Anti-Inflammatory and Pro-Regenerative Effects of Hyaluronan-Chitlac Mixture in Human Dermal Fibroblasts: A Skin Ageing Perspective
Abstract
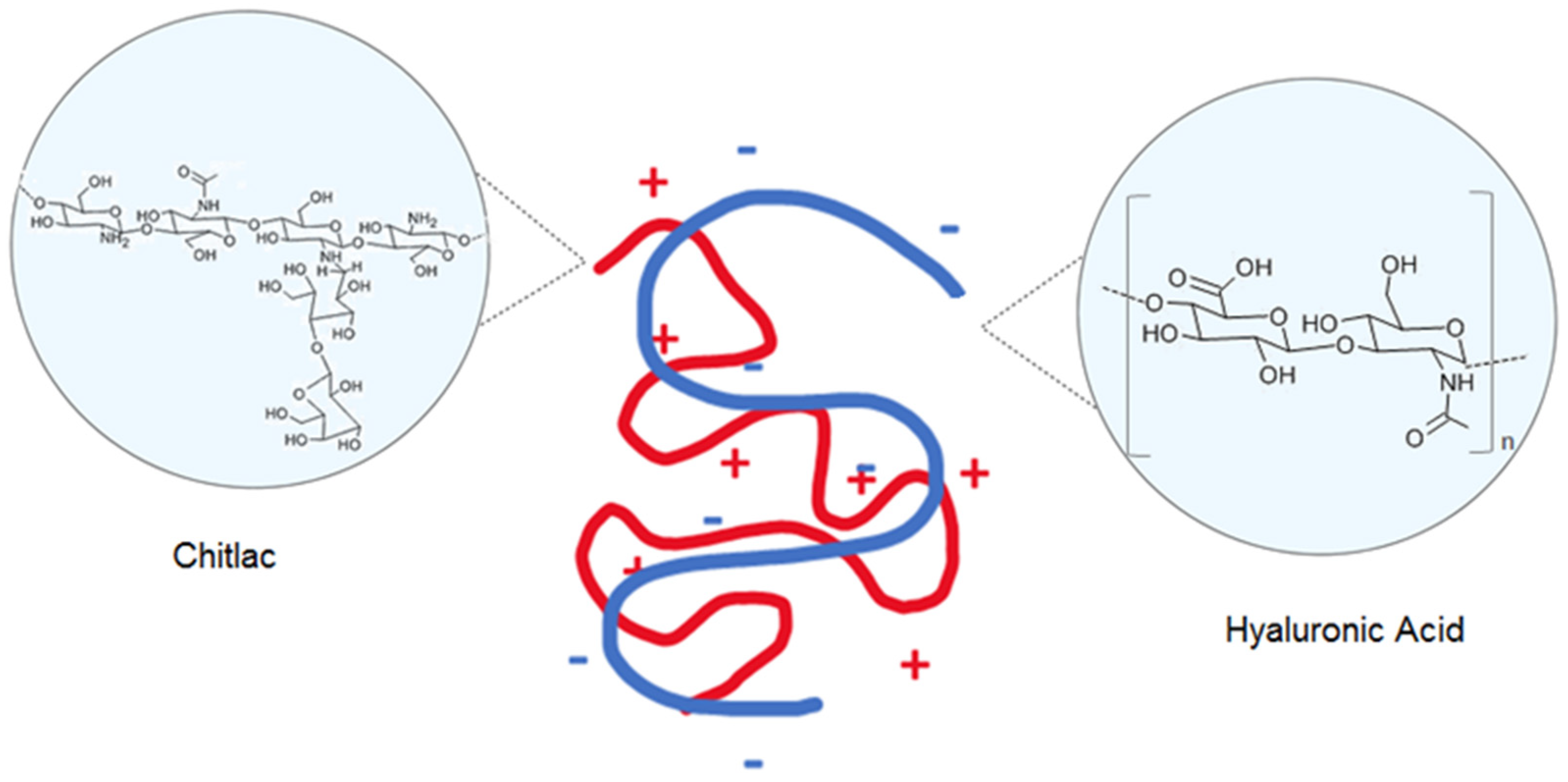
:1. Introduction
2. Materials and Methods
2.1. Drugs, Chemicals and Cells
2.2. Activated U937 Monocyte Conditioned Medium
2.3. Evaluation of the Effects of HA and Chitlac (CTL) on Human Dermal Fibroblasts Viability
2.4. Analysis of the Anti-Inflammatory, Antioxidant and Regenerative Effects Induced by HA and CTL
2.4.1. Intracellular ROS Generation
2.4.2. RNA Isolation and qPCR Analysis
2.4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Analysis
3. Results
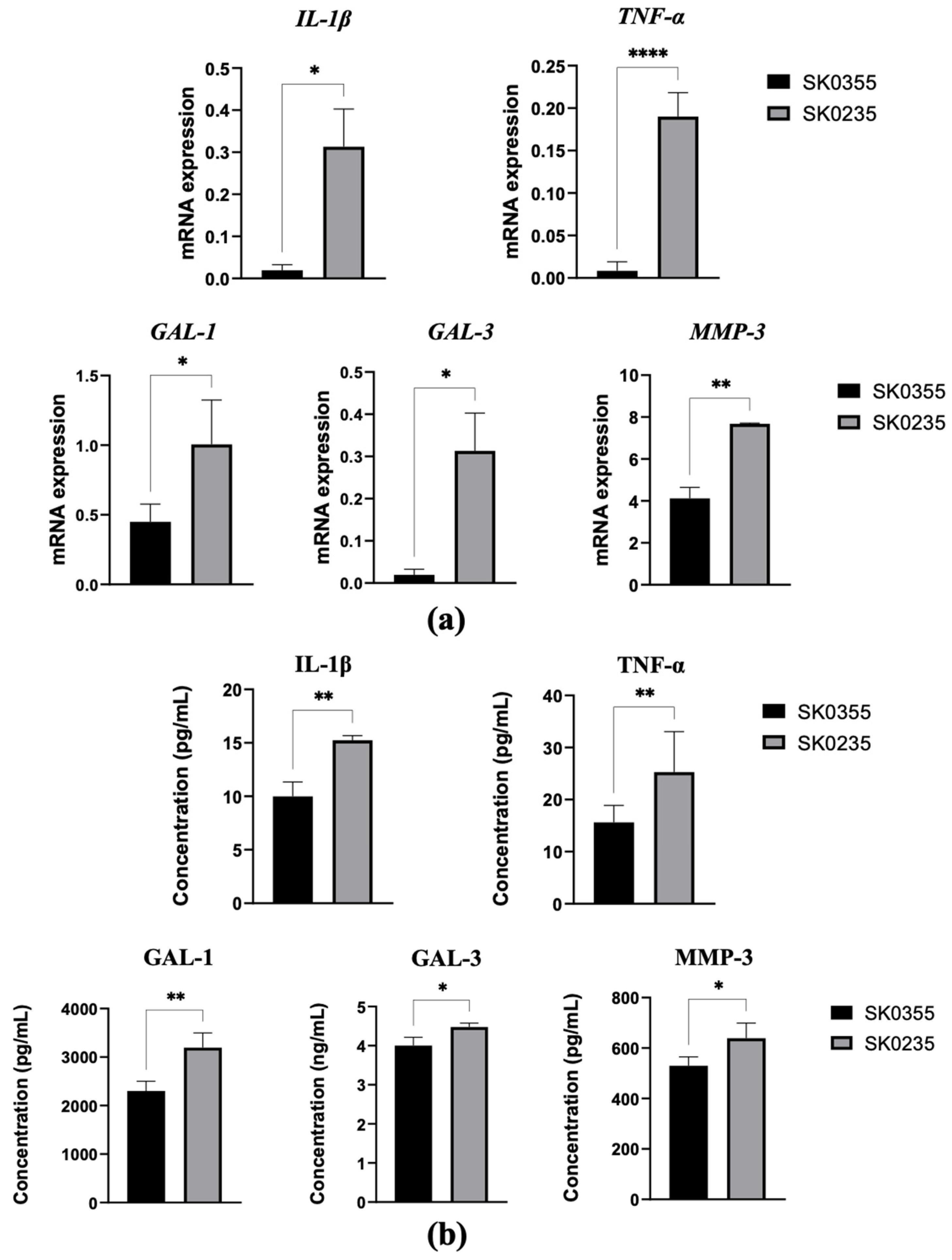
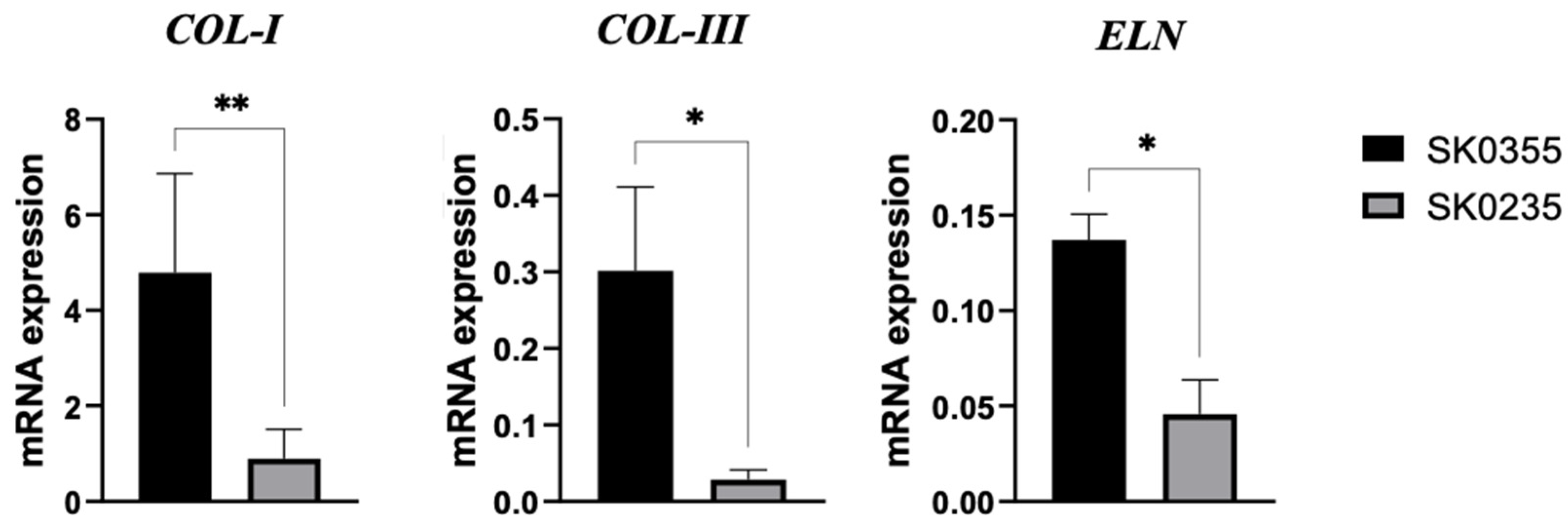
3.1. Gene Expressions of Pro-Inflammatory Molecules, Galectins, MMP-3 and ECM Molecules Are Age-Related in Cultured Dermal Fibroblasts
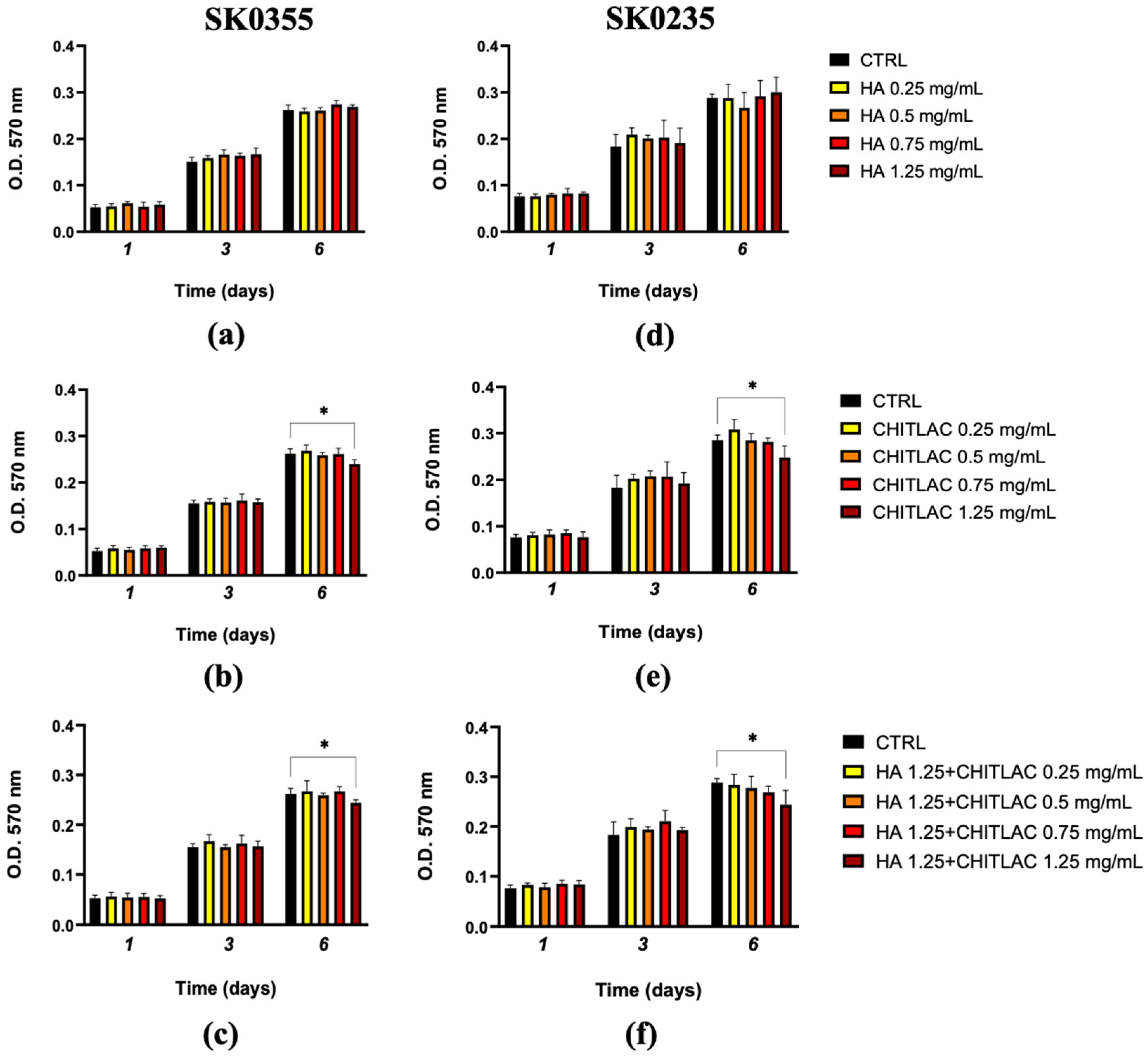
3.2. Viability of Fibroblasts Is Not Affected by Low Doses of HA, CTL and Their Mixture
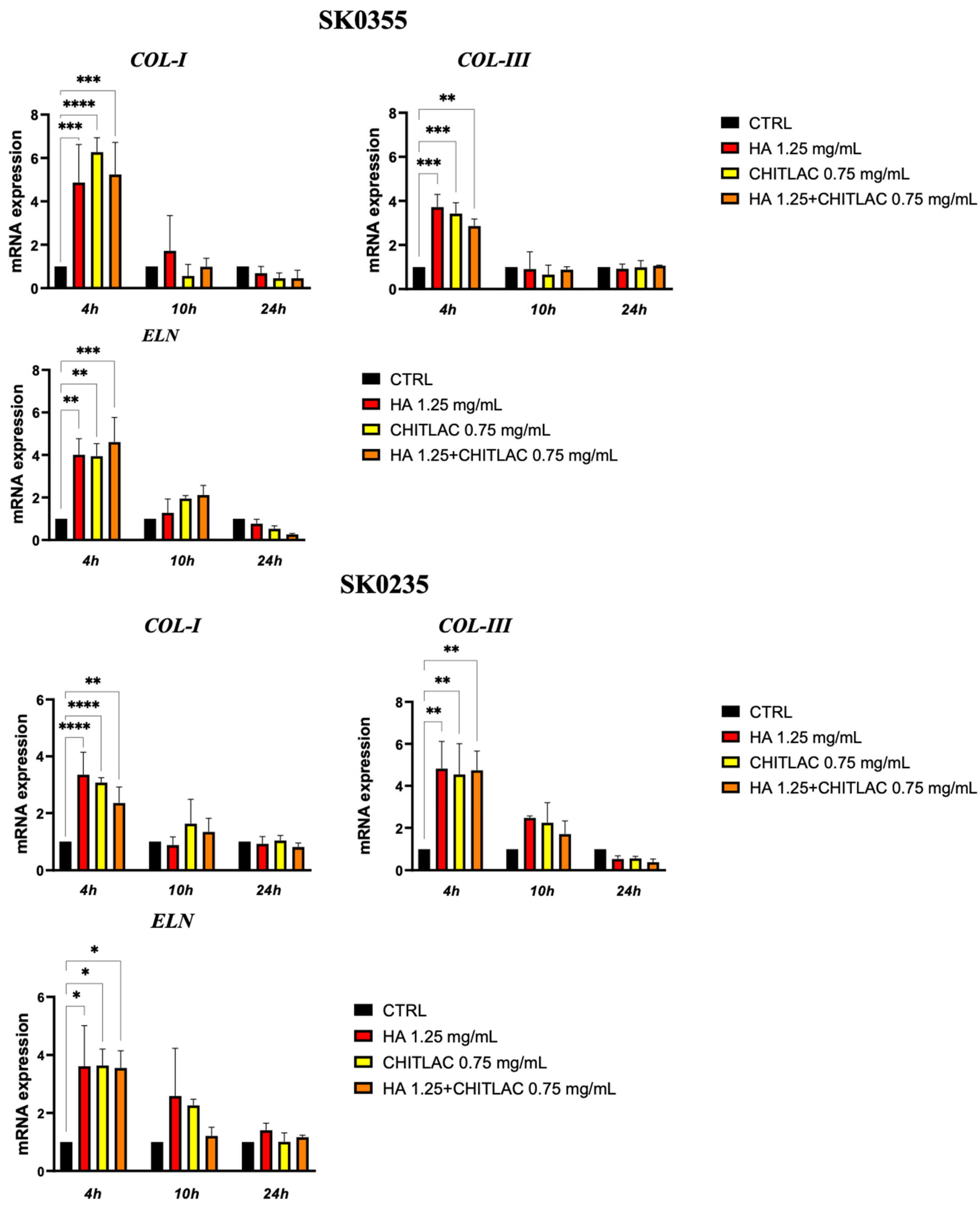
3.3. HA and CTL Upregulate the Synthesis of Collagens and Elastin
3.4. HA-CTL Mixture Induced a Reduction of ROS Formation in Human Dermal Fibroblasts Exposed to CM of Activated U937 Cells
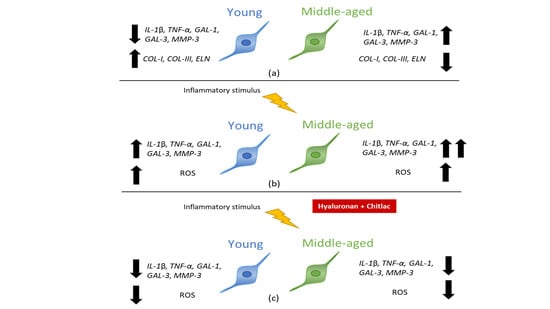
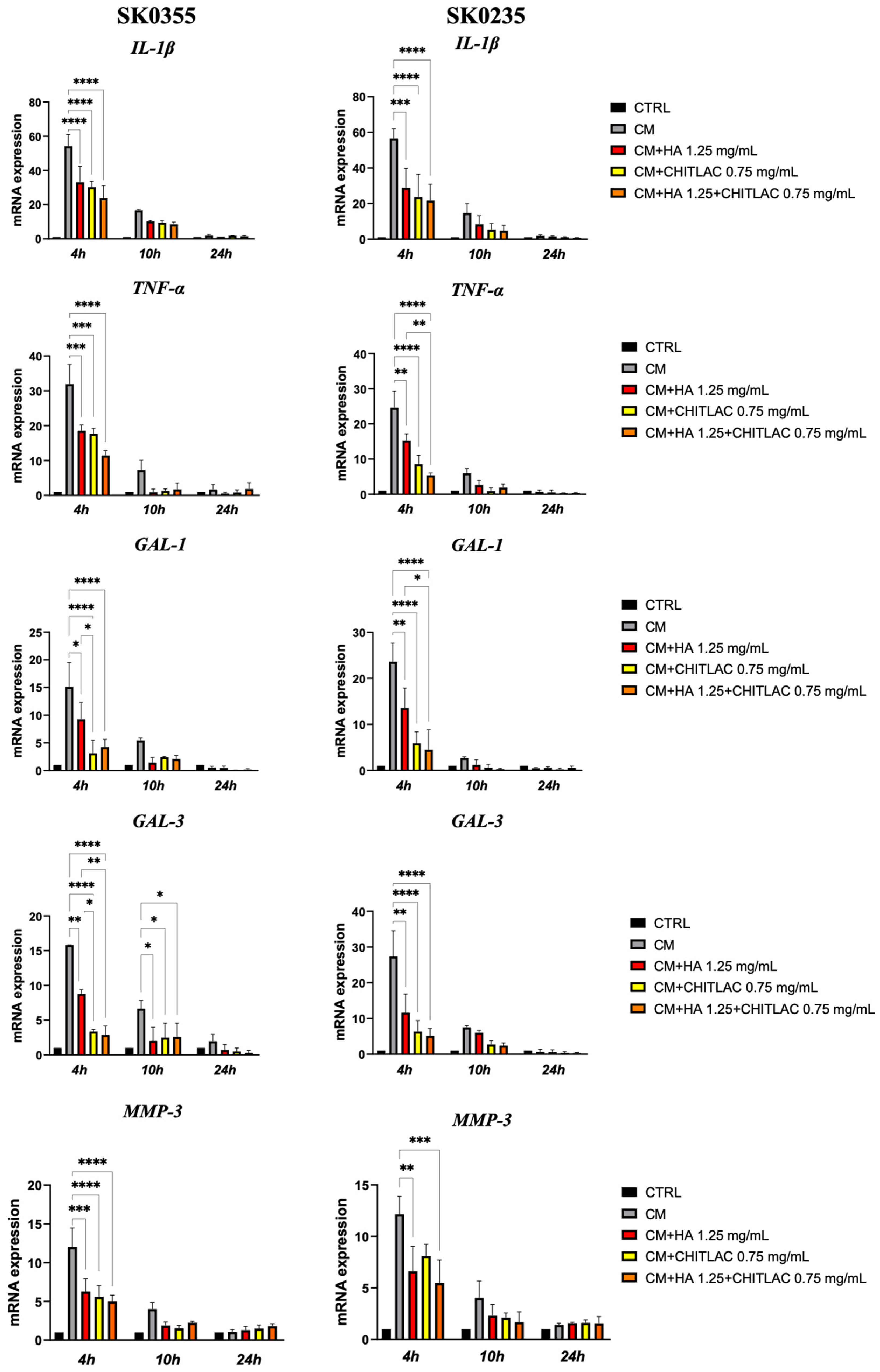
