Fluorescence Labeling of Cellulose Nanocrystals—A Facile and Green Synthesis Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Titration Experiments
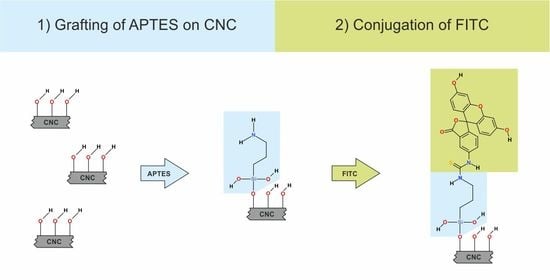
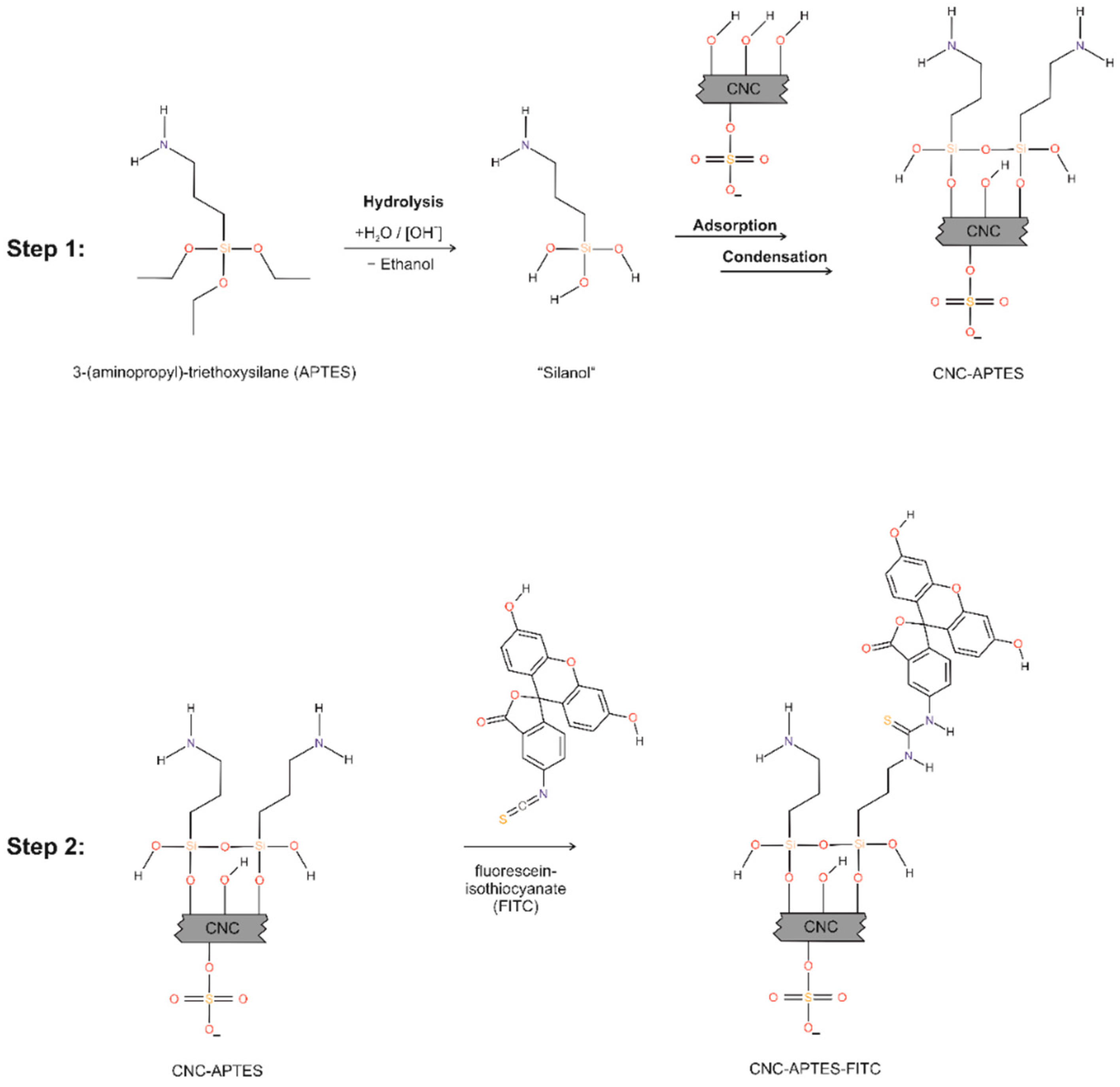
2.2.2. Preparation of CNC-APTES
2.2.3. Preparation of CNC-APTES-FITC
2.2.4. Thermogravimetry
2.2.5. Calculation of Degree of Substitution (DoS)
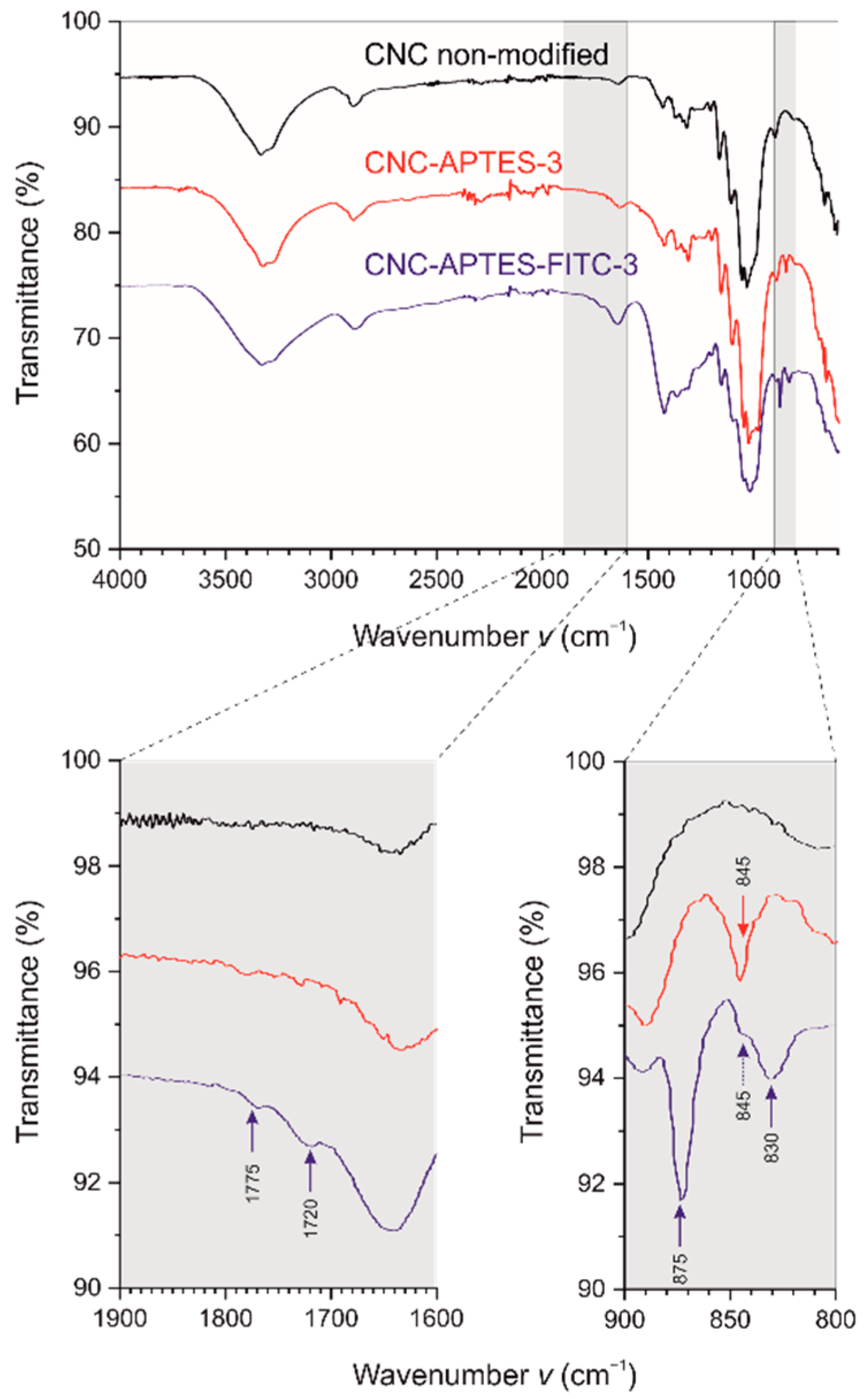
2.2.6. FTIR Analysis
2.2.7. Photometry
2.2.8. Dynamic Light Scattering (DLS)
2.2.9. Asymmetrical Flow Field-Flow Fractionation—Multi-Angle Light Scattering (AF4-MALS)
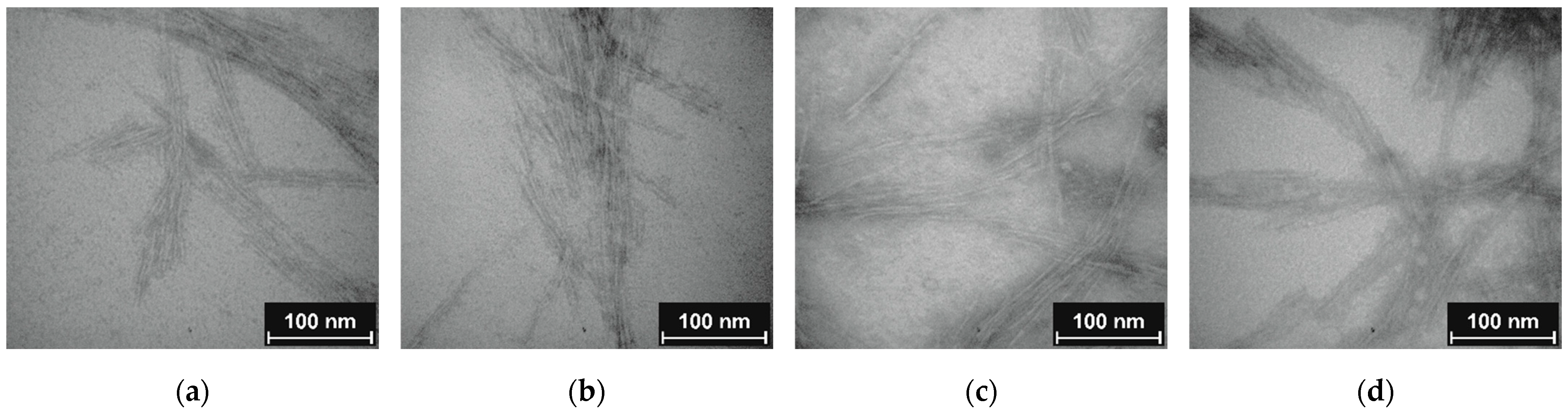
2.2.10. Transmission Electron Microscopy (TEM)
3. Results and Discussion
3.1. Overview and Discussion of Existing Routes for Cellulose Labelling with FITC
| Substrate | Grafted Molecule (1) | Reaction Conditions (1) | Fluorophore (2) | Reaction Conditions (2) | Comment | DoS | Ref. |
|---|---|---|---|---|---|---|---|
| CNC | - | - | FITC, RBITC | dark reaction/0.1 mol L−1 NaOH/72 h | 0.031 | [37] | |
| CNC | ECH | NaOH/60 °C, amination with NH4OH/60 °C | FITC | dark reaction/borate buffer/overnight | ECH is toxic | 0.024 | [63] |
| CNC | ECH | FITC, RBITC | 0.024 | [39] | |||
| CNC, CNF | ECH | FITC | [65] | ||||
| CNC | ECH * | ECH + NH4OH first form 2-hydroxy-3-chloro propylamine; DMSO/TBAH/50 °C | - | - | 0.357 | [64] | |
| CNC | APTES | DMF/2 h RT | FITC | DMF/19 h RT | DMF is toxic | [38] | |
| CNC | APTES | modification with PDDA, Fe3O4; TEOS/APTES at alkaline pH | FITC | ethanol, 24 h/RT | superparamagnetic core-shell structure | [67] | |
| CNC | APTES | hydrolysis of APTES at pH 4, + CNC, stirring 2 h/RT, precipitate cured at 105 °C | - | - | thermal curing | 0.180 | [68] |
| CNF | APTES | water/ethanol, pH 5.5, 1 h, RT; curing 110 °C | - | - | 0.458 | [69] | |
| CNF | AzPTES, VTES, MPTES | acidic silane hydrolysis (HCl, 30 min, RT), alkaline condensation (NaOH, 3 h, RT) | - | - | aqueous protocol, pH induced hydrolysis | 0.195 | [75] |
| CNC | APTES | FITC | bicarbonate buffer, pH 9, overnight | 0.040 | this work |
3.2. Preparation and Optimization of Aqueous Protocol
3.2.1. Influence of Substrate and Silane Type on pH
3.2.2. Increased Reaction Temperature
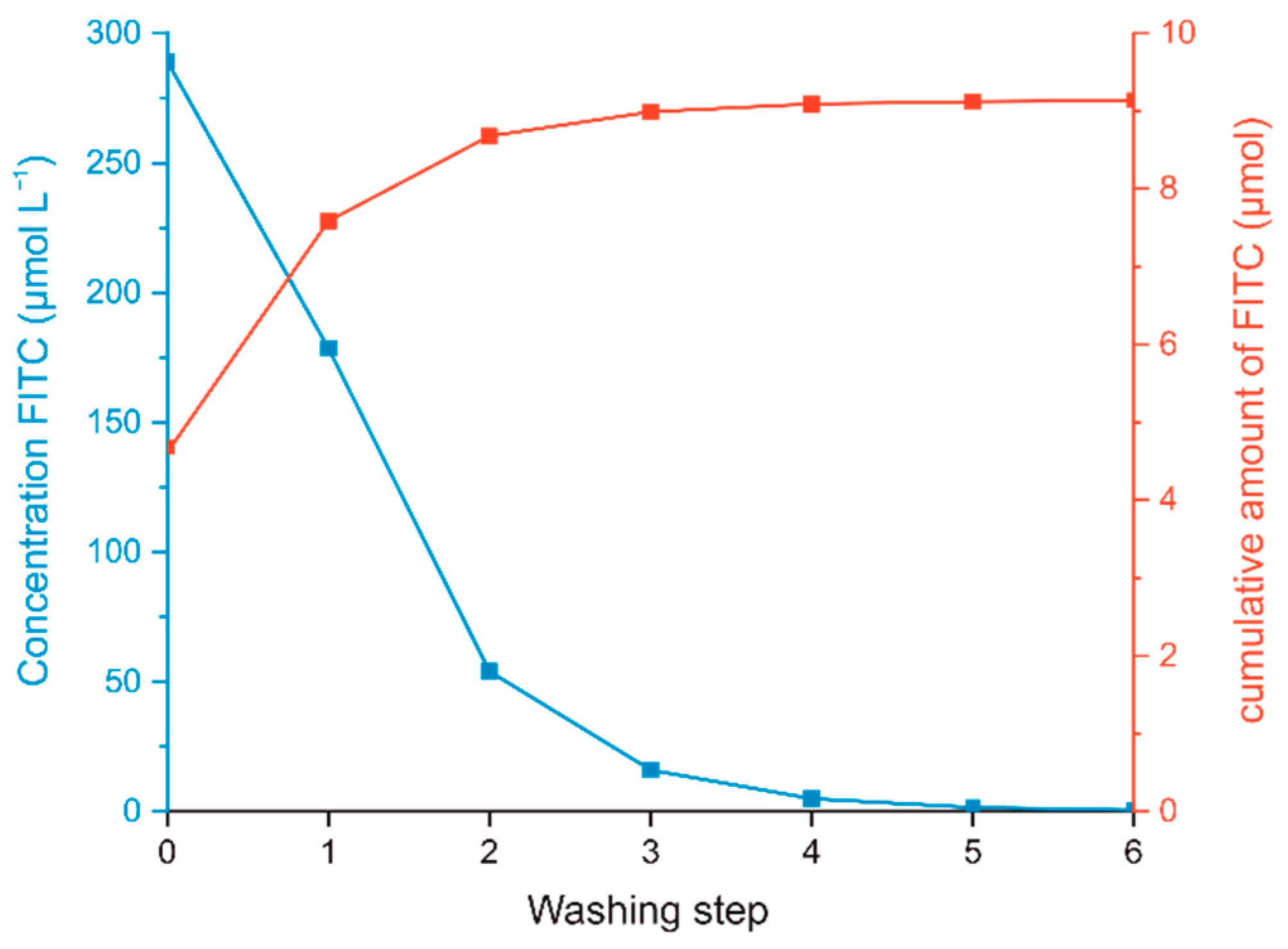
3.2.3. Separation of CNC-APTES and CNC-APTES-FITC from Supernatants
3.3. Proof of Functionalization and DoS
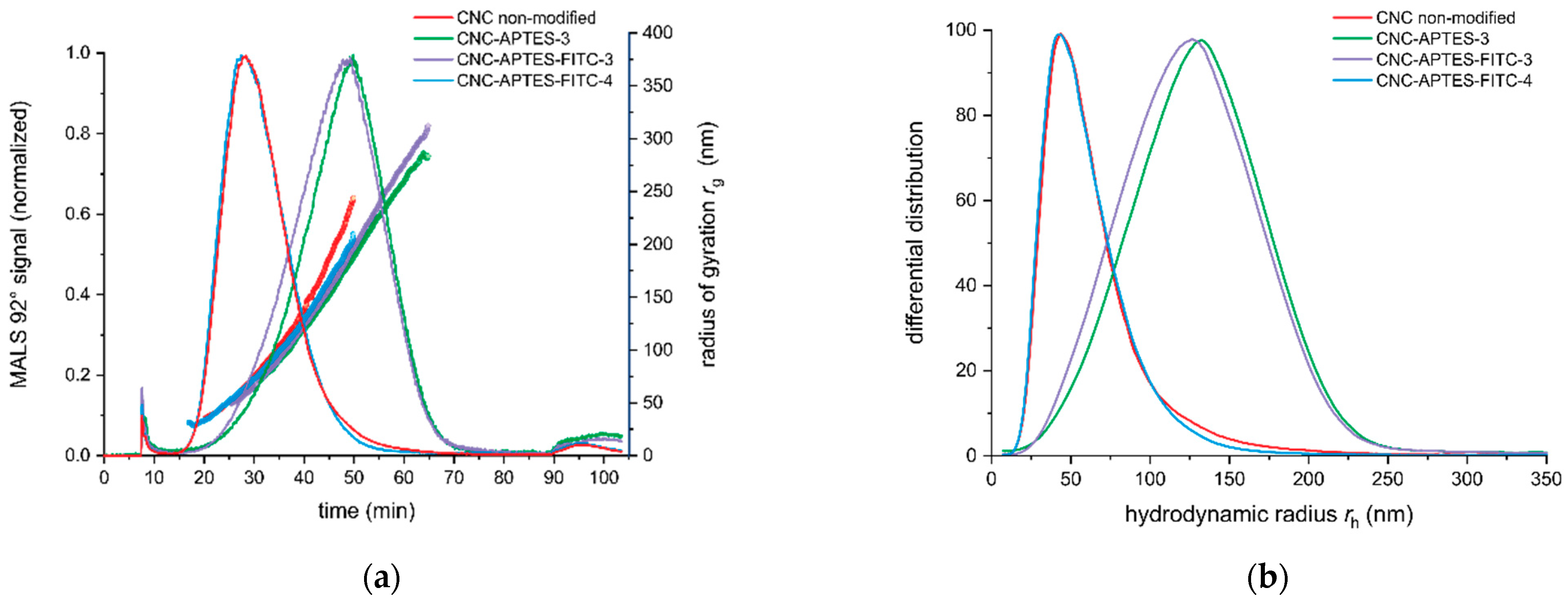
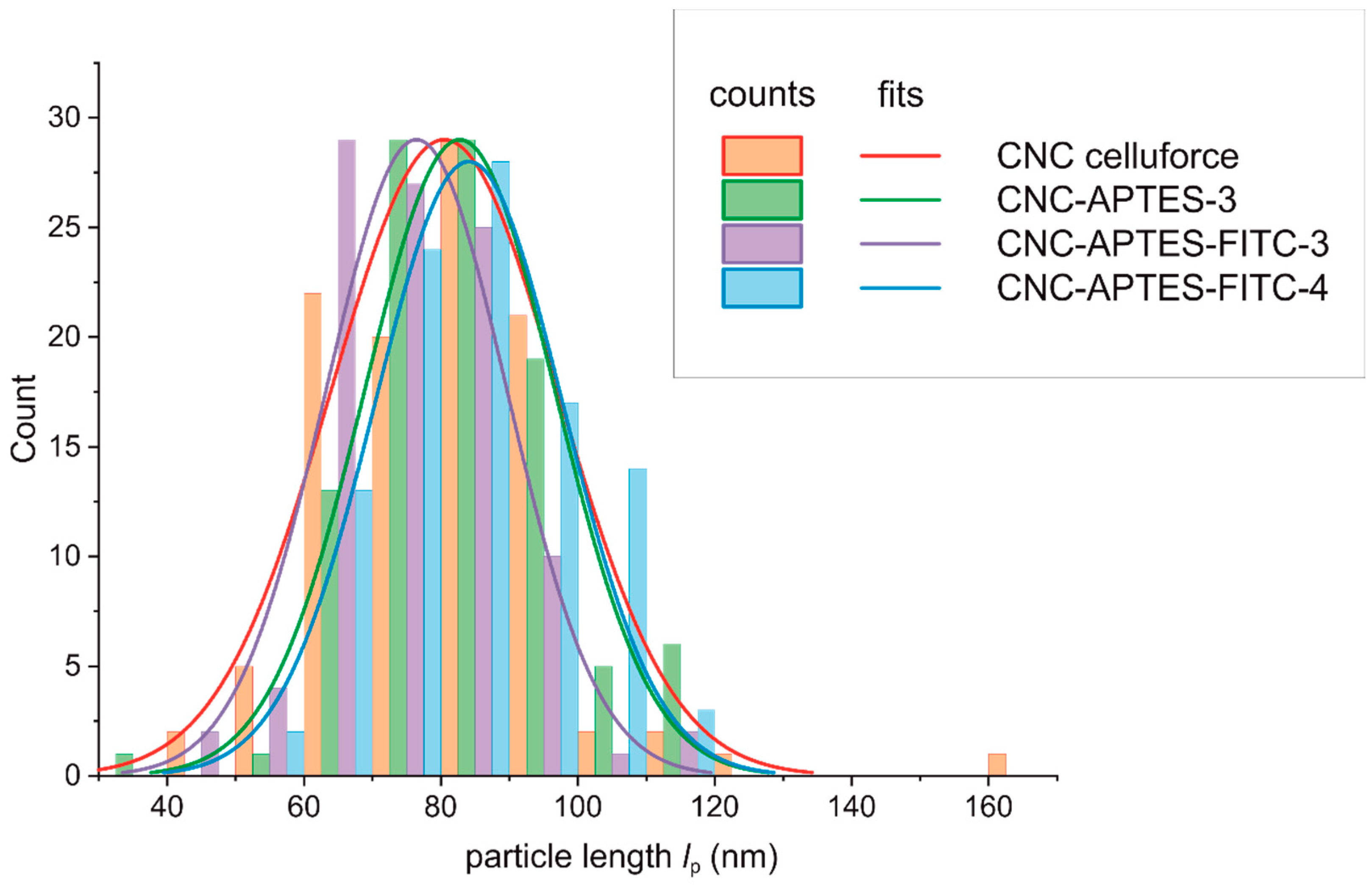
3.4. Particle Size Characterization after Grafting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ververis, C.; Georghiou, K.; Christodoulakis, N.; Santas, P.; Santas, R. Fiber dimensions, lignin and cellulose content of various plant materials and their suitability for paper production. Ind. Crops Prod. 2004, 19, 245–254. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based Lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Huber, T.; Müssig, J.; Curnow, O.; Pang, S.; Bickerton, S.; Staiger, M.P. A critical review of all-cellulose composites. J. Mater. Sci. 2012, 47, 1171–1186. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Tech. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Türk, O. Stoffliche Nutzung Nachwachsender Rohstoffe; Springer: Wiesbaden, Germany, 2014. [Google Scholar]
- Dufresne, A. Nanocellulose; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Tang, J.T.; Sisler, J.; Grishkewich, N.; Tam, K.C. Functionalization of cellulose nanocrystals for advanced applications. J. Colloid Interface Sci. 2017, 494, 397–409. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in thin films, coatings, and plies for packaging applications: A review. Bioresources 2017, 12, 2143–2233. [Google Scholar] [CrossRef] [Green Version]
- Metzger, C.; Sanahuja, S.; Behrends, L.; Sängerlaub, S.; Lindner, M.; Briesen, H. Efficiently extracted cellulose nanocrystals and starch nanoparticles and techno-functional properties of films made thereof. Coatings 2018, 8, 142. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer aerogels and foams: Chemistry, properties, and applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Yuen, A.C.Y.; Li, A.; Lin, B.; Chen, T.B.Y.; Yang, W.; Lu, H.-D.; Yeoh, G.H. Recent progress in bio-based aerogel absorbents for oil/water separation. Cellulose 2019, 26, 6449–6476. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Fürtauer, S.; Hassan, M.; Elsherbiny, A.; Gabal, S.A.; Mehanny, S.; Abushammala, H. Current status of cellulosic and nanocellulosic materials for oil spill cleanup. Polymers 2021, 13, 2739. [Google Scholar] [CrossRef]
- Lei, X.; Li, H.; Luo, Y.; Sun, X.; Guo, X.; Hu, Y.; Wen, R. Novel fluorescent nanocellulose hydrogel based on gold nanoclusters for the effective adsorption and sensitive detection of mercury ions. J. Taiwan Inst. Chem. Eng. 2021, 123, 79–86. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Jackson, J.C.; Camargos, C.H.M.; Noronha, V.T.; Paula, A.J.; Rezende, C.A.; Faria, A.F. Sustainable cellulose nanocrystals for improved antimicrobial properties of thin film composite membranes. ACS Sustain. Chem. Eng. 2021, 9, 6534–6540. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Nurazzi, N.M.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Syed Najmuddin, S.U.F.; Ilyas, R.A. Emerging development of nanocellulose as an antimicrobial material: An overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Nicole, L.; Rosario, G.; Diego, B.-M.; Gabriela, M.d.O.-V.; Pablo, C.; Fernanda, G.M.; José, V.-B.; Hugo, C.; Mary, L. Preparation and characterization of a novel nanocellulose-derivative as a potential radiopharmaceutical agent. Waste Biomass Valoriz. 2022, 13, 173–183. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Naficy, S.; Chandrawati, R.; Dehghani, F. Nanocellulose for sensing applications. Adv. Mater. Interfaces 2019, 6, 1900424. [Google Scholar] [CrossRef]
