Cutting-Edge Progress in Stimuli-Responsive Bioadhesives: From Synthesis to Clinical Applications
Abstract
:1. Introduction
2. Principal Criteria in Bioadhesive
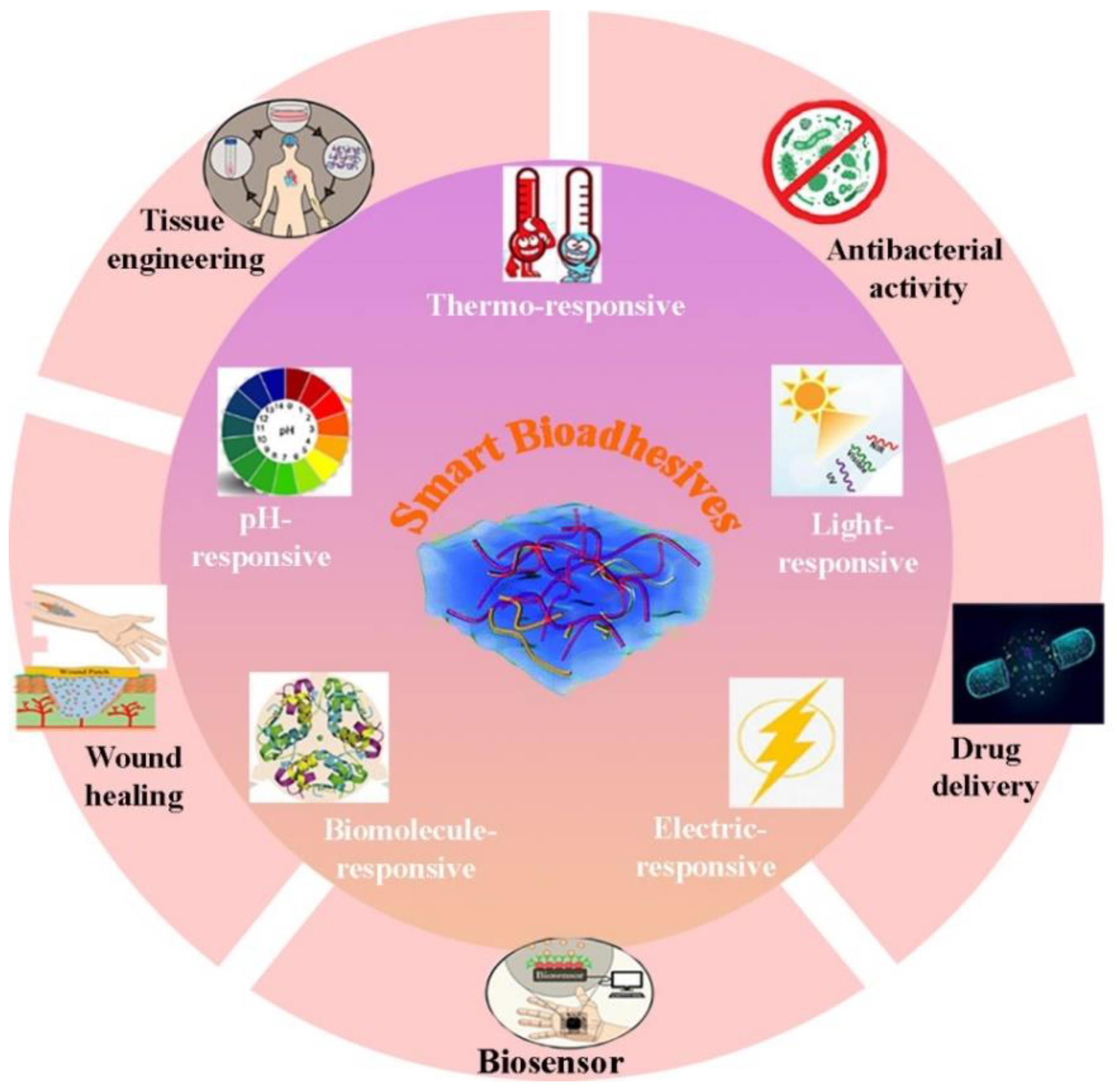
3. Smart Bioadhesives and Their Applications
3.1. Light-Responsive Bioadhesives
3.2. Thermo-Responsive Bioadhesives
3.3. pH-Responsive Bioadhesives
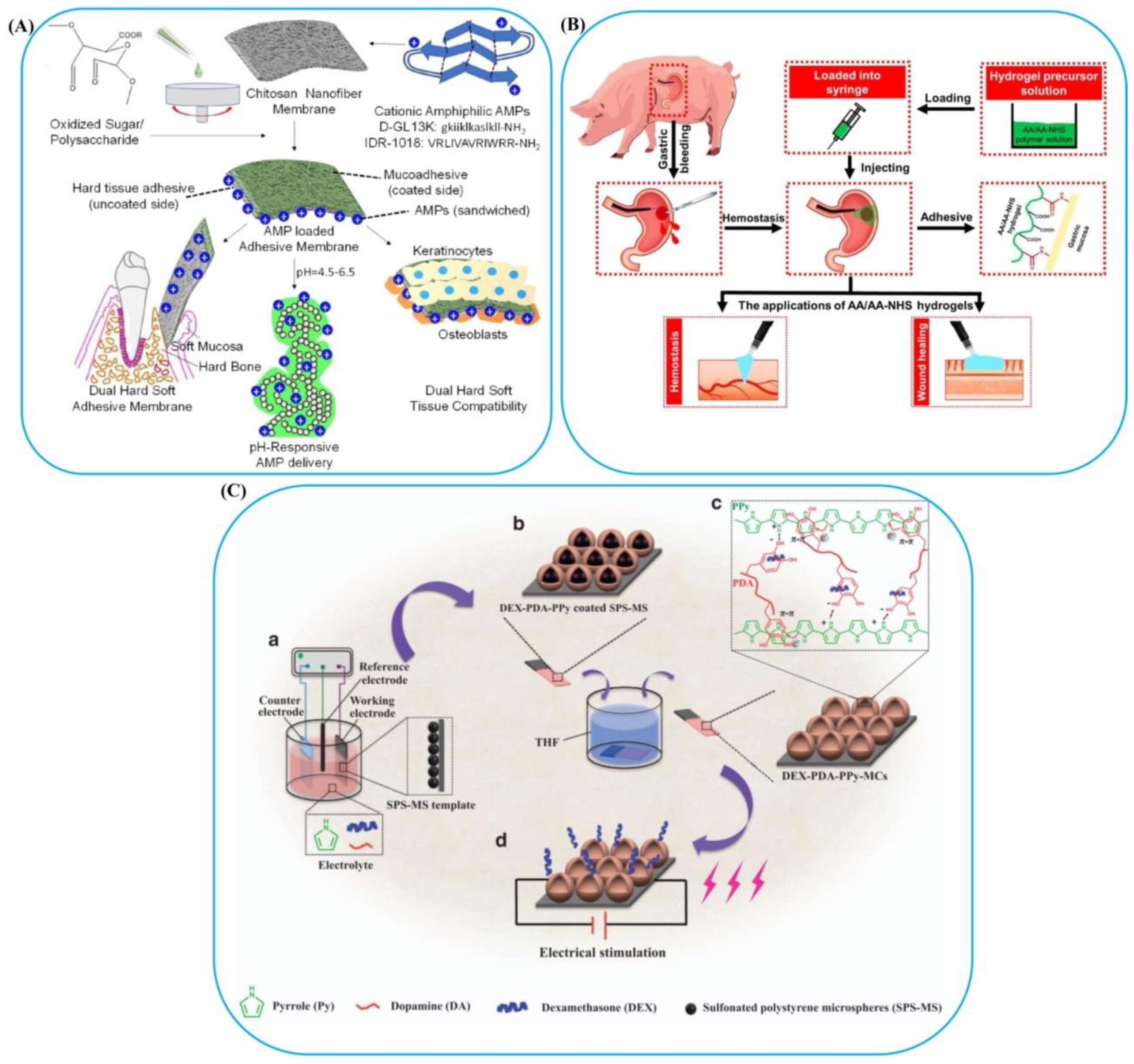
3.4. Electromagnetic-Responsive Bioadhesives
3.5. Biomolecule-Responsive Bioadhesive
3.6. Multi-Responsive Bioadhesives
4. Clinical Applications of Stimuli-Responsive Bioadhesives
4.1. Wound Healing of Soft and Hard Tissues
4.2. Drug Delivery
4.3. Leak Sealants in Medical
4.4. Wearable Medical Devices
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Su, X. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- Khanlari, S.; Dubé, M.A. Bioadhesives: A review. Macromol. React. Eng. 2013, 7, 573–587. [Google Scholar] [CrossRef]
- Ramesh, M.; Kumar, L.R. Bioadhesives, Green Adhesives: Preparation, Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 145–164. [Google Scholar]
- Chopra, H.; Kumar, S.; Singh, I. Bioadhesive Hydrogels and Their Applications. In Bioadhesives in Drug Delivery; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 147–170. [Google Scholar]
- Pinnaratip, R.; Bhuiyan, M.S.A.; Meyers, K.; Rajachar, R.M.; Lee, B.P. Multifunctional biomedical adhesives. Adv. Healthc. Mater. 2019, 8, 1801568. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Mokhtari, H.; Kharaziha, M.; Kermanpur, A.; Talebi, A.; Moshtaghian, J. A multifunctional nanocomposite spray dressing of Kappa-carrageenan-polydopamine modified ZnO/L-glutamic acid for diabetic wounds. Mater. Sci. Eng. C 2020, 111, 110837. [Google Scholar] [CrossRef]
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite® as a potential surgical sealant. J. Colloid Interface Sci. 2020, 564, 155–169. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- El-Sherbiny, I.M.; Khalil, I.A.; Ali, I.H. Updates on stimuli-responsive polymers: Synthesis approaches and features. In Polymer Gels; Springer: New York, NY, USA, 2018; pp. 129–146. [Google Scholar]
- Bruschi, M.L.; Borghi-Pangoni, F.B.; Junqueira, M.V.; de Souza Ferreira, S.B. Nanostructured therapeutic systems with bioadhesive and thermoresponsive properties. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherland, 2017; pp. 313–342. [Google Scholar]
- Dey, A.; Bhattacharya, P.; Neogi, S. Bioadhesives in Biomedical Applications: A Critical Review. Rev. Adhes. Adhes. 2020, 8, 130–152. [Google Scholar] [CrossRef]
- Duan, W.; Bian, X.; Bu, Y. Applications of bioadhesives: A mini review. Front. Bioeng. Biotechnol. 2021, 9, 716035. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.; Chun, B.; Seo, J. Rational engineering and applications of functional bioadhesives in biomedical engineering. Biotechnol. J. 2021, 16, 2100231. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Bratek-Skicki, A. Towards a new class of stimuli-responsive polymer-based materials–Recent advances and challenges. Appl. Surf. Sci. Adv. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Beaussart, A.; Ngo, T.C.; Derclaye, S.; Kalinova, R.; Mincheva, R.; Dubois, P.; Leclère, P.; Dufrêne, Y.F. Chemical force microscopy of stimuli-responsive adhesive copolymers. Nanoscale 2014, 6, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, G.; Hosseinkhani, H. Biomolecule-responsive hydrogels in medicine. Adv. Healthc. Mater. 2017, 6, 1700801. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.R.; San Román, J. Introduction to smart polymers and their applications. In Smart Polymers and Their Applications; Elsevier: Amsterdam, The Netherland, 2019; pp. 1–11. [Google Scholar]
