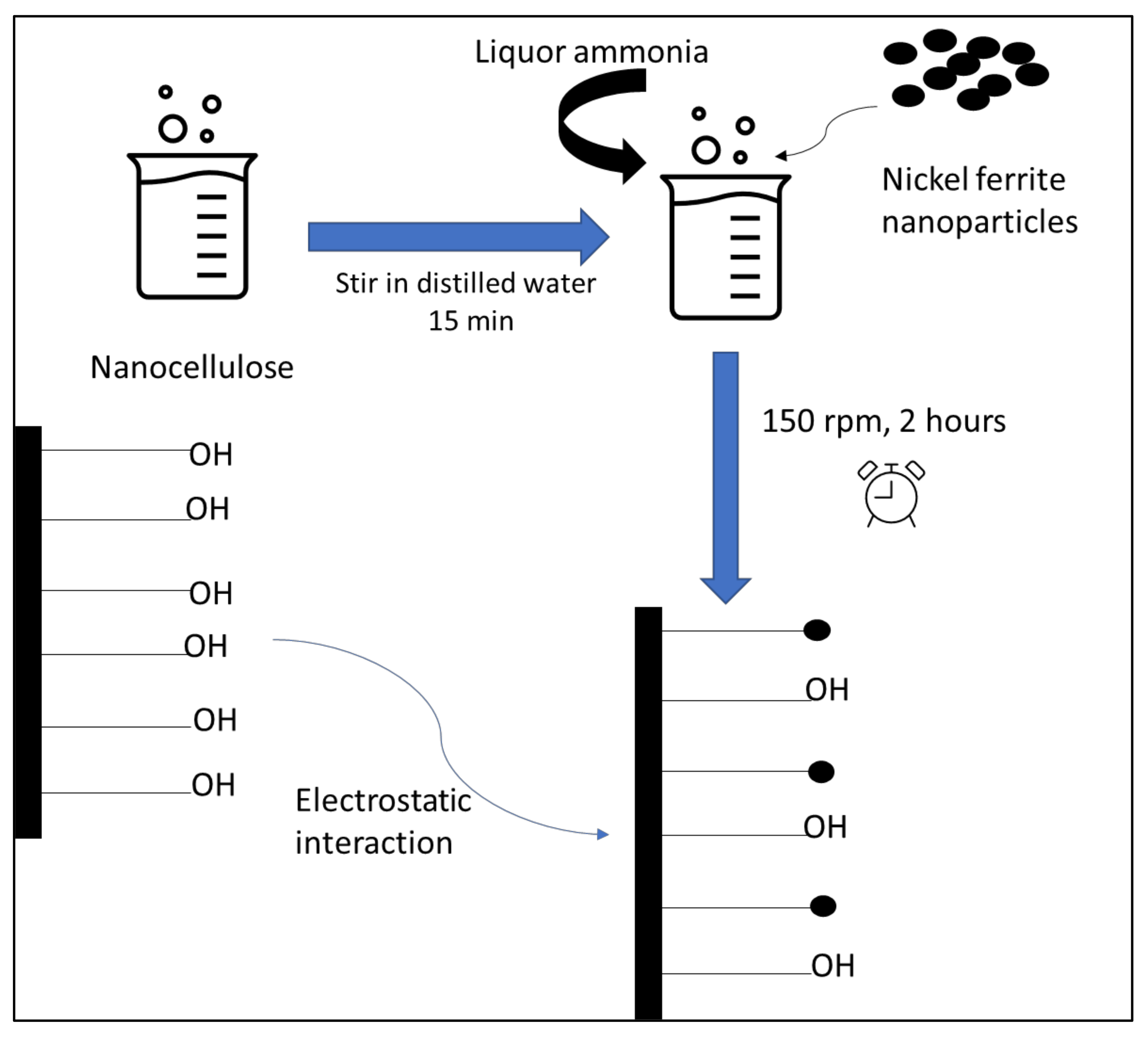
2.2. Nanocomposite Preparation
2.3. Characterisation of Hybrid Nanocomposites
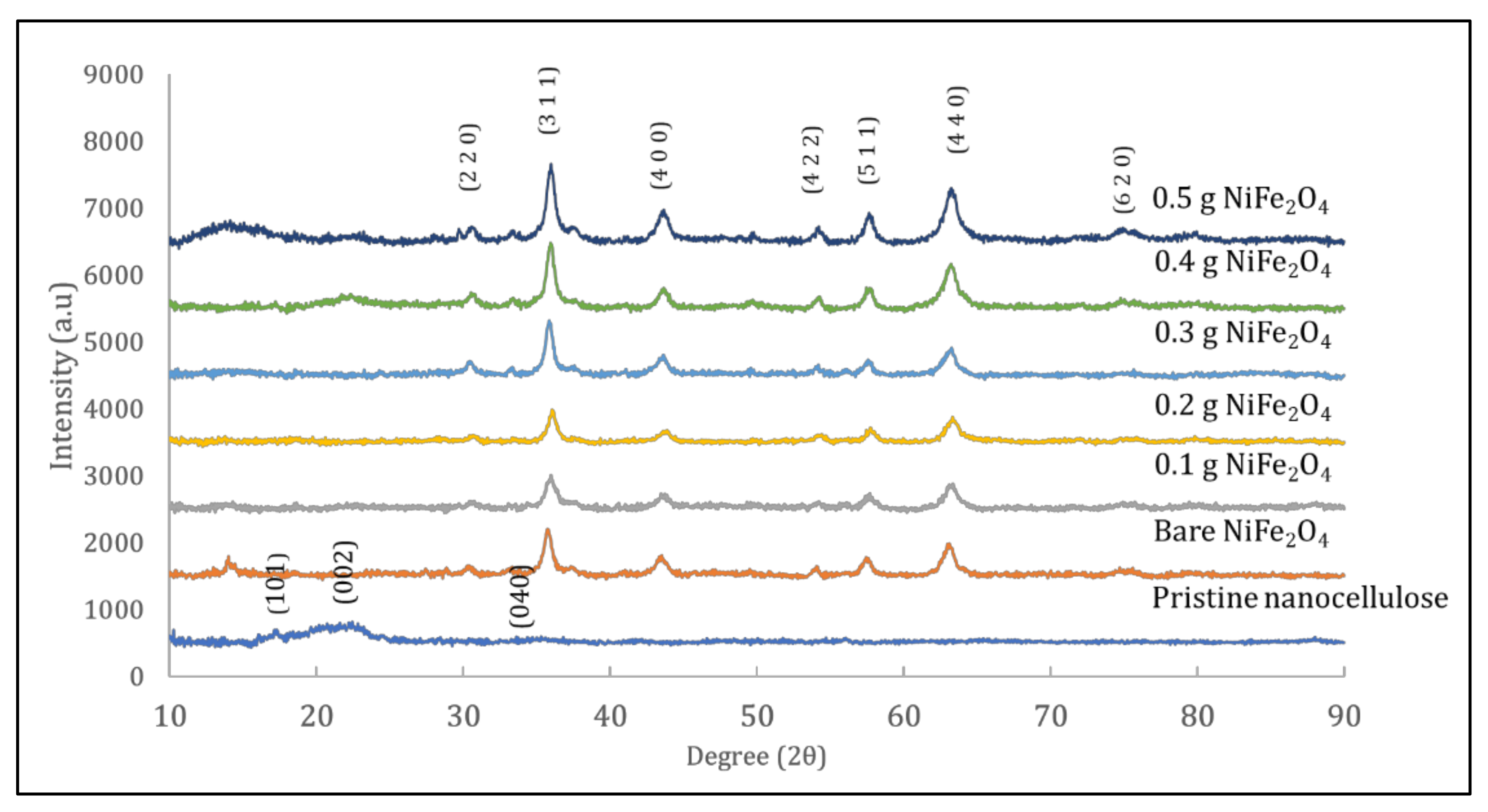
2.3.1. X-ray Diffraction Analysis
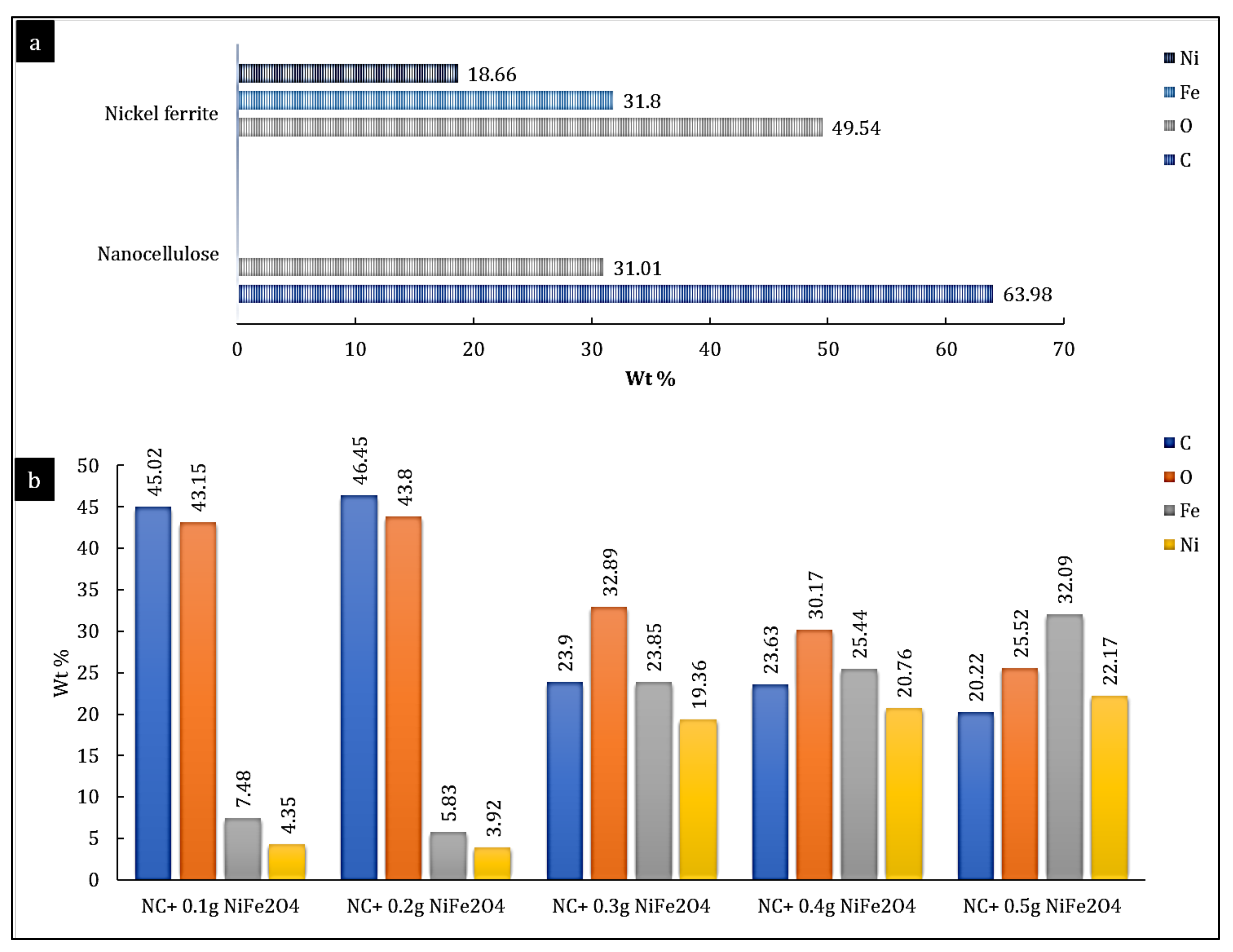
2.3.2. Energy-Dispersive X-ray Analysis
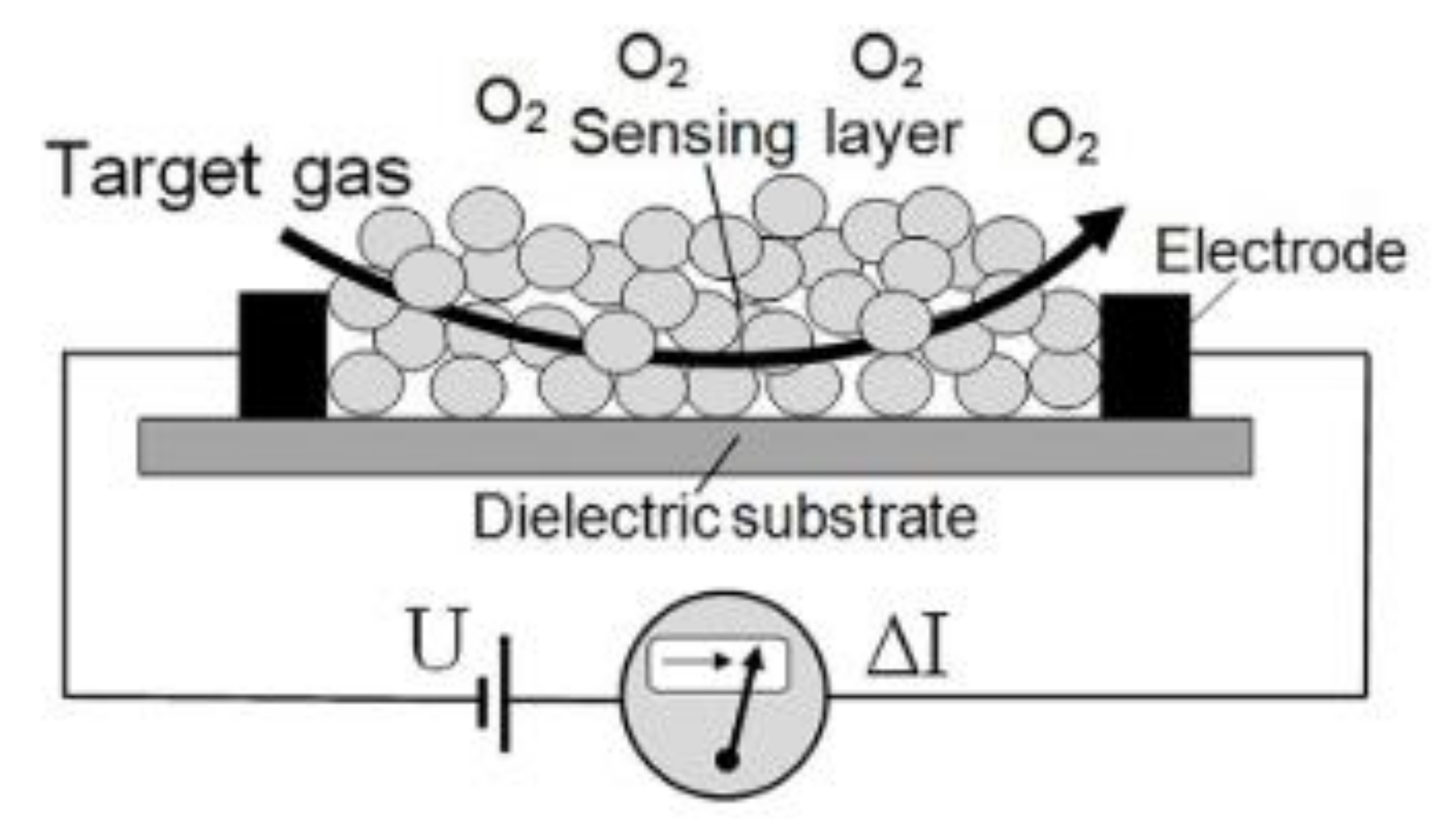
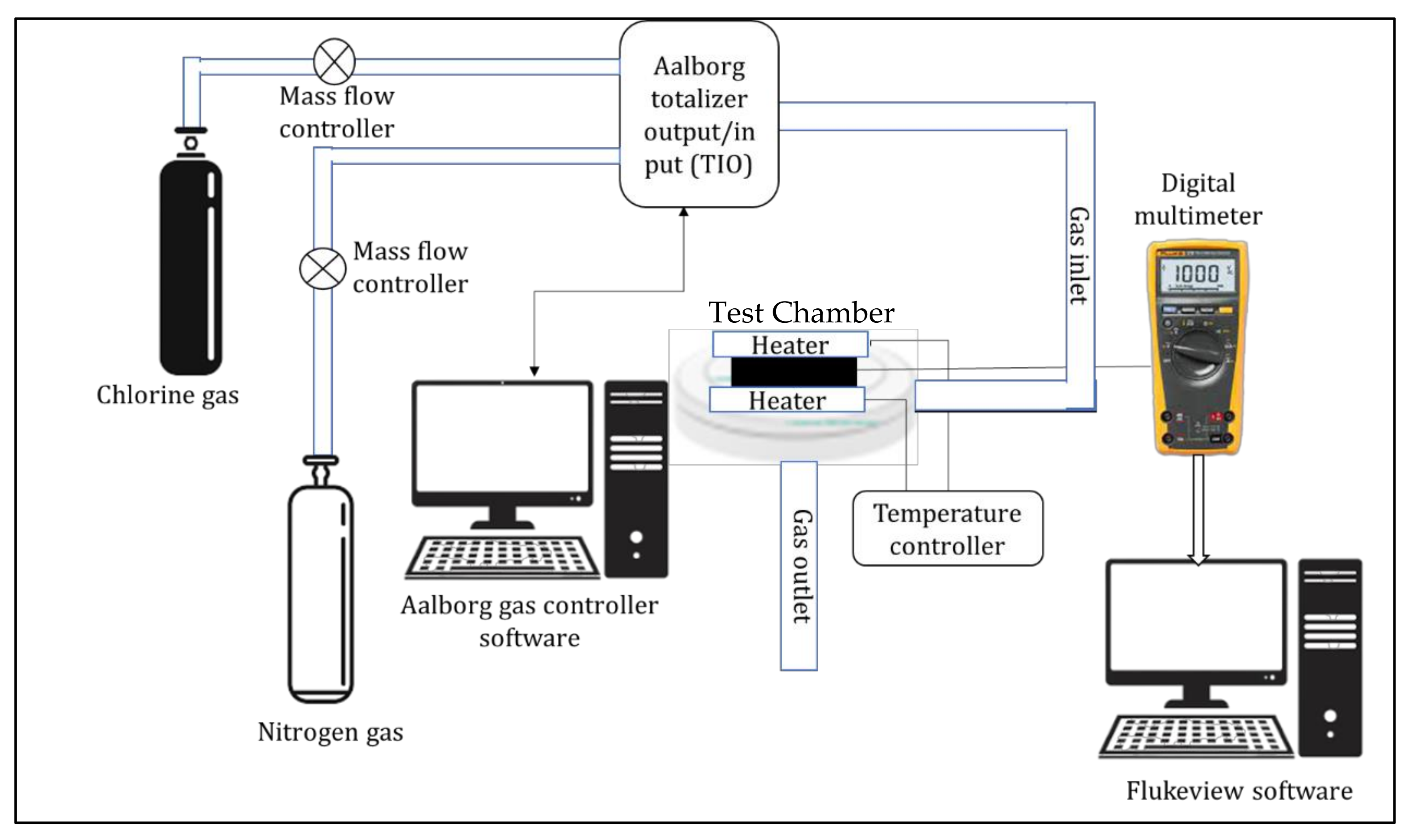
2.4. Sensing Measurements
2.4.1. Preparation of Thin-Film Sensing Materials
2.4.2. Detection of Chlorine Gas
3. Results and Discussion
3.1. Characterisation
3.2. Sensing Characteristics
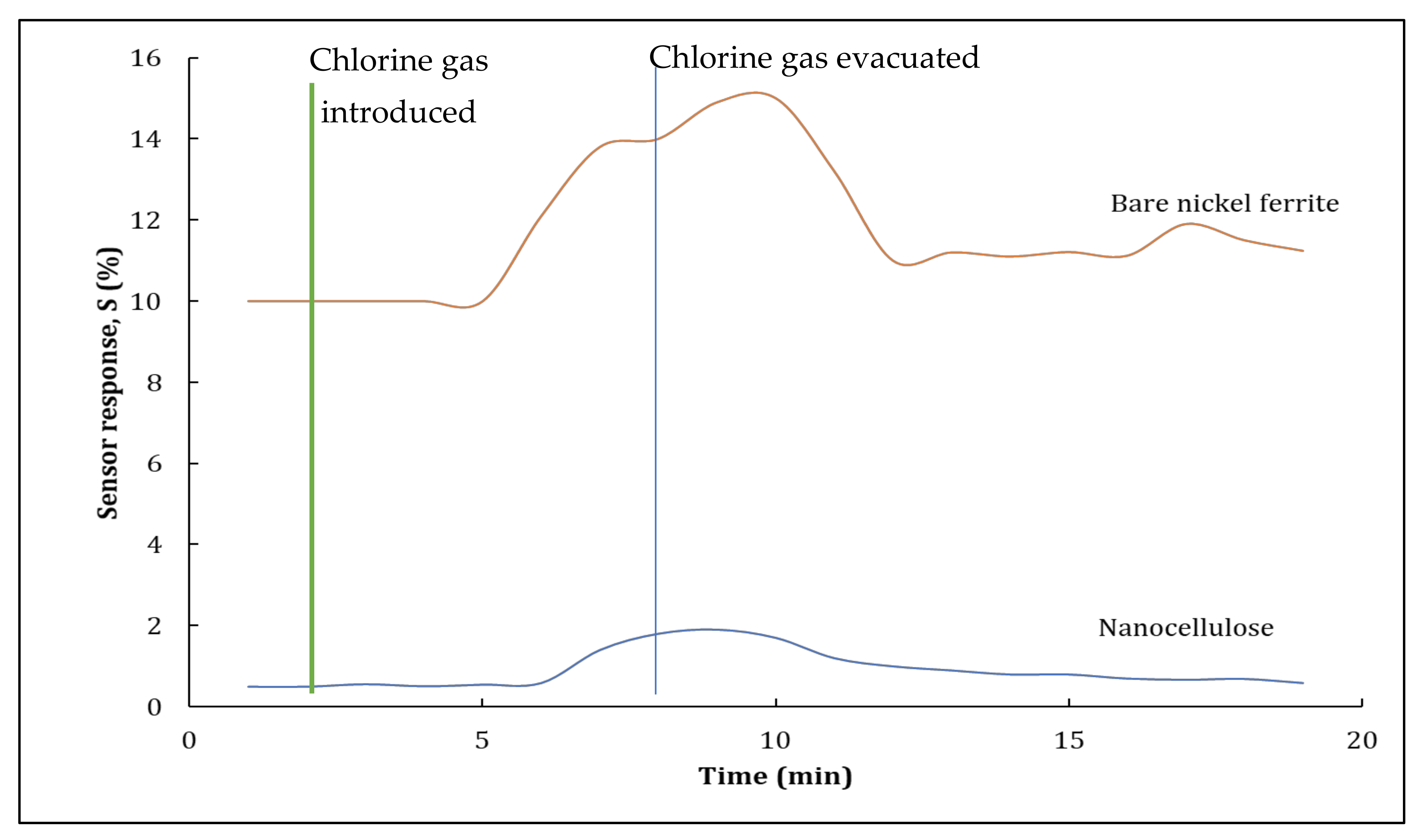
3.2.1. Pristine Nanocellulose and Nickel Ferrite
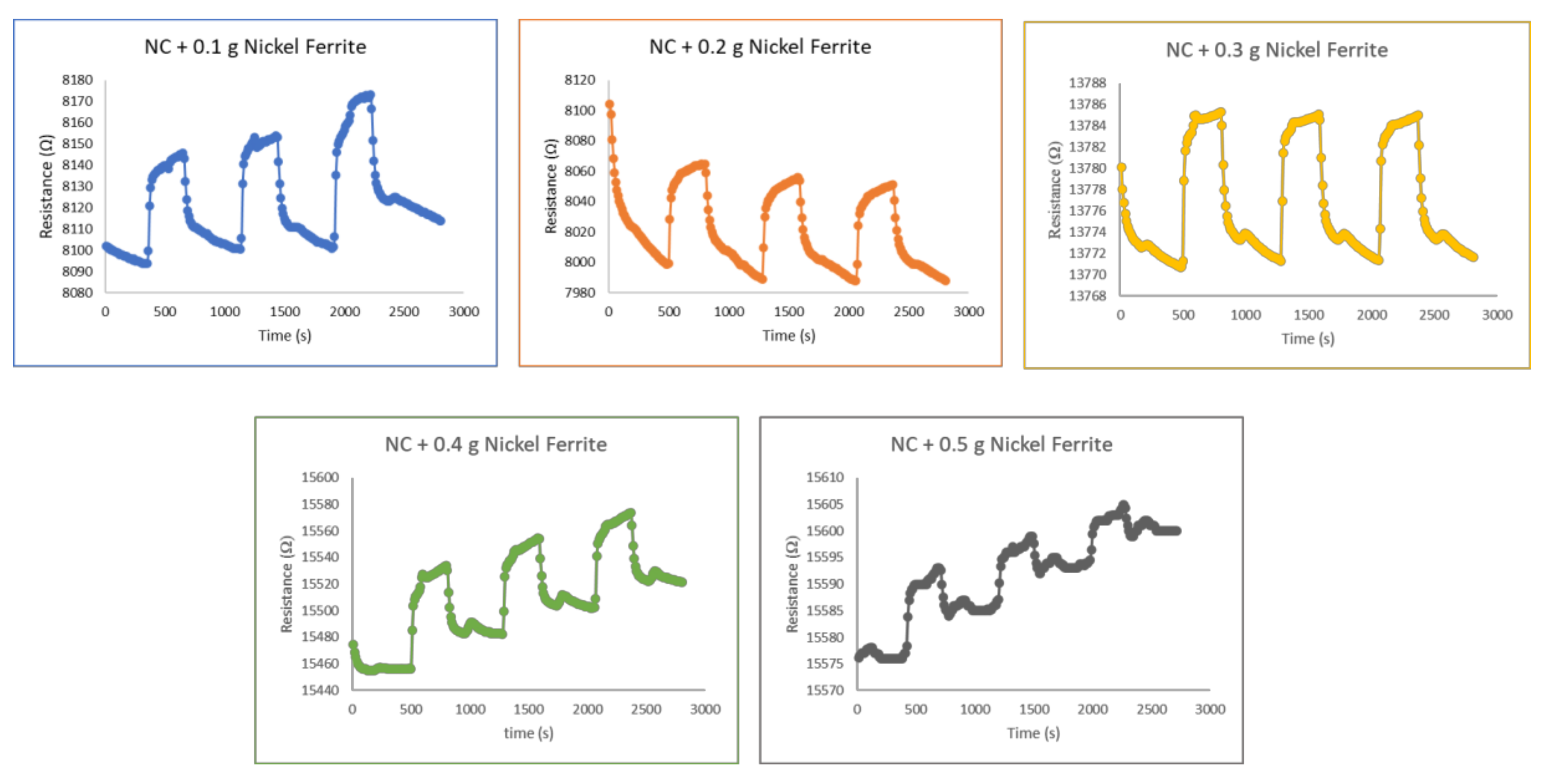
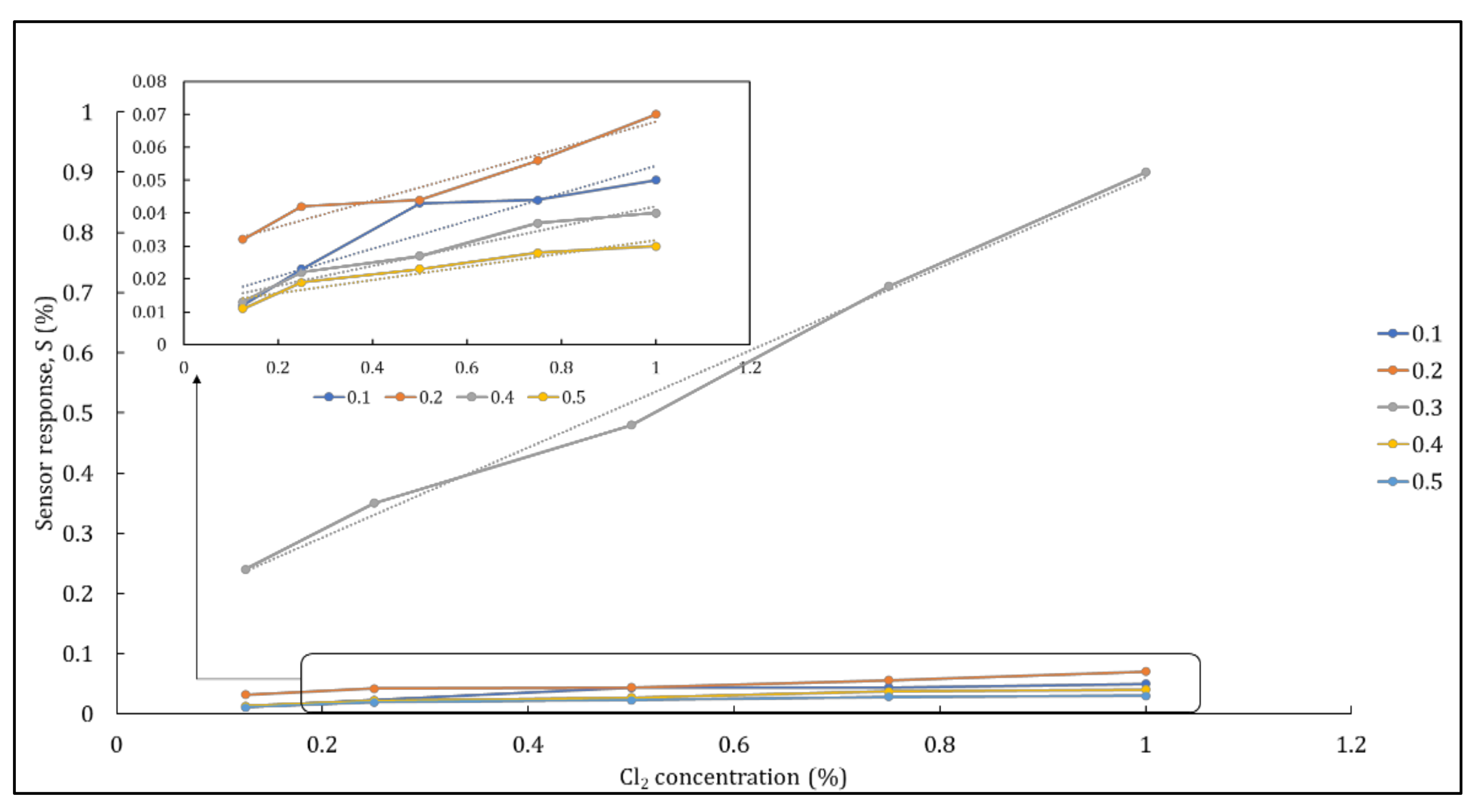
3.2.2. Nickel ferrite–nanocellulose nanocomposites
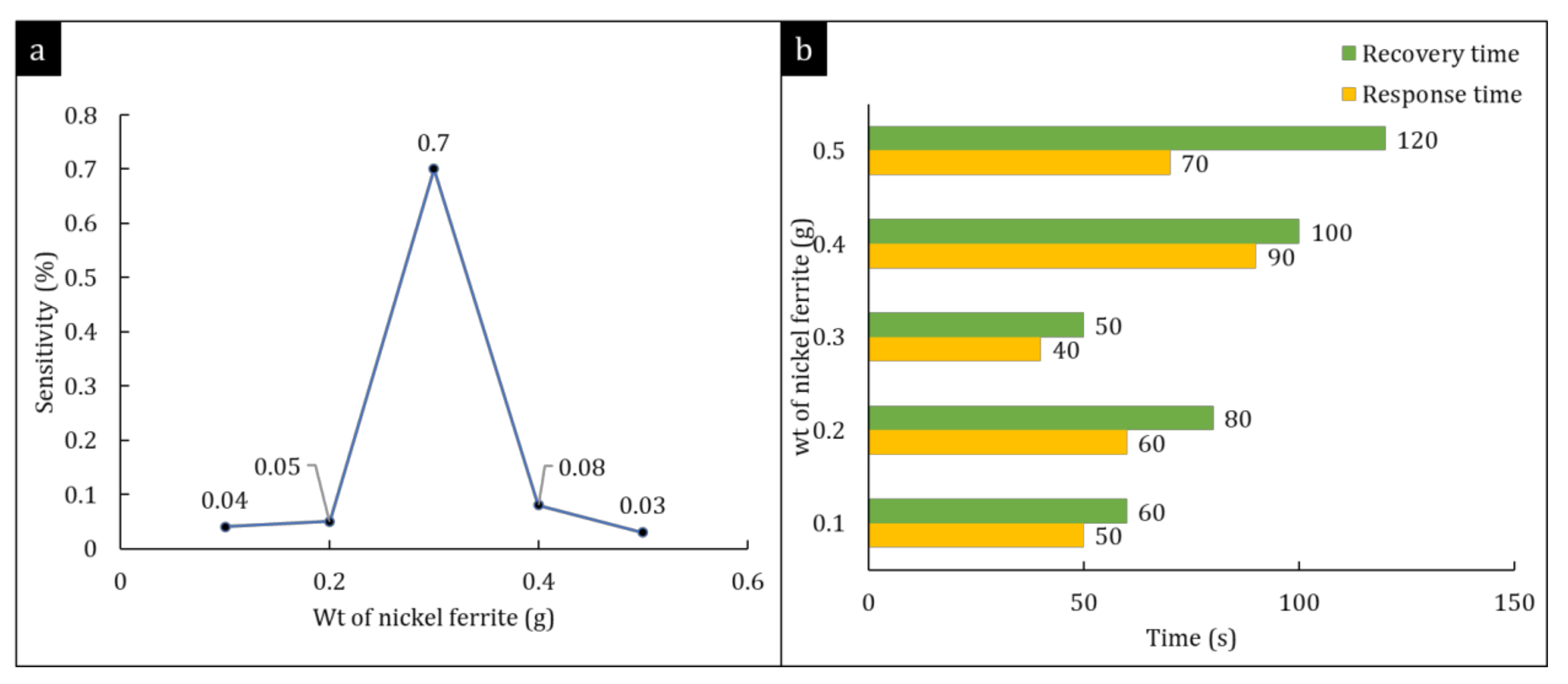
3.2.3. Sensitivity Study of Hybrid Nanocomposites
| Metal Oxides | Reaction Barrier | Ref. |
|---|---|---|
| Metal atoms (X = Cu, Zn, Cd, Ga, Al, Au, or Hg) with YH4 molecules (Y = C, Si, or Ge) | 85–109.8 | [55] |
| Ga-21.5In-10Sn/Cu | 98–106.7 | [56] |
| MgCl2 | 290.2 | [57] |
| NiFe2O4 | ~7 | [58] |
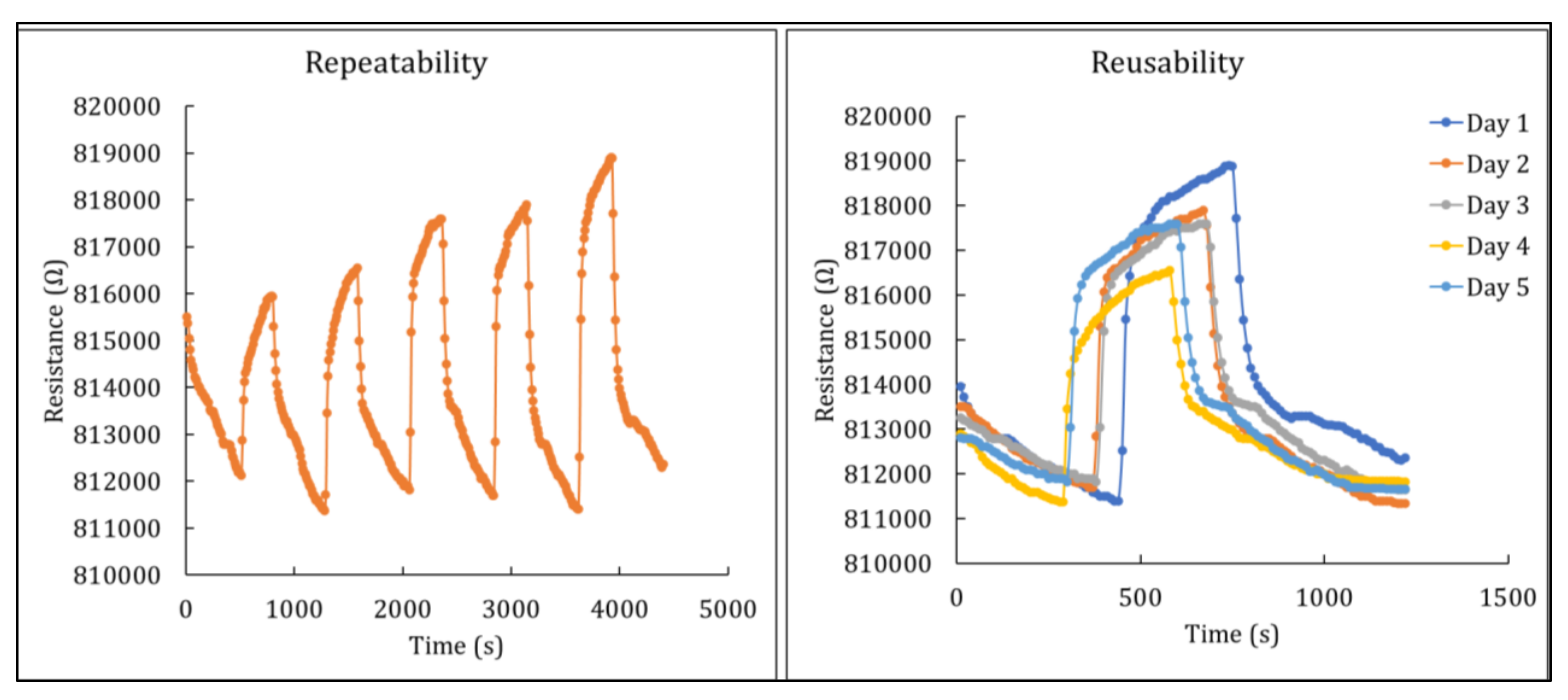
3.2.4. Repeatability and Reusability of Hybrid Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Al Madhoun, W.; Ramli, N.; Yahaya, A. Monitoring the Total Volatile Organic Compounds (TVOCs) and Benzene Emitted at Different Locations in Malaysia. J. Eng. Sci. 2012, 8, 61–69. [Google Scholar]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. The effect of hydrogen sulfide, carbon monoxide and water on the performance of a PDMS membrane in carbon dioxide/nitrogen separation. J. Membr. Sci. 2010, 350, 189–199. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Wang, F.; Qian, L.; Zhou, Y.; Qi, W.; Chen, J. Effect of disinfectant residual on the interaction between bacterial growth and assimilable organic carbon in a drinking water distribution system. Chemosphere 2018, 202, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, T.; Li, Z.; Qiu, J.; Zhao, Y.; Zhang, H.; Wang, J. Exceptional High and Reversible Ammonia Uptake by Two Dimension Few-layer BiI3 Nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 25918–25925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, P.; Ahmed, R.; Wang, J.; Akin, D.; Soto, F.; Liu, B.-F.; Li, P.; Demirci, U. Advanced Point-of-Care Testing Technologies for Human Acute Respiratory Virus Detection. Adv. Mater. 2022, 34, e2103646. [Google Scholar] [CrossRef]
