Polysaccharides Composite Materials as Carbon Nanoparticles Carrier
Abstract
:1. Introduction
2. Carbon-Based Nanostructures
2.1. Graphene
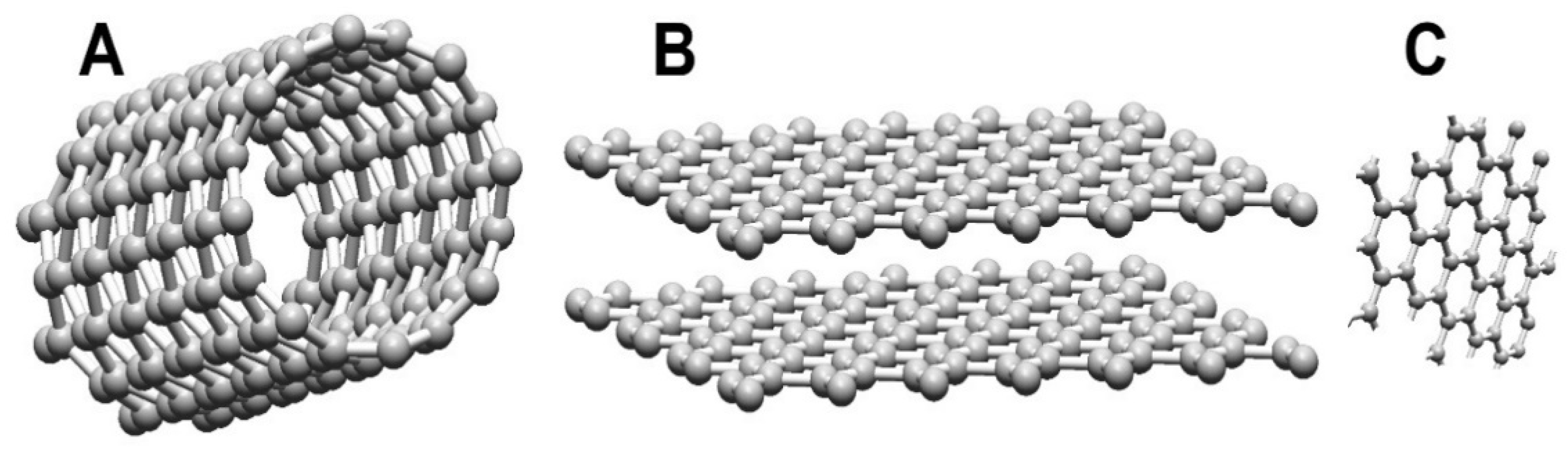
2.2. Carbon Nanotubes
2.3. Carbon Quantum Dots (CQDs)
3. Application of Carbon Nanoparticles
4. Carbon Nanoparticles/Nanostructures in Polysaccharides Matrices
4.1. Hydrogels
4.2. Foams
4.3. Films
5. Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, K.; Sen, S.; Biswas, P. A review paper—On the use of nanotechnology in construction industry. In Proceedings of the Industry Interactive Innovations in Science, Engineering & Technology (I3SET2K19), Kalyani, India, 13–14 December 2019. [Google Scholar] [CrossRef]
- Feynman, R. There’s Plenty of Room at the Bottom. Eng. Sci. 1960, 23, 22–36. Available online: http://resolver.caltech.edu/CaltechES:23.5.1960Bottom (accessed on 29 January 2022).
- Srivastava, S.; Bhargava, A. Green Nanotechnology: An Overview; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–13. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, M. Size and shape dependence of optical properties of nanostructures. Appl. Phys. A 2020, 126, 1–8. [Google Scholar] [CrossRef]
- Feynman, R.P.; Gilbert, D. Miniaturization; Reinhold: New York, NY, USA, 1961; pp. 282–296. [Google Scholar]
- Jablonska, A.; Jaworska, A.; Kasztelan, M.; Berbec, S.; Palys, B. Graphene and Graphene Oxide Applications for SERS Sensing and Imaging. Curr. Med. Chem. 2019, 26, 6878–6895. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R. Nanotechnology. Caltechs Eng. Sci. 1960, 23.5, 22–36. [Google Scholar]
- Neri, G.; Fazio, E.; Mineo, P.G.; Scala, A.; Piperno, A. SERS Sensing Properties of New Graphene/Gold Nanocomposite. Nanomaterials 2019, 9, 1236. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Palencia, J.D.; Wang, N.; Jiang, Y.; Wang, D.-Y. Nanocarbon-Based Flame Retardant Polymer Nanocomposites. Molecules 2021, 26, 4670. [Google Scholar] [CrossRef]
- Khachatryan, K.; Khachatryan, L.; Krzan, M.; Krystyjan, M.; Krzemińska-Fiedorowicz, L.; Lenart-Boroń, A.; Koronowicz, A.; Drozdowska, M.; Khachatryan, G. Formation and Investigation of Physicochemical, Biological and Bacteriostatic Properties of Nanocomposite Foils Containing Silver Nanoparticles and Graphene Oxide in Hyaluronic Acid Matrix. Materials 2021, 14, 3377. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, Bacteriostatic, and Biological Properties of Starch/Chitosan Polymer Composites Modified by Graphene Oxide, Designed as New Bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef]
- Zheng, Y.; Monty, J.; Linhardt, R.J. Polysaccharide-based nanocomposites and their applications. Carbohydr. Res. 2015, 405, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, L.; Gao, J.; Huang, J.; Yang, Y.; Xu, Y.; Liu, S.; Yu, W. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydr. Polym. 2021, 260, 117796. [Google Scholar] [CrossRef]
- Abuduwaili, A.; Nuerxiati, R.; Mutailifu, P.; Gao, Y.; Lu, C.; Yili, A. Isolation, structural modification, characterization, and bioactivity of polysaccharides from Folium Isatidis. Ind. Crop. Prod. 2021, 176, 114319. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.K.; Chakraborty, I.; Mandal, A.K.; Bhanja, S.K.; Patra, S.; Maity, P. A review on antiviral and immunomodulatory polysaccharides from Indian medicinal plants, which may be beneficial to COVID-19 infected patients. Int. J. Biol. Macromol. 2021, 181, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef]
- Qian, W.-W.; Yang, S.-Q.; Hu, S.-M.; Wang, X.-L.; Zhu, Y.; Zhou, T. Enzymatic degradation, antioxidant and immunoregulatory activities of polysaccharides from brown algae Sargassum fusiforme. J. Food Meas. Charact. 2021, 15, 1960–1972. [Google Scholar] [CrossRef]
- Dzyazko, Y.; Ogenko, V. Polysaccharides: An Efficient Tool for Fabrication of Carbon Nanomaterials. Polysaccharides 2021, 337–366. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K. Starch based nanocomposites as sensors for heavy metals–detection of Cu2+ and Pb2+ ions. Int. Agrophys. 2019, 33, 121–126. [Google Scholar] [CrossRef]
- Itami, K.; Maekawa, T. Molecular Nanocarbon Science: Present and Future. Nano Lett. 2020, 20, 4718–4720. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Moosa, A.A.; Abed, M.S. Graphene preparation and graphite exfoliation. Turk. J. Chem. 2021, 45, 493–519. [Google Scholar] [CrossRef]
- Draude, A.P.; Dierking, I. Thermotropic liquid crystals with low-dimensional carbon allotropes. Nano Express 2021, 2, 012002. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials 2021, 11, 3258. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2019, 8, 1198–1224. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, İ.A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2020, 33, 2001105. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Hossain, K.; Rafatullah, M.; Abbas, S.Z.; Ahmad, A.; Ismail, N.; Maruthi, A.Y. Antimicrobial activity of graphene-based nanomaterials. In Graphene-Based Nanotechnologies for Energy and Environment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–314. [Google Scholar]
- Frindy, S.; Primo, A.; Ennajih, H.; Qaiss, A.E.K.; Bouhfid, R.; Lahcini, M.; Essassi, E.M.; Garcia, H.; El Kadib, A. Chitosan–graphene oxide films and CO 2 -dried porous aerogel microspheres: Interfacial interplay and stability. Carbohydr. Polym. 2017, 167, 297–305. [Google Scholar] [CrossRef]
- Garg, A.; Chalak, H.; Belarbi, M.-O.; Zenkour, A.; Sahoo, R. Estimation of carbon nanotubes and their applications as reinforcing composite materials—An engineering review. Compos. Struct. 2021, 272, 114234. [Google Scholar] [CrossRef]
- Ghalandari, M.; Maleki, A.; Haghighi, A.; Shadloo, M.S.; Nazari, M.A.; Tlili, I. Applications of nanofluids containing carbon nanotubes in solar energy systems: A review. J. Mol. Liq. 2020, 313, 113476. [Google Scholar] [CrossRef]
- Basheer, B.V.; George, J.J.; Siengchin, S.; Parameswaranpillai, J. Polymer grafted carbon nanotubes—Synthesis, properties, and applications: A review. Nano-Struct. Nano-Objects 2020, 22, 100429. [Google Scholar] [CrossRef]
- Jena, S.K.; Chakraverty, S.; Malikan, M.; Tornabene, F. Effects of surface energy and surface residual stresses on vibro-thermal analysis of chiral, zigzag, and armchair types of SWCNTs using refined beam theory. Mech. Based Des. Struct. Mach. 2020, 1–15. [Google Scholar] [CrossRef]
- Lönnecke, K.; Eberhardt, O.; Wallmersperger, T. Electrostatic charge distribution in armchair and zigzag carbon nanotubes: A numerical comparison of CNT charge models. Acta Mech. 2021, 1–16. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Xie, Y.; Wei, Y.; Yang, Y.; Liu, X.; Chen, Y.; Xu, B. An Efficient Synthesis and Photoelectric Properties of Green Carbon Quantum Dots with High Fluorescent Quantum Yield. Nanomaterials 2020, 10, 82. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; Zhang, Q.; Wen, M.; Mao, Z.; Wei, H.; Chang, H.-J.; Niu, X. Solvent regulation synthesis of single-component white emission carbon quantum dots for white light-emitting diodes. Nanotechnol. Rev. 2021, 10, 465–477. [Google Scholar] [CrossRef]
- Mai, X.-D.; Phan, Y.T.H.; Nguyen, V.-Q. Excitation-Independent Emission of Carbon Quantum Dot Solids. Adv. Mater. Sci. Eng. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Wang, Q.; Wu, Z.; Zhang, X.; Li, B.; Lin, L. Recent advances in quantum dots-based biosensors for antibiotic detection. J. Pharm. Anal. 2021. In Press. [Google Scholar] [CrossRef]
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-Based Quantum Dots for Supercapacitors: Recent Advances and Future Challenges. Nanomaterials 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, L.; Tong, Y.; Wang, S.; Hou, X.; An, X.; Dou, S.X.; Liang, J. Photo-rechargeable batteries and supercapacitors: Critical roles of carbon-based functional materials. Carbon Energy 2021, 3, 225–252. [Google Scholar] [CrossRef]
- Azam, N.; Ali, M.N.; Khan, T.J. Carbon Quantum Dots for Biomedical Applications: Review and Analysis. Front. Mater. 2021, 8, 272. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [Green Version]
- Chahal, S.; Macairan, J.-R.; Yousefi, N.; Tufenkji, N.; Naccache, R. Green synthesis of carbon dots and their applications. RSC Adv. 2021, 11, 25354–25363. [Google Scholar] [CrossRef]
- Zhou, L.; Qiao, M.; Zhang, L.; Sun, L.; Zhang, Y.; Liu, W. Green and efficient synthesis of carbon quantum dots and their luminescent properties. J. Lumin. 2019, 206, 158–163. [Google Scholar] [CrossRef]
- Yuan, M.; Zhong, R.; Gao, H.; Li, W.; Yun, X.; Liu, J.; Zhao, X.; Zhao, G.; Zhang, F. One-step, green and economic synthesis of water-soluble photoluminescent carbon dots by hydrothermal treatment of wheat straw and their bio-applications in labeling, imaging and sensing. Appl. Surf. Sci. 2015, 355, 1136–1144. [Google Scholar] [CrossRef]
- Tejwan, N.; Saha, S.K.; Das, J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv. Colloid Interface Sci. 2020, 275, 102046. [Google Scholar] [CrossRef]
- Radnia, F.; Mohajeri, N.; Zarghami, N. New insight into the engineering of green carbon dots: Possible applications in emerging cancer theranostics. Talanta 2020, 209, 120547. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S.K.; Nyodu, R.; Kumar, S.; Maurya, V.K. Current Advances in Nanotechnology and Medicine. In NanoBioMedicine; Saxena, S.K., Khurana, S.M.P., Eds.; Springer: Singapore, 2020; pp. 3–16. [Google Scholar]
- Laird, N.Z.; Acri, T.M.; Chakka, J.L.; Quarterman, J.C.; Malkawi, W.I.; Elangovan, S.; Salem, A.K. Applications of nanotechnology in 3D printed tissue engineering scaffolds. Eur. J. Pharm. Biopharm. 2021, 161, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Hashemi, S.A.; Yousefi, K.; Mousavi, S.M.; Chiang, W.-H.; Ramakrishna, S.; Mazraedoost, S.; Alizadeh, A.; Omidifar, N.; Behbudi, G.; et al. 3D Nanostructures for Tissue Engineering, Cancer Therapy, and Gene Delivery. J. Nanomater. 2020, 2020, 1–24. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Devi, P.; Saini, S.; Kim, K.-H. The advanced role of carbon quantum dots in nanomedical applications. Biosens. Bioelectron. 2019, 141, 111158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Leng, J.; Liu, H.; Zhang, Y.; Wang, C.; An, W.; Bao, C.; Lei, H. The green synthesis of carbon quantum dots and applications for sulcotrione detection and anti-pathogen activities. J. Saudi Chem. Soc. 2021, 25, 101373. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Dera, M.W.; Teseme, W.B. Review on the Application of Food Nanotechnology in Food Processing. Am. J. Eng. Technol. Manag. 2020, 5, 41. [Google Scholar] [CrossRef]
- Jagtiani, E. Advancements in nanotechnology for food science and industry. Food Front. 2021, 1–27. [Google Scholar] [CrossRef]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Khachatryan, G.; Khachatryan, K.; Konieczna-Molenda, A.; Krzan, M.; Grzyb, J. Synthesis of Silver and Gold Nanoparticles in Sodium Alginate Matrix Enriched with Graphene Oxide and Investigation of Properties of the Obtained Thin Films. Appl. Sci. 2021, 11, 3857. [Google Scholar] [CrossRef]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, L.; Wang, Y.; Ou, Y.; Wang, X.; Pan, Y.; Wang, Y.; Huang, L.; Cheng, G.; Xie, S.; et al. Construction of an Electrochemical Receptor Sensor Based on Graphene/Thionine for the Sensitive Determination of β-Lactam Antibiotics Content in Milk. Int. J. Mol. Sci. 2020, 21, 3306. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. [Google Scholar] [CrossRef] [Green Version]
- Özcan, N.; Karaman, C.; Atar, N.; Karaman, O.; Yola, M.L. A Novel Molecularly Imprinting Biosensor Including Graphene Quantum Dots/Multi-Walled Carbon Nanotubes Composite for Interleukin-6 Detection and Electrochemical Biosensor Validation. ECS J. Solid State Sci. Technol. 2020, 9, 121010. [Google Scholar] [CrossRef]
- Kiss, É. Nanotechnology in Food Systems: A Review. Acta Aliment. 2020, 49, 460–474. [Google Scholar] [CrossRef]
- Luo, X.; Han, Y.; Chen, X.; Tang, W.; Yue, T.; Li, Z. Carbon dots derived fluorescent nanosensors as versatile tools for food quality and safety assessment: A review. Trends Food Sci. Technol. 2019, 95, 149–161. [Google Scholar] [CrossRef]
- Kousheh, S.; Moradi, M.; Tajik, H.; Molaei, R. Preparation of antimicrobial/ultraviolet protective bacterial nanocellulose film with carbon dots synthesized from lactic acid bacteria. Int. J. Biol. Macromol. 2020, 155, 216–225. [Google Scholar] [CrossRef]
- Kandra, R.; Bajpai, S. Synthesis, mechanical properties of fluorescent carbon dots loaded nanocomposites chitosan film for wound healing and drug delivery. Arab. J. Chem. 2020, 13, 4882–4894. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Pan, H.; Xu, N.; Mei, C.; Mao, H.; Zhang, W.; Cai, J.; Xu, C. Preparation and Performance of Radiata-Pine-Derived Polyvinyl Alcohol/Carbon Quantum Dots Fluorescent Films. Materials 2020, 13, 67. [Google Scholar] [CrossRef] [Green Version]
- Salimi, F.; Moradi, M.; Tajik, H.; Molaei, R. Optimization and characterization of eco-friendly antimicrobial nanocellulose sheet prepared using carbon dots of white mulberry (Morus alba L.). J. Sci. Food Agric. 2021, 101, 3439–3447. [Google Scholar] [CrossRef]
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-doped and hybrid carbon dots: A comprehensive review on their synthesis and biomedical applications. J. Control. Release 2021, 330, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lu, X.; Wang, W.; Hubbe, M.A.; Liu, Y.; Mu, J.; Wang, J.; Sun, J.; Rojas, O.J. Recent developments in colorimetric and optical indicators stimulated by volatile base nitrogen to monitor seafood freshness. Food Packag. Shelf Life 2021, 28, 100634. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Kousheh, S.A.; Guimarães, J.T.; McClements, D.J. Carbon dots synthesized from microorganisms and food by-products: Active and smart food packaging applications. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Avouris, P.; Chen, Z.; Perebeinos, V. Carbon-based electronics. Nat. Nanotechnol. 2007, 2, 605–615. [Google Scholar] [CrossRef]
- Report by Raghu Das, Carbon Nanotubes (CNT) for Electronics & Electrics 2013–2023: Forecasts, Applications, Technologies. Available online: https://www.idtechex.com/en/research-report/carbon-nanotubes-cnt-for-electronics-and-electrics-2013-2023-forecasts-applications-technologies/342 (accessed on 29 January 2022).
- Sung, J.; Hamilton, I.; Kamiya, G. Energy Efficiency 2020; Energy Efficiency Division of the International Energy Agency (IEA): Paris, France, 2020. [Google Scholar]
- Available online: https://www.idtechex.com/de/research-report/flexible-printed-and-thin-film-batteries-2015-2025-technologies-forecasts-players/410 (accessed on 29 January 2022).
- Available online: https://toronto.ctvnews.ca/seemed-like-pandemonium-cellphone-catches-fire-on-board-air-canada-flight-1.3824410 (accessed on 29 January 2022).
- Available online: https://www.transportation.gov/briefing-room/dot-bans-all-samsung-galaxy-note7-phones-airplanes (accessed on 29 January 2022).
- Niu, J.; Kang, S. Chapter 1: New High-energy Anode Materials. Future Lithium-Ion Batter. 2019, 1–25. [Google Scholar] [CrossRef]
- Zarska, S.; Kulawik, D.; Pavlyuk, V.; Tomasik, P.; Bachmatiuk, A.; Szukiewicz, R.; Ciesielski, W. A Facile and Efficient Bromination of Multi-Walled Carbon Nanotubes. Materials 2021, 14, 3161. [Google Scholar] [CrossRef]
- Żarska, S.; Kulawik, D.; Drabowicz, J.; Ciesielski, W. A review of procedures of purification and chemical modification of carbon nanotubes with bromine. Full Nanotub. Carbon Nanostruct. 2017, 25, 563–569. [Google Scholar] [CrossRef]
- Ciesielski, W.; Drabowicz, J.; Kulawik, D. The Process of Preparing the Brominated Multi-Walled Carbon Nanotubes (MWCNT) Containing Bromine Atoms and the Way of Purification Them. EP 3002253B1, 9 September 2020. [Google Scholar]
- Ciesielski, W.; Kulawik, D.; Żarska, S.; Folentarska, A.; Drabowicz, J. Functionalized Multi-Wall Carbon Nanotubes, Method of Their Production and Their Application. P.428772, 31 January 2019. [Google Scholar]
- Misztal, R.; Balinska, A.; Kulawik, D.; Pavlyuk, V.; Ciesielski, W. Chromium substitution effect on structural and electrochemical behavior of Li-Cr-Ni-O oxides. Ionics 2015, 21, 3039–3049. [Google Scholar] [CrossRef]
- Zdanowska, S.; Pyzalska, M.; Kulawik, D.; Pavlyuk, V.; Drabowicz, J.; Ciesielski, W. Właściwości fizykochemiczne bromowanych wielościennych nanorurek węglowych funkcjonalizowanych tiofosforanem O-metylo-O-2-naftylo-L-N-metyloefedryniowym. Przemysł Chem. 2015, 94, 2189–2194. [Google Scholar]
- Pavlyuk, V.; Dmytriv, G.; Chumak, I.; Gutfleisch, O.; Lindemann, I.; Ehrenberg, H. High hydrogen content super-lightweight intermetallics from the Li–Mg–Si system. Int. J. Hydrogen Energy 2013, 38, 5724–5737. [Google Scholar] [CrossRef]
- Pavlyuk, V.; Ciesielski, W.; Kulawik, D.; Dmytriv, G. Enhancement of hydrogen storage properties of Li12+xMg3-xSi4-ySny (x = y = 0.48) phase by modification with LixZnO/La2O3-CNT composites. Int. J. Hydrogen Energy 2021, 46, 22864–22876. [Google Scholar] [CrossRef]
- Di Lecce, D.; Andreotti, P.; Boni, M.; Gasparro, G.; Rizzati, G.; Hwang, J.-Y.; Sun, Y.-K.; Hassoun, J. Multiwalled Carbon Nanotubes Anode in Lithium-Ion Battery with LiCoO2, Li[Ni1/3Co1/3Mn1/3]O2, and LiFe1/4Mn1/2Co1/4PO4 Cathodes. ACS Sustain. Chem. Eng. 2018, 6, 3225–3232. [Google Scholar] [CrossRef]
- Pavlyuk, V.; Kulawik, D.; Ciesielski, W.; Pavlyuk, N.; Dmytriv, G. New quaternary carbide Mg1.52Li0.24Al0.24C0.86 as a disorder derivative of the family of hexagonal close-packed (hcp) structures and the effect of structure modification on the electrochemical behaviour of the electrode. Acta Crystallogr. Sect. C Struct. Chem. 2018, 74, 360–365. [Google Scholar] [CrossRef]
- Liu, J.-H.; Miao, H.-Y.; Lakshmanan, S.; Wang, L.-C.; Tsai, R.-H. Fabrication of Metal Alloy-Deposited Flexible MWCNT Buckypaper for Thermoelectric Applications. J. Nanomater. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Okajima, M.K.; Kumar, A.; Fujiwara, A.; Mitsumata, T.; Kaneko, D.; Ogawa, T.; Kurata, H.; Isoda, S.; Kaneko, T. Anionic complexes of MWCNT with supergiant cyanobacterial polyanions. Biopolymers 2012, 99, 1–9. [Google Scholar] [CrossRef]
- Schlemmer, W.; Selinger, J.; Hobisch, M.A.; Spirk, S. Polysaccharides for sustainable energy storage—A review. Carbohydr. Polym. 2021, 265, 118063. [Google Scholar] [CrossRef]
- Alam, N.; Maria, K.H.; Rahman, M.J.; Sultana, P.; Mieno, T. A Wet Chemical Synthesis and Characterization of Mwcnt-Starch Biocomposites. J. Bangladesh Acad. Sci. 2020, 44, 43–52. [Google Scholar] [CrossRef]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Nowak, N.; Khachatryan, G.; Krzan, M.; Krystyjan, M.; Kosiński, J.; Khachatryan, K. The Preparation and Characterization of Quantum Dots in Plysaccharide Carriers (Starch/Chitosan) as Elements of Smart Packaging and Their Impact on the Growth of Microorganisms in Food. Materials 2021, 14, 7732. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Dykas, K.; Felkle, D.; Karnas, K.; Khachatryan, G.; Karewicz, A. Hyaluronic Acid-Silver Nanocomposites and Their Biomedical Applications: A Review. Materials 2022, 15, 234. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.A.; Benito, M.; Pérez, E.; Maria Teijon, J.; Dolores Blanco, M. The role of anionic polysaccharides in the prep-aration of nanomedicines with anticancer applications. Curr. Pharm. Des. 2016, 22, 3364–3379. [Google Scholar] [CrossRef] [PubMed]
- Folentarska, A.; Łagiewka, J.; Krystyjan, M.; Ciesielski, W. Biodegradable Binary and Ternary Complexes from Renewable Raw Materials. Polymers 2021, 13, 2925. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.; Silvestre, A.; Barros, C.S. Polysaccharide Based Hybrid Materials: Metals and Metal Oxides, Graphene and Carbon Nanotubes; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Zavareh, H.S.; Pourmadadi, M.; Moradi, A.; Yazdian, F.; Omidi, M. Chitosan/carbon quantum dot/aptamer complex as a potential anticancer drug delivery system towards the release of 5-fluorouracil. Int. J. Biol. Macromol. 2020, 165, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Anjali, J.; Jose, V.K.; Lee, J.-M. Carbon-based hydrogels: Synthesis and their recent energy applications. J. Mater. Chem. A 2019, 7, 15491–15518. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Javanbakht, S.; Nazari, N.; Rakhshaei, R.; Namazi, H. Cu-crosslinked carboxymethylcellulose/naproxen/graphene quantum dot nanocomposite hydrogel beads for naproxen oral delivery. Carbohydr. Polym. 2018, 195, 453–459. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Roshani, S.; Bagheri, Z.; Yaghoubi-Avini, M. Carbon dots—Sodium alginate hydrogel: A novel tetracycline fluorescent sensor and adsorber. J. Environ. Chem. Eng. 2019, 7, 103419. [Google Scholar] [CrossRef]
- Saeednia, L.; Yao, L.; Cluff, K.; Asmatulu, R. Sustained releasing of methotrexate from injectable and thermosensitive chi-tosan–carbon nanotube hybrid hydrogels effectively controls tumor cell growth. ACS Omega 2019, 4, 4040–4048. [Google Scholar] [CrossRef]
- Kosowska, K.; Domalik-Pyzik, P.; Sekuła-Stryjewska, M.; Noga, S.; Jagiełło, J.; Baran, M.; Lipińska, L.; Zuba-Surma, E.; Chłopek, J. Gradient Chitosan Hydrogels Modified with Graphene Derivatives and Hydroxyapatite: Physiochemical Properties and Initial Cytocompatibility Evaluation. Int. J. Mol. Sci. 2020, 21, 4888. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-Synthesized Polysaccharide-Derived Carbon Dots as Therapeutic Cargoes and Toughening Agents for Elastomeric Gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef]
- Wang, H.; Biswas, S.K.; Zhu, S.; Lu, Y.; Yue, Y.; Han, J.; Xu, X.; Wu, Q.; Xiao, H. Self-Healable Electro-Conductive Hydrogels Based on Core-Shell Structured Nanocellulose/Carbon Nanotubes Hybrids for Use as Flexible Supercapacitors. Nanomaterials 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.; Prasad, S.R.; Mandal, D.; Das, P. Carbon dot cross-linked polyvinylpyrrolidone hybrid hydrogel for simultaneous dye adsorption, photodegradation and bacterial elimination from waste water. J. Hazard. Mater. 2020, 392, 122287. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, K.; Eid, K.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Rational synthesis, characterization, and application of environmentally friendly (polymer–carbon dot) hybrid composite film for fast and efficient UV-assisted Cd2+ removal from water. Environ. Sci. Eur. 2020, 32, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, K.; Suneetha, M.; Rao, K.M.; Han, S.S. Antibacterial reduced graphene oxide reinforces polyelectrolyte hydrogels with polysaccharides via a green method. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127340. [Google Scholar] [CrossRef]
- Krzan, M.; Kulawik-Pióro, A.; Tyliszczak, B. Chapter 5. Foams stabilized by particles. In Foam Films and Foams: Fundamentals and Application; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; Progress in colloids and Interfacial Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–294. ISBN 9781466587724. [Google Scholar]
- Zhang, Y.; Zhu, J.-Y.; Ren, H.-B.; Bi, Y.-T.; Zhang, L. Facile synthesis of nitrogen-doped graphene aerogels functionalized with chitosan for supercapacitors with excellent electrochemical performance. Chin. Chem. Lett. 2017, 28, 935–942. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, E.F.; Yan, H.; Kono, Y.; Wen, B.; Bai, L.; Shi, F.; Zhang, J.; Kenney-Benson, C.; Park, C.; et al. Nanoarchitectured materials composed of fullerene-like spheroids and disordered graphene layers with tunable mechanical properties. Nat. Commun. 2015, 6, 6212. [Google Scholar] [CrossRef] [Green Version]
- Thangavelu, S.A.G.; Mukherjee, M.; Layana, K.; Kumar, C.D.; Sulthana, Y.R.; Kumar, R.R.; Ananthan, A.; Muthulakshmi, V.; Mandal, A.B. Biodegradable polyurethanes foam and foam fullerenes nanocomposite strips by one-shot moulding: Physicochemical and mechanical properties. Mater. Sci. Semicond. Process. 2020, 112, 105018. [Google Scholar] [CrossRef]
- Zhang, M. United State Patent Application. U.S. Patent 2018/0201508A1, 19 July 2018. [Google Scholar]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Structure and properties of thermomechanically processed chitosan/carboxymethyl cellulose/graphene oxide polyelectrolyte complexed bionanocomposites. Int. J. Biol. Macromol. 2020, 158, 420–429. [Google Scholar] [CrossRef]
- Zarate-Triviño, D.; Prokhorov, E.; Luna-Bárcenas, G.; Mendez-Nonell, J.; González-Campos, J.; Elizalde-Peña, E.; Mota-Morales, J.D.; Santiago-Jacinto, P.; Terrones, M.; Gómez-Salazar, S.; et al. The effect of CNT functionalization on electrical and relaxation phenomena in MWCNT/chitosan composites. Mater. Chem. Phys. 2015, 155, 252–261. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alsohaimi, I.H.; Alshehri, S.; Alawady, A.R.; El-Aassar, M.; Nghiem, L.D.; Panhuis, M.I.H. Nanofiltration membranes prepared from pristine and functionalised multiwall carbon nanotubes/biopolymer composites for water treatment applications. J. Mater. Res. Technol. 2020, 9, 9080–9092. [Google Scholar] [CrossRef]
- Yamakawa, A.; Suzuki, S.; Oku, T.; Enomoto, K.; Ikeda, M.; Rodrigue, J.; Tateiwa, K.; Terada, Y.; Yano, H.; Kitamura, S. Nanostructure and physical properties of cellulose nanofiber-carbon nanotube composite films. Carbohydr. Polym. 2017, 171, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Khachatryan, G.; Kopel, P.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Kucharek, M.; Bębenek, Z.; Zimowska, M. Furcellaran nanocomposite films: The effect of nanofillers on the structural, thermal, mechanical and antimicrobial properties of biopolymer films. Carbohydr. Polym. 2020, 240, 116244. [Google Scholar] [CrossRef]
- Keleshteri, A.R.; Basiri, H.; Mohseni, S.S.; Farokhi, M.; Mehrizi, A.A.; Moztarzadeh, F. Preparation of microfluidic-based pectin microparticles loaded carbon dots conjugated with BMP-2 embedded in gelatin-elastin-hyaluronic acid hydrogel scaffold for bone tissue engineering application. Int. J. Biol. Macromol. 2021, 184, 29–41. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Z.; Sun, J.; Geng, C.; Bu, Q.; Wu, D.; Xia, Y. Electrospinning Highly Concentrated Sodium Alginate Nanofibres without Surfactants by Adding Fluorescent Carbon Dots. Nanomaterials 2020, 10, 565. [Google Scholar] [CrossRef] [Green Version]
- Kesavan, S.; Meena, K.S.; Dhakshinamoorthy, R. Bioactive Polysaccharides Based Graphene Oxide Nanoparticle as a Promising Carrier for Anticancer Drug Delivery. Biointerface Res. Appl. Chem. 2022, 12, 3429–3445. [Google Scholar] [CrossRef]
- Low, Y.Z.; Li, L.; Tan, L.P. Investigating the Behavior of Mucoadhesive Polysaccharide-Functionalized Graphene Oxide in Bladder Environment. ACS Appl. Bio Mater. 2021, 4, 630–639. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Y.; Zhang, M.; Cui, B.; Hu, Q.; Wang, L. A novel electrochemical strategy based on porous 3D graphene-starch architecture and silver deposition for ultrasensitive detection of neuron-specific enolase. Analyst 2019, 144, 2186–2194. [Google Scholar] [CrossRef]
- Wei, Q.; Fu, T.; Yue, Q.; Liu, H.; Ma, S.; Cai, M.; Zhou, F. Graphene oxide/brush-like polysaccharide copolymer nanohybrids as eco-friendly additives for water-based lubrication. Tribol. Int. 2021, 157, 106895. [Google Scholar] [CrossRef]
- Dacrory, S. Antimicrobial Activity, DFT Calculations, and Molecular Docking of Dialdehyde Cellulose/Graphene Oxide Film Against Covid. J. Polym. Environ. 2021, 29, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Huang, Y.; Ouyang, S.; Huang, J.; Shi, Y.; Tong, Y.-J.; Zhao, X.; Li, N.; Zheng, J.; Zheng, J.; et al. Efficient solid phase microextraction of organic pollutants based on graphene oxide/chitosan aerogel. Anal. Chim. Acta 2022, 1195, 339462. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deng, W.; Zhou, W.; Luo, J. Novel magnetic polysaccharide/graphene oxide @Fe3O4 gel beads for adsorbing heavy metal ions. Carbohydr. Polym. 2019, 216, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dai, G.; Gao, Q. Starch Nanoparticles–Graphene Aerogels with High Supercapacitor Performance and Efficient Adsorption. ACS Sustain. Chem. Eng. 2019, 7, 14064–14073. [Google Scholar] [CrossRef]
- Mishra, R.; Singh, S.K.; Gupta, H.; Srivastava, N.; Meghnani, D.; Tiwari, R.K.; Patel, A.; Tiwari, A.; Tiwari, V.K.; Singh, R.K. Surface modification of nano Na[Ni0.60Mn0.35Co0.05]O2 cathode material by dextran functionalized RGO via hydrothermal treatment for high performance sodium batteries. Appl. Surf. Sci. 2020, 535, 147695. [Google Scholar] [CrossRef]
- Hsiao, L.-Y.; Jing, L.; Li, K.; Yang, H.; Li, Y.; Chen, P.-Y. Carbon nanotube-integrated conductive hydrogels as multifunctional robotic skin. Carbon 2020, 161, 784–793. [Google Scholar] [CrossRef]
- Wang, Y.; Han, L.; Zhang, X.; Cao, L.; Hu, K.; Li, L.; Wei, Y. 3D bioprinting of an electroactive and self-healing polysaccharide hydrogels. J. Tissue Eng. Regen. Med. 2022, 16, 76–85. [Google Scholar] [CrossRef]
- Garnica-Palafox, I.; Estrella-Monroy, H.; Vázquez-Torres, N.; Álvarez-Camacho, M.; Castell-Rodríguez, A.; Sánchez-Arévalo, F. Influence of multi-walled carbon nanotubes on the physico-chemical and biological responses of chitosan-based hybrid hydrogels. Carbohydr. Polym. 2020, 236, 115971. [Google Scholar] [CrossRef]
- Ou, Y.; Tsen, W.-C.; Gong, C.; Wang, J.; Liu, H.; Zheng, G.; Qin, C.; Wen, S. Chitosan-based composite membranes containing chitosan-coated carbon nanotubes for polymer electrolyte membranes. Polym. Adv. Technol. 2018, 29, 612–622. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Song, A.; Goodman, S.M.; He, D.; Bai, J. Smart papers comprising carbon nanotubes and cellulose microfibers for multifunctional sensing applications. J. Mater. Chem. A 2017, 5, 20161–20169. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kwon, S.; Lee, H.L.; Toivakka, M.; Oh, K. Cellulose nanofibril/carbon nanotube composite foam-stabilized paraffin phase change material for thermal energy storage and conversion. Carbohydr. Polym. 2021, 273, 118585. [Google Scholar] [CrossRef] [PubMed]
- El Badawi, N.; Ramadan, A.R.; Esawi, A.M.; El-Morsi, M. Novel carbon nanotube–cellulose acetate nanocomposite membranes for water filtration applications. Desalination 2014, 344, 79–85. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V.; Mallakpour, F. Synthesis of alginate/carbon nanotube/carbon dot/fluoroapatite/TiO2 beads for dye photocatalytic degradation under ultraviolet light. Carbohydr. Polym. 2019, 224, 115138. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Roy, S.; Rhim, J.-W. Pectin/gelatin-based bioactive composite films reinforced with sulfur functionalized carbon dots. Colloids Surf. A Physicochem. Eng. Asp. 2021, 634, 128123. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.-W. Gelatin/Carrageenan-Based Functional Films with Carbon Dots from Enoki Mushroom for Active Food Packaging Applications. ACS Appl. Polym. Mater. 2021, 3, 6437–6445. [Google Scholar] [CrossRef]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control 2021, 126, 108039. [Google Scholar] [CrossRef]
- Won, H.J.; Ryplida, B.; Kim, S.G.; Lee, G.; Ryu, J.H.; Park, S.Y. Diselenide-Bridged Carbon-Dot-Mediated Self-Healing, Conductive, and Adhesive Wireless Hydrogel Sensors for Label-Free Breast Cancer Detection. ACS Nano 2020, 14, 8409–8420. [Google Scholar] [CrossRef]
| Carbon Nanoparticles | Polysaccharides | Application | Ref. |
|---|---|---|---|
| Graphene and its derivatives | Chitosan/xyloglucan | Nanocarrier for biomedical applications | [132] |
| Chitosan/carboxymethylcellulose | [133] | ||
| Starch | Immunoassay to detect neuron-specific enolase with a triple signal amplification strategy | [134] | |
| Chitosan | Additives for water-based lubrication | [135] | |
| Dialdehyde cellulose | Film against COVID-19 | [136] | |
| Chitosan | Microextraction of organic pollutants | [137] | |
| Chitosan/sodium alginate | Wastewater treatment | [138] | |
| Starch | Supercapacitor electrodes and efficient adsorbents | [139] | |
| Dextran | High-performance sodium batteries | [140] | |
| Carbon nanotubes | Gelatin | Multifunctional robotic skin | [141] |
| Konjac glucomannan | Scaffolds for muscle and cardiac nerve tissue regeneration | [142] | |
| Chitosan | Tissue engineering and biomedical applications | [143] | |
| Electrolyte membranes | [144] | ||
| Cellulose | Smart papers for multifunctional sensing | [145] | |
| Scaffold for bone regeneration | [146] | ||
| Thermal energy storage | [147] | ||
| Alginate | Membranes for water filtration | [148] | |
| [149] | |||
| Carbon quantum dots | Starch/chitosan | Elements of smart and active packaging | [102] |
| Pectin/gelatin | [150] | ||
| Gelatin/carrageenan | [151] | ||
| Sodium alginate | Tetracycline fluorescent sensor and adsorber | [111] | |
| Starch | Foil for monitoring spoilage of pork | [152] | |
| Gelatin | Sensors for label-free breast cancer detection | [153] | |
| Chitosan | Wound healing and drug delivery system | [73,107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krystyjan, M.; Khachatryan, G.; Khachatryan, K.; Krzan, M.; Ciesielski, W.; Żarska, S.; Szczepankowska, J. Polysaccharides Composite Materials as Carbon Nanoparticles Carrier. Polymers 2022, 14, 948. https://doi.org/10.3390/polym14050948
Krystyjan M, Khachatryan G, Khachatryan K, Krzan M, Ciesielski W, Żarska S, Szczepankowska J. Polysaccharides Composite Materials as Carbon Nanoparticles Carrier. Polymers. 2022; 14(5):948. https://doi.org/10.3390/polym14050948
Chicago/Turabian StyleKrystyjan, Magdalena, Gohar Khachatryan, Karen Khachatryan, Marcel Krzan, Wojciech Ciesielski, Sandra Żarska, and Joanna Szczepankowska. 2022. "Polysaccharides Composite Materials as Carbon Nanoparticles Carrier" Polymers 14, no. 5: 948. https://doi.org/10.3390/polym14050948
APA StyleKrystyjan, M., Khachatryan, G., Khachatryan, K., Krzan, M., Ciesielski, W., Żarska, S., & Szczepankowska, J. (2022). Polysaccharides Composite Materials as Carbon Nanoparticles Carrier. Polymers, 14(5), 948. https://doi.org/10.3390/polym14050948