- Tortorella, S.; Vetri Buratti, V.; Maturi, M.; Sambri, L.; Comes Franchini, M.; Locatelli, E. Surface-modified nanocellulose for application in biomedical engineering and nanomedicine: A review. Int. J. Nanomed. 2020, 15, 9909–9937. [Google Scholar] [CrossRef]
- Shi, Y.; Jiao, H.; Sun, J.; Lu, X.; Yu, S.; Cheng, L.; Wang, Q.; Liu, H.; Biranje, S.; Wang, J.; et al. Functionalization of nanocellulose applied with biological molecules for biomedical application: A review. Carbohydr. Polym. 2022, 285, 119208. [Google Scholar] [CrossRef]
- Rodionov, P.V.; Veselova, I.A.; Shekhovtsova, T.N. A solid-phase fluorescent biosensor for the determination of phenolic compounds and peroxides in samples with complex matrices. Anal. Bioanal. Chem. 2014, 406, 1531–1540. [Google Scholar] [CrossRef]
- Li, H.; Tong, W.; Gao, C. Photo-responsive polyethyleneimine microcapsules cross-linked by ortho-nitrobenzyl derivatives. J. Colloid Interface Sci. 2016, 463, 22–28. [Google Scholar] [CrossRef]
- Joshi, A.; Rashmi, C.; Rohit, S. FITC-tagged macromolecule-based alginate microspheres for urea sensoring. In Nanosensors, Biosensors, and Info-Tech Sensors and Systems 2014, Proceedings of the SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, San Diego, CA, USA, 9–13 March 2014; SPIE: Bellingham, WA, USA, 2014. [Google Scholar]
- Chaudhari, R.; Joshi, A.; Pandya, K. pH based urea biosensing using fluorescein isothiocyanate (FITC)-dextran encapsulated micro-carriers of calcium alginate. Sens. Lett. 2016, 14, 451–459. [Google Scholar] [CrossRef]
- Afsharipour, R.; Haji Shabani, A.M.; Dadfarnia, S. A selective off–on fluorescent aptasensor for alpha-fetoprotein determination based on n-carbon quantum dots and oxidized nanocellulose. J. Photochem. Photobiol. A Chem. 2022, 428, 113872. [Google Scholar] [CrossRef]
- Do, T.T.A.; Grijalvo, S.; Imae, T.; Garcia-Celma, M.J.; Rodríguez-Abreu, C. A nanocellulose-based platform towards targeted chemo-photodynamic/photothermal cancer therapy. Carbohydr. Polym. 2021, 270, 118366. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, L.J.; Eyley, S.; Thielemans, W.; Aylott, J.W. Dual fluorescent labelling of cellulose nanocrystals for pH sensing. Chem. Commun. 2010, 46, 8929–8931. [Google Scholar] [CrossRef]
- Yang, Q.A.; Pan, X.J. A facile approach for fabricating fluorescent cellulose. J. Appl. Polym. Sci. 2010, 117, 3639–3644. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J.H.T. Effect of surface charge on the cellular uptake and cytotoxicity of fluorescent labeled cellulose nanocrystals. ACS Appl. Mater. Inter. 2010, 2, 2924–2932. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Z.; Li, Q.; Zhou, J.P.; Zhang, L.N. Synthesis and photophysical behavior of pyrene-bearing cellulose nanocrystals for Fe3+ sensing. Macromol. Chem. Phys. 2012, 213, 1612–1617. [Google Scholar] [CrossRef]
- Hassan, M.L.; Moorefield, C.M.; Elbatal, H.S.; Newkome, G.R.; Modarelli, D.A.; Romano, N.C. Fluorescent cellulose nanocrystals via supramolecular assembly of terpyridine-modified cellulose nanocrystals and terpyridine-modified perylene. Mater. Sci. Eng. B Adv. 2012, 177, 350–358. [Google Scholar] [CrossRef]
- Drogat, N.; Granet, R.; Le Morvan, C.; Begaud-Grimaud, G.; Krausz, P.; Sol, V. Chlorin-PEI-labeled cellulose nanocrystals: Synthesis, characterization and potential application in PDT. Bioorg. Med. Chem. Lett. 2012, 22, 3648–3652. [Google Scholar] [CrossRef]
- Abitbol, T.; Palermo, A.; Moran-Mirabal, J.M.; Cranston, E.D. Fluorescent labeling and characterization of cellulose nanocrystals with varying charge contents. Biomacromolecules 2013, 14, 3278–3284. [Google Scholar] [CrossRef]
- Huang, J.L.; Li, C.J.; Gray, D.G. Cellulose nanocrystals incorporating fluorescent methylcoumarin groups. ACS Sustain. Chem. Eng. 2013, 1, 1160–1164. [Google Scholar] [CrossRef]
- Harrington, B.J.; Hageage, G.J., Jr. Calcofluor white: A review of its uses and applications in clinical mycology and parasitology. Lab. Med. 2003, 34, 361–367. [Google Scholar] [CrossRef]