- Rafiei, N.; Fatehizadeh, A.; Amin, M.M.; Pourzamani, H.R.; Ebrahimi, A.; Taheri, E.; Aminabhavi, T.M. Application of UV/chlorine processes for the DR83:1 degradation from wastewater: Effect of coexisting anions. J. Environ. Manag. 2021, 297, 113349. [Google Scholar] [CrossRef]
- Wen, G.; Cao, R.; Wan, Q.; Tan, L.; Xu, X.; Wang, J.; Huang, T. Development of fungal spore staining methods for flow cytometric quantification and their application in chlorine-based disinfection. Chemosphere 2020, 243, 125453. [Google Scholar] [CrossRef]
- Kongkaew, S.; Joonyong, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Fabrication and characterization of Prussian blue screen-printed working electrode and their application for free chlorine monitoring in swimming pool water. Electrochim. Acta 2021, 388, 138558. [Google Scholar] [CrossRef]
- Al-Mutlaq, S.; Mahal, E. Amelioration of Bitumen Characteristics Via Natural Rubber Latex and Chlorine Gas. Egypt. J. Chem. 2021, 64, 2–3. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Zhang, H.; Zhao, W.; Qu, L.; Chen, S.; Wu, S. Electrospun strong, bioactive, and bioabsorbable silk fibroin/poly (L-lactic-acid) nanoyarns for constructing advanced nanotextile tissue scaffolds. Mater. Today Bio 2022, 14, 100243. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Khajeh Ebrahimi, A.; Yarahmadi, A.; Zarrintaj, P.; Barani, M. Development of a multifunctional system based on CoFe2O4@polyacrylic acid NPs conjugated to folic acid and loaded with doxorubicin for cancer theranostics. Nanotechnology 2021, 32, 305101. [Google Scholar] [CrossRef] [PubMed]