- Shoukat, H.; Buksh, K.; Noreen, S.; Pervaiz, F.; Maqbool, I. Hydrogels as potential drug-delivery systems: Network design and applications. Ther. Deliv. 2021, 12, 375–396. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-stimuli-responsive hydrogels and their medical applications. New J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Ding, M.; Jing, L.; Yang, H.; Machnicki, C.; Fu, X.; Li, K.; Wong, I.; Chen, P.-Y. Multifunctional soft machines based on stimuli-responsive hydrogels: From freestanding hydrogels to smart integrated systems. Mater. Today Adv. 2020, 8, 100088. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, H.N.; Seong, M.; Lee, S.H.; Kang, M.; Yi, H.; Bae, W.G.; Kwak, M.K.; Jeong, H.E. Multifunctional smart skin adhesive patches for advanced health care. Adv. Healthc. Mater. 2018, 7, 1800275. [Google Scholar] [CrossRef]
- Guillon, E.; Das, D.; Jülich, D.; Hassan, A.-R.; Geller, H.; Holley, S. Fibronectin is a smart adhesive that both influences and responds to the mechanics of early spinal column development. Elife 2020, 9, e48964. [Google Scholar] [CrossRef]
- Pathak, K.; Malviya, R. Introduction, Theories and Mechanisms of Bioadhesion. In Bioadhesives in Drug Delivery; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 1–27. [Google Scholar]
- Lenaerts, V.M.; Gurny, R. Bioadhesive Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Kaurav, H.; HariKumar, S.; Kaur, A. Mucoadhesive microspheres as carriers in drug delivery: A review. Int. J. Drug Dev. Res. 2012, 4, 21–34. [Google Scholar]
- Masareddy, R.S.; Patil, A.S.; Gadad, A.P. Bioadhesive Nanoparticulate Drug Delivery System. In Nanopharmaceutical Advanced Delivery Systems; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 309–331. [Google Scholar]
- Bouten, P.J.; Zonjee, M.; Bender, J.; Yauw, S.T.; van Goor, H.; van Hest, J.C.; Hoogenboom, R. The chemistry of tissue adhesive materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, T.; Newland, B.; Duffy, P.; Annaidh, A.N.; O’Cearbhaill, E.D.; Wang, W. On-demand and negative-thermo-swelling tissue adhesive based on highly branched ambivalent PEG–catechol copolymers. J. Mater. Chem. B 2015, 3, 6420–6428. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G.; Kim, K.S.; Kim, D.W.; Kim, D.S.; Seo, Y.G.; Go, T.G.; Youn, Y.S.; Kim, J.O.; Yong, C.S.; Choi, H.-G. Development of a novel sodium fusidate-loaded triple polymer hydrogel wound dressing: Mechanical properties and effects on wound repair. Int. J. Pharm. 2016, 497, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Korde, J.M.; Kandasubramanian, B. Biocompatible alkyl cyanoacrylates and their derivatives as bio-adhesives. Biomater. Sci. 2018, 6, 1691–1711. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chuah, Y.J.; Wang, D.-A. Bioadhesives for internal medical applications: A review. Acta Biomater. 2018, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schricker, S.R.; Palacio, M.L.; Bhushan, B. Designing nanostructured block copolymer surfaces to control protein adhesion. Philos. Trans. Royal Soc. A 2012, 370, 2348–2380. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Karolewicz, B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm. J. 2016, 24, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Ebara, M.; Kotsuchibashi, Y.; Narain, R.; Idota, N.; Kim, Y.-J.; Hoffman, J.M.; Uto, K.; Aoyagi, T. Smart Biomaterials; Springer: New York, NY, USA, 2014. [Google Scholar]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Michal, B.T.; Spencer, E.J.; Rowan, S.J. Stimuli-responsive reversible two-level adhesion from a structurally dynamic shape-memory polymer. ACS Appl. Mater. Interfaces 2016, 8, 11041–11049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, Z.; Field, R.; Moloney, M.G.; Rimmer, S.; Ye, H. Thermo-responsive microcarriers based on poly (N-isopropylacrylamide). Eur. Polym. J. 2015, 67, 346–364. [Google Scholar] [CrossRef]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly (acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 2016, 12, 2542–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martella, D.; Nocentini, S.; Micheletti, F.; Wiersma, D.S.; Parmeggiani, C. Polarization-dependent deformation in light responsive polymers doped by dichroic dyes. Soft Matter 2019, 15, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, V.; Saadaoui, H.; Rodriguez-Hernandez, J.; Drummond, C. Electro-responsive polyelectrolyte-coated surfaces. Faraday Discuss. 2017, 199, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Meng, J.; Wang, S. Photo-responsive polymer materials for biological applications. Chin. Chem. Lett. 2017, 28, 2085–2091. [Google Scholar] [CrossRef]
