Biodegradation of Polymers Used in Oil and Gas Operations: Towards Enzyme Biotechnology Development and Field Application
Abstract
1. Introduction
2. Approaches Used to Study Polymer Biodegradation
3. Polymer Biodegradation—Current State of Knowledge
3.1. Guar Gum
3.2. Cellulose-Based Polymers
3.3. PAM and HPAM
4. Advantages and Challenges of Applying Enzyme Biotechnologies to Oil and Gas Recovery Operations
5. Summary and Gaps in Knowledge
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mouser, P.J.; Borton, M.; Darrah, T.H.; Hartsock, A.; Wrighton, K.C. Hydraulic fracturing offers view of microbial life in the deep terrestrial subsurface. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [PubMed]
- Economides, M.J.; Mikhailov, D.N.; Nikolaevskiy, V.N. On the problem of fluid leakoff during hydraulic fracturing. Transp. Porous Media 2006, 67, 487–499. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.-T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 40735. [Google Scholar] [CrossRef]
- Fink, J. Water-Based Chemicals and Technology for Drilling, Completion, and Workover Fluids; Elsevier Science & Technology: Saint Louis, MO, USA, 2015. [Google Scholar]
- Horst, P.M.V.D. Use of CMC in drilling fluids. U.S. Patent 7,939,469 B2, 10 May 2011. [Google Scholar]
- Carlson, J.; Gurley, D.; King, G.; Price-Smith, C.; Waters, F. Sand control: Why and how. Oilfield Rev. 1992, 4, 41–53. [Google Scholar]
- Montgomery, C. Fracturing Fluid Components. In Effective and Sustainable Hydraulic Fracturing; Jeffrey, R., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Fink, J. Hydraulic Fracturing Chemicals and Fluids Technology; Gulf professional publishing: Saint Louis, MO, USA, 2013. [Google Scholar]
- Charoenwongsa, S.; Kazemi, H.; Fakcharoenphol, P.; Miskimins, J.L. Simulation of Gel Filter Cake Formation, Gel Cleanup, and Post-Frac Well Performance in Hydraulically Fractured Gas Wells. SPE Prod. Oper. 2013, 28, 235–245. [Google Scholar] [CrossRef]
- Brannon, H.D.; Tjon-Joe-Pin, R.M. Biotechnological Breakthrough Improves Performance of Moderate to High-Temperature Fracturing Applications. SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 25–28 September; 1994; pp. 515–530. [Google Scholar] [CrossRef]
- Armstrong, C.D.; Stevens, R.F.; Van Le, H.; Stephenson, C.J.; Qu, Q. The Next Generation of Regenerative Catalytic Breakers for Use in Alkaline and High-Temperature Fracturing Fluids. SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 10–12 February; 2010; pp. 1–13. [Google Scholar]
- Cooper, G.M. The central role of enzymes as biological catalysts. In The Cell: A Molecular Approach; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Brannon, H.D.; Tjon-Joe-Pin, R.M.; Carman, P.S.; Wood., W.D. Enzyme breaker technologies: A decade of improved well stimulation. SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October; 2003. [Google Scholar]