- Samal, A.; Honovar, J.; White, C.R.; Patel, R.P. Potential for Chlorine Gas-induced Injury in the Extrapulmonary Vasculature. Proc. Am. Thorac. Soc. 2010, 7, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.F. Chapter Thirty-Two—Chlorine Dioxide. In Microbiology of Waterborne Diseases, 2nd ed.; Percival, S.L., Yates, M.V., Williams, D.W., Chalmers, R.M., Gray, N.F., Eds.; Academic Press: London, UK, 2014; pp. 591–598. ISBN 978-0-12-415846-7. [Google Scholar]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Singh, G.; Choudhary, A.; Haranath, D.; Joshi, A.G.; Singh, N.; Singh, S.; Pasricha, R. ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon N. Y. 2012, 50, 385–394. [Google Scholar] [CrossRef]
- Bouchikhi, B.; Chludziński, T.; Saidi, T.; Smulko, J.; Bari, N.E.; Wen, H.; Ionescu, R. Formaldehyde detection with chemical gas sensors based on WO3 nanowires decorated with metal nanoparticles under dark conditions and UV light irradiation. Sens. Actuators B Chem. 2020, 320, 128331. [Google Scholar] [CrossRef]
- Kalidoss, R.; Umapathy, S.; Sivalingam, Y. An investigation of GO-SnO2-TiO2 ternary nanocomposite for the detection of acetone in diabetes mellitus patient’s breath. Appl. Surf. Sci. 2017, 449, 677–684. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. Synthesis of Nanostructured Tungsten Oxide By Thermal Oxidation Method and Its Integration in Sensor for VOCs Detection. Adv. Mater. Lett. 2016, 7, 697–701. [Google Scholar] [CrossRef]
- Tasaltin, C.; Ebeoglu, M.A.; Ozturk, Z.Z. Acoustoelectric effect on the responses of saw sensors coated with electrospun ZnO nanostructured thin film. Sensors 2012, 12, 12006–12015. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Y.; Ni, Q.Q. Carbon nanotube template-assisted synthesis of zinc ferrite nanochains. Mater. Chem. Phys. 2010, 124, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Al-Rawi, N.N.; Anwer, B.A.; Al-Rawi, N.H.; Uthman, A.T.; Ahmed, I.S. Magnetism in drug delivery: The marvels of iron oxides and substituted ferrites nanoparticles. Saudi Pharm. J. 2020, 28, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Chethan, B.; Ravikiran, Y.T.; Vijayakumari, S.C.; Rajprakash, H.G.; Thomas, S. Nickel substituted cadmium ferrite as room temperature operable humidity sensor. Sens. Actuators A Phys. 2018, 280, 466–474. [Google Scholar] [CrossRef]