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic-and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Hao, Y.; Cui, H.; Meng, J.; Wang, S. Photo-responsive smart surfaces with controllable cell adhesion. J. Photochem. Photobiol. A Chem. 2018, 355, 202–211. [Google Scholar] [CrossRef]
- He, F.; Yang, G.; Yang, P.; Yu, Y.; Lv, R.; Li, C.; Dai, Y.; Gai, S.; Lin, J. A new single 808 nm NIR light-induced imaging-guided multifunctional cancer therapy platform. Adv. Funct. Mater. 2015, 25, 3966–3976. [Google Scholar] [CrossRef]
- Kasiński, A.; Zielińska-Pisklak, M.; Oledzka, E.; Sobczak, M. Smart hydrogels–synthetic stimuli-responsive antitumor drug release systems. Int. J. Nanomed. 2020, 15, 4541. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Z.; Zhou, L.; Li, Z.; Ren, J.; Qu, X. Noninvasive and reversible cell adhesion and detachment via single-wavelength near-infrared laser mediated photoisomerization. J. Am. Chem. Soc. 2015, 137, 8199–8205. [Google Scholar] [CrossRef] [PubMed]
- Bian, Q.; Chen, S.; Xing, Y.; Yuan, D.; Lv, L.; Wang, G. Host-guest self-assembly toward reversible visible-light-responsive switching for bacterial adhesion. Acta Biomater. 2018, 76, 39–45. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.; Zhang, Q. Injectable redox and light responsive MnO2 hybrid hydrogel for simultaneous melanoma therapy and multidrug-resistant bacteria-infected wound healing. Biomaterials 2020, 260, 120314. [Google Scholar] [CrossRef] [PubMed]
- Ryplida, B.; Lee, K.D.; In, I.; Park, S.Y. Light-Induced Swelling-Responsive Conductive, Adhesive, and Stretchable Wireless Film Hydrogel as Electronic Artificial Skin. Adv. Funct. Mater. 2019, 29, 1903209. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Z.; Jiang, Q.; Feng, J.; Wu, S.; Del Campo, A. Near-infrared-light regulated angiogenesis in a 4D hydrogel. Nanoscale 2020, 12, 13654–13661. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Ren, J.; Qu, X. 3D graphene oxide–polymer hydrogel: Near-infrared light-triggered active scaffold for reversible cell capture and on-demand release. Adv. Mater. 2013, 25, 6737–6743. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine nanoparticles modulating stimuli-responsive PNIPAM hydrogels with cell/tissue adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef]
- Abueva, C.D.; Chung, P.-S.; Ryu, H.-S.; Park, S.-Y.; Woo, S.H. Photoresponsive Hydrogels as Drug Delivery Systems. Med. Lasers Eng. Basic Res. Clin. Appl. 2020, 9, 6–11. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, Y.; Xie, M.; Wang, J.; Yan, X.-W.; Li, Z.; Dong, W.-G.; Huang, W.-H. Near-infrared light-responsive hydrogel for specific recognition and photothermal site-release of circulating tumor cells. ACS Nano 2016, 10, 6201–6210. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Tan, M.L.; Taheri, M.; Yan, Q.; Tsuzuki, T.; Gardiner, M.G.; Diggle, B.; Connal, L.A. Strong, Self-Healable, and Recyclable Visible-Light-Responsive Hydrogel Actuators. Angew. Chem. 2020, 132, 7115–7122. [Google Scholar] [CrossRef]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 10, 2001806. [Google Scholar] [CrossRef]
- Bi, J.; Song, K.; Wu, S.; Zhang, Y.; Wang, Y.; Liu, T. Effect of thermal-responsive surfaces based on PNIPAAm on cell adsorption/desorption. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 145–151. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive Polymers and Thermo-Responsive Liposomal Drug Delivery Systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Zhang, R.; Ruan, H.; Fu, Q.; Zhu, X.; Yao, Y. A high strain, adhesive, self-healable poly (acrylic acid) hydrogel with temperature sensitivity as an epidermal sensor. Mater. Adv. 2020, 1, 329–333. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X. Injectable Self-Healing Natural Biopolymer-Based Hydrogel Adhesive with Thermoresponsive Reversible Adhesion for Minimally Invasive Surgery. Adv. Funct. Mater. 2021, 31, 2007457. [Google Scholar] [CrossRef]