- Gao, C. Viscosity of partially hydrolyzed polyacrylamide under shearing and heat. J. Pet. Explor. Prod. Technol. 2013, 3, 203–206. [Google Scholar] [CrossRef]
- Sang, G.; Pi, Y.; Bao, M.; Li, Y.; Lu, J. Biodegradation for hydrolyzed polyacrylamide in the anaerobic baffled reactor combined aeration tank. Ecol. Eng. 2015, 84, 121–127. [Google Scholar] [CrossRef]
- Whitaker, J.R. Determination of molecular weights of proteins by gel filtration of Sephadex. Anal. Chem. 1963, 35, 1950–1953. [Google Scholar] [CrossRef]
- Yu, F.; Fu, R.; Xie, Y.; Chen, W. Isolation and characterization of polyacrylamide-degrading bacteria from dewatered sludge. Int. J. Environ. Res. Public Health 2015, 12, 4214–4230. [Google Scholar] [CrossRef] [PubMed]
- Gharfeh, S.G.; Moradi-Araghi, A. Determination of anionic high-molecular-weight water-soluble polymers by size-exclusion chromatography. J. Chromatogr. A 1986, 366, 343–350. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Lin, K.; Cai, W. Microbial degradation of polyacrylamide by aerobic granules. Environ. Technol. 2012, 33, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Nakamiya, K.; Kinoshita, S. Isolation of polyacrylamide-degrading bacteria. J. Ferment. Bioeng. 1995, 80, 418–420. [Google Scholar] [CrossRef]
- Sorbie, K.S. Polymer-Improved Oil Recovery; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Scoggins, M.; Miller, J. Determination of Water-soluble Polymers Containing Primary Amide Groups Using the Starch-triiodide Method. SPE Annu. Tech. Conf. Exhib. 1979, 19, 151–154. [Google Scholar] [CrossRef]
- Lu, J.H.; Wu, L. Spectrophotometric determination of polyacrylamide in waters containing dissolved organic matter. J. Agric. Food Chem. 2001, 49, 4177–4182. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sharrock, K. Cellulase assay methods: A review. J. Biochem. Biophys. Methods 1988, 17, 81–105. [Google Scholar] [CrossRef]
- Gusakov, A.V.; Kondratyeva, E.G.; Sinitsyn, A.P. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int. J. Anal. Chem. 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Deshpande, M.V.; Eriksson, K.-E.; Göran Pettersson, L. An assay for selective determination of exo-1,4,-β-glucanases in a mixture of cellulolytic enzymes. Anal. Biochem. 1984, 138, 481–487. [Google Scholar] [CrossRef]
- Bao, M.; Chen, Q.; Li, Y.; Jiang, G. Biodegradation of partially hydrolyzed polyacrylamide by bacteria isolated from production water after polymer flooding in an oil field. J. Hazard. Mater. 2010, 184, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wei, L.; Wang, L.; Chang, C.C. Isolation and identification of the sulphate-reducing bacteria strain H1 and its function for hydrolysed polyacrylamide degradation. Int. J. Biotechnol. 2008, 10, 55–63. [Google Scholar] [CrossRef]
- Scheffer, G.; Berdugo-Clavijo, C.; Sen, A.; Gieg, L.M. Enzyme biotechnology development for treating polymers in hydraulic fracturing operations. Microb. Biotechnol. 2021, 14, 953–966. [Google Scholar] [CrossRef]