- Sathisha, I.C.; Manjunatha, K.; Bajorek, A.; Rajesh Babu, B.; Chethan, B.; Ranjeth Kumar Reddy, T.; Ravikiran, Y.T.; Jagadeesha Angadi, V. Enhanced humidity sensing and magnetic properties of bismuth doped copper ferrites for humidity sensor applications. J. Alloys Compd. 2020, 848, 156577. [Google Scholar] [CrossRef]
- Manikandan, V.; Singh, M.; Yadav, B.C.; Mane, R.S.; Vigneselvan, S.; Mirzaei, A.; Chandrasekaran, J. Room temperature LPG sensing properties of tin substituted copper ferrite (Sn−CuFe2O4) thin film. Mater. Chem. Phys. 2020, 240, 122265. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pal, M. Highly efficient novel carbon monoxide gas sensor based on bismuth ferrite nanoparticles for environmental monitoring. New J. Chem. 2018, 42, 7188–7196. [Google Scholar] [CrossRef]
- Wu, J.; Gao, D.; Sun, T.; Bi, J.; Zhao, Y.; Ning, Z.; Fan, G.; Xie, Z. Highly selective gas sensing properties of partially inversed spinel zinc ferrite towards H2S. Sens. Actuators B Chem. 2016, 235, 258–262. [Google Scholar] [CrossRef]
- Koli, P.B.; Kapadnis, K.H.; Deshpande, U.G. Nanocrystalline-modified nickel ferrite films: An effective sensor for industrial and environmental gas pollutant detection. J. Nanostruct. Chem. 2019, 9, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yuan, Z.; Meng, F. Spinel-type materials used for gas sensing: A review. Sensors 2020, 20, 5413. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Rao, P.; Godbole, R.V.; Bhagwat, S. Chlorine gas sensing performance of palladium doped nickel ferrite thin films. J. Magn. Magn. Mater. 2016, 405, 219–224. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Suman, S.; Sahaw, A.; Balaji, U.; Sakthivel, R. Improved LPG sensing properties of nickel doped cobalt ferrites derived from metallurgical wastes. J. Magn. Magn. Mater. 2021, 537, 168231. [Google Scholar] [CrossRef]
- Manikandan, V.; Kim, J.H.; Mirzaei, A.; Kim, S.S.; Vigneselvan, S.; Singh, M.; Chandrasekaran, J. Effect of temperature on gas sensing properties of lithium (Li) substituted (NiFe2O4) nickel ferrite thin film. J. Mol. Struct. 2019, 1177, 485–490. [Google Scholar] [CrossRef]
- Reddy, L.P.B.; Prakash, H.G.R.; Ravikiran, Y.T.; Ganiger, S.K.; Angadi, V.J. Structural and humidity sensing properties of niobium pentoxide-mixed nickel ferrite prepared by mechano-chemical mixing method. J. Mater. Sci. Mater. Electron. 2020, 31, 21981–21999. [Google Scholar] [CrossRef]