- Lee, Y.B.; Shin, Y.M.; Kim, E.M.; Lee, J.-Y.; Lim, J.; Kwon, S.K.; Shin, H. Mussel adhesive protein inspired coatings on temperature-responsive hydrogels for cell sheet engineering. J. Mater. Chem. B 2016, 4, 6012–6022. [Google Scholar] [CrossRef]
- Sultana, T.; Gwon, J.-G.; Lee, B.-T. Thermal stimuli-responsive hyaluronic acid loaded cellulose based physical hydrogel for post-surgical de novo peritoneal adhesion prevention. Mater. Sci. Eng. C 2020, 110, 110661. [Google Scholar] [CrossRef]
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Guo, Q.; Ji, F.; Tian, X.; Cui, J.; Song, Y.; Sun, H.; Li, J.; Yao, F. Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomater. 2018, 74, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Hatakeyama, Y.; Shimizu, T.; Matsuura, K.; Yamato, M.; Takeda, N.; Okano, T. Hydrophobized thermoresponsive copolymer brushes for cell separation by multistep temperature change. Biomacromolecules 2013, 14, 3423–3433. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Liu, X.; Miller, A.L., II; Cheng, Y.-S.; Yeh, M.-L.; Lu, L. Strengthening injectable thermo-sensitive NIPAAm-g-chitosan hydrogels using chemical cross-linking of disulfide bonds as scaffolds for tissue engineering. Carbohydr. Polym. 2018, 192, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.D.; Carvalho, S.M.; Mansur, H.S.; Pereira, M.M. Thermogelling chitosan–collagen–bioactive glass nanoparticle hybrids as potential injectable systems for tissue engineering. Mater. Sci. Eng. C 2016, 58, 1207–1216. [Google Scholar] [CrossRef]
- Umapathi, R.; Reddy, P.M.; Rani, A.; Venkatesu, P. Influence of additives on thermoresponsive polymers in aqueous media: A case study of poly (N-isopropylacrylamide). Phys. Chem. Chem. Phys. 2018, 20, 9717–9744. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
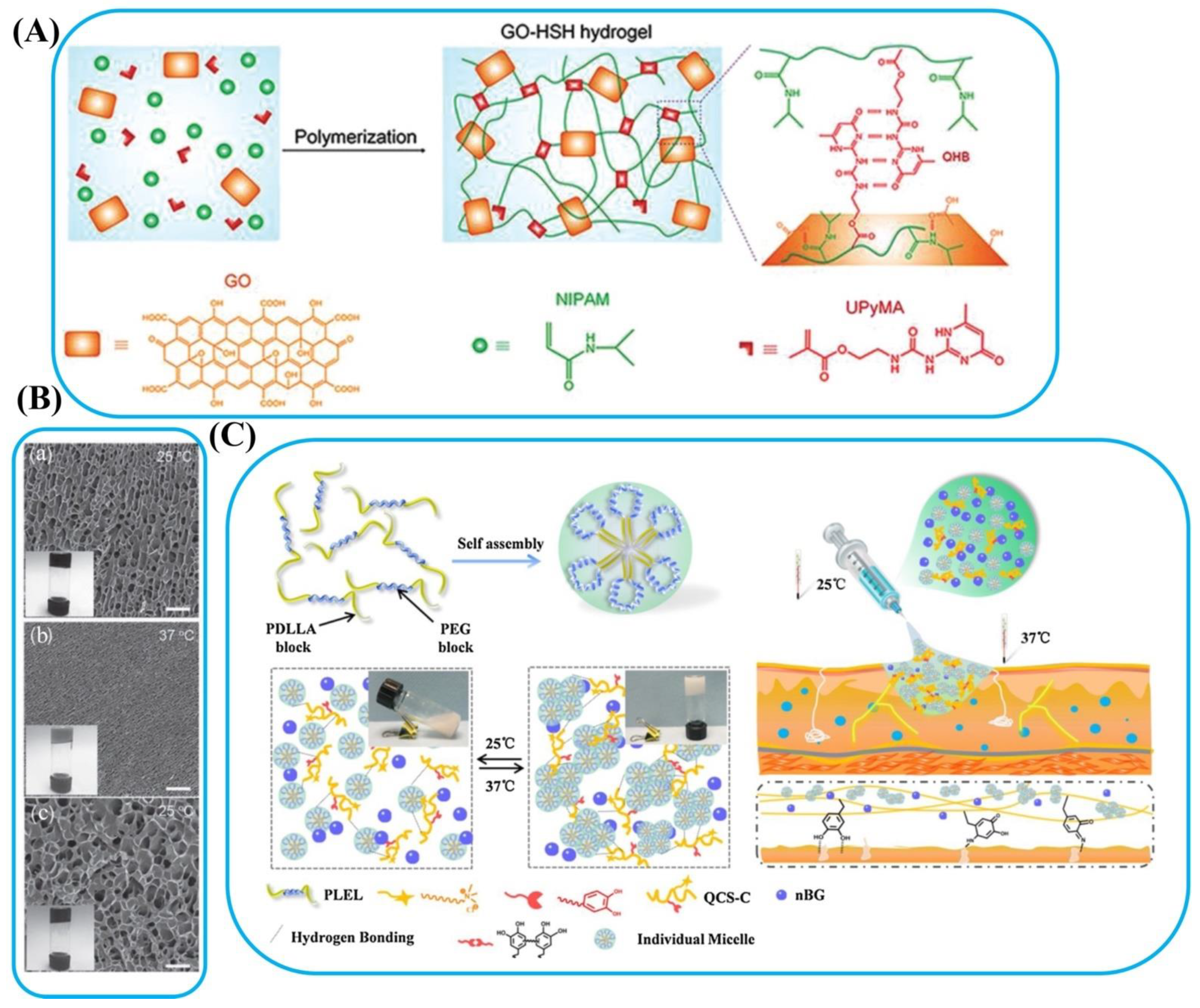
- Chen, Y.; Cheng, W.; Teng, L.; Jin, M.; Lu, B.; Ren, L.; Wang, Y. Graphene oxide hybrid supramolecular hydrogels with self-healable, bioadhesive and stimuli-responsive properties and drug delivery application. Macromol. Mater. Eng. 2018, 303, 1700660. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Thiagarajan, L.; Braim, S.; Saunders, B.R.; Shakesheff, K.M.; Alexander, C. Upper critical solution temperature thermo-responsive polymer brushes and a mechanism for controlled cell attachment. J. Mater. Chem. B 2017, 5, 4926–4933. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Uchikawa, N.; Hirotani, T.; Akimoto, A.M.; Kanazawa, H. Thermoresponsive anionic copolymer brush-grafted surfaces for cell separation. Colloids Surf. B Biointerfaces 2020, 185, 110565. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, G.; Ma, Y.; Yu, D.; Sun, J.; Li, Z. Smart surfaces based on thermo-responsive polymer brushes prepared from L-alanine derivatives for cell capture and release. Soft Matter 2015, 11, 7502–7506. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Anuradha, D. Chitosan based thermoresponsive hydrogel containing graphene oxide for bone tissue repair. Biomed. Pharmacother. 2018, 107, 908–917. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, Y.; He, M.; Huang, Y.; Zhao, W.; Zhao, C. Thermoresponsive Antibacterial Surfaces Switching from Bacterial Adhesion to Bacterial Repulsion. Macromol. Mater. Eng. 2018, 303, 1700590. [Google Scholar] [CrossRef]
- Sutton, A.; Shirman, T.; Timonen, J.V.; England, G.T.; Kim, P.; Kolle, M.; Ferrante, T.; Zarzar, L.D.; Strong, E.; Aizenberg, J. Photothermally triggered actuation of hybrid materials as a new platform for In Vitro cell manipulation. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Tyagi, P.; Agate, S.; McCord, M.G.; Lucia, L.A.; Pal, L. Highly tunable bioadhesion and optics of 3D printable PNIPAm/cellulose nanofibrils hydrogels. Carbohydr. Polym. 2020, 234, 115898. [Google Scholar] [CrossRef]
