Structural Characterization of Nanocellulose/Fe3O4 Hybrid Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NC/Fe3O4
2.3. Characterization Techniques
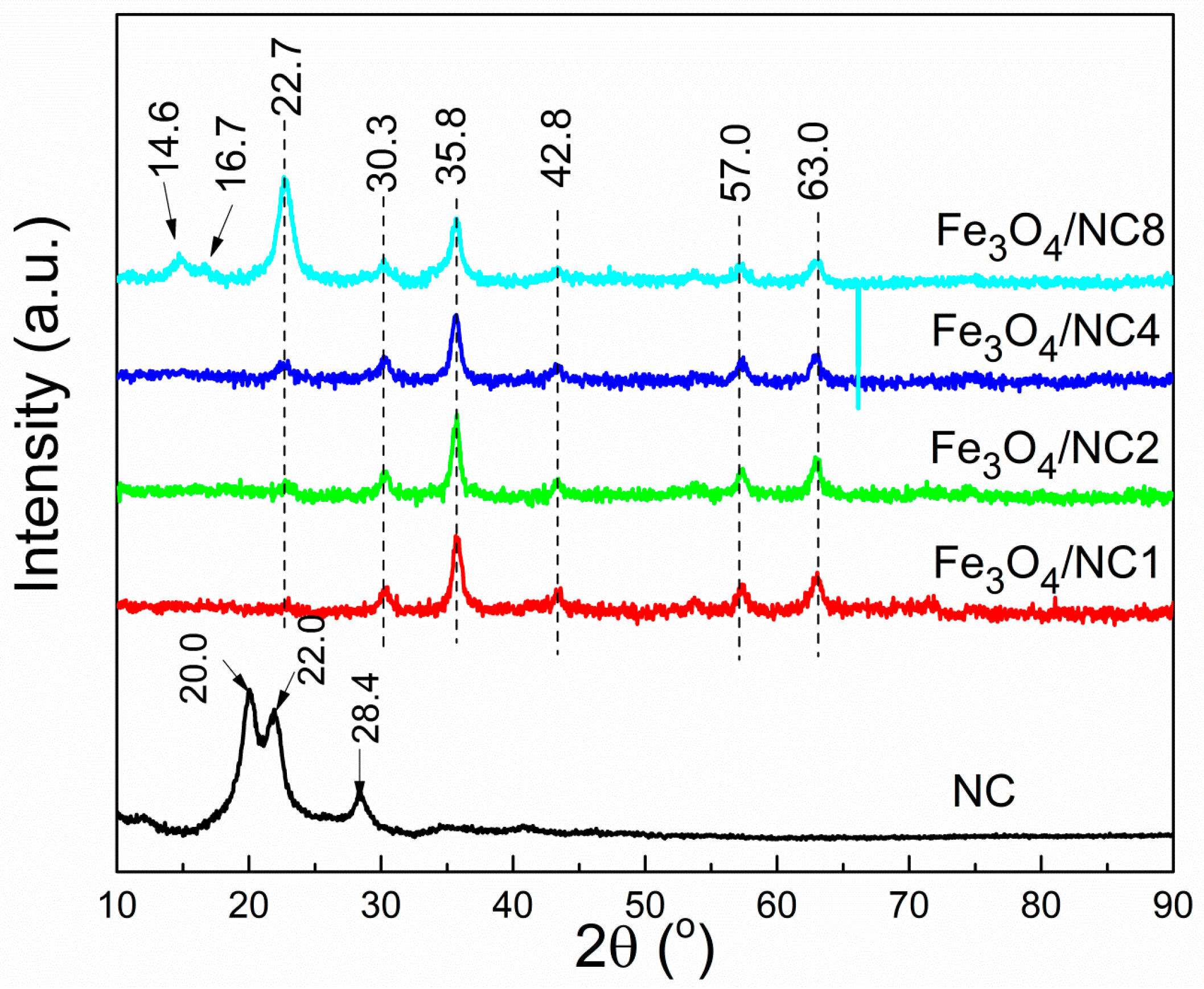
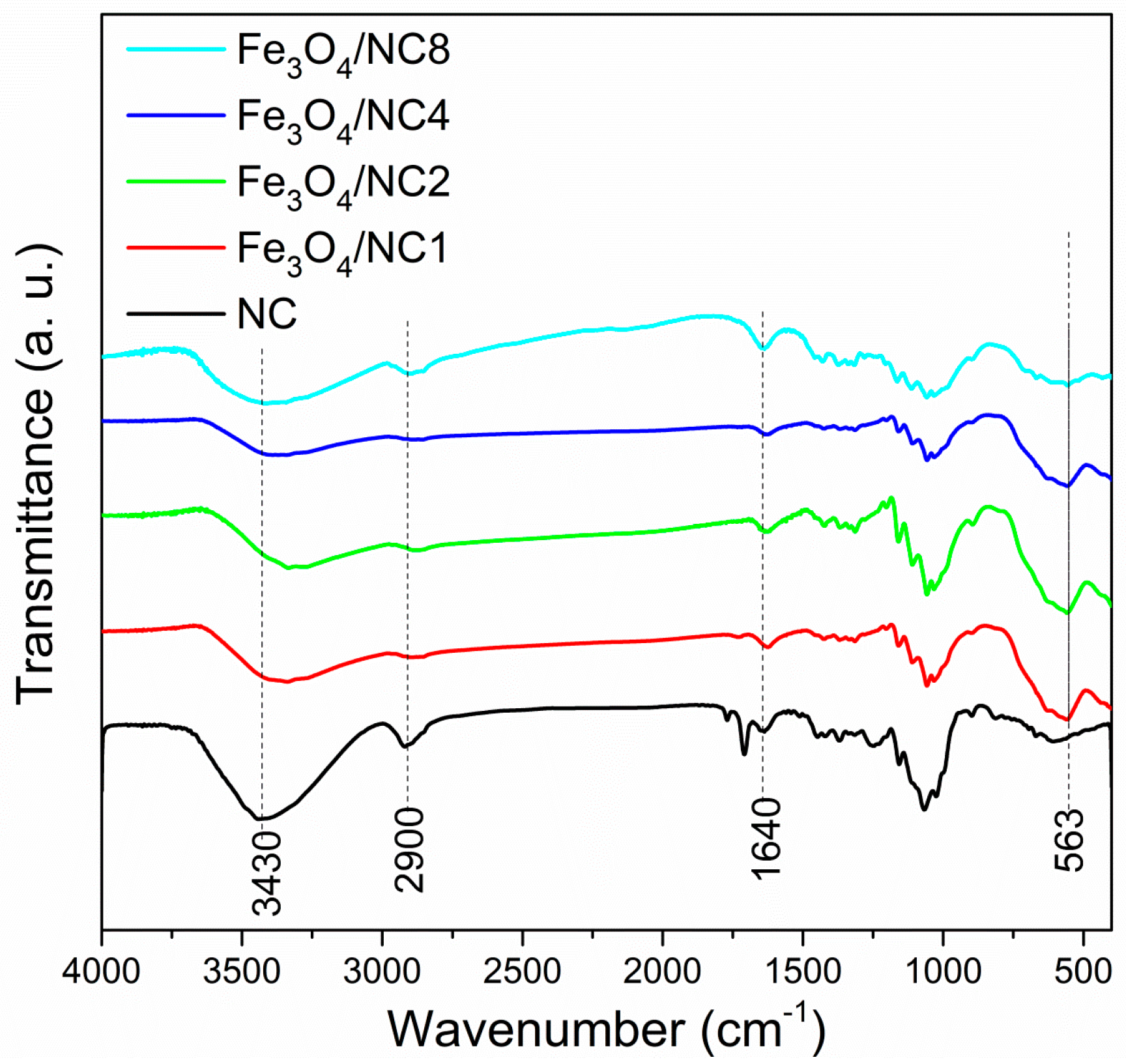
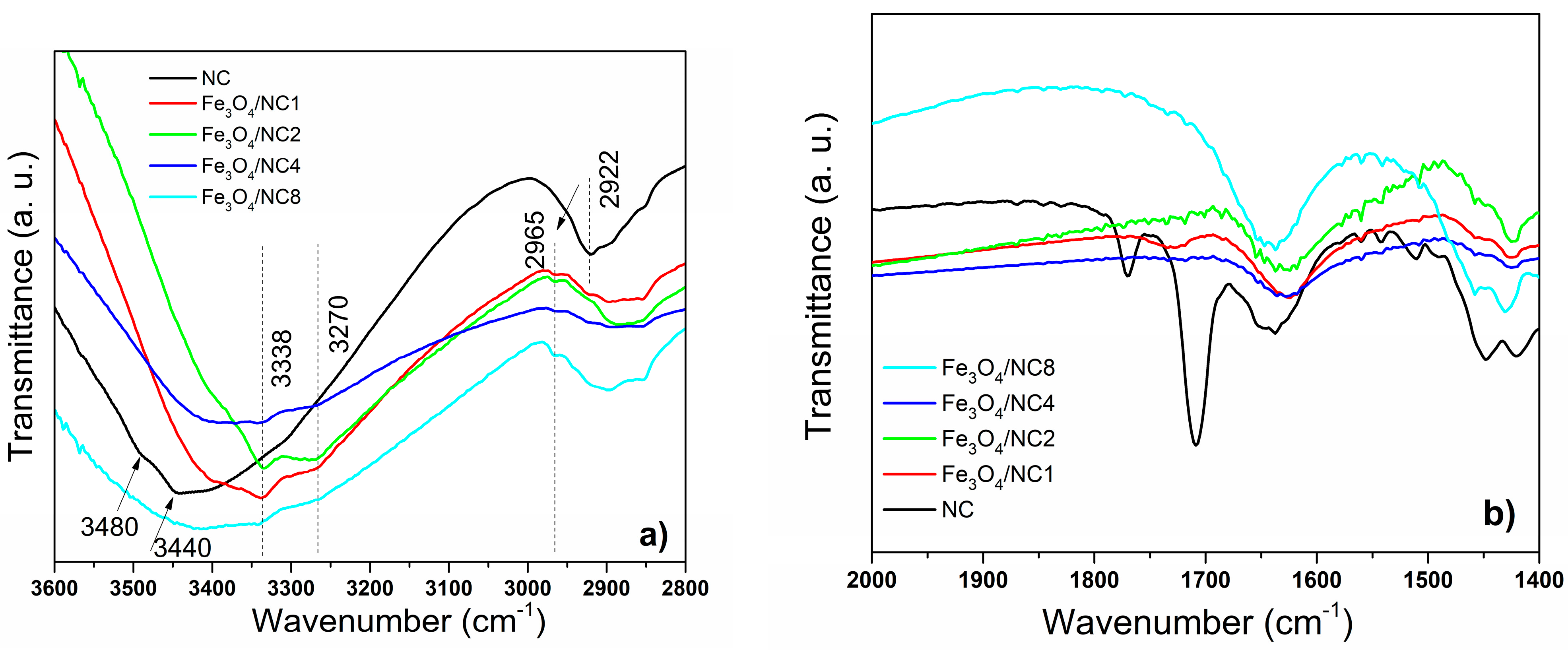
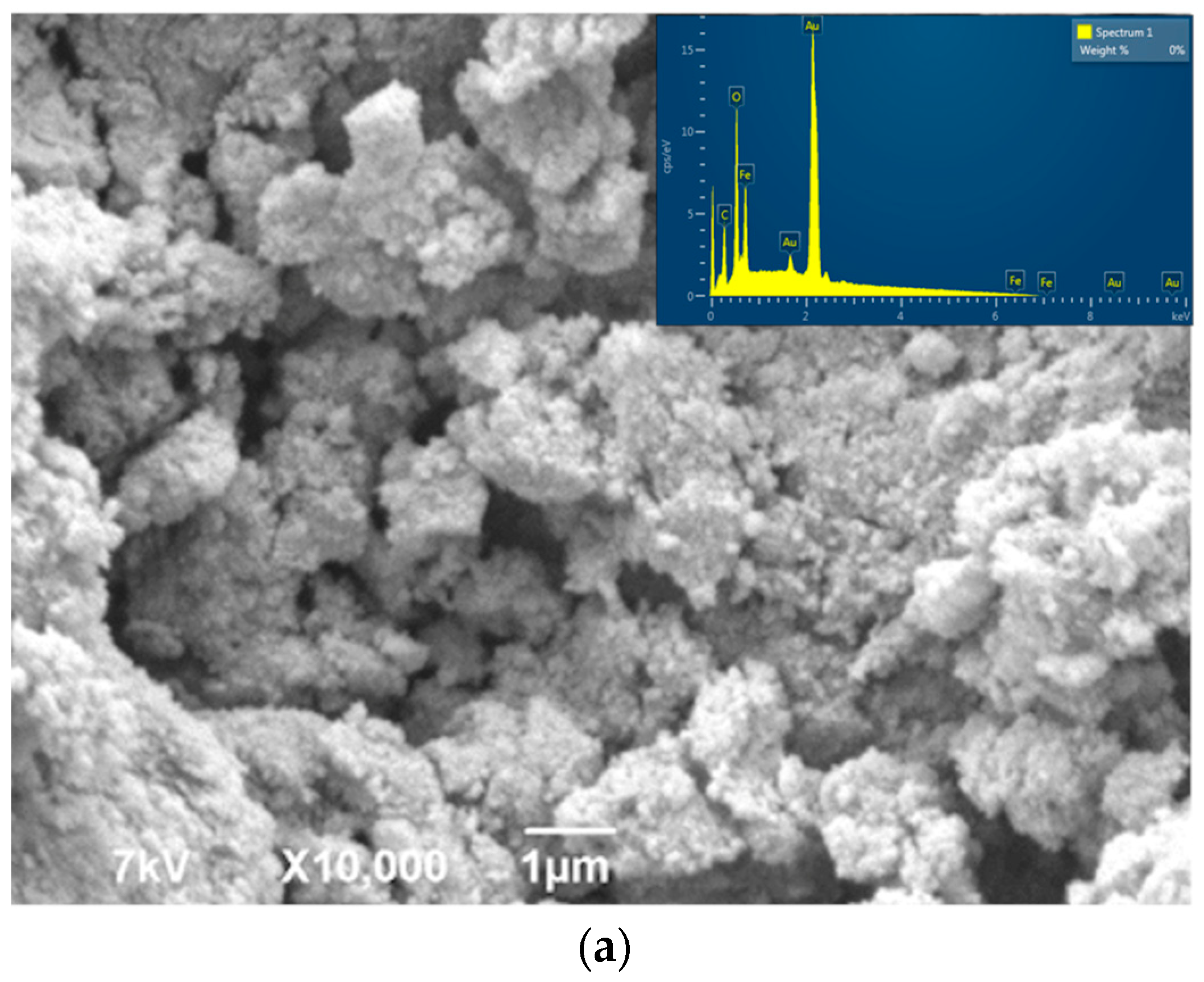
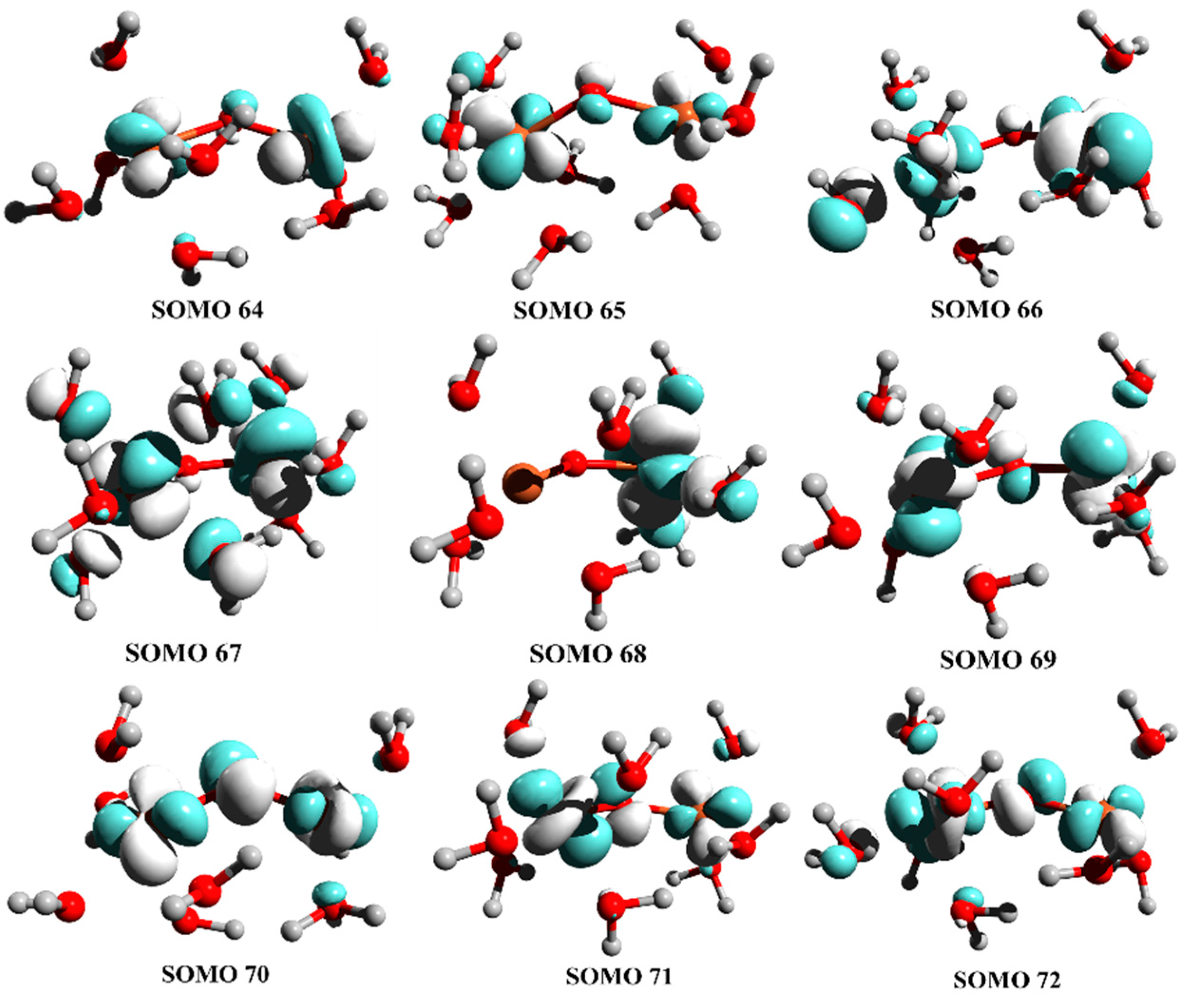
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rejab, M.R.B.M.; Hamdan, M.H.B.M.; Quanjin, M.; Siregar, J.P.; Bachtiar, D.; Muchlis, Y. Historical Development of Hybrid Materials. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 445–455. [Google Scholar]
- Gu, H.; Liu, C.; Zhu, J.; Gu, J.; Wujcik, E.K.; Shao, L.; Wang, N.; Wei, H.; Scaffaro, R.; Zhang, J.; et al. Introducing advanced composites and hybrid materials. Adv. Compos. Hybrid Mater. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Ashby, M.F. Hybrids to fill holes in material property space. Philos. Mag. 2005, 85, 3235–3257. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, R.K.; Samanta, S.B.; Rastogi, R.C.; Singh, R. Single-step magnetic patterning of iron nanoparticles in a semiconducting polymer matrix. Macromol. Chem. Phys. 2006, 207, 1584–1588. [Google Scholar] [CrossRef]
- Gonçalves, R.; Martins, P.M.; Caparrós, C.; Martins, P.; Benelmekki, M.; Botelho, G.; Lanceros-Mendez, S.; Lasheras, A.; Gutiérrez, J.; Barandiarán, J.M. Nucleation of the electroactive b-phase, dielectric and magnetic response of poly(vinylidene fluoride) composites with Fe2O3 nanoparticles. J. Non-Cryst. Solids 2013, 361, 93–99. [Google Scholar] [CrossRef]
- Jayakumar, O.D.; Mandal, B.P.; Majeed, J.; Lawes, G.; Naik, R.; Tyagi, K. Inorganic-organic multiferroic hybrid films of Fe3O4 and PVDF with significant magneto-dielectric coupling. J. Mater. Chem. C 2013, 1, 3710–3715. [Google Scholar] [CrossRef]
- Shabanian, M.; Khoobi, M.; Hemati, F.; Khonakdar, H.A.; Esmaeil, S.; Ebrahimi, S.; Wagenknecht, U.; Shafiee, A. New PLA/PEI-functionalized Fe3O4 nanocomposite: Preparation and characterization. J. Ind. Eng. Chem. 2015, 24, 211–218. [Google Scholar] [CrossRef]
- Huo, X.; Li, W.; Zhu, J.; Li, L.; Li, Y.; Luo, L.; Zhu, Y. Composite Based on Fe3O4@BaTiO3 Particles and Polyvinylidene Fluoride with Excellent Dielectric Properties and High Energy Density. J. Phys. Chem. C 2015, 119, 25786–25791. [Google Scholar] [CrossRef]
- Tsonos, C.; Soin, N.; Tomara, G.; Yang, B.; Psarras, G.C.; Kanapitsas, A.; Siores, E. Electromagnetic wave absorption properties of ternary poly(vinylidene fluoride)/magnetite nanocomposites with carbon nanotubes and graphene. RSC Adv. 2016, 6, 1919–1924. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Gonzales, K.N.; Sari, R.M.; Gea, S. Bacterial cellulose-based biosensors. Med. Devices Sens. 2020, 3, e10102. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent advances in cellulose-based biosensors for medical diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef]