- Manikandan, V.; Mirzaei, A.; Vigneselvan, S.; Kavita, S.; Mane, R.S.; Kim, S.S.; Chandrasekaran, J. Role of ruthenium in the dielectric, magnetic properties of nickel ferrite (Ru-NiFe2O4) nanoparticles and their application in hydrogen sensors. ACS Omega 2019, 4, 12919–12926. [Google Scholar] [CrossRef] [Green Version]
- Zhai, C.; Zhang, H.; Du, L.; Wang, D.; Xing, D.; Zhang, M. Nickel/iron-based bimetallic MOF-derived nickel ferrite materials for triethylamine sensing. Cryst. Eng. Comm. 2020, 22, 1286–1293. [Google Scholar] [CrossRef]
- Virlan, C.; Tudorache, F.; Pui, A. Increased sensibility of mixed ferrite humidity sensors by subsequent heat treatment. Int. J. Appl. Ceram. Technol. 2017, 14, 1174–1182. [Google Scholar] [CrossRef]
- Lizundia, E.; Rincón-Iglesias, M.; Lanceros-Méndez, S. Combining cobalt ferrite and graphite with cellulose nanocrystals for magnetically active and electrically conducting mesoporous nanohybrids. Carbohydr. Polym. 2020, 236, 116001. [Google Scholar] [CrossRef]
- Gupta, K.; Kaushik, A.; Tikoo, K.B.; Kumar, V.; Singhal, S. Enhanced catalytic activity of composites of NiFe2O4 and nano cellulose derived from waste biomass for the mitigation of organic pollutants. Arab. J. Chem. 2020, 13, 783–798. [Google Scholar] [CrossRef]
- Fatima, A.; Yasir, S.; Khan, M.S.; Manan, S.; Ullah, M.W.; Ul-Islam, M. Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 2021, 6, 26–32. [Google Scholar] [CrossRef]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A Mussel-Inspired Polydopamine-Filled Cellulose Aerogel for Solar-Enabled Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Zheng, C.; Lin, H.; Xu, R.; Zhu, H.; Bao, L.; Xu, X. Lanthanum carbonate grafted ZSM-5 for superior phosphate uptake: Investigation of the growth and adsorption mechanism. Chem. Eng. J. 2022, 430, 133166. [Google Scholar] [CrossRef]
- Pawar, C.S.; Gujar, M.P.; Mathe, V.L. Synthesis and Characterization of Nano crystalline Nickel Zinc Ferrite for Chlorine * as 6 ensor at 5 oom 7 emperature. AIP Conf. Proc. 2015, 2015, 050060. [Google Scholar] [CrossRef]