3.5. HA, CTL and Their Mixture Exert Anti-Inflammatory Effects on Human Dermal Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Ishii, T.; Miyazawa, M.; Hartman, P.S.; Ishii, N. Mitochondrial superoxide anion (O2−) inducible “mev-1” animal models for aging research. BMB Rep. 2011, 44, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Lu, C.-H.; Wu, C.-H.; Li, K.-J.; Kuo, Y.-M.; Hsieh, S.-C.; Yu, C.-L. Molecular Basis of Accelerated Aging with Immune Dysfunction-Mediated Inflammation (Inflamm-Aging) in Patients with Systemic Sclerosis. Cells 2021, 10, 3402. [Google Scholar] [CrossRef]
- Guimarães, G.R.; Almeida, P.P.; de Oliveira Santos, L.; Rodrigues, L.P.; de Carvalho, J.L.; Boroni, M. Hallmarks of Aging in Macrophages: Consequences to Skin Inflammaging. Cells 2021, 10, 1323. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Cole, M.A.; Quan, T.; Voorhees, J.J.; Fisher, G.J. Extracellular matrix regulation of fibroblast function: Redefining our perspective on skin aging. J. Cell Commun. Signal. 2018, 12, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Arciniegas, E.; Carrillo, L.M.; Rojas, H.; Ramírez, R.; Chopite, M. Galectin-1 and Galectin-3 and Their Potential Binding Partners in the Dermal Thickening of Keloid Tissues. Am. J. Dermatopathol. 2019, 41, 193–204. [Google Scholar] [CrossRef]
- McLeod, K.; Walker, J.T.; Hamilton, D.W. Galectin-3 regulation of wound healing and fibrotic processes: Insights for chronic skin wound therapeutics. J. Cell Commun. Signal. 2018, 12, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Ladiè, R.; Cosentino, C.; Tagliaro, I.; Antonini, C.; Bianchini, G.; Bertini, S. Supramolecular Structuring of Hyaluronan-Lactose-Modified Chitosan Matrix: Towards High-Performance Biopolymers with Excellent Biodegradation. Biomolecules 2021, 11, 389. [Google Scholar] [CrossRef]
- Donati, I.; Borgogna, M.; Turello, E.; Cesàro, A.; Paoletti, S. Tuning supramolecular structuring at the nanoscale level: Nonstoichiometric soluble complexes in dilute mixed solutions of alginate and lactose-modified chitosan (chitlac). Biomacromolecules 2007, 8, 1471–1479. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Parrilli, A.; Martini, L.; Nicoli Aldini, N.; Abatangelo, G.; Frizziero, A.; Fini, M. Effects of intra-articular hyaluronic acid associated to Chitlac (arty-duo®) in a rat knee osteoarthritis model. J. Orthop. Res. 2019, 37, 867–876. [Google Scholar] [CrossRef]
- Tarricone, E.; Mattiuzzo, E.; Belluzzi, E.; Elia, R.; Benetti, A.; Venerando, R.; Vindigni, V.; Ruggieri, P.; Brun, P. Anti-Inflammatory Performance of Lactose-Modified Chitosan and Hyaluronic Acid Mixtures in an In Vitro Macrophage-Mediated Inflammation Osteoarthritis Model. Cells 2020, 9, 1328. [Google Scholar] [CrossRef]
- Mattiuzzo, E.; Faggian, A.; Venerando, R.; Benetti, A.; Belluzzi, E.; Abatangelo, G.; Ruggieri, P.; Brun, P. In Vitro Effects of Low Doses of β-Caryophyllene, Ascorbic Acid and d-Glucosamine on Human Chondrocyte Viability and Inflammation. Pharmaceuticals 2021, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lyga, J. Inflammaging in skin and other tissues—The roles of complement system and macrophage. Inflamm. Allergy Drug Targets 2014, 13, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heino, J.; Heinonen, T. Interleukin-1 beta prevents the stimulatory effect of transforming growth factor-beta on collagen gene expression in human skin fibroblasts. Biochem. J. 1990, 271, 827–830. [Google Scholar] [CrossRef]
- García Caballero, G.; Kaltner, H.; Kutzner, T.J.; Ludwig, A.K.; Manning, J.C.; Schmidt, S.; Sinowatz, F.; Gabius, H.J. How galectins have become multifunctional proteins. Histol. Histopathol. 2020, 35, 509–539. [Google Scholar] [CrossRef]
- Stowell, S.R.; Qian, Y.; Karmakar, S.; Koyama, N.S.; Dias-Baruffi, M.; Leffler, H.; McEver, R.P.; Cummings, R.D. Differential Roles of Galectin-1 and Galectin-3 in Regulating Leukocyte Viability and Cytokine Secretion. J. Immunol. 2008, 180, 3091–3102. [Google Scholar] [CrossRef] [Green Version]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M.F.; Robinson, M.K.; Baron, E.D.; Cooper, K.D. Skin immune systems and inflammation: Protector of the skin or promoter of aging? J. Investig. Dermatol. Symp. Proc. 2008, 13, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Makrantonaki, E.; Zouboulis, C.C. Molecular mechanisms of skin aging: State of the art. Ann. N. Y. Acad. Sci. 2007, 1119, 40–50. [Google Scholar] [CrossRef]
- Uitto, J.; Bernstein, E.F. Molecular mechanisms of cutaneous aging: Connective tissue alterations in the dermis. J. Investig. Dermatol. Symp. Proc. 1998, 3, 41–44. [Google Scholar]
- Jia, W.; Wang, Z.; Gao, C.; Wu, J.; Wu, Q. Trajectory modeling of endothelial-to-mesenchymal transition reveals galectin-3 as a mediator in pulmonary fibrosis. Cell Death Dis. 2021, 12, 327. [Google Scholar] [CrossRef]
- Maeda, N.; Kawada, N.; Seki, S.; Arakawa, T.; Ikeda, K.; Iwao, H.; Okuyama, H.; Hirabayashi, J.; Kasai, K.; Yoshizato, K. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J. Biol. Chem. 2003, 278, 18938–18944. [Google Scholar] [CrossRef] [Green Version]
- Miljković, M.; Stefanović, A.; Bogavac-Stanojević, N.; Simić-Ogrizović, S.; Dumić, J.; Černe, D.; Jelić-Ivanović, Z.; Kotur-Stevuljević, J. Association of Pentraxin-3, Galectin-3 and Matrix Metalloproteinase-9/Timp-1 with Cardiovascular Risk in Renal Disease Patients. Acta Clin. Croat. 2017, 56, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Pichler, K.M.; Weinmann, D.; Schmidt, S.; Kubista, B.; Lass, R.; Martelanz, L.; Alphonsus, J.; Windhager, R.; Gabius, H.J.; Toegel, S. The Dysregulated Galectin Network Activates NF-κB to Induce Disease Markers and Matrix Degeneration in 3D Pellet Cultures of Osteoarthritic Chondrocytes. Calcif. Tissue Int. 2021, 108, 377–390. [Google Scholar] [CrossRef]
- Wang, L.; Friess, H.; Zhu, Z.; Frigeri, L.; Zimmermann, A.; Korc, M.; Berberat, P.O.; Büchler, M.W. Galectin-1 and Galectin-3 in Chronic Pancreatitis. Lab. Investig. 2000, 80, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.; Gilliam, A.C.; Chen, G.; Tootell, E.; Cooper, K.D. In human skin, UVB initiates early induction of IL-10 over IL-12 preferentially in the expanding dermal monocytic/macrophagic population. J. Investig. Dermatol. 1998, 111, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Nissinen, L.M.; Kähäri, V.M. Collagen Turnover in Wound Repair—A Macrophage Connection. J. Investig. Dermatol. 2015, 135, 2350–2352. [Google Scholar] [CrossRef] [Green Version]
- Brun, C.; Jean-Louis, F.; Oddos, T.; Bagot, M.; Bensussan, A.; Michel, L. Phenotypic and functional changes in dermal primary fibroblasts isolated from intrinsically aged human skin. Exp. Dermatol. 2016, 25, 113–119. [Google Scholar] [CrossRef]
- Boraldi, F.; Annovi, G.; Tiozzo, R.; Sommer, P.; Quaglino, D. Comparison of ex vivo and in vitro human fibroblast ageing models. Mech. Ageing Dev. 2010, 131, 625–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, J.; Weinberger, B.; Arnold, C.R.; Maier, A.B.; Westendorp, R.G.; Grubeck-Loebenstein, B. The effect of chronological age on the inflammatory response of human fibroblasts. Exp. Gerontol. 2012, 47, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Z.; Robichaud, P.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Oxidant Exposure Induces Cysteine-Rich Protein 61 (CCN1) via c-Jun/AP-1 to Reduce Collagen Expression in Human Dermal Fibroblasts. PLoS ONE 2014, 9, e115402. [Google Scholar] [CrossRef] [Green Version]
- Quan, T.; Little, E.; Quan, H.; Qin, Z.; Voorhees, J.J.; Fisher, G.J. Elevated matrix metalloproteinases and collagen fragmentation in photodamaged human skin: Impact of altered extracellular matrix microenvironment on dermal fibroblast function. J. Investig. Dermatol. 2013, 133, 1362–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.E.; Chung, M.Y.; Lim, T.G.; Huh, W.B.; Lee, H.J.; Lee, K.W. Quercetin suppresses intracellular ROS formation, MMP activation, and cell motility in human fibrosarcoma cells. J. Food Sci. 2013, 78, H1464–H1469. [Google Scholar] [CrossRef] [PubMed]


| Gene (Accession Number) | Name | Primer Sequences |
|---|---|---|
| COL1A1 (NM_000088.4) | Collagen type I alpha 1 chain | Fw 5′-CATAAAGGGTCACCGTGGCT-3′ Rv 5′-GGGACCTTGTTCACCAGGAG- 3′ |
| IL-1β (NM_000576.3) | Interleukin 1 beta | Fw 5′-GAATCTCCGACCACCACTACAG-3′ Rv 5′-TGATCGTACAGGTGCATCGTG-3′ |
| LSGALS1 (NM_002305.4) | Galectin 1 | Fw 5′-TCTCGGGTGGAGTCTTCTGA-3′ Rv 5′-GTTCAGCACGAAGCTCTTAGC-3′ |
| LSGALS3 (NM_002306.4) | Galectin 3 | Fw 5′-CTGCTGGGGCACTGATTGT-3′ Rv 5′-TGTTTGCATTGGGCTTCACC-3′ |
| MMP-3 (NM_002422.5) | Matrix metalloproteinase 3 | Fw 5′- TCACTCACAGACCTGACTCG-3′ Rv 5′-AAAGCAGGATCACAGTTGGC-3′ |
| ELN (NM_000501.4) | Elastin | Fw 5′-CAGCTAAATACGGTGCTGCTG-3′ Rv 5′-AATCCGAAGCCAGGTCTTG-3′ |
| COL3A1 (NM_000090.4) | Collagen type III alpha 1 chain | Fw 5′-CTTCTCTCCAGCCGAGCTTC-3′ Rv 5′-CCAGTGTGTTTCGTGCAACC-3′ |
| PPIA (NM_021130.5) | Peptidylprolyl Isomerase A | Fw 5′-GGGCTTTAGGCTGTAGGTCAA-3′ Rv 5′-AACCAAAGCTAGGGAGAGGC-3′ |
| TNF-α (NM_000594.3) | TNF- alpha | Fw 5′-AAGCCTGTAGCCCATGTTGT-3′ Rv 5′-GGACCTGGGAGTAGATGAGGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donato, A.; Belluzzi, E.; Mattiuzzo, E.; Venerando, R.; Cadamuro, M.; Ruggieri, P.; Vindigni, V.; Brun, P. Anti-Inflammatory and Pro-Regenerative Effects of Hyaluronan-Chitlac Mixture in Human Dermal Fibroblasts: A Skin Ageing Perspective. Polymers 2022, 14, 1817. https://doi.org/10.3390/polym14091817
Donato A, Belluzzi E, Mattiuzzo E, Venerando R, Cadamuro M, Ruggieri P, Vindigni V, Brun P. Anti-Inflammatory and Pro-Regenerative Effects of Hyaluronan-Chitlac Mixture in Human Dermal Fibroblasts: A Skin Ageing Perspective. Polymers. 2022; 14(9):1817. https://doi.org/10.3390/polym14091817
Chicago/Turabian StyleDonato, Alice, Elisa Belluzzi, Elena Mattiuzzo, Rina Venerando, Massimiliano Cadamuro, Pietro Ruggieri, Vincenzo Vindigni, and Paola Brun. 2022. "Anti-Inflammatory and Pro-Regenerative Effects of Hyaluronan-Chitlac Mixture in Human Dermal Fibroblasts: A Skin Ageing Perspective" Polymers 14, no. 9: 1817. https://doi.org/10.3390/polym14091817
APA StyleDonato, A., Belluzzi, E., Mattiuzzo, E., Venerando, R., Cadamuro, M., Ruggieri, P., Vindigni, V., & Brun, P. (2022). Anti-Inflammatory and Pro-Regenerative Effects of Hyaluronan-Chitlac Mixture in Human Dermal Fibroblasts: A Skin Ageing Perspective. Polymers, 14(9), 1817. https://doi.org/10.3390/polym14091817