- Gu, H.; Zhou, X.; Lyu, S.; Pan, D.; Dong, M.; Wu, S.; Ding, T.; Wei, X.; Seok, I.; Wei, S.; et al. Magnetic nanocellulose-magnetite aerogel for easy oil adsorption. J. Colloid Interface Sci. 2020, 560, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Amiralian, N.; Mustapic, M.; Hossain, S.A.; Wang, C.; Konarova, M.; Tang, J.; Na, J.; Khan, A.; Rowan, A. Magnetic nanocellulose: A potential material for removal of dye from water. J. Hazard Mater. 2020, 394, 122571. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 924, 79–2498. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R. Aminated β-Cyclodextrin-Modified-Carboxylated Magnetic Cobalt/Nanocellulose Composite for Tumor-Targeted Gene Delivery. J. Appl. Chem. 2014, 2014, 184153. [Google Scholar] [CrossRef]
- Hernández-Flores, J.A.; Morales-Cepeda, A.B.; Castro-Guerrero, C.F.; Delgado-Arroyo, F.; Díaz-Guillén, M.R.; de la Cruz-Soto, J.; Magallón-Cacho, L.; León-Silva, U. Morphological and Electrical Properties of Nanocellulose Compounds and Its Application on Capacitor Assembly. Int. J. Polym. Sci. 2020, 2020, e1891064. [Google Scholar] [CrossRef]
- Tuukkanen, S.; Rajala, S. Nanocellulose as a Piezoelectric Material, Piezoelectricity—Organic and Inorganic Materials and Applications, 1st ed.; Intech Open: London, UK, 2018. [Google Scholar]
- El-Nahas, A.M.; Salaheldin, T.A.; Zaki, T.; El-Maghrabi, H.H.; Marie, A.M.; Morsy, S.M.; Allam, N.K. Functionalized cellulose-magnetite nanocomposite catalysts for efficient biodiesel production. Chem. Eng. J. 2017, 322, 167–180. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Liu, S. Preparation of entangled nanocellulose fibers from APMP and its magnetic functional property as matrix. Carbohydr. Polym. 2013, 94, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Nypelö, T.; Rodriguez-Abreu, C.; Kolen’ko, Y.V.; Rivas, J.; Rojas, O.J. Microbeads and Hollow Microcapsules Obtained by Self-Assembly of Pickering Magneto-Responsive Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2014, 6, 16851–16858. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Kaku, Y.; Kimura, S.; Saito, T. Magnetically Collectable Nanocellulose-Coated Polymer Microparticles by Emulsion Templating. Langmuir 2020, 36, 9235–9240. [Google Scholar] [CrossRef] [PubMed]
- Taleb, K.; Markovski, J.; Veličković, Z.; Rusmirović, J.; Rančić, M.; Pavlović, V.; Marinković, A. Arsenic removal by magnetite-loaded amino modified nano/microcellulose adsorbents: Effect of functionalization and media size. Arab. J. Chem. 2019, 12, 4675–4693. [Google Scholar] [CrossRef]
- Luna-Martínez, J.F.; Reyes-Melo, E.; González-González, V.; Guerrero-Salazar, C.; Torres-Castro, A.; Sepúlveda-Guzmán, S. Synthesis and characterization of a magnetic hybrid material consisting of iron oxide in a carboxymethyl cellulose matrix. J. Appl. Polym. Sci. 2013, 127, 2325–2331. [Google Scholar] [CrossRef]
- Chen, L.; Berry, R.M.; Tam, K.C. Synthesis of β-Cyclodextrin-Modified Cellulose Nanocrystals (CNCs)@Fe3O4@SiO2 Superparamagnetic Nanorods. ACS Sustain. Chem. Eng. 2014, 2, 951–958. [Google Scholar] [CrossRef]
- Baniukevic, J.; Boyaci, I.H.; Bozkurt, A.G.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic gold nanoparticles in SERS-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosens. Bioelectron. 2013, 43, 281–288. [Google Scholar] [CrossRef]
- Sundar, S.T.; Sain, M.M.; Oksman, K. Characterization of microcrystalline cellulose and cellulose long fiber modified by iron salt. Carbohydr. Polym. 2010, 80, 35–43. [Google Scholar] [CrossRef]
- Zarei, S.; Niad, M.; Raanaei, H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J. Hazard. Mater. 2018, 344, 258–273. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Noodleman, L.; Norman, J.G.; Osborne, J.H.; Aizman, A.; Case, D.A. Models for ferredoxins: Electronic structures of iron-sulfur clusters with one, two, and four iron atoms. J. Am. Chem. Soc. 1985, 107, 3418–3426. [Google Scholar] [CrossRef]
- Noodleman, L.; Case, D.A.; Aizman, A. Broken symmetry analysis of spin coupling in iron-sulfur clusters. J. Am. Chem. Soc. 1988, 110, 1001–1005. [Google Scholar] [CrossRef]
- Noodleman, L.; Peng, C.Y.; Case, D.A.; Mouesca, J.-M. Orbital interactions, electron delocalization and spin coupling in iron-sulfur clusters. Coord. Chem. Rev. 1995, 144, 199–244. [Google Scholar] [CrossRef]
- Noodleman, L. Valence bond description of antiferromagnetic coupling in transition metal dimers. J. Chem. Phys. 1981, 74, 5737–5743. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional calculations of molecular bond energies. J. Chem. Phys. 1986, 84, 4524–4529. [Google Scholar] [CrossRef]
- Perdew, J.P.; Yue, W. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B 1986, 33, 8800–8802. [Google Scholar] [CrossRef]
- Swart, M.; Ehlers, A.W.; Lammertsma, K. Performance of the OPBE exchange-correlation functional. Mol. Phys. 2004, 102, 2467–2474. [Google Scholar] [CrossRef]
- Yue, Y. A Comparative Study of Cellulose I and II and Fibers and Nanocrystals. Master’s Theses, Louisiana State University, Baton Rouge, LA, USA, 2011. [Google Scholar]
- Mansikkamäki, P.; Lahtinen, M.; Rissanen, K. Structural Changes of Cellulose Crystallites Induced by Mercerisation in Different Solvent Systems; Determined by Powder X-ray Diffraction Method. Cellulose 2005, 12, 233–242. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Johari, A.; Jusoh, M. Isolation, Characterization, and Application of Nanocellulose from Oil Palm Empty Fruit Bunch Fiber as Nanocomposites. J. Nanomater. 2014, 2014, e702538. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R. Poly(methacrylic acid-co-vinyl sulfonic acid)-grafted-magnetite/nanocellulose superabsorbent composite for the selective recovery and separation of immunoglobulin from aqueous solutions. Sep. Purif. Technol. 2013, 119, 82–93. [Google Scholar]
- Djordjevic, N.; Marinkovic, A.; Nikolic, J.; Drmanic, S.; Rancic, M.; Brkovic, D.; Uskokovic, P. A study of the barrier properties of polyethylene coated with nanocellulose/magnetite composite film. J. Serb. Chem. Soc. 2016, 81, 589–605. [Google Scholar]
- Nyquist, R.O.; Kagel, R.A. Infrared Spectra of Inorganic Compounds (3800–45 cm−1); Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Anggraini Yossy, H. Nanocomposites Comprising Cellulose and Nanomagnetite as Heterogeneous Catalysts for the Synthesis of Biodiesel from Oleic Acid. Int. J. Technol. 2019, 10, 798–807. [Google Scholar]
- Rusmirović, J.D.; Ivanović, J.Z.; Pavlović, V.B.; Rakić, V.M.; Rančić, M.P.; Djokić, V.; Marinković, A.D. Novel modified nanocellulose applicable as reinforcement in high-performance nanocomposites. Carbohydr. Polym. 2017, 164, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Khor, S.; Petinakis, E.; Yu, L.; Simon, G.; Dean, K.; Bateman, S. Effects of hydrophilic fillers on the thermal degradation of poly(lactic acid). Thermochim. Acta 2010, 509, 147–151. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar]
- Zheng, D.; Zhang, Y.; Guo, Y.; Yue, J. Isolation and Characterization of Nanocellulose with a Novel Shape from Walnut (Juglans Regia L.) Shell Agricultural Waste. Polymers 2019, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Y.; Deng, P.; Zhang, T. Fe3O4@rGO hybrids intercalated nanocellulose-based aerogels for enhanced ferromagnetic and mechanical properties. J. App. Polym. Sci. 2020, 137, 48564. [Google Scholar]
- Heisenberg, W. Zur Theorie des Ferromagnetismus. Z. Für Phys. 1928, 49, 619–636. [Google Scholar]
- Dirac, P.A.M. The Principles of Quantum Mechanics; Clarendon Press: Oxford, UK, 1947. [Google Scholar]
- Soda, T.; Kitagawa, Y.; Onishi, T.; Takano, Y.; Shigeta, Y.; Nagao, H.; Yoshioka, Y.; Yamaguchi, K. Ab initio computations of effective exchange integrals for H–H, H–He–H and Mn2O2 complex: Comparison of broken-symmetry approaches. Chem. Phys. Lett. 2000, 319, 223–230. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahara, Y.; Fueno, T. Ab-Initio Molecular Orbital Studies of Structure and Reactivity of Transition Metal-OXO Compounds. In Applied Quantum Chemistry; Smith, V.H., Schaefer, H.F., Morokuma, K., Eds.; Springer: Dordrecht, The Netherlands, 1986; pp. 155–184. [Google Scholar]
- Neese, F. ORCA—An ab Initio, DFT and Semiempirical Electronic Structure Package, version 2.8, revision 15; Max-Planck-Institute für Bioanorganische Chemi: Mülheim an der Ruhr, Germany, 2009. [Google Scholar]



| Structure | JOPBE | JB3LYP | Fe2+-O | Fe3+-O |
|---|---|---|---|---|
| Fe3O4/NC1 | 48.7 | 46.7 | 2.081 | 1.846 |
| Fe3O4/NC2 | 49.8 | 48.1 | 2.069 | 1.835 |
| Fe3O4/NC4 | 49.3 | 47.7 | 2.073 | 1.839 |
| Fe3O4/NC8 | 49.5 | 47.8 | 2.071 | 1.837 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janićijević, A.; Pavlović, V.P.; Kovačević, D.; Perić, M.; Vlahović, B.; Pavlović, V.B.; Filipović, S. Structural Characterization of Nanocellulose/Fe3O4 Hybrid Nanomaterials. Polymers 2022, 14, 1819. https://doi.org/10.3390/polym14091819
Janićijević A, Pavlović VP, Kovačević D, Perić M, Vlahović B, Pavlović VB, Filipović S. Structural Characterization of Nanocellulose/Fe3O4 Hybrid Nanomaterials. Polymers. 2022; 14(9):1819. https://doi.org/10.3390/polym14091819
Chicago/Turabian StyleJanićijević, Aleksandra, Vera P. Pavlović, Danijela Kovačević, Marko Perić, Branislav Vlahović, Vladimir B. Pavlović, and Suzana Filipović. 2022. "Structural Characterization of Nanocellulose/Fe3O4 Hybrid Nanomaterials" Polymers 14, no. 9: 1819. https://doi.org/10.3390/polym14091819
APA StyleJanićijević, A., Pavlović, V. P., Kovačević, D., Perić, M., Vlahović, B., Pavlović, V. B., & Filipović, S. (2022). Structural Characterization of Nanocellulose/Fe3O4 Hybrid Nanomaterials. Polymers, 14(9), 1819. https://doi.org/10.3390/polym14091819