- Silva, G.M.G.; Leão, V.N.S.; Pereira, M.F.G.; Faia, P.M.; Araújo, E.S. Zn2+-Doped TiO2:WO3 Films Prepared by Electrospinning and Sintering: Microstructural Characterization and Electrical Signature to Moisture Sensing. Ceramics 2021, 4, 576–591. [Google Scholar] [CrossRef]
- Rahimi Kord Sofla, M.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Asiri, S.; Sertkol, M.; Güngüneş, H.; Amir, M.; Manikandan, A.; Ercan, I.; Baykal, A. The Temperature Effect on Magnetic Properties of NiFe2O4 Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1587–1597. [Google Scholar] [CrossRef]
- Virlan, C.; Tudorache, F.; Pui, A. Tertiary NiCuZn ferrites for improved humidity sensors: A systematic study. Arab. J. Chem. 2020, 13, 2066–2075. [Google Scholar] [CrossRef]
- Soleimanpour, A.M.; Jayatissa, A.H.; Sumanasekera, G. Surface and gas sensing properties of nanocrystalline nickel oxide thin films. Appl. Surf. Sci. 2013, 276, 291–297. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Kasim, N.A.M.; Knight, V.F.; Ong, K.K.; Noor, S.A.M.; Halim, N.A.; Shah, N.A.A.; Jamal, S.H.; Janudin, N.; Misenan, M.S.M.; et al. Emerging developments regarding nanocellulose-based membrane filtration material against microbes. Polymers 2021, 13, 3249. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mahajan, A.; Kumar, S.; Debnath, A.K.; Aswal, D.K. Tailoring of the chlorine sensing properties of substituted metal phthalocyanines non-covalently anchored on single-walled carbon nanotubes. RSC Adv. 2018, 8, 32719–32730. [Google Scholar] [CrossRef] [Green Version]
- Šutka, A.; Gross, K.A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B Chem. 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Wang, C.; Ma, J.; Peng, H.; Wen, Y.; Fan, H. High sensitivity and selectivity chlorine gas sensors based on 3D open porous SnO2 synthesized by solid-state method. Ceram. Int. 2019, 45, 20566–20574. [Google Scholar] [CrossRef]
- Bedi, R.K.; Saini, R.; Mahajan, A. Room temperature ppb level chlorine gas sensor based on copper (II) 1,4,8,11,15,18,22,25-octabutoxy-29H,31H-phthalocyanine films. AIP Conf. Proc. 2010, 1313, 205–208. [Google Scholar] [CrossRef]
- Novaro, O.; Pacheco-Blas, M.D.A.; Pacheco-Sánchez, J.H. Potential energy surfaces for reactions of X metal atoms (X = Cu, Zn, Cd, Ga, Al, Au, or Hg) with YH 4 Molecules (Y = C, Si, or Ge) and transition probabilities at avoided crossings in some cases. Adv. Phys. Chem. 2012, 2012, 720197. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Wang, C.; Gao, N.; Guo, S.; Chen, Y.; Chai, Z.; Wang, Y.; Ma, H. Electrodeposited Ni-W coatings as the effective reaction barrier at Ga-21.5In-10Sn/Cu interfaces. Surf. Interfaces 2022, 30, 101838. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Hu, J.; Zheng, Y.; Huang, M.; Guo, K.; Li, C. Reversible Mg metal anode in conventional electrolyte enabled by durable heterogeneous SEI with low surface diffusion barrier. Energy Storage Mater. 2022, 46, 1–9. [Google Scholar] [CrossRef]
- Rák, Z.; Brenner, D.W. Negative surface energies of nickel ferrite nanoparticles under hydrothermal conditions. J. Nanomater. 2019, 2019, 5268415. [Google Scholar] [CrossRef]
- Kamble, R.B.; Mathe, V.L. Nanocrystalline nickel ferrite thick film as an efficient gas sensor at room temperature. Sens. Actuators B Chem. 2008, 131, 205–209. [Google Scholar] [CrossRef]
| Sensing Material | Target Gas | Operating T (°C) | Concentration of Gas (ppm) | RT and RC | S (%) | Ref. |
|---|---|---|---|---|---|---|
| Pd-doped NiFe2O4 | Cl2 | 325 | 15 | 2 s and 6 s | 6.9 | [31] |
| Co-doped NiFe2O4 | LPG | 250 | 5000 | 25 s and 240 s | 86 | [32] |
| Li-doped NiFe2O4 | H2 | 200 | 100 | Not mentioned | 1.09 | [33] |
| Nb2O5 mixed with NiFe2O4 | Humidity | 25 | 11–97 % | 20 s and 30 s | 1190 | [34] |
| Ru−NiFe2O4 | H2 | 100 | 50 | Not mentioned | 1.36 | [35] |
| NiFe2O4 | Triethylamine | 190 | 50 | 6 s and 585 s | 18.9 | [36] |
| Sample | Lattice Parameters (Å) | Average Crystallite Size, D (nm) | |
|---|---|---|---|
| Nanocellulose | NiFe2O4 | ||
| Pristine NC | − | 1.40 | − |
| Bare NiFe2O4 | 8.2690 | − | 0.83 |
| NC + 0.1 g NiFe2O4 | 8.1810 | 1.34 | 0.96 |
| NC + 0.2 g NiFe2O4 | 8.2010 | 1.28 | 0.97 |
| NC + 0.3 g NiFe2O4 | 8.2125 | 1.22 | 0.98 |
| NC + 0.4 g NiFe2O4 | 8.2183 | 1.33 | 0.83 |
| NC + 0.5 g NiFe2O4 | 8.2203 | 1.25 | 0.93 |
| Hybrid Nanocomposite | Concentration (%) | Fitting Function | R2 | ||||
|---|---|---|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 0.75 | 1.0 | |||
| NC + 0.1 g NiFe2O4 | 0.012 | 0.023 | 0.043 | 0.044 | 0.05 | y = 0.0419x + 0.0124 | 0.8622 |
| NC + 0.2 g NiFe2O4 | 0.032 | 0.042 | 0.044 | 0.056 | 0.07 | y = 0.0398x + 0.0279 | 0.9522 |
| NC + 0.3 g NiFe2O4 | 0.240 | 0.350 | 0.480 | 0.710 | 0.90 | y = 0.7473x + 0.1437 | 0.9934 |
| NC + 0.4 g NiFe2O4 | 0.013 | 0.022 | 0.027 | 0.037 | 0.04 | y = 0.0300x + 0.012 | 0.9506 |
| NC + 0.5 g NiFe2O4 | 0.011 | 0.019 | 0.023 | 0.028 | 0.03 | y = 0.0202x + 0.0116 | 0.9056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janudin, N.; Kasim, N.A.M.; Feizal Knight, V.; Norrrahim, M.N.F.; Razak, M.A.I.A.; Abdul Halim, N.; Mohd Noor, S.A.; Ong, K.K.; Yaacob, M.H.; Ahmad, M.Z.; et al. Fabrication of a Nickel Ferrite/Nanocellulose-Based Nanocomposite as an Active Sensing Material for the Detection of Chlorine Gas. Polymers 2022, 14, 1906. https://doi.org/10.3390/polym14091906
Janudin N, Kasim NAM, Feizal Knight V, Norrrahim MNF, Razak MAIA, Abdul Halim N, Mohd Noor SA, Ong KK, Yaacob MH, Ahmad MZ, et al. Fabrication of a Nickel Ferrite/Nanocellulose-Based Nanocomposite as an Active Sensing Material for the Detection of Chlorine Gas. Polymers. 2022; 14(9):1906. https://doi.org/10.3390/polym14091906
Chicago/Turabian StyleJanudin, Nurjahirah, Noor Azilah Mohd Kasim, Victor Feizal Knight, Mohd Nor Faiz Norrrahim, Mas Amira Idayu Abdul Razak, Norhana Abdul Halim, Siti Aminah Mohd Noor, Keat Khim Ong, Mohd Hanif Yaacob, Muhammad Zamharir Ahmad, and et al. 2022. "Fabrication of a Nickel Ferrite/Nanocellulose-Based Nanocomposite as an Active Sensing Material for the Detection of Chlorine Gas" Polymers 14, no. 9: 1906. https://doi.org/10.3390/polym14091906
APA StyleJanudin, N., Kasim, N. A. M., Feizal Knight, V., Norrrahim, M. N. F., Razak, M. A. I. A., Abdul Halim, N., Mohd Noor, S. A., Ong, K. K., Yaacob, M. H., Ahmad, M. Z., & Yunus, W. M. Z. W. (2022). Fabrication of a Nickel Ferrite/Nanocellulose-Based Nanocomposite as an Active Sensing Material for the Detection of Chlorine Gas. Polymers, 14(9), 1906. https://doi.org/10.3390/polym14091906