- Herth, W.; Schnepf, E. The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 1980, 105, 129–133. [Google Scholar] [CrossRef]
- Peretz, R.; Mamane, H.; Sterenzon, E.; Gerchman, Y. Rapid quantification of cellulose nanocrystals by calcofluor white fluorescence staining. Cellulose 2019, 26, 971–977. [Google Scholar] [CrossRef]
- Edwards, J.V.; Fontenot, K.R.; Haldane, D.; Prevost, N.T.; Condon, B.D.; Grimm, C. Human neutrophil elastase peptide sensors conjugated to cellulosic and nanocellulosic materials: Part I, synthesis and characterization of fluorescent analogs. Cellulose 2016, 23, 1283–1295. [Google Scholar] [CrossRef]
- Tang, L.R.; Li, T.; Zhuang, S.Y.; Lu, Q.L.; Li, P.F.; Huang, B. Synthesis of pH-sensitive fluorescein grafted cellulose nanocrystals with an amino acid spacer. ACS Sustain. Chem. Eng. 2016, 4, 4842–4849. [Google Scholar] [CrossRef]
- Cateto, C.A.; Ragauskas, A. Amino acid modified cellulose whiskers. RSC Adv. 2011, 1, 1695–1697. [Google Scholar] [CrossRef]
- Barazzouk, S.; Daneault, C. Spectroscopic characterization of oxidized nanocellulose grafted with fluorescent amino acids. Cellulose 2011, 18, 643–653. [Google Scholar] [CrossRef]
- Alimohammadzadeh, R.; Rafi, A.A.; Goclik, L.; Tai, C.-W.; Cordova, A. Direct organocatalytic thioglycolic acid esterification of cellulose nanocrystals: A simple entry to click chemistry on the surface of nanocellulose. Carbohydr. Polym. Technol. Appl. 2022, 3, 100205. [Google Scholar] [CrossRef]
- Anastas, P.T.W.J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK; New York, NY, USA, 1998. [Google Scholar]
- Hermanson, G. Bioconjugate Techniques; Elsevier: Amsterdam, The Netherlands, 2008; p. 1323. [Google Scholar]
- Celluforce. Product Specification Celluforce NCV100; Celluforce: Montreal, QC, Canada, 2019. [Google Scholar]
- ISO 22412:2017; Particle Size Analysis—Dynamic Light Scattering (DLS). Standardization, I.O.F.: Geneva, Switzerland, 2017.
- Metzger, C.; Drexel, R.; Meier, F.; Briesen, H. Effect of ultrasonication on the size distribution and stability of cellulose nanocrystals in suspension: An asymmetrical flow field-flow fractionation study. Cellulose 2021, 28, 10221–10238. [Google Scholar] [CrossRef]
- ISO/TS 21362:2018; Nanotechnologies—Analysis of Nanoobjects Using Asymmetrical-Flow and Centrifugal Field-Flow Fractionation. International Organization for Standardization: Geneva, Switzerland, 2018.
- Novaanalysis; Postnova Analytics GmbH: Landsberg am Lech, Germany, 2007.
- Laaser, T.; Soppa, K.; Krekel, C. The migration of hydroxy propyl cellulose during consolidation of a painted wallpaper: A case study using a fluorescent-labelled consolidant. In Proceedings of the ICOM-CC Graphic Document Working Group—Interim Meeting, Austrian National Library, Vienna, Austria, 17–19 April 2013; Watteeuw, L., Hofmann, C., Eds.; International Council of Museums (ICOM): Paris, France; Austrian National Library: Vienna, Austria, 2013; pp. 88–90. [Google Scholar]
- Jia, R.N.; Tian, W.G.; Bai, H.T.; Zhang, J.M.; Wang, S.; Zhang, J. Amine-responsive cellulose-based ratiometric fluorescent materials for real-time and visual detection of shrimp and crab freshness. Nat. Commun. 2019, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Meier, R.J.; Wolfbeis, O.S.; Landthaler, M.; Szeimies, R.M.; Babilas, P. 2D luminescence imaging of pH in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 2432–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Roman, M. Fluorescently labeled cellulose nanocrystals for bioimaging applications. J. Am. Chem. Soc. 2007, 129, 13810–13811. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Zaman, M.; Mohammed, N.; Brinatti, C.; Batmaz, R.; Berry, R.; Loh, W.; Tam, K.C. Synthesis of amine functionalized cellulose nanocrystals: Optimization and characterization. Carbohyd. Res. 2015, 409, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Salari, M.; Bitounis, D.; Bhattacharya, K.; Pyrgiotakis, G.; Zhang, Z.Y.; Purington, E.; Gramlich, W.; Grondin, Y.; Rogers, R.; Bousfield, D.; et al. Development & characterization of fluorescently tagged nanocellulose for nanotoxicological studies. Environ. Sci. Nano 2019, 6, 1516–1526. [Google Scholar]
- Tian, M.; Wu, X.-L.; Zhang, B.; Li, J.-L.; Shi, Z. Synthesis of chlorinated fluoresceins for labeling proteins. Bioorg. Med. Chem. Lett. 2008, 18, 1977–1979. [Google Scholar] [CrossRef]
- Ren, S.X.; Zhang, X.Q.; Dong, L.L.; Lei, T.Z.; Teng, Z.G.; Song, K.L.; Sun, X.X.; Wu, Q.L. Cellulose nanocrystal supported superparamagnetic nanorods with aminated silica shell: Synthesis and properties. J. Mater. Sci. 2017, 52, 6432–6441. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Robles, E.; Csoka, L.; Labidi, J. Effect of reaction conditions on the surface modification of cellulose nanofibrils with aminopropyl triethoxysilane. Coatings 2018, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, T.; Maiti, T.K.; Behera, B.; Ghosh, S.K.; Apoorva, A.; Padmavati, M. Biofunctionalized cellulose paper matrix for cell delivery applications. Int. J. Biol. Macromol. 2019, 139, 114–127. [Google Scholar] [CrossRef]
- Cai, C.X.; We, B.X.; Jin, Z.Y.; Tian, Y.Q. Facile method for fluorescent labeling of starch nanocrystal. ACS Sustain. Chem. Eng. 2017, 5, 3751–3761. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Sadocco, P.; Aonso-Varona, A.; Palomares, T.; Eceiza, A.; Silvestre, A.J.D.; Mondragon, I.; Freire, C.S.R. Bioinspired antimicrobial and biocompatible bacterial cellulose membranes obtained by surface functionalization with aminoalkyl groups. ACS Appl. Mater. Inter. 2013, 5, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Abdussalam-Mohammed, W.; Ali, A.; Errayes, A. Green chemistry: Principles, applications and disadvantages. Chem. Methodol. 2020, 4, 408–423. [Google Scholar]
- Hettegger, H.; Beaumont, M.; Potthast, A.; Rosenau, T. Aqueous modification of nano- and microfibrillar cellulose with a click synthon. ChemSusChem 2016, 9, 75–79. [Google Scholar] [CrossRef]
- Beaumont, M.; Bacher, M.; Opietnik, M.; Gindl-Altmutter, W.; Potthast, A.; Rosenau, T. A general aqueous silanization protocol to introduce vinyl, mercapto or azido functionalities onto cellulose fibers and nanocelluloses. Molecules 2018, 23, 1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hettegger, H.; Sumerskii, I.; Sortino, S.; Potthast, A.; Rosenau, T. Silane meets click chemistry: Towards the functionalization of wet bacterial cellulose sheets. ChemSusChem 2015, 8, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Tingaut, P.; Hauert, R.; Zimmermann, T. Highly efficient and straightforward functionalization of cellulose films with thiol-ene click chemistry. J. Mater. Chem. 2011, 21, 16066–16076. [Google Scholar] [CrossRef]
- ChemicalBook. 3-Aminopropyltriethoxysilane (cas 919-30-2). Available online: https://www.chemicalbook.com/ProductMSDSDetailCB8686147_EN.htm (accessed on 14 March 2022).
- Riedel, E. Anorganische Chemie; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Preparation of Solid Catalysts; John Wiley & Sons: Weinheim, Germany, 2008. [Google Scholar]
- Thomas, J. Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Band 1–8. 2., Vollständig Überarbeitete und Erweiterte Auflage; Wiley-VCH: Weinheim, Germany, 2009; Volume 121, pp. 3421–3591. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., McKelvy, M.L., Eds.; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Kamel, S.; Salama, A.; Sarhan, H.-A. Preparation and infrared study of cellulose based amphiphilic materials. Cellulose Chem. Technol. 2018, 52, 193–200. [Google Scholar]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Nakamura, M.; Mochizuki, Y.; Usami, K.; Itoh, Y.; Nozaki, T. Infrared absorption spectra and compositions of evaporated silicon oxides (SiOx). Solid State Commun. 1984, 50, 1079–1081. [Google Scholar] [CrossRef]
- Harwood, L.M.; Moody, C.J.; Percy, J.M. Experimental Organic Chemistry: Standard and Microscale, 2nd ed.; Wiley-Blackwell Publishing: London, UK, 1999. [Google Scholar]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-VIS spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 60. [Google Scholar] [CrossRef] [Green Version]
- Schimpf, M.E.; Caldwell, K.; Giddings, J.C. Field-Flow Fractionation Handbook; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
| Sample | Preparation | Combustion Residue from Silane (wt.%) | DoStotal | DoSsurface |
|---|---|---|---|---|
| CNC-APTES-1 | protocol according to [75] | 3.68 * ± 0.63 | 0.106 * ± 0.020 | 0.155 * ± 0.030 |
| CNC-APTES-2 | pH establishment during hydrolysis | 2.06 * ± 0.00 | 0.058 * ± 0.005 | 0.085 * ± 0.007 |
| CNC-APTES-3 | optimized protocol (25 °C) | 1.42 ± 0.16 | 0.040 ± 0.005 | 0.058 ± 0.007 |
| CNC-APTES-4 | increased condensation temperature (40 °C) | 1.69 ± 0.14 | 0.047 ± 0.004 | 0.069 ± 0.006 |
| DLS | ||
|---|---|---|
| dh (nm) | Ð | |
| CNC non-modified | 163.4 ± 2.3 | 0.211 ± 0.005 |
| CNC-APTES-3 | 190.4 ± 1.3 | 0.139 ± 0.011 |
| CNC-APTES-FITC-3 | 182.8 ± 2.5 | 0.168 ± 0.012 |
| CNC-APTES-FITC-4 | 178.3 ± 0.5 | 0.195 ± 0.018 |
| AF4-MALS | |||
|---|---|---|---|
| rg (nm) | rh (nm) | rg/rh | |
| CNC non-modified | 63.5 ± 1.0 | 42.8 ± 2.2 | 1.49 ± 0.06 |
| CNC-APTES-3 | 183.5 ± 1.5 | 133.7 ± 1.4 | 1.37 ± 0.02 |
| CNC-APTES-FITC-3 | 179.8 ± 2.6 | 125.9 ± 3.3 | 1.43 ± 0.02 |
| CNC-APTES-FITC-4 | 60.7 ± 1.4 | 41.8 ± 2.3 | 1.45 ± 0.09 |
| lp (nm) | n | |
|---|---|---|
| CNC non-modified | 80.3 ± 16.7 | 105 |
| CNC-APTES-3 | 82.7 ± 13.9 | 103 |
| CNC-APTES-FITC-3 | 74.6 ± 13.2 | 100 |
| CNC-APTES-FITC-4 | 84.1 ± 13.7 | 101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campora, L.D.; Metzger, C.; Dähnhardt-Pfeiffer, S.; Drexel, R.; Meier, F.; Fürtauer, S. Fluorescence Labeling of Cellulose Nanocrystals—A Facile and Green Synthesis Route. Polymers 2022, 14, 1820. https://doi.org/10.3390/polym14091820
Campora LD, Metzger C, Dähnhardt-Pfeiffer S, Drexel R, Meier F, Fürtauer S. Fluorescence Labeling of Cellulose Nanocrystals—A Facile and Green Synthesis Route. Polymers. 2022; 14(9):1820. https://doi.org/10.3390/polym14091820
Chicago/Turabian StyleCampora, Lorenzo Donato, Christoph Metzger, Stephan Dähnhardt-Pfeiffer, Roland Drexel, Florian Meier, and Siegfried Fürtauer. 2022. "Fluorescence Labeling of Cellulose Nanocrystals—A Facile and Green Synthesis Route" Polymers 14, no. 9: 1820. https://doi.org/10.3390/polym14091820
APA StyleCampora, L. D., Metzger, C., Dähnhardt-Pfeiffer, S., Drexel, R., Meier, F., & Fürtauer, S. (2022). Fluorescence Labeling of Cellulose Nanocrystals—A Facile and Green Synthesis Route. Polymers, 14(9), 1820. https://doi.org/10.3390/polym14091820