Chitosan Catalyzed Novel Piperidinium Dicoumarol: Green Synthesis, X-ray Diffraction, Hirshfeld Surface and DFT Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of Piperidinium 3-[(4-Hydroxy-2-oxo-2H-chromen-3-yl)-(4-oxo-4H-chromen-3-yl)-methyl]-2-oxo-2H-chromen-4-olate Monohydrate (4) under Different Solvent Assisted Conditions Using Chitosan as Catalyst
2.3. Chitosan Catalyzed Green Synthesis of Piperidinium 3-[(4-Hydroxy-2-oxo-2H-chromen-3-yl)-(4-oxo-4H-chromen-3-yl)-methyl]-2-oxo-2H-chromen-4-olate Monohydrate (4)
2.4. Recycling and Reuse of Chitosan Catalyst for the Synthesis of Piperidinium 3-[(4-Hydroxy-2-oxo-2H-chromen-3-yl)-(4-oxo-4H-chromen-3-yl)-methyl]-2-oxo-2H-chromen-4-olate Monohydrate (4)
2.5. Characterization Data of Piperidinium 3-[(4-Hydroxy-2-oxo-2H-chromen-3-yl)-(4-oxo-4H-chromen-3-yl)-methyl]-2-oxo-2H-chromen-4-olate Monohydrate (4)
2.6. Single Crystal X-ray Crystallography
2.7. Computational Study
3. Results and Discussion
3.1. Chemistry
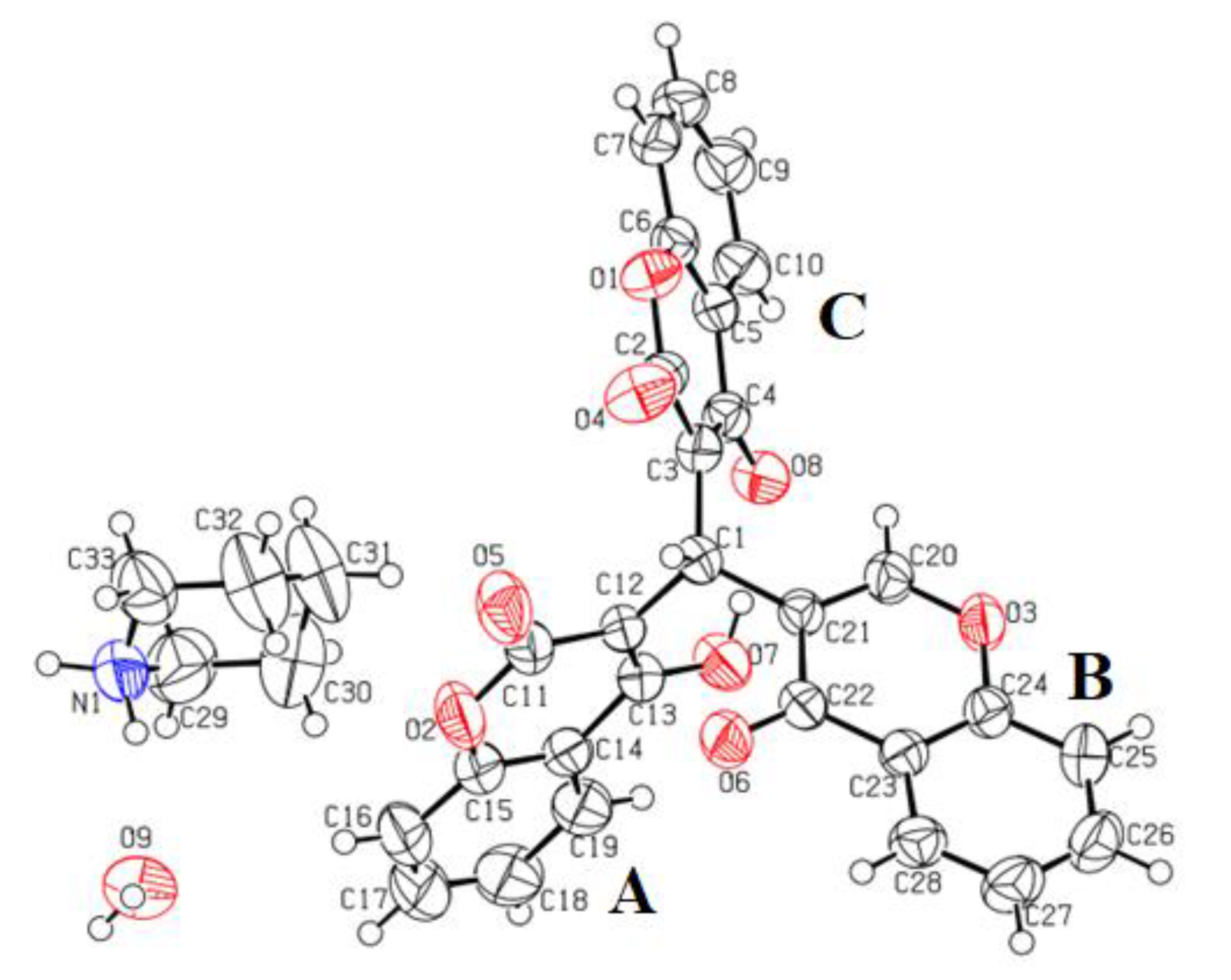
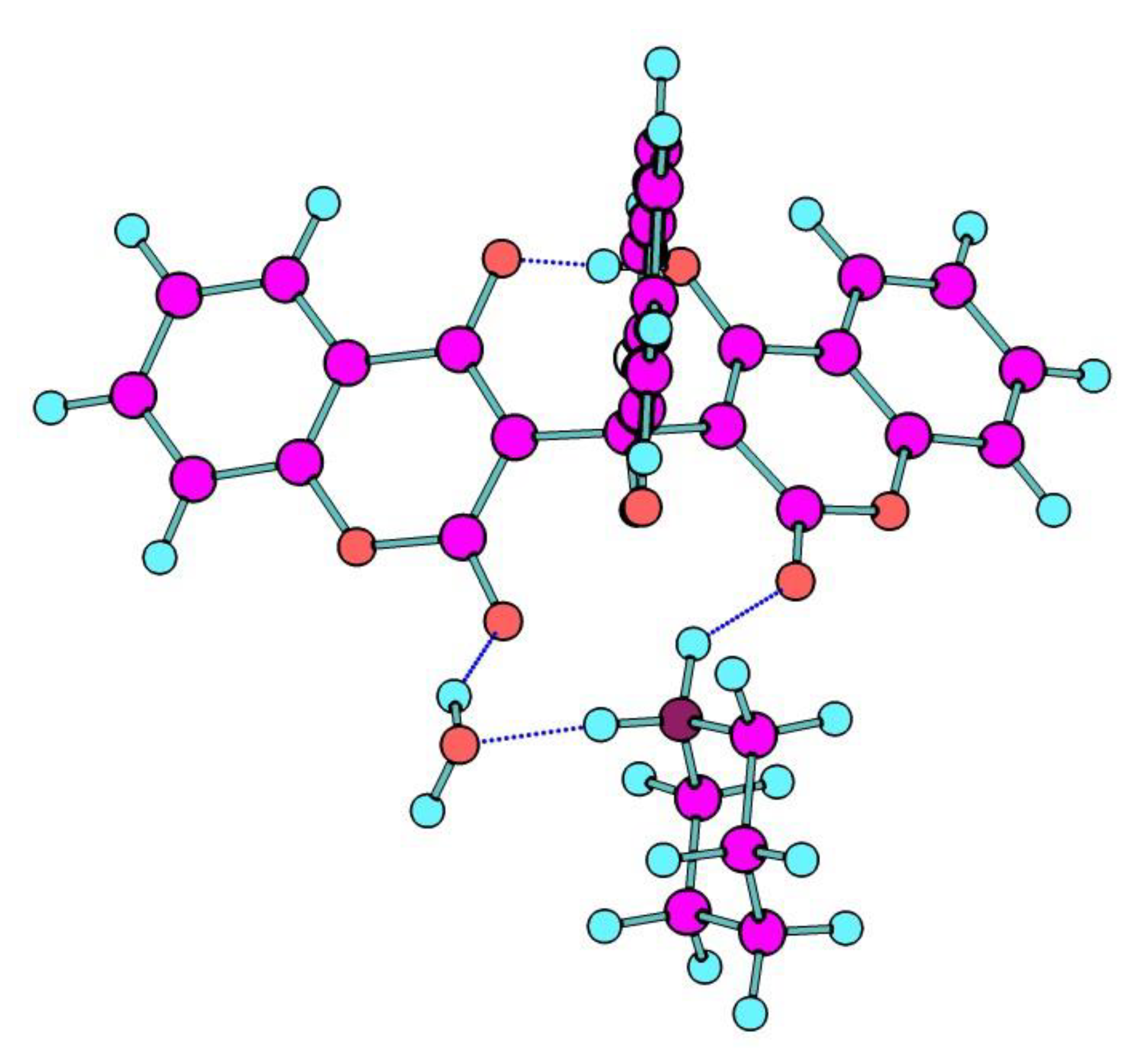
3.2. Crystal Structure Description
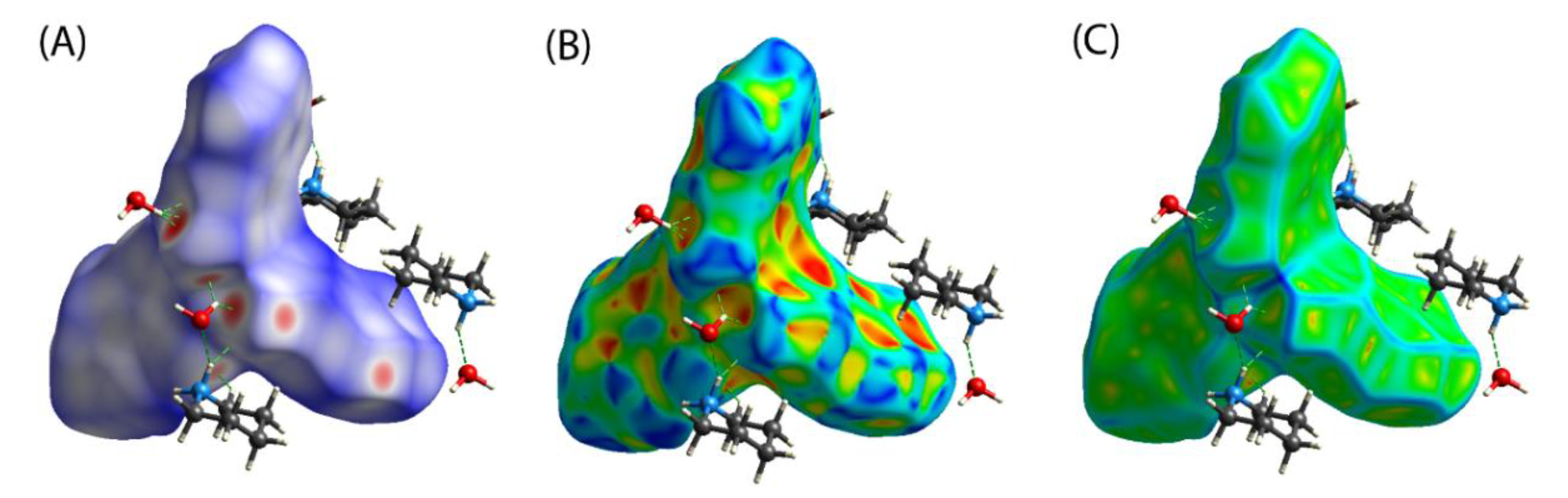
3.3. Hirshfeld Surface Analyses
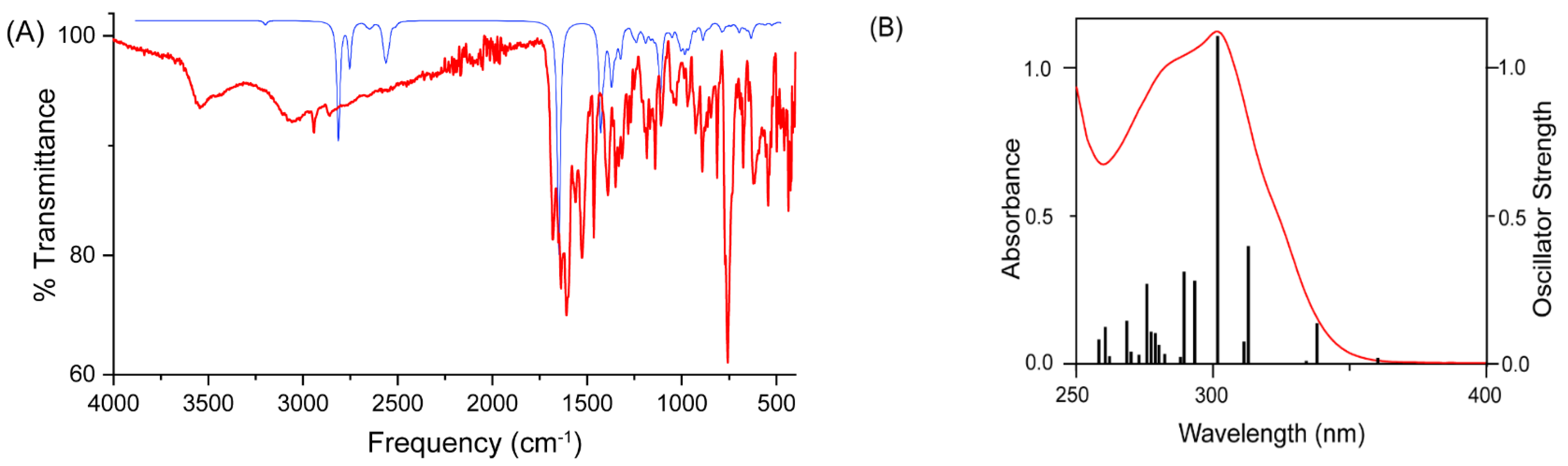
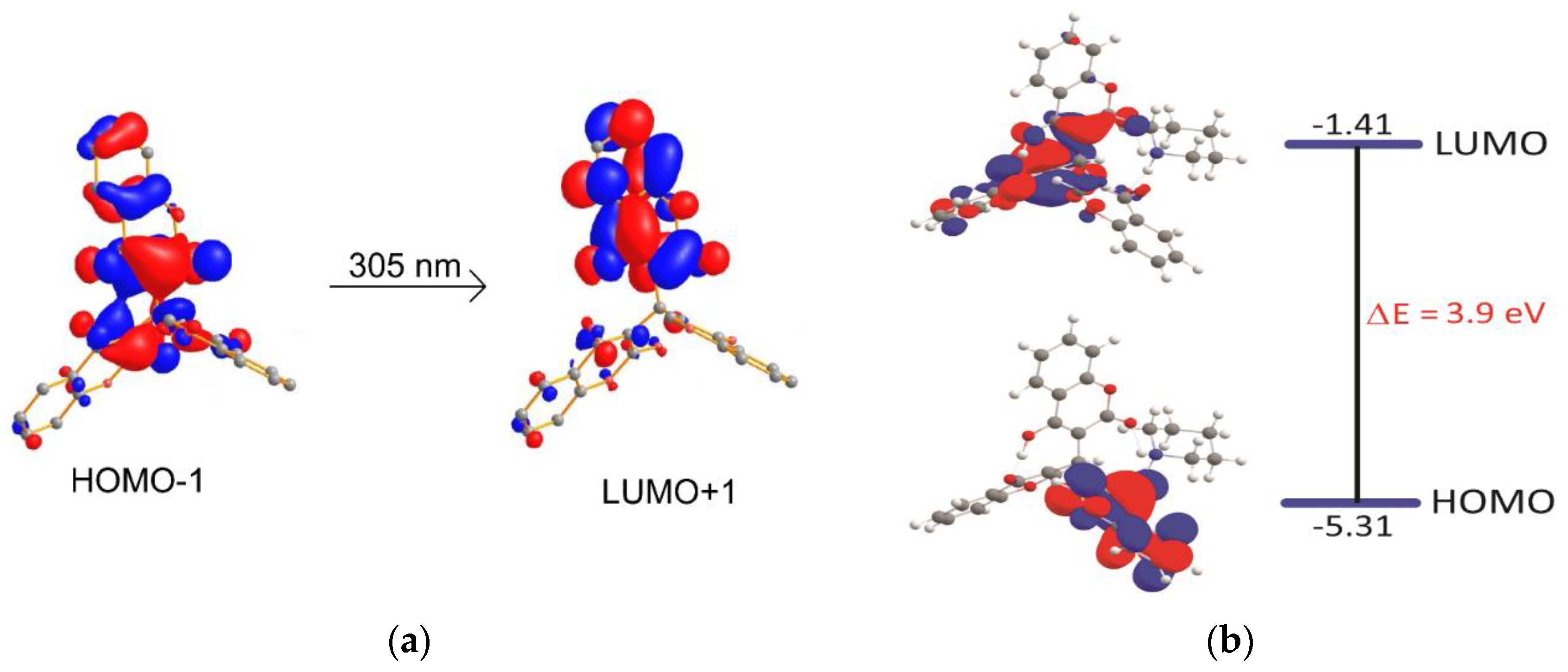
3.4. Density Functional Theory
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, Z.N.; Asad, M.; Praveen, S. Synthesis and biological activity of heterocycles from chalcone. Med. Chem. Res. 2008, 17, 318–325. [Google Scholar] [CrossRef]
- Saquib, M.; Baig, M.H.; Khan, M.F.; Azmi, S.; Khatoon, S.; Rawat, A.K.; Dong, J.J.; Asad, M.; Arshad, M.; Hussain, M.K. Design and synthesis of bioinspired benzocoumarin-chalcones chimeras as potential anti-breast cancer agents. ChemistrySelect 2021, 6, 8754–8765. [Google Scholar] [CrossRef]
- Kumar, D.; Kaur, N.; Giri, R.; Singh, N. A biscoumarin scaffold as an efficient anti-Zika virus lead with NS3-helicase inhibitory potential: In vitro and in silico investigations. New J. Chem. 2020, 44, 1872–1880. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Musthafa, T.N.M.; Praveen, S. Solvent- and catalyst-free synthesis of bis-adducts of 3-formylchromone as potential antimicrobial agents. Med. Chem. Res. 2013, 22, 127–133. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; TN, M.M.; Ahmad, A.; Khan, A.U. Synthesis of 4-hydroxycoumarin heteroarylhybrids as potential antimicrobial agents. Arch. Pharm. 2011, 344, 394–401. [Google Scholar] [CrossRef]
- Gaspar, A.; Matos, M.J.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef] [Green Version]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; Teissier García, A.G.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef]
- Yu, J.; Shi, F.; Gong, L.-Z. Brønsted-acid catalyzed asymmetric multicomponent reactions for the facile synthesis of highly enantioenriched structurally diverse nitrogenous heterocycles. Acc. Chem. Res. 2011, 44, 1156–1171. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Hamdi, N.; Puerta, M.C.; Valerga, P. Synthesis, structure, antimicrobial and antioxidant investigations of dicoumarol and related compounds. Eur. J. Med. Chem. 2008, 43, 2541–2548. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, D.; Singh, A.; Banerjee, B. Camphor sulfonic acid catalyzed facile and general method for the synthesis of 3,3′-(arylmethylene)bis(4-hydroxy-2H-chromen-2-ones), 3,3′-(arylmethylene)bis(2-hydroxynaphthalene-1,4-diones) and 3,3′-(2-oxoindoline-3,3-diyl)bis(2-hydroxynaphthalene-1,4-dione) derivatives at room temperature. Synth. Commun. 2021, 51, 1045–1057. [Google Scholar] [CrossRef]
- Hodge, P.; Sherrington, D.C. Polymer-Supported Reactions in Organic Synthesis; John Wiley & Sons: New York, NY, USA, 1980; ISBN 0471277126. [Google Scholar]
- Sherrington, D.C.; Hodge, P. Synthesis and Separations Using Functional Polymers. Adv. Mater. 1988, 1, 93–94. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A.; El Meray, M. Influence of the nature of the metal ions on the complexation with chitosan. Eur. Polym. J. 2002, 38, 1523–1530. [Google Scholar] [CrossRef]
- Boldrini, D.E.; Angeletti, S.; Cervellini, P.M.; Reinoso, D.M. Highly ordered mesoporous Al-MCM-41 synthesis through valorization of natural sediment. ACS Sustain. Chem. Eng. 2019, 7, 4684–4691. [Google Scholar] [CrossRef]
- Sahu, P.K. A green approach to the synthesis of a nano catalyst and the role of basicity, calcination, catalytic activity and aging in the green synthesis of 2-aryl bezimidazoles, benzothiazoles and benzoxazoles. RSC Adv. 2017, 7, 42000–42012. [Google Scholar] [CrossRef] [Green Version]
- Agilent. CrysAlisPRO; Agilent Technologies Ltd.: Yarnton, England, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sec. A. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. App. Crayst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON—A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2005. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Frisch, M.J.T.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.C.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, G.A.N.B.; Petersson, H.; et al. Fox Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Becke, A.D. Perspective on “Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Hariharan, P.C.; Pople, J.A. The effect of d-functions on molecular orbital energies for hydrocarbons. Chem. Phys. Lett. 1972, 16, 217–219. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19. [Google Scholar] [CrossRef]
- Martin de Antonino, R.S.C.; Fook, B.R.P.L.; Lima, V.A.; Rached, R.I.; Lima, E.P.N.; Lima, R.J.; Covas, C.A.P.; Fook, M.V.L. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asad, M.; Oo, C.-W.; Osman, H.; Yeap, C.S.; Fun, H.-K. 4-Hydroxy-3-[(4-hydroxy-6,7-dimethyl2-oxo-2H-chromen-3-yl)(4-oxo-4Hchromen-3-yl)methyl]-6,7-dimethyl-2H-chromen-2-one. Acta Crystallogr. 2010, E66, o2859–o2860. [Google Scholar] [CrossRef]




| Solvents | Time | Yield |
|---|---|---|
| PEG-400 | 7 h | 52% |
| Water | 7 h | 65% |
| Methanol | 3 h | 69% |
| Ethanol | 2.5 h | 76% |
| Solvent-free | 30 min | 88% |
| Cycle | Time | Yield |
|---|---|---|
| I | 30 min | 88% |
| II | 30 min | 88% |
| III | 30 min | 88% |
| IV | 30 min | 88% |
| V | 28 min | 85% |
| Identification Code | 19050ccdcolx |
|---|---|
| Empirical formula | C33H29NO9 |
| Formula weight | 583.57 |
| Temperature/K | 296(2) |
| Crystal system | monoclinic |
| Space group | P21/c |
| a/Å | 13.8247(6) |
| b/Å | 12.6540(5) |
| c/Å | 16.4037(8) |
| α/° | 90 |
| β/° | 102.255(4) |
| γ/° | 90 |
| Volume/Å3 | 2804.2(2) |
| Z | 4 |
| ρcalc g/cm3 | 1.382 |
| μ/mm−1 | 0.101 |
| F(000) | 1224.0 |
| Crystal size/mm3 | 0.42 × 0.37 × 0.32 |
| Radiation | MoKα (λ = 0.71073) |
| 2θ range for data collection/° | 6.016 to 58.078 |
| Index ranges | −17 ≤ h ≤ 18, −16 ≤ k ≤ 16, −20 ≤ l ≤ 20 |
| Reflections collected | 16568 |
| Independent reflections | 6632 [Rint = 0.0359, Rsigma = 0.0489] |
| Data/restraints/parameters | 6632/0/400 |
| Goodness-of-fit on F2 | 1.028 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0537, wR2 = 0.1134 |
| Final R indexes [all data] | R1 = 0.1084, wR2 = 0.1414 |
| Largest diff. peak/hole/e Å−3 | 0.26/−0.17 |
| D | H | A | d(D-H)/Å | d(H-A)/Å | d(D-A)/Å | D-H-A/° |
|---|---|---|---|---|---|---|
| C16 | H16 | O5 1 | 0.93 | 2.41 | 3.215(3) | 145.1 |
| O7 | H1O | O8 | 0.82 | 1.66 | 2.466(2) | 168.2 |
| C33 | H33B | O6 1 | 0.97 | 2.44 | 3.215(3) | 136.4 |
| N1 | H1N | O8 2 | 1.05(4) | 1.94(4) | 2.933(3) | 156(3) |
| N1 | H2N | O9 | 0.95(4) | 2.01(4) | 2.854(3) | 146(3) |
| O9 | H2O | O4 1 | 0.85 | 2.36 | 2.972(2) | 129 |
| O9 | H2O | O5 1 | 0.85 | 2.21 | 2.859(1) | 134 |
| O9 | H3O | O4 3 | 0.85 | 2.14 | 2.974(2) | 166 |
| 1 1 − X,1 − Y,1 − Z; 2 1 − X,1/2 + Y,3/2 − Z, 3 x-1,y,z | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asad, M.; Arshad, M.N.; T.N., M.M.; Asiri, A.M. Chitosan Catalyzed Novel Piperidinium Dicoumarol: Green Synthesis, X-ray Diffraction, Hirshfeld Surface and DFT Studies. Polymers 2022, 14, 1854. https://doi.org/10.3390/polym14091854
Asad M, Arshad MN, T.N. MM, Asiri AM. Chitosan Catalyzed Novel Piperidinium Dicoumarol: Green Synthesis, X-ray Diffraction, Hirshfeld Surface and DFT Studies. Polymers. 2022; 14(9):1854. https://doi.org/10.3390/polym14091854
Chicago/Turabian StyleAsad, Mohammad, Muhammad Nadeem Arshad, Mohammed Musthafa T.N., and Abdullah M. Asiri. 2022. "Chitosan Catalyzed Novel Piperidinium Dicoumarol: Green Synthesis, X-ray Diffraction, Hirshfeld Surface and DFT Studies" Polymers 14, no. 9: 1854. https://doi.org/10.3390/polym14091854
APA StyleAsad, M., Arshad, M. N., T.N., M. M., & Asiri, A. M. (2022). Chitosan Catalyzed Novel Piperidinium Dicoumarol: Green Synthesis, X-ray Diffraction, Hirshfeld Surface and DFT Studies. Polymers, 14(9), 1854. https://doi.org/10.3390/polym14091854