- Ferber, S.; Behrens, A.M.; McHugh, K.J.; Rosenberg, E.M.; Linehan, A.R.; Sugarman, J.L.; Jayawardena, H.S.N.; Langer, R.; Jaklenec, A. Evaporative cooling hydrogel packaging for storing biologics outside of the cold chain. Adv. Healthc. Mater. 2018, 7, 1800220. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [Green Version]
- Luckanagul, J.A.; Pitakchatwong, C.; Bhuket, P.R.N.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef]
- Kumar, T.M.; Paul, W.; Sharma, C.P.; Kuriachan, M. Bioadhesive, pH responsive micromatrix for oral delivery of insulin. Trends Biomater. Artif. Organs 2005, 18, 198–202. [Google Scholar]
- Dai, S.; Ravi, P.; Tam, K.C. pH-Responsive polymers: Synthesis, properties and applications. Soft Matter 2008, 4, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dahlström, C.; Edlund, H.; Lindman, B.; Norgren, M. pH-responsive cellulose—Chitosan nanocomposite films with slow release of chitosan. Cellulose 2019, 26, 3763–3776. [Google Scholar] [CrossRef] [Green Version]
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagniere, E.; Mangin, D.; Elaïssari, A. pH-sensitive polymers: Classification and some fine potential applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Li, A.; Li, T.; Liu, M.; von Klitzing, R.; Ober, C.K.; Kayitmazer, A.B.; Li, L.; Guo, X. Zinc induced polyelectrolyte coacervate bioadhesive and its transition to a self-healing hydrogel. RSC Adv. 2015, 5, 66871–66878. [Google Scholar] [CrossRef]
- Garriga, R.; Jurewicz, I.; Seyedin, S.; Bardi, N.; Totti, S.; Matta-Domjan, B.; Velliou, E.G.; Alkhorayef, M.A.; Cebolla, V.L.; Razal, J.M. Multifunctional, biocompatible and pH-responsive carbon nanotube-and graphene oxide/tectomer hybrid composites and coatings. Nanoscale 2017, 9, 7791–7804. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef]
- Agrawal, P.; Singh, R.P.; Sharma, G.; Mehata, A.K.; Singh, S.; Rajesh, C.V.; Pandey, B.L.; Koch, B.; Muthu, M.S. Bioadhesive micelles of d-α-tocopherol polyethylene glycol succinate 1000: Synergism of chitosan and transferrin in targeted drug delivery. Colloids Surf. B Biointerfaces 2017, 152, 277–288. [Google Scholar] [CrossRef]
- Wu, D.; Shi, X.; Zhao, F.; Chilengue, S.T.F.; Deng, L.; Dong, A.; Kong, D.; Wang, W.; Zhang, J. An injectable and tumor-specific responsive hydrogel with tissue-adhesive and nanomedicine-releasing abilities for precise locoregional chemotherapy. Acta Biomater. 2019, 96, 123–136. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Y.; Su, D.; Wu, S.; Zhou, J.; Chen, J. Injectable, Self-healing and pH Responsive Stem Cell Factor Loaded Collagen Hydrogel as Dynamic Bioadhesive Dressing for Diabetic Wound Repair. J. Mater. Chem. B 2021, 9, 5887–5897. [Google Scholar] [CrossRef] [PubMed]
- Boda, S.K.; Fischer, N.G.; Ye, Z.; Aparicio, C. Dual Oral Tissue Adhesive Nanofiber Membranes for pH-Responsive Delivery of Antimicrobial Peptides. Biomacromolecules 2020, 21, 4945–4961. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef]
- Yadav, V.; Jaimes-Lizcano, Y.A.; Dewangan, N.K.; Park, N.; Li, T.-H.; Robertson, M.L.; Conrad, J.C. Tuning bacterial attachment and detachment via the thickness and dispersity of a pH-responsive polymer brush. ACS Appl. Mater. Interfaces 2017, 9, 44900–44910. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Z.; Yang, Y.; Ren, F.; Li, J.; Zhu, S.; Ma, F.; Wu, R.; Lv, Y.; He, G. Injectable Self-Healing Adhesive pH-Responsive Hydrogels Accelerate Gastric Hemostasis and Wound Healing. Nano-Micro Lett. 2021, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Baloğlu, E.; Özyazıcı, M.; Hızarcıoğlu, S.Y.; Karavana, H.A. An in vitro investigation for vaginal bioadhesive formulations: Bioadhesive properties and swelling states of polymer mixtures. Il Farmaco 2003, 58, 391–396. [Google Scholar] [CrossRef]
- Pan, G.; Li, F.; He, S.; Li, W.; Wu, Q.; He, J.; Ruan, R.; Xiao, Z.; Zhang, J.; Yang, H. Mussel-and Barnacle Cement Proteins-Inspired Dual-Bionic Bioadhesive with Repeatable Wet-Tissue Adhesion, Multimodal Self-Healing, and Antibacterial Capability for Nonpressing Hemostasis and Promoted Wound Healing. Adv. Funct. Mater. 2022, 2200908. [Google Scholar] [CrossRef]
- Xie, C.; Li, P.; Han, L.; Wang, Z.; Zhou, T.; Deng, W.; Wang, K.; Lu, X. Electroresponsive and cell-affinitive polydopamine/polypyrrole composite microcapsules with a dual-function of on-demand drug delivery and cell stimulation for electrical therapy. NPG Asia Mater. 2017, 9, e358. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Xudong, L.; Xiaoqing, C.; Zhiwei, J.; Pervaiz, M.; Weimin, Y.; Haoyi, L.; Sain, M. A review of electro-stimulated gels and their applications: Present state and future perspectives. Mater. Sci. Eng. C 2019, 103, 109852. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive smart polymers for biomedical applications. Materials 2019, 12, 277. [Google Scholar] [CrossRef] [Green Version]
- Romasanta, L.J.; López-Manchado, M.A.; Verdejo, R. Increasing the performance of dielectric elastomer actuators: A review from the materials perspective. Prog. Polym. Sci. 2015, 51, 188–211. [Google Scholar] [CrossRef] [Green Version]
- Adesanya, K.; Vanderleyden, E.; Embrechts, A.; Glazer, P.; Mendes, E.; Dubruel, P. Properties of electrically responsive hydrogels as a potential dynamic tool for biomedical applications. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Yan, H.; Li, L.; Wang, Z.; Wang, Y.; Guo, M.; Shi, X.; Yeh, J.-M.; Zhang, P. Mussel-inspired conducting copolymer with aniline tetramer as intelligent biological adhesive for bone tissue engineering. ACS Biomater. Sci. Eng. 2019, 6, 634–646. [Google Scholar] [CrossRef]
- di Luca, M.; Vittorio, O.; Cirillo, G.; Curcio, M.; Czuban, M.; Farfalla, A.; Hampel, S.; Nicoletta, F.P.; Iemma, F. Electro-responsive graphene oxide hydrogels for skin bandages: The outcome of gelatin and trypsin immobilization. Int. J. Pharm. 2018, 546, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kumar, A.; Han, S.S. Polysaccharide-based magnetically responsive polyelectrolyte hydrogels for tissue engineering applications. J. Mater. Sci. Technol. 2018, 34, 1371–1377. [Google Scholar] [CrossRef]
- Birajdar, R.P.; Patil, S.B.; Alange, V.V.; Kulkarni, R.V. Electro-responsive polyacrylamide-grafted-gum ghatti copolymer for transdermal drug delivery application. J. Macromol. Sci. Part A 2019, 56, 306–315. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-responsive conductive nanocomposite hydrogels with high stretchability, self-healing, adhesiveness, and 3D printability for human motion sensing. ACS Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef]
- Bagheri, B.; Zarrintaj, P.; Samadi, A.; Zarrintaj, R.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M.; Park, O.O.; Kim, Y.C. Tissue engineering with electrospun electro-responsive chitosan-aniline oligomer/polyvinyl alcohol. Int. J. Biol. Macromol. 2020, 147, 160–169. [Google Scholar] [CrossRef]
- Qiao, K.; Guo, S.; Zheng, Y.; Xu, X.; Meng, H.; Peng, J.; Fang, Z.; Xie, Y. Effects of graphene on the structure, properties, electro-response behaviors of GO/PAA composite hydrogels and influence of electro-mechanical coupling on BMSC differentiation. Mater. Sci. Eng. C 2018, 93, 853–863. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Willner, I. Stimuli-Responsive Biomolecule-Based Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2020, 59, 15342–15377. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, H.; Long, R.; Wang, S.; Huang, H.; Xia, Y.; Wang, P.; Lei, Y.; Cai, Y.; Cai, D. Oral delivery of insulin with intelligent glucose-responsive switch for blood glucose regulation. J. Nanobiotech. 2020, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.T.; Gu, J.; Li, C.H.; Lee, T.R.; Xie, L.; Chen, S.; Cao, P.Y.; Jiang, S.; Yuan, Y.; Hong, X. A nanoparticle-decorated biomolecule-responsive polymer enables robust signaling cascade for biosensing. Adv. Mater. 2017, 29, 1702090. [Google Scholar] [CrossRef] [PubMed]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable self-healing glucose-responsive hydrogels with pH-regulated mechanical properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Huang, Q.; Tong, Z.; Yao, J.; Kong, X. A composite hydrogel system containing glucose-responsive nanocarriers for oral delivery of insulin. Mater. Sci. Eng. C 2016, 69, 37–45. [Google Scholar] [CrossRef]
- Sigen, A.; Xu, Q.; Johnson, M.; Creagh-Flynn, J.; Venet, M.; Zhou, D.; Lara-Sáez, I.; Tai, H.; Wang, W. An injectable multi-responsive hydrogel as self-healable and on-demand dissolution tissue adhesive. Appl. Mater. Today 2021, 22, 100967. [Google Scholar]
- Aminu, N.; Toh, S. Applicability of nanoparticles-hydrogel composite in treating periodontal diseases and beyond. Asian J. Pharm. Clin. Res. 2017, 10, 65–70. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009, 85, 848–860. [Google Scholar] [CrossRef]
- Le, T.M.D.; Duong, H.T.T.; Thambi, T.; Giang Phan, V.; Jeong, J.H.; Lee, D.S. Bioinspired pH-and temperature-responsive injectable adhesive hydrogels with polyplexes promotes skin wound healing. Biomacromolecules 2018, 19, 3536–3548. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, S.; Park, J.P.; Shin, M.; Kim, K.; Ryu, J.H.; Lee, H. Dynamic bonds between boronic acid and alginate: Hydrogels with stretchable, self-healing, stimuli-responsive, remoldable, and adhesive properties. Biomacromolecules 2018, 19, 2053–2061. [Google Scholar] [CrossRef]
- Abebe, M.W.; Appiah-Ntiamoah, R.; Kim, H. Gallic acid modified alginate self-adhesive hydrogel for strain responsive transdermal delivery. Int. J. Biol. Macromol. 2020, 163, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Yao, G.; Liu, H. Self-adhesive, self-healable, and triple-responsive hydrogel doped with polydopamine as an adsorbent toward methylene blue. Ind. Eng. Chem. Res. 2019, 58, 17075–17087. [Google Scholar] [CrossRef]
- Shan, M.; Gong, C.; Li, B.; Wu, G. A pH, glucose, and dopamine triple-responsive, self-healable adhesive hydrogel formed by phenylborate–catechol complexation. Polym. Chem. 2017, 8, 2997–3005. [Google Scholar] [CrossRef]
- Di, X.; Kang, Y.; Li, F.; Yao, R.; Chen, Q.; Hang, C.; Xu, Y.; Wang, Y.; Sun, P.; Wu, G. Poly (N-isopropylacrylamide)/ polydopamine/clay nanocomposite hydrogels with stretchability, conductivity, and dual light-and thermo-responsive bending and adhesive properties. Colloids Surf. B Biointerfaces 2019, 177, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, P.; Köwitsch, A.; Liedmann, A.; Menzel, M.; Fuhrmann, B.; Schmidt, G.; Klehm, J.; Groth, T. Stimuli-responsive multilayers based on thiolated polysaccharides that affect fibroblast cell adhesion. ACS Appl. Mater. Interfaces 2018, 10, 8507–8518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Deng, F.; Tang, L.; Wu, H.; Ni, Y.; Chen, L.; Huang, L.; Hu, X.; Lin, S.; Ding, C. Super-ductile, injectable, fast self-healing collagen-based hydrogels with multi-responsive and accelerated wound-repair properties. Chem. Eng. J. 2021, 405, 126756. [Google Scholar] [CrossRef]
- Che, Y.; Li, D.; Liu, Y.; Yue, Z.; Zhao, J.; Ma, Q.; Zhang, Q.; Tan, Y.; Yue, Q.; Meng, F. Design and fabrication of a triple-responsive chitosan-based hydrogel with excellent mechanical properties for controlled drug delivery. J. Polym. Res. 2018, 25, 1–17. [Google Scholar] [CrossRef]
- Wang, Z.; Si, Y.; Zhao, C.; Yu, D.; Wang, W.; Sun, G. Flexible and washable poly (ionic liquid) nanofibrous membrane with moisture proof pressure sensing for real-life wearable electronics. ACS Appl. Mater. Interfaces 2019, 11, 27200–27209. [Google Scholar] [CrossRef]
- Qu, X.; Zhao, Y.; Chen, Z.A.; Wang, S.; Ren, Y.; Wang, Q.; Shao, J.; Wang, W.; Dong, X. Thermoresponsive Lignin-Reinforced Poly (Ionic Liquid) Hydrogel Wireless Strain Sensor. Research 2021, 2021, 9845482. [Google Scholar] [CrossRef]
