Comprehensive Characterization of Solution-Cast Pristine and Reduced Graphene Oxide Composite Polyvinylidene Fluoride Films for Sensory Applications
Abstract
:1. Introduction
2. Materials and Method
2.1. PVDF Film Preparation
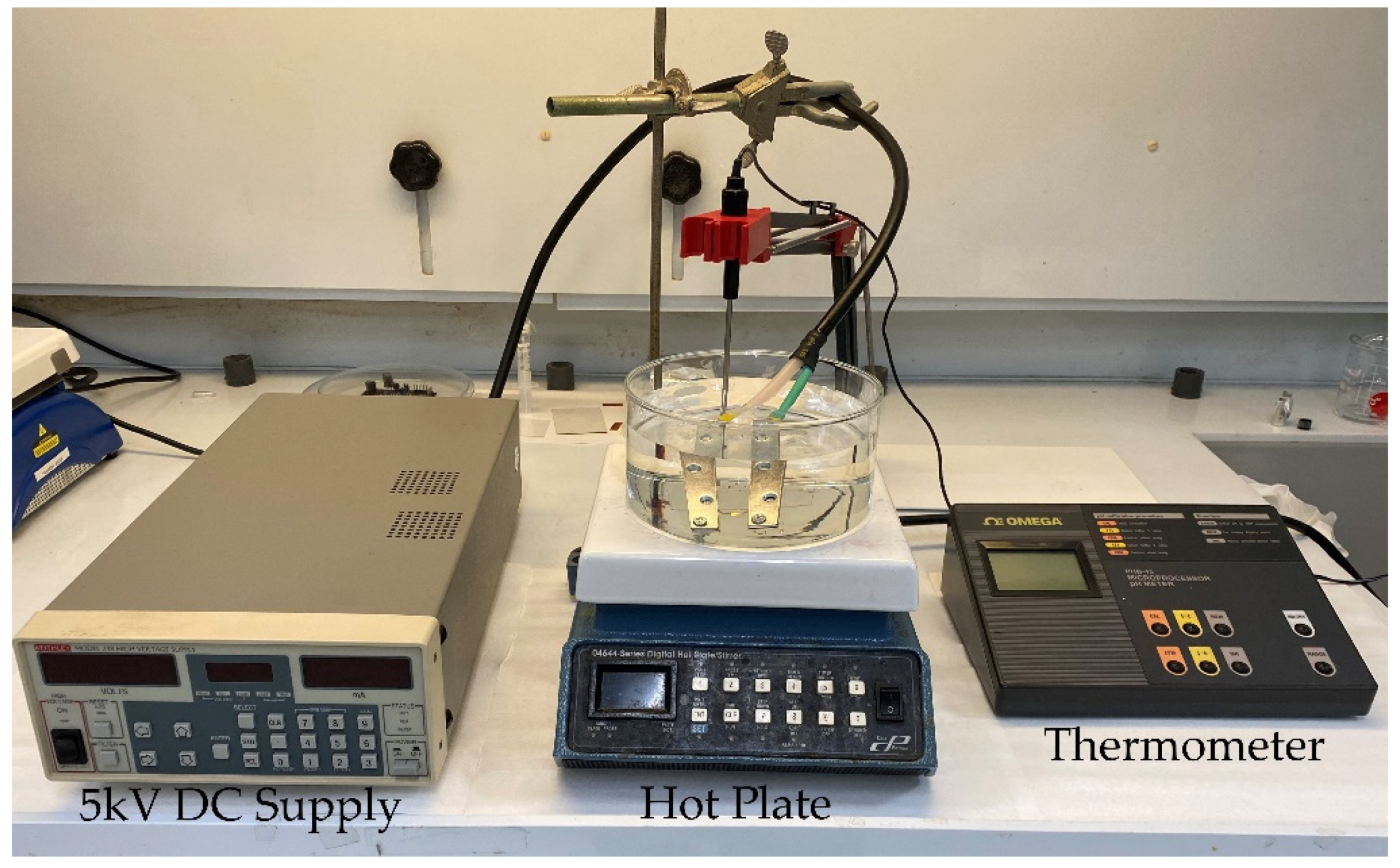
2.2. Methods Used for Film Characterization
2.2.1. Chemical Characterization Using FTIR-ATR, XRD, and DSC
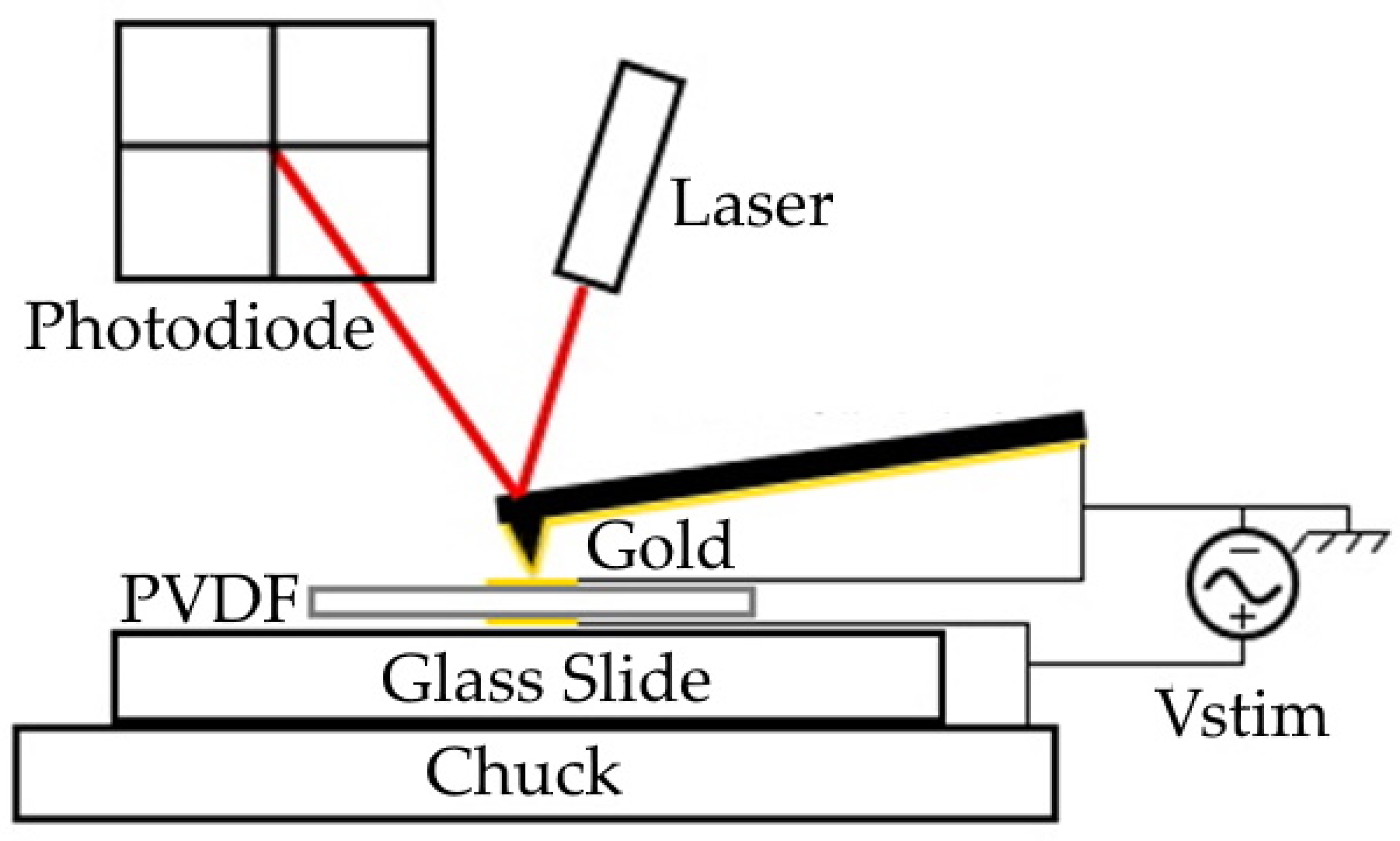
2.2.2. Electromechanical Characterization Using Static AFM
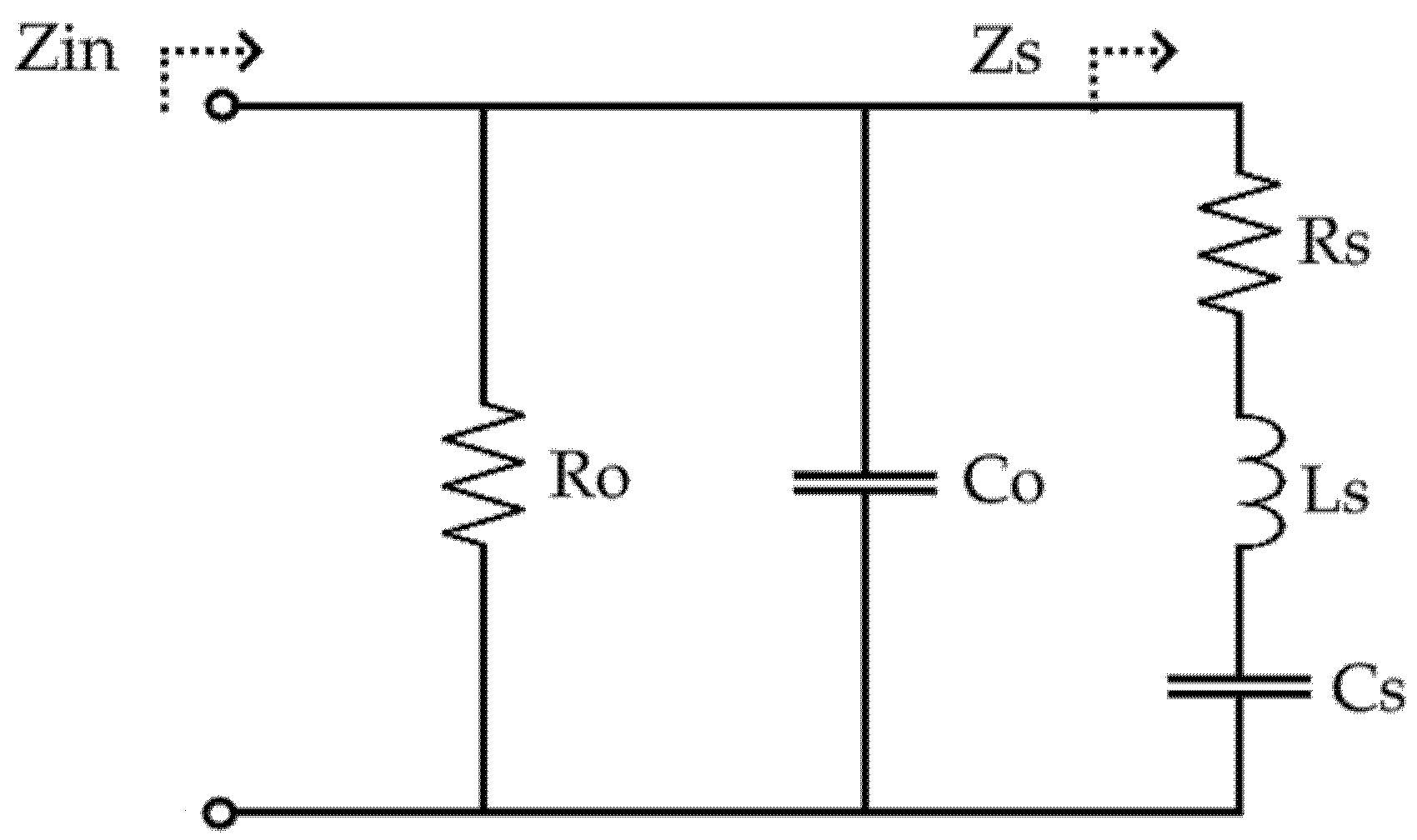
2.2.3. Electrical Characterization Using the Mason Model
3. Results
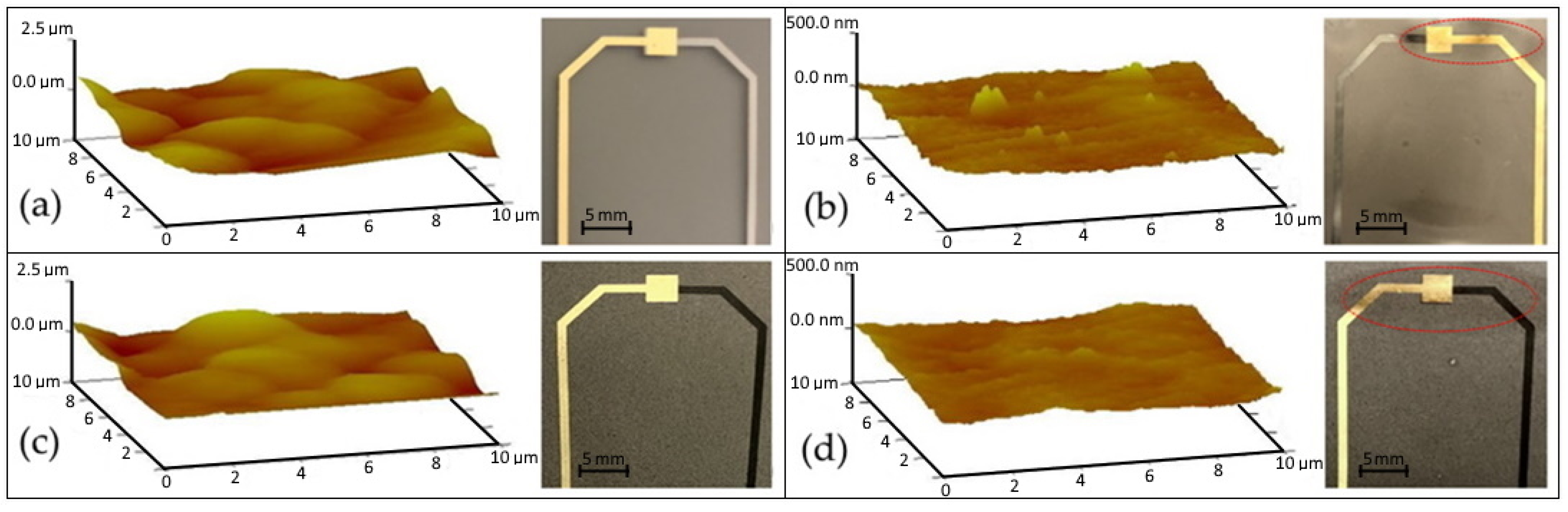
3.1. Physical Characteristics
3.2. Chemical Characteristics
3.3. Electromechancial Characteristics
3.4. Electrical Characteristics
4. Discussion
4.1. Chemical Characterization
4.2. Electromechanical Characterization
4.3. Electrical Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaczmarek, H.; Królikowski, B.; Klimiec, E.; Chylińska, M.; Bajer, D. Advances in the Study of Piezoelectric Polymers. Russ. Chem. Rev. 2019, 88, 749–774. [Google Scholar] [CrossRef]
- Sun, C.; Shi, J.; Bayerl, D.J.; Wang, X. PVDF Microbelts for Harvesting Energy from Respiration. Energy Environ. Sci. 2011, 4, 4508–4512. [Google Scholar] [CrossRef]
- Tamjidi, N.; Sato, K.; Sakurai, J.; Hata, S. PVDF Actuator for High-Frequency Fatigue Test of Thin-Film Metals. IEEJ Trans. Electr. Electron. Eng. 2013, 8, 199–205. [Google Scholar] [CrossRef]
- Uma, G.; Umapathy, M.; Jose, S.; Natarajan, V.; Kathiresan, M. Design and Simulation of PVDF-MOSFET Based MEMS Hydrophone. Instrum. Sci. Technol. 2007, 35, 329–339. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.; Li, K. Preparation of Porous PVDF Hollow Fibre Membrane via a Phase Inversion Method Using Lithium Perchlorate (LiClO4) as an Additive. J. Membr. Sci. 2005, 258, 16–22. [Google Scholar] [CrossRef]
- Shirinov, A.V.; Schomburg, W.K. Pressure Sensor from a PVDF Film. Sens. Actuators A Phys. 2008, 142, 48–55. [Google Scholar] [CrossRef]
- Shen, Y.; Xi, N.; Li, W.J.; Tan, J. A High Sensitivity Force Sensor for Microassembly: Design and Experiments. In Proceedings of the IEEE/ASME International Conference on Advanced Intelligent Mechatronics, AIM, Kobe, Japan, 20–24 July 2003; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2003; Volume 2, pp. 703–708. [Google Scholar]
- Roy, K.; Ghosh, S.K.; Sultana, A.; Garain, S.; Xie, M.; Bowen, C.R.; Henkel, K.; Schmeiβer, D.; Mandal, D. A Self-Powered Wearable Pressure Sensor and Pyroelectric Breathing Sensor Based on GO Interfaced PVDF Nanofibers. ACS Appl. Nano Mater. 2019, 2, 2013–2025. [Google Scholar] [CrossRef]
- Chinaglia, D.L.; Gregorio, R.; Stefanello, J.C.; Altafim, R.A.P.; Wirges, W.; Wang, F.; Gerhard, R. Influence of the Solvent Evaporation Rate on the Crystalline Phases of Solution-Cast Poly(Vinylidene Fluoride) Films. J. Appl. Polym. Sci. 2010, 116, 785–791. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Minas, G.; Lanceros-Méndez, S. Multilayer Spin-Coating Deposition of Poly(Vinylidene Fluoride) Films for Controlling Thickness and Piezoelectric Response. Sens. Actuators A Phys. 2013, 192, 76–80. [Google Scholar] [CrossRef]
- Kalimuldina, G.; Turdakyn, N.; Abay, I.; Medeubayev, A.; Nurpeissova, A.; Adair, D.; Bakenov, Z. A Review of Piezoelectric Pvdf Film by Electrospinning and Its Applications. Sensors 2020, 20, 5214. [Google Scholar] [CrossRef]
- Pei, H.; Xie, Y.; Xiong, Y.; Lv, Q.; Chen, Y. A Novel Polarization-Free 3D Printing Strategy for Fabrication of Poly (Vinylidene Fluoride) Based Nanocomposite Piezoelectric Energy Harvester. Compos. Part B Eng. 2021, 225, 378–387. [Google Scholar] [CrossRef]
- Rietveld, I.B.; Kobayashi, K.; Honjo, T.; Ishida, K.; Yamada, H.; Matsushige, K. Electrospray Induced Ferroelectricity in Poly(Vinylidene Fluoride) Thin Films. R. Soc. Chem. 2010, 20, 8272–8278. [Google Scholar] [CrossRef]
- Taleb, S.; Badillo-Ávila, M.A.; Acuautla, M. Fabrication of Poly (Vinylidene Fluoride) Films by Ultrasonic Spray Coating; Uniformity and Piezoelectric Properties. Mater. Des. 2021, 212, 110273. [Google Scholar] [CrossRef]
- Soulestin, T.; Ladmiral, V.; dos Santos, F.D.; Améduri, B. Vinylidene Fluoride- and Trifluoroethylene-Containing Fluorinated Electroactive Copolymers. How Does Chemistry Impact Properties? Prog. Polym. Sci. 2017, 72, 16–60. [Google Scholar] [CrossRef]
- Ramanathan, A.K.; Headings, L.M.; Dapino, M.J. Design Optimization of Flexible Piezoelectric PVDF Unimorphs for Surface Pressure Transducer Applications. In Proceedings of the SPIE Smart Structures + Nondestructive Evaluation, Denver, CO, USA, 18 March 2019; p. 6. [Google Scholar]
- Kang, K.; Park, J.; Kim, K.; Yu, K.J. Recent Developments of Emerging Inorganic, Metal and Carbon-Based Nanomaterials for Pressure Sensors and Their Healthcare Monitoring Applications. Nano Res. 2021, 14, 3096–3111. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High Performance Piezoelectric Devices Based on Aligned Arrays of Nanofibers of Poly(Vinylidenefluoride-Co-Trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef]
- Dzedzickis, A.; Sutinys, E.; Bucinskas, V.; Samukaite-Bubniene, U.; Jakstys, B.; Ramanavicius, A.; Morkvenaite-Vilkonciene, I. Polyethylene-Carbon Composite (Velostat®) Based Tactile Sensor. Polymers 2020, 12, 2905. [Google Scholar] [CrossRef]
- Lovinger, A.J. Annealing of Poly(Vinylidene Fluoride) and Formation of Fifth Phase. Macromolecules 1982, 15, 40–44. [Google Scholar] [CrossRef]
- Sencadas, V.; Gregorio, R.; Lanceros-Méndez, S. α to β Phase Transformation and Microestructural Changes of PVDF Films Induced by Uniaxial Stretch. J. Macromol. Sci. Part B Phys. 2009, 48, 514–525. [Google Scholar] [CrossRef]
- Pan, H.; Na, B.; Lv, R.; Li, C.; Zhu, J.; Yu, Z. Polar Phase Formation in Poly(Vinylidene Fluoride) Induced by Melt Annealing. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1433–1437. [Google Scholar] [CrossRef]
- Muduli, S.P.; Parida, S.; Rout, S.K.; Rajput, S.; Kar, M. Effect of Hot Press Temperature on β-Phase, Dielectric and Ferroelectric Properties of Solvent Casted Poly(Vinyledene Fluoride) Films. Mater. Res. Express 2019, 6, 025006. [Google Scholar] [CrossRef]
- Soin, N.; Boyer, D.; Prashanthi, K.; Sharma, S.; Narasimulu, A.A.; Luo, J.; Shah, T.H.; Siores, E.; Thundat, T. Exclusive Self-Aligned β-Phase PVDF Films with Abnormal Piezoelectric Coefficient Prepared via Phase Inversion. Chem. Commun. 2015, 51, 8257–8260. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Duan, X.; Xie, M.; Aw, K.C.; Xue, Q. Composites, Fabrication and Application of Polyvinylidene Fluoride for Flexible Electromechanical Devices: A Review. Micromachines 2020, 11, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent Advances in Graphene Based Polymer Composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Barkauskas, J.; Mikoliunaite, L.; Paklonskaite, I.; Genys, P.; Petroniene, J.J.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Samukaite-Bubniene, U.; Ramanavicius, A. Single-Walled Carbon Nanotube Based Coating Modified with Reduced Graphene Oxide for the Design of Amperometric Biosensors. Mater. Sci. Eng. C 2019, 98, 515–523. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, H.; Ding, H.; Pan, N.; Wang, X. Fabrication of Low-Cost and Highly Sensitive Graphene-Based Pressure Sensors by Direct Laser Scribing Polydimethylsiloxane. ACS Appl. Mater. Interfaces 2019, 11, 6195–6200. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, W.; Zhang, N.; Zhang, Q.; Xia, X.; Gu, X.; Wang, Y.; Xie, S. All-Carbon Pressure Sensors with High Performance and Excellent Chemical Resistance. Small 2019, 15, 1804779. [Google Scholar] [CrossRef]
- Uyor, U.O.; Popoola, A.P.; Popoola, O.; Aigbodion, V.S. Energy Storage and Loss Capacity of Graphene-Reinforced Poly(Vinylidene Fluoride) Nanocomposites from Electrical and Dielectric Properties Perspective: A Review. Adv. Polym. Technol. 2018, 37, 2838–2858. [Google Scholar] [CrossRef]
- Mahapatra, S.D.; Mohapatra, P.C.; Aria, A.I.; Christie, G.; Mishra, Y.K.; Hofmann, S.; Thakur, V.K. Piezoelectric Materials for Energy Harvesting and Sensing Applications: Roadmap for Future Smart Materials. Adv. Sci. 2021, 8, 2100864. [Google Scholar] [CrossRef]
- Pariy, I.O.; Ivanova, A.A.; Shvartsman, V.V.; Lupascu, D.C.; Sukhorukov, G.B.; Ludwig, T.; Bartasyte, A.; Mathur, S.; Surmeneva, M.A.; Surmenev, R.A. Piezoelectric Response in Hybrid Micropillar Arrays of Poly(Vinylidene Fluoride) and Reduced Graphene Oxide. Polymers 2019, 11, 1065. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Chung, G.S. Synthesis of PVDF-Graphene Nanocomposites and Their Properties. J. Alloys Compd. 2013, 581, 724–730. [Google Scholar] [CrossRef]
- Huang, L.; Lu, C.; Wang, F.; Wang, L. Preparation of PVDF/Graphene Ferroelectric Composite Films by in Situ Reduction with Hydrobromic Acids and Their Properties. RSC Adv. 2014, 4, 45220–45229. [Google Scholar] [CrossRef]
- Lee, J.; Lim, S. Polarization Behavior of Polyvinylidene Fluoride Films with the Addition of Reduced Graphene Oxide. J. Ind. Eng. Chem. 2018, 67, 478–485. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly(Vinylidene Fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Berlincourt, D.; Krueger, H.H.A. Domain Processes in Lead Titanate Zirconate and Barium Titanate Ceramics. J. Appl. Phys. 1959, 30, 1804–1810. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Bonnell, D.A. Contrast Mechanism Maps for Piezoresponse Force Microscopy. J. Mater. Res. 2002, 17, 936–939. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.; Cain, M.G. Direct Piezoelectric Measurement: The Berlincourt Method. In Characterisation of Ferroelectric Bulk Materials and Thin Films; Springer: Berlin/Heidelberg, Germany, 2014; pp. 37–38. [Google Scholar]
- Peter, F.; Rüdiger, A.; Szot, K.; Waser, R.; Reichenberg, B. Sample-Tip Interaction of Piezoresponse Force Microscopy in Ferroelectric Nanostructures. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 2253–2259. [Google Scholar] [CrossRef] [Green Version]
- Seol, D.; Kim, B.; Kim, Y. Non-Piezoelectric Effects in Piezoresponse Force Microscopy. Curr. Appl. Phys. 2017, 17, 661–674. [Google Scholar] [CrossRef] [Green Version]
- Darestani, M.T.; Coster, H.G.L.; Chilcott, T.C.; Fleming, S.; Nagarajan, V.; An, H. Piezoelectric Membranes for Separation Processes: Fabrication and Piezoelectric Properties. J. Membr. Sci. 2013, 434, 184–192. [Google Scholar] [CrossRef]
- Jahan, N.; Mighri, F.; Rodrigue, D.; Ajji, A. Enhanced Electroactive β Phase in Three Phase PVDF/CaCO3/Nanoclay Composites: Effect of Micro-CaCO3 and Uniaxial Stretching. J. Appl. Polym. Sci. 2017, 134, 3–4. [Google Scholar] [CrossRef]
- Karan, S.K.; Mandal, D.; Khatua, B.B. Self-Powered Flexible Fe-Doped RGO/PVDF Nanocomposite: An Excellent Material for a Piezoelectric Energy Harvester. Nanoscale 2015, 7, 10655–10666. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Q.; Xia, W.; Qiu, X.; Cheng, Q.; Zhu, G. Electroactive PVDF Thin Films Fabricated via Cooperative Stretching Process. J. Appl. Polym. Sci. 2018, 135, 46324. [Google Scholar] [CrossRef]
- Zhu, G.D.; Zeng, Z.G.; Zhang, L.; Yan, X.J. Piezoelectricity in β-Phase PVDF Crystals: A Molecular Simulation Study. Comput. Mater. Sci. 2008, 44, 224–229. [Google Scholar] [CrossRef]
- Gebrekrstos, A.; Madras, G.; Bose, S. Piezoelectric Response in Electrospun Poly(Vinylidene Fluoride) Fibers Containing Fluoro-Doped Graphene Derivatives. ACS Omega 2018, 3, 5317–5326. [Google Scholar] [CrossRef] [PubMed]
- Sim, L.N.; Majid, S.R.; Arof, A.K. FTIR Studies of PEMA/PVdF-HFP Blend Polymer Electrolyte System Incorporated with LiCF3SO3 Salt. Vib. Spectrosc. 2012, 58, 57–66. [Google Scholar] [CrossRef]
- Romanyuk, K.; Costa, C.M.; Luchkin, S.Y.; Kholkin, A.L.; Lanceros-Méndez, S. Giant Electric-Field-Induced Strain in PVDF-Based Battery Separator Membranes Probed by Electrochemical Strain Microscopy. Langmuir 2016, 32, 5267–5276. [Google Scholar] [CrossRef]
- Garrett, J.T.; Roland, C.M.; Petchsuk, A.; Chung, T.C. Electrostrictive Behavior of Poly(Vinylidene Fluoride-Trifluoroethylene-Chlorotrifluoroethylene). Appl. Phys. Lett. 2003, 83, 1190–1192. [Google Scholar] [CrossRef] [Green Version]
- Klapetek, P. Force and Mechanical Properties. In Quantitative Data Processing in Scanning Probe Microscopy; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 127–172. [Google Scholar] [CrossRef]
- Christman, J.A.; Woolcott, R.R.; Kingon, A.I.; Nemanich, R.J. Piezoelectric Measurements with Atomic Force Microscopy. Appl. Phys. Lett. 1998, 73, 3851–3853. [Google Scholar] [CrossRef]
- Rhyne, T.L. An Improved Interpretation of Mason’s Model for Pizoelectric Plate Transducers. IEEE Trans. Sonics Ultrason. 1978, 25, 98–103. [Google Scholar] [CrossRef]
- Brown Lewis, F.; Carlson Carlson, D. Ultrasound Transducer Models for Piezoelectric Polymer Films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1989, 36, 313–316. [Google Scholar] [CrossRef]
- Rathod, V.T. A Review of Acoustic Impedance Matching Techniques for Piezoelectric Sensors and Transducers. Sensors 2020, 20, 4051. [Google Scholar] [CrossRef]
- Satapathy, S.; Pawar, S.; Gupta, P.K.; Varma, B.R. Effect of Annealing on Phase Transition in Poly(Vinylidene Fluoride) Films Prepared Using Polar Solvent; Springer: Berlin/Heidelberg, Germany, 2011; Volume 34. [Google Scholar]
- Guan, J.; Xing, C.; Wang, Y.; Li, Y.; Li, J. Poly (Vinylidene Fluoride) Dielectric Composites with Both Ionic Nanoclusters and Well Dispersed Graphene Oxide. Compos. Sci. Technol. 2017, 138, 98–105. [Google Scholar] [CrossRef]
- Muduli, S.P.; Parida, S.; Nayak, S.; Rout, S.K. Effect of Graphene Oxide Loading on Ferroelectric and Dielectric Properties of Hot Pressed Poly(Vinylidene Fluoride) Matrix Composite Film. Polym. Compos. 2020, 41, 2855–2865. [Google Scholar] [CrossRef]
- Gregorio, R. Determination of the α, β, and γ Crystalline Phases of Poly(Vinylidene Fluoride) Films Prepared at Different Conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- el Achaby, M.; Arrakhiz, F.Z.; Vaudreuil, S.; Essassi, E.M.; Qaiss, A. Piezoelectric β-Polymorph Formation and Properties Enhancement in Graphene Oxide-PVDF Nanocomposite Films. Appl. Surf. Sci. 2012, 258, 7668–7677. [Google Scholar] [CrossRef]
- Gregorio, R.; da Silva, A.B.; Wisniewski, C.; Esteves, J.V.A. Effect of Drawing on the Dielectric Properties and Polarization of Pressed Solution Cast β-PVDF Films. J. Mater. Sci. 2010, 45, 4206–4215. [Google Scholar] [CrossRef]
- Ismail, A.M.; Mohammed, M.I.; Fouad, S.S. Optical and Structural Properties of Polyvinylidene Fluoride (PVDF)/Reduced Graphene Oxide (RGO) Nanocomposites. J. Mol. Struct. 2018, 1170, 51–59. [Google Scholar] [CrossRef]
- Gradys, A.; Sajkiewicz, P. Determination of the Melting Enthalpy of β Phase of Poly(Vinylidene Fluoride). e-Polymers 2013, 13, 203–216. [Google Scholar] [CrossRef]
- He, L.; Cui, B.; Jia, N.; Sun, J.; Xia, G.; Zhang, H.; Song, R. Enhanced β Crystalline Phase in Poly(Vinylidene Fluoride) via the Incorporation of Graphene Oxide Sheets Assisted by Supercritical CO2 Treatment. J. Macromol. Sci. Part B Phys. 2016, 55, 503–517. [Google Scholar] [CrossRef]
- Chiu, F.C.; Chen, Y.J. Evaluation of Thermal, Mechanical, and Electrical Properties of PVDF/GNP Binary and PVDF/PMMA/GNP Ternary Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Buckley, J.; Cebe, P.; Cherdack, D.; Crawford, J.; Ince, B.S.; Jenkins, M.; Pan, J.; Reveley, M.; Washington, N.; Wolchover, N. Nanocomposites of Poly(Vinylidene Fluoride) with Organically Modified Silicate. Polymer 2006, 47, 2411–2422. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ishida, Y. Dielectric Relaxations and Molecular Motions in Poly(Vinylidene Fluoride) with Crystal Form II. J. Polym. Sci. Polym. Phys. Ed. 1973, 11, 1503. [Google Scholar] [CrossRef]
- Rodriguez, B.J.; Callahan, C.; Kalinin, S.V.; Proksch, R. Dual-Frequency Resonance-Tracking Atomic Force Microscopy. Nanotechnology 2007, 18, 727. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Weiss, D.N.; Li, J. Rapid Nanoimprinting and Excellent Piezoresponse of Polymeric Ferroelectric Nanostructures. ACS Nano 2010, 4, 83–90. [Google Scholar] [CrossRef]
- Sharma, M.; Madras, G.; Bose, S. Process Induced Electroactive β-Polymorph in PVDF: Effect on Dielectric and Ferroelectric Properties. Phys. Chem. Chem. Phys. 2014, 16, 14792–14799. [Google Scholar] [CrossRef]
- He, F.; Lau, S.; Chan, H.L.; Fan, J. High Dielectric Permittivity and Low Percolation Threshold in Nanocomposites Based on Poly(Vinylidene Fluoride) and Exfoliated Graphite Nanoplates. Adv. Mater. 2009, 21, 710–715. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, Y.; Yu, L.; Shen, B.; Lv, F.; Chu, P.K. Fabrication and Dielectric Properties of Oriented Polyvinylidene Fluoride Nanocomposites Incorporated with Graphene Nanosheets. Mater. Chem. Phys. 2012, 134, 867–874. [Google Scholar] [CrossRef]

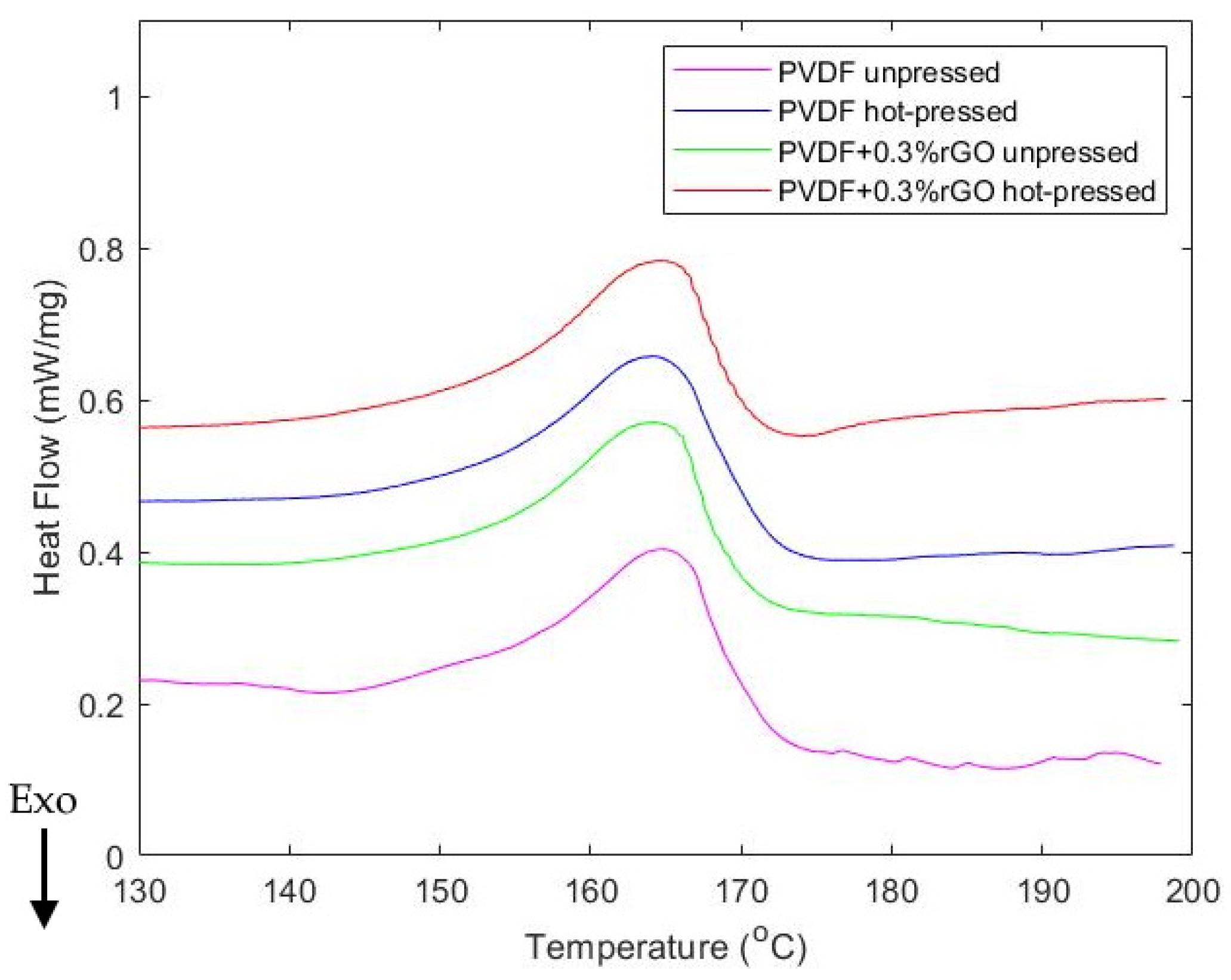

| Sample | -Phase Fraction (%) | Crystallinity (%) | Melt Temperature (°C) |
|---|---|---|---|
| PVDF unpressed | 80.1 | 39.4 | 165.0 |
| PVDF hot-pressed | 83.7 | 42.4 | 164.3 |
| 0.3%w.t. rGO doped and unpressed | 80.8 | 37.2 | 164.5 |
| 0.3%w.t. rGO doped and hot-pressed | 81.7 | 37.3 | 164.5 |
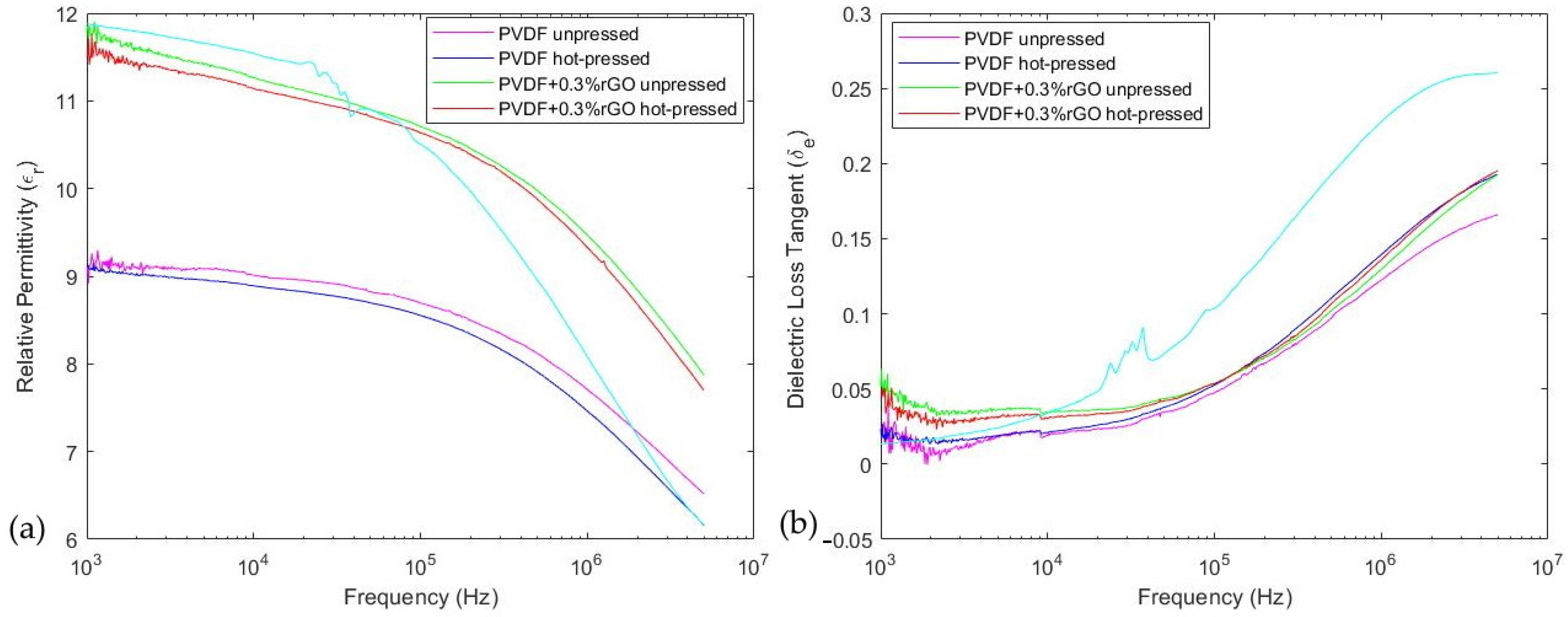
| Sample | Relative Permittivity @ 1 kHz | Loss Tangent @ 1 kHz |
|---|---|---|
| PVDF unpressed | 9.15 | 0.025 |
| PVDF hot pressed | 9.01 | 0.017 |
| 0.3%w.t. rGO doped and unpressed | 11.70 | 0.053 |
| 0.3%w.t. rGO doped and hot pressed | 11.52 | 0.045 |
| PVDF commercial film | 11.87 | 0.013 |
| Fabrication Procedure | Technique | Source | ||
|---|---|---|---|---|
| PVDF solution cast, hot pressed, thermally poled | 83.67 | 45 | Static AFM | Current study |
| PVDF + 0.3%w.t. rGO solution-cast, hot pressed, thermally poled | 81.69 | 58 | Static AFM | Current Study |
| PVDF solution-cast, poled | 100 | 22 | Piezometer | [34] |
| PVDF + 0.3%rGO in situ, solution-cast, poled | ~100 | 37 | Piezometer | [34] |
| PVDF poled, solution cast | 2 | 13 | Piezometer | [35] |
| PVDF + 0.25%w.t. rGO in situ, solution-cast | 37 | 25 | Piezometer | [35] |
| PVDF spin coated, thermally poled | 80 | 20 | Piezometer | [10] |
| PVDF spin coated, stretched, poled | 75 | 37 | Static AFM | [45] |
| PVDF solution-cast, thermally poled | - | 46.1 | Static AFM | [46] |
| PVDF spin coated, quenched at −20 °C | 98 | 49.6 | PFM | [24] |
| PVDF micropillar, hot pressed, thermally poled | 16 | 64 | PFM | [32] |
| PVDF + 0.4%w.t. rGO in situ, micropillar, thermally pressed | 23 | 66 | PFM | [32] |
| PVDF + 0.1%w.t. rGO in situ, micropillar, thermally pressed | 20 | 75 | PFM | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hintermueller, D.; Prakash, R. Comprehensive Characterization of Solution-Cast Pristine and Reduced Graphene Oxide Composite Polyvinylidene Fluoride Films for Sensory Applications. Polymers 2022, 14, 2546. https://doi.org/10.3390/polym14132546
Hintermueller D, Prakash R. Comprehensive Characterization of Solution-Cast Pristine and Reduced Graphene Oxide Composite Polyvinylidene Fluoride Films for Sensory Applications. Polymers. 2022; 14(13):2546. https://doi.org/10.3390/polym14132546
Chicago/Turabian StyleHintermueller, Dane, and Ravi Prakash. 2022. "Comprehensive Characterization of Solution-Cast Pristine and Reduced Graphene Oxide Composite Polyvinylidene Fluoride Films for Sensory Applications" Polymers 14, no. 13: 2546. https://doi.org/10.3390/polym14132546
APA StyleHintermueller, D., & Prakash, R. (2022). Comprehensive Characterization of Solution-Cast Pristine and Reduced Graphene Oxide Composite Polyvinylidene Fluoride Films for Sensory Applications. Polymers, 14(13), 2546. https://doi.org/10.3390/polym14132546