- Tjon-Joe-Pin, R.M. Enzyme breaker for galactomannan based fracturing fluids. U.S. Patent 5,806,597 A, 13 April 1993. [Google Scholar]
- Meng, Y.; Zhao, F.; Jin, X.; Feng, Y.; Sun, G.; Lin, J.; Jia, B.; Li, P. Performance evaluation of enzyme breaker for fracturing applications under simulated reservoir conditions. Molecules 2021, 26, 3133. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Abdelrahim, M.; Belhaj., H. Delayed breaker systems to remove residual polymer damage in hydraulically fractured reservoirs. ACS Omega 2021, 6, 31646–31657. [Google Scholar] [CrossRef] [PubMed]
- Fridjonsson, O.; Watzlawick, H.; Gehmeiler, A.; Rohrhirsch, T.; Mattes, R. Cloning of the gene encoding a novel thermostable alpha-galactosidase from Thermus brockianus ITI360. Appl. Environ. Microbiol. 1999, 64, 3955–3963. [Google Scholar] [CrossRef]
- Liebl, W.; Wagner, B.; Schellhase, J. Properties of an α-galactosidase, and structure of its gene galA, within an α-and β-galactoside utilization gene cluster of the hyperthermophilic bacterium Thermotoga maritima. Syst. Appl. Microbiol. 1998, 21, 1–11. [Google Scholar] [CrossRef]
- McCutchen, C.M.; Duffaud, G.D.; Leduc, P.; Petersen, A.R.H.; Tayal, A.; Khan, S.A.; Kelly, R.M. Characterization of extremely thermostable enzymatic breakers (α-1,6-galactosidase and β-1,4-mannanase) from the hyperthermophilic bacterium Thermotoga neapolitana 5068 for hydrolysis of guar gum. Biotechnol. Bioeng. 1996, 52, 332–339. [Google Scholar] [CrossRef]
- You, J.; Liu, J.-F.; Yang, S.-Z.; Mu, B.-Z. Low-temperature-active and salt-tolerant β-mannanase from a newly isolated Enterobacter sp. strain N18. J. Biosci. Bioeng. 2016, 121, 140–146. [Google Scholar] [CrossRef]
- Politz, O.; Krah, M.; Thomsen, K.K.; Borriss, R. A highly thermostable endo-(1,4)- β-mannanase from the marine bacterium Rhodothermus marinus. Appl. Microbiol. Biotechnol. 2000, 53, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Chopade, P.; Fontenelle, L.; Reddy, B.R.; Coria, B. Novel Stabilized Enzyme Breaker for Fracturing Applications. SPE Prod. Oper. Symp. 2015, 1–9. [Google Scholar] [CrossRef]
- Cobianco, S.; Albonico, P.; Battistel, E.; Bianchi, D.; Fornaroli, M. Thermophilic Enzymes for Filtercake Removal at High Temperature. SPE Eur. Form. Damage Conf. 2007, 1–9. [Google Scholar] [CrossRef]
- Gilbert, W.J.R.; Johnson, S.J.; Tsau, J.-S.; Liang, J.-T.; Scurto, A.M. Enzymatic degradation of polyacrylamide in aqueous solution with peroxidase and H2O2. J. Appl. Polym. Sci. 2016, 134, 1–10. [Google Scholar] [CrossRef]
- Nakamiya, K.; Ooi, T.; Kinoshita, S. Degradation of synthetic water-soluble polymers by hydroquinone peroxidase. J. Ferment. Bioeng. 1997, 84, 213–218. [Google Scholar] [CrossRef]
- Ramsden, D.; Fielding, S.; Atkinson, N.; Boota, M. The degradation of polyacrylamide in aqueous solution induced by chemically generated hydroxyl radicals—Part III: Xanthine/xanthine oxidase. Polym. Degrad. Stab. 1987, 17, 49–55. [Google Scholar] [CrossRef]
- Zhao, L.; Song, T.; Han, D.; Bao, M.; Lu, J. Hydrolyzed polyacrylamide biotransformation in an up-flow anaerobic sludge blanket reactor system: Key enzymes, functional microorganisms, and biodegradation mechanisms. Bioprocess Biosyst. Eng. 2019, 42, 941–951. [Google Scholar] [CrossRef]
- Ma, L.; Hu, T.; Liu, Y.; Liu, J.; Wang, Y.; Zhou, J.; Chen, M.; Yang, B.; Li, L. Combination of biochar and immobilized bacteria accelerates polyacrylamide biodegradation in soil by both bio-augmentation and bio-stimulation strategies. J. Hazard. Mater. 2021, 405, 1–12. [Google Scholar] [CrossRef]
- Song, T.; Li, S.; Lu, Y.; Yan, D.; Sun, P.; Bao, M.; Li, Y. Biodegradation of hydrolyzed polyacrylamide by a Bacillus megaterium strain SZK-5: Functional enzymes and antioxidant defense mechanism. Chemosphere 2019, 231, 183–193. [Google Scholar] [CrossRef]
- Gupta, D.V.S. Method of using asparaginase as a polyacrylamide enzyme breaker. U.S. Patent 9,090,815 B2, 28 July 2015. [Google Scholar]
- Rickards, A.R.; Tjon-Joe-Pin, R.M.; Boles, J.L. Enzymatic breaker system for nondamaging removal of cellulose-based blocking gels. SPE Production Operations Symposium, Oklahoma City, OK, USA, 21–23 March; 1993; pp. 1–12. [Google Scholar] [CrossRef]
- Scheffer, G.; Rachel, N.M.; Ng, K.K.; Sen, A.; Gieg, L.M. Preparation and identification of carboxymethyl cellulose-degrading enzyme candidates for oilfield applications. J. Biotechnol. 2022, 347, 18–25. [Google Scholar] [CrossRef]
- Trabelsi, S.; Kakadjian, S. Comparative Study Between Guar and Carboxymethylcellulose Used as Gelling Systems in Hydraulic Fracturing Application. SPE Prod. Oper. Symp. 2013, 1–20. [Google Scholar] [CrossRef]
- Hasan, A.M.; Abdel-Raouf, M.E. Applications of guar gum and its derivatives in petroleum industry: A review. Egypt. J. Pet. 2018, 27, 1043–1050. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications-A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Clark, A.H.; Dea, I.C.; Rees, D.A. The fine structures of carob and guar galactomannans. Carbohydr. Res. 1985, 139, 237–260. [Google Scholar] [CrossRef]
- Comfort, D.A.; Chhabra, S.R.; Conners, S.B.; Chou, C.-J.; Epting, K.L.; Johnson, M.R.; Jones, K.L.; Sehgal, A.C.; Kelly, R.M. Strategic biocatalysis with hyperthermophilic enzymes. Green Chem. 2004, 6, 459–465. [Google Scholar] [CrossRef]
- Zhou, J.; Legemah, M.; Beall, B.; Sun, H.; Qu, Q. Alternative Polysaccharide Fracturing Fluids for Harsh Reservoir Conditions. SPE Unconv. Resour. Conf. Exhib. Asia Pac. 2013, 1–8. [Google Scholar] [CrossRef]
- Azizov, E.; Quintero, H.J.; Saxton, K.; Sessarego, S. Carboxymethylcellulose a Cost Effective Alternative to Guar, CMHPG and Surfactant-Based Fluid Systems. SPECSUR Unconv. Resour. Conf. 2015, 1–31. [Google Scholar] [CrossRef]
- Aubert, J.P.; Béguin, P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar]
- Sieger, C.; Kroon, A.; Batelaan, J.; van Ginkel, C. Biodegradation of carboxymethyl celluloses by Agrobacterium CM-1. Carbohydr. Polym. 1995, 27, 137–143. [Google Scholar] [CrossRef]
- Lavanya, C.; Kulkarni, P.; Dixit, P.K.; Raavi, L.N.V. Krishna, Sources of cellulose and their applications-A review. Int. J. Drug Formul. Res. 2011, 2, 19–38. [Google Scholar]
- Ryu, D.D.; Mandels, M. Cellulases: Biosynthesis and applications. Enzym. Microb. Technol. 1980, 2, 91–102. [Google Scholar] [CrossRef]
- Bae, J.; Morisaka, H.; Kuroda, K.; Ueda, M. Cellulosome complexes: Natural biocatalysts as arming microcompartments of enzymes. J. Mol. Microbiol. Biotechnol. 2013, 23, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.V.; Willink, F.W.; Ingvorsen, K. Aerobic and anaerobic cellulase production by Cellulomonas uda. Arch. Microbiol. 2016, 198, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, G.T.; Zhekova, B.Y. Biosynthesis, purification and characterization of endoglucanase from a xylanase producing strain Aspergillus niger B03. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2012, 43, 70–77. [Google Scholar] [CrossRef][Green Version]
- Saraihom, S.; Kobayashi, D.Y.; Lotrakul, P.; Prasongsuk, S.; Eveleigh, D.E.; Punnapayak, H. First report of a tropical Lysobacter enzymogenes producing bifunctional endoglucanase activity towards carboxymethylcellulose and chitosan. Ann. Microbiol. 2016, 66, 907–919. [Google Scholar] [CrossRef]
- Tjon-Joe-Pin, R.M.; Rickards, A.R. Enzyme complex used for breaking crosslinked cellulose based blocking gels at low to moderate temperatures. U.S. Patent 5,224,544 A, 7 July 1993. [Google Scholar]
- Freeman, M.; Norman, M.; Ballard, D.; Jiang, P.; Symes, K.; Mistry, K. Method and composition for the triggered release of polymer-degrading agents for oil field use. U.S. Patent 20,050,130,845 A1, 16 June 2005. [Google Scholar]
- Gupta, D.V.S.; Prasek, B.B. Method for fracturing subterranean formations using controlled release breakers and compositions useful therein. U.S. Patent 5,437,331 A, August 15, 1995. [Google Scholar]
- Hong, J.; Ye, Y.; Wang, Y.; Zhang, Y. Bioseparation of recombinant cellulose-binding module-proteins by affinity adsorption on an ultra-high-capacity cellulosic adsorbent. Anal. Chim. Acta 2008, 621, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ling, Z.; Khan, A.; Virk, A.; Kulshrestha, S.; Li, X. Improvements of thermophilic enzymes: From genetic modifications to applications. Bioresour. Technol. 2019, 279, 350–361. [Google Scholar] [CrossRef]
- Goldsmith, M.; Tawfik, D.S. Directed enzyme evolution: Beyond the low-hanging fruit. Curr. Opin. Struct. Biol. 2012, 22, 406–412. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. Npj Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Qiao, G.G.; Solomon, D.H. Some aspects of the properties and degradation of polyacrylamides. Chem. Rev. 2002, 102, 3067–3084. [Google Scholar] [CrossRef]
- Kay-Shoemake, J.L.; Watwood, M.E.; Lentz, R.D.; Sojka, R.E. Polyacrylamide as an organic nitrogen source for soil microorganisms with potential effects on inorganic soil nitrogen in agricultural soil. Soil Biol. Biochem. 1998, 30, 1045–1052. [Google Scholar] [CrossRef]
- Grula, M.M.; Huang, M.-L.; Sewell, G. Interactions of certain polyacrylamides with soil bacteria. Soil Sci. 1994, 158, 291–300. [Google Scholar] [CrossRef]
- Haveroen, M.E.; MacKinnon, M.D.; Fedorak, P.M. Polyacrylamide added as a nitrogen source stimulates methanogenesis in consortia from various wastewaters. Water Res. 2005, 39, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, J.-F.; Li, C.-Y.; Yang, S.-Z.; Gu, J.-D.; Mu, B.-Z. Anaerobic biodegradation of partially hydrolyzed polyacrylamide in long-term methanogenic enrichment cultures from production water of oil reservoirs. Biodegradation 2018, 29, 233–243. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, Z.; Zhao, Y.; Zhang, H.; Feng, Y. Biodegradation of polyacrylamide by bacteria isolated from activated sludge and oil-contaminated soil. J. Hazard. Mater. 2010, 175, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Kay-Shoemake, J.L.; Watwood, M.E.; Sojka, R.E.; Lentz, R.D. Polyacrylamide as a substrate for microbial amidase in culture and soil. Soil Biol. Biochem. 1998, 30, 1647–1654. [Google Scholar] [CrossRef]
- Dai, X.; Luo, F.; Zhang, D.; Dai, L.; Chen, Y.; Dong, B. Waste-activated sludge fermentation for in biological polyacrylamide removal. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Luo, F.; Yi, J.; He, Q.; Dong, B. Biodegradation of polyacrylamide by anaerobic digestion under mesophilic condition and its performance in actual dewatered sludge system. Bioresour. Technol. 2013, 153, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhao, L.; Bao, M.; Lu, J. Hydrolyzed polyacrylamide biodegradation and mechanism in sequencing batch biofilm reactor. Bioresour. Technol. 2016, 207, 315–321. [Google Scholar] [CrossRef]
- Song, T.; Li, S.; Ding, W.; Li, H.; Bao, M.; Li, Y. Biodegradation of hydrolyzed polyacrylamide by the combined expanded granular sludge bed reactor-aerobic biofilm reactor biosystem and key microorganisms involved in this bioprocess. Bioresour. Technol. 2018, 263, 153–162. [Google Scholar] [CrossRef]
- Berdugo-Clavijo, C.; Sen, A.; Seyyedi, M.; Quintero, H.; O’Neil, B.; Gieg, L.M. High temperature utilization of PAM and HPAM by microbial communities enriched from oilfield produced water and activated sludge. AMB Exp. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Harris, R.; McKay, I. New Applications for Enzymes in Oil and Gas Production. Eur. Pet. Conf. 1998, 1–9. [Google Scholar] [CrossRef]
- Sarwar, M.U.; Cawiezel, K.E.; Nasr-El-Din, H.A. Gel Degradation Studies of Oxidative and Enzyme Breakers to Optimize Breaker Type and Concentration for Effective Break Profiles at Low and Medium Temperature Ranges. SPE Hydraul. Fract. Technol. Conf. 2011, 1–21. [Google Scholar] [CrossRef]
- Kyaw, A.; Azahar, B.S.B.N.; Tunio, S.Q. Fracturing fluid (guar polymer gel) degradation study by using oxidative and enzyme breaker. Res. J. Appl. Sci. Eng. Technol. 2012, 4, 1167–1671. [Google Scholar]
- Yennamalli, R.M.; Rader, A.J.; Kenny, A.J.; Wolt, J.D.; Sen, T.Z. Endoglucanases: Insights into thermostability for biofuel applications. Biotechnol. Biofuels 2013, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Barati, R.; Johnson, S.J.; Mccool, S.; Green, D.W.; Willhite, G.P.; Liang, J. Fracturing fluid cleanup by controlled release of enzymes from polyelectrolyte complex nanoparticles. J. Appl. Polym. Sci. 2011, 121, 1292–1298. [Google Scholar] [CrossRef]
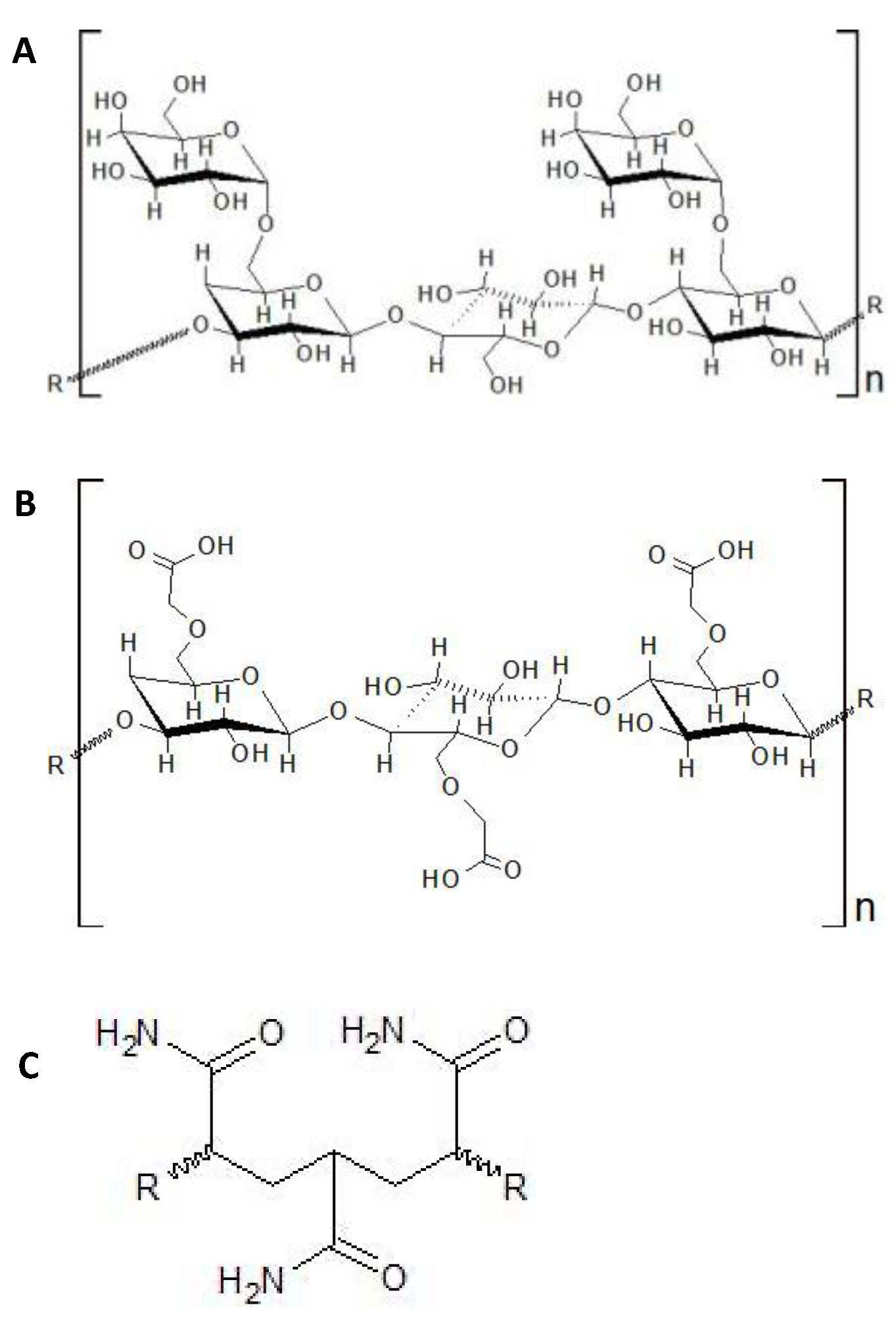

| Type of Polymer | Gelling Agent | Use | References |
|---|---|---|---|
| Cellulose-based | Proppant delivery agent | [8] | |
| Hydroxyethyl cellulose | Fluid loss additive | ||
| Gravel packing | |||
| Thickener | |||
| Hydroxypropyl cellulose | Proppant delivery agent | [8] | |
| Thickener | |||
| Carboxymethyl cellulose | Proppant delivery agent | [8] | |
| Gravel packing | |||
| Thickener | |||
| Carboxymethylhydroxyethyl cellulose | Proppant delivery agent | [8] | |
| Methyl cellulose | Thickener | [8] | |
| Guar-based | Guar gum | Proppant delivery agent | [8] |
| Thickener | |||
| Hydropropyl guar | Proppant delivery agent | [8] | |
| Gravel packing | |||
| Carboxymethyl guar | Proppant delivery agent | [8] | |
| Carboxymethylhydropropyl guar | Proppant delivery agent | [8] | |
| Acrylamide & acrylic acid-based | Polyacrylamide | Friction reducer | [7,8] |
| Thickener | |||
| Polyacrylate | Friction reducer | [8] | |
| Methylacrylamide | Thickener | [8] | |
| Acrylic acid | Thickener | [8] | |
| Methylacrylic acid | Thickener | [8] | |
| Copolymers from acrylamide & acrylic acid | Friction reducer | [8] | |
| Thickener | |||
| Others | Xanthan gum | Proppant delivery agent | [4,8] |
| Foaming agent | |||
| Gravel packing | |||
| Drilling muds | |||
| Thickener | |||
| Starch & its derivatives | Fluid-loss additive | [4] | |
| Scleroglucan | Proppant delivery agent | [8] | |
| Polyurethanes, Polyesters, | Thickeners | [8] | |
| Locust bean gum, Gum Ghatti | |||
| Gum karaya, Tragacanth gum | |||
| Tamarind gum | |||
| Welan gum | Foaming agent | [8] | |
| Thickener | |||
| Polycationic quaternary amine polymer, | Clay stabilizers | [8] | |
| Guanidyl copolymer, Anionic polymer, | |||
| Copolymer of styrene & maleic anhydride | |||
| with polyethylene glycol | |||
| Lignosulfonate | Fluid-loss additive | [8] | |
| 2-Acrylamino-2-methy-1-propane sulfonic acid (AMPS) derivatives & N-Vinylpyridine | Thickeners | [8] |
| Polymer | Enzyme Name | Activity Conditions (Temperature, pH, Salinity) | Source of Enzyme | Reference |
|---|---|---|---|---|
| Guar gum | 1,6-α-d-galactosidase | 10 to 82 °C, pH 2 to 11 | Aspergillus niger | [32] |
| Mannan endo-1,4-mannosidase | ||||
| Mannanase II | 40 to 70 °C, pH 7 to 8.5 | Not specified | [33] | |
| Galacto-mannanase | up to 120 °C | Not specified, but gene expressed in E. coli | [34] | |
| α-1,6-galactosidase | 93 °C, pH 5.5 to 6.5 | Thermus brockianus | [35] | |
| α-1,6-galactosidase | 85 °C | Thermotoga maritima | [36] | |
| α-1,6-galactosidase | 85 to 100 °C pH 7.4 | Thermotoga neapolitana | [37] | |
| β-1,4-mannanase | ||||
| Mannanase | 50 °C, pH 3 to 8, up to 4 M NaCl | Enterobacter sp. N18 | [38] | |
| Mannanase | 85 °C, pH 5.4 | Rhodothermus marimus | [39] | |
| Mannanase | 60 to 70 °C, pH up to 10.5 | Not specified | [40] | |
| Starch | α-amylase | 50 to 90 °C, pH 5–9 | Not specified | [41] |
| Xanthan gum | β-glucanase | |||
| PAM/HPAM | Horseradish peroxidase | 37 °C | Amoracia rusticana | [42] |
| Hydroquinone peroxidase | 30 °C, pH 7 | Azotobacter beijerinckii HM121 | [43] | |
| Xanthine oxidase | 20 °C | Bovine milk | [44] | |
| Phosphatase, Urease, Dehydrogenase | 33 °C, pH 7.5 | Activated sludge * | [45] | |
| Amidase | 38 °C, pH 6.6 | Klebsiella sp. | [46] | |
| Urease | 24 °C, pH 8.19 | Bacillus megaterium | [47] | |
| Asparaginase | 20 to 120 °C | Aspergillus oryzae | [48] | |
| Laccase | 35 °C | Wastewater enrichment * | [36] | |
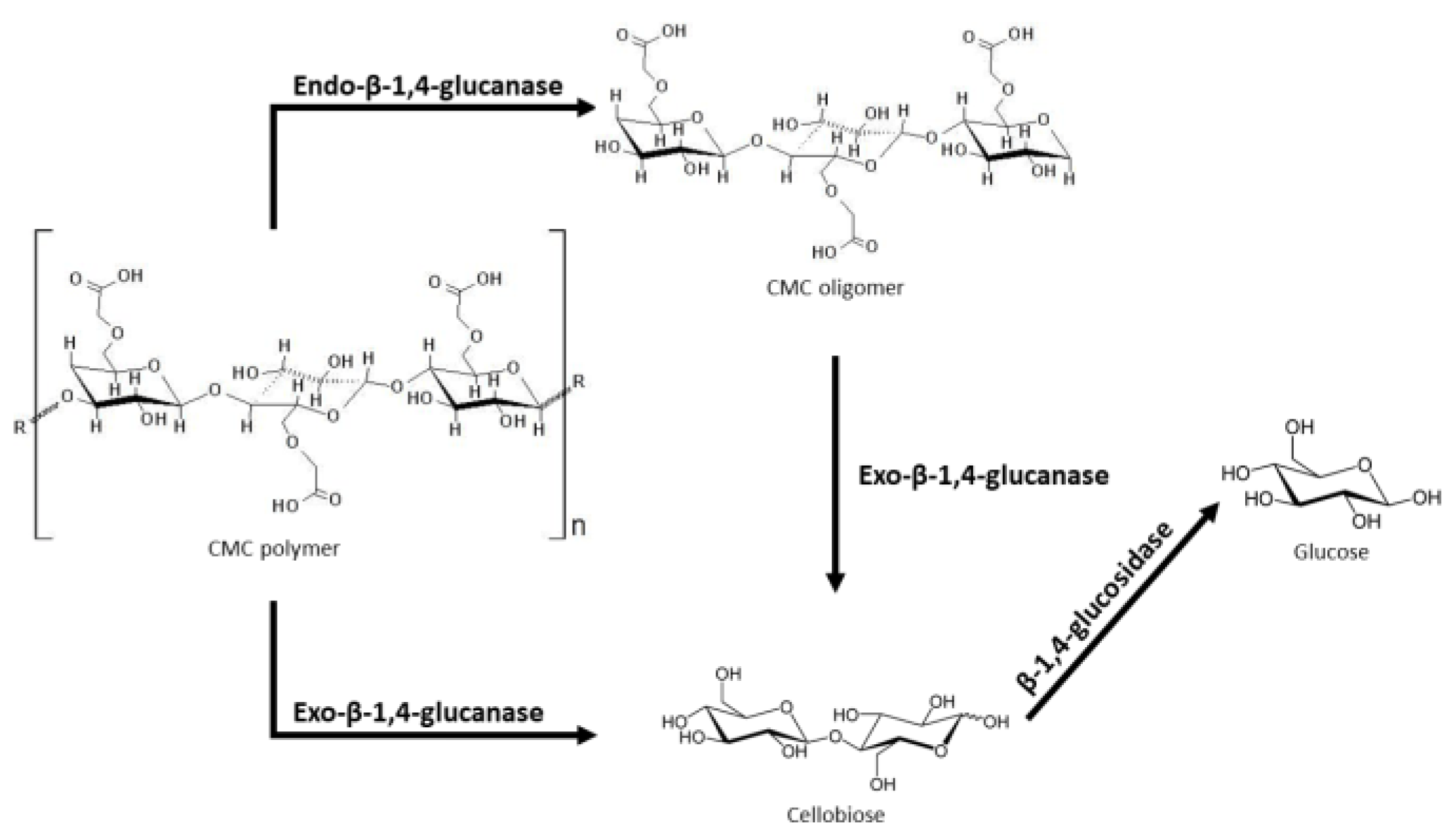
| CMC | Endo(1,4)-glucanase-d-xylanase | 15 to 60 °C, pH 1 to 8 | Not specified | [49] |
| Exo(1,4)-glucanase-d-xylanase | ||||
| Xylanase | 50 to 80 °C, pH 6 to 8, up to 20% (w/v) NaCl | Caldicoprobacter faecalis | [31,50] | |
| Enzyme 1 and enzyme 2 | 49 °C, pH 4.75 | Not specified | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berdugo-Clavijo, C.; Scheffer, G.; Sen, A.; Gieg, L.M. Biodegradation of Polymers Used in Oil and Gas Operations: Towards Enzyme Biotechnology Development and Field Application. Polymers 2022, 14, 1871. https://doi.org/10.3390/polym14091871
Berdugo-Clavijo C, Scheffer G, Sen A, Gieg LM. Biodegradation of Polymers Used in Oil and Gas Operations: Towards Enzyme Biotechnology Development and Field Application. Polymers. 2022; 14(9):1871. https://doi.org/10.3390/polym14091871
Chicago/Turabian StyleBerdugo-Clavijo, Carolina, Gabrielle Scheffer, Arindom Sen, and Lisa M. Gieg. 2022. "Biodegradation of Polymers Used in Oil and Gas Operations: Towards Enzyme Biotechnology Development and Field Application" Polymers 14, no. 9: 1871. https://doi.org/10.3390/polym14091871
APA StyleBerdugo-Clavijo, C., Scheffer, G., Sen, A., & Gieg, L. M. (2022). Biodegradation of Polymers Used in Oil and Gas Operations: Towards Enzyme Biotechnology Development and Field Application. Polymers, 14(9), 1871. https://doi.org/10.3390/polym14091871