- Kuddushi, M.; Ray, D.; Aswal, V.; Hoskins, C.; Malek, N. Poly (vinyl alcohol) and functionalized ionic liquid-based smart hydrogels for doxorubicin release. ACS Appl. Bio Mater. 2020, 3, 4883–4894. [Google Scholar] [CrossRef]
- Xiang, S.; Zheng, F.; Chen, S.; Lu, Q. Self-healable, recyclable, and ultrastrong adhesive ionogel for multifunctional strain sensor. ACS Appl. Mater. Interfaces 2021, 13, 20653–20661. [Google Scholar] [CrossRef] [PubMed]
- Kuddushi, M.; Patel, N.K.; Rajput, S.; El Seoud, O.A.; Mata, J.P.; Malek, N.I. Temperature-Responsive Low Molecular Weight Ionic Liquid Based Gelator: An Approach to Fabricate an Anti-Cancer Drug-Loaded Hybrid Ionogel. ChemSystemsChem 2020, 2, e1900053. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, C.; Chen, S.; Meng, L.; Zhao, H.; Xu, F.; Yang, J. Adhesive Ionohydrogels Based on Ionic Liquid/Water Binary Solvents with Freezing Tolerance for Flexible Ionotronic Devices. Chem. Mater. 2022, 34, 1065–1077. [Google Scholar] [CrossRef]
- Rahimi, M.; Shafiei-Irannejad, V.; Safa, K.D.; Salehi, R. Multi-branched ionic liquid-chitosan as a smart and biocompatible nano-vehicle for combination chemotherapy with stealth and targeted properties. Carbohydr. Polym. 2018, 196, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic liquid—Polymer composites: A new platform for multifunctional applications. Adv. Funct. Mater. 2020, 30, 1909736. [Google Scholar] [CrossRef]
- Hu, X.; Huang, J.; Zhang, W.; Li, M.; Tao, C.; Li, G. Photonic Ionic Liquids Polymer for Naked-Eye Detection of Anions. Adv. Mater. 2008, 20, 4074–4078. [Google Scholar] [CrossRef]
- Usuba, M.; Hongo, C.; Matsumoto, T.; Nishino, T. On-demand easy peeling of acrylic adhesives containing ionic liquids through a microwave irradiation stimulus. Polym. J. 2018, 50, 1051–1056. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Hu, R.; Lv, C.; Zheng, J. A highly ionic conductive, healable, and adhesive polysiloxane—Supported ionogel. Macromol. Rapid Commun. 2019, 40, 1800776. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, F.; Gao, X.; Zheng, L. Double-network hydrogels with adjustable surface morphology and multifunctional integration for flexible strain sensors. Soft Matter 2021, 17, 4352–4362. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Zhang, Y.; Yang, F.; Liu, Y.; Wang, X.; Yang, X.; Zheng, J. Highly stretchable, self-adhesive, biocompatible, conductive hydrogels as fully polymeric strain sensors. J. Mater. Chem. A 2020, 8, 20474–20485. [Google Scholar] [CrossRef]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R.; et al. Bioadhesive Polymeric Films Based on Red Onion Skins Extract for Wound Treatment: An Innovative and Eco-Friendly Formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, K.; Gu, Q.; Zhou, D.; Zhou, M.; Zhang, L. Recent progress in surgical adhesives for biomedical applications. Smart Mater. Med. 2021, 3, 41–65. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A review of the properties and applications of bioadhesive hydrogels. Polym. Chem. 2021, 12, 3721–3739. [Google Scholar] [CrossRef]
- Brahmbhatt, D. Bioadhesive drug delivery systems: Overview and recent advances. Int. J. Chem. Life Sci. 2017, 6, 2016. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Pang, X.; Liu, M.; Zhang, B.; Li, L.; Zhai, G. Recent progresses in bioadhesive microspheres via transmucosal administration. Colloids Surf. B Biointerfaces 2016, 140, 361–372. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2020, 20, 229–236. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Gan, D.; Soubrier, M.; Chiang, H.-Y.; Yan, L.; Li, Y.; Li, J.; Yu, S.; Xia, Y.; et al. Bioadhesive and conductive hydrogel-integrated brain-machine interfaces for conformal and immune-evasive contact with brain tissue. Matter 2022, 5, 1204–1223. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, Y.; Huang, Y.; He, J.; Han, Y.; Guo, B. Physical double—Network hydrogel adhesives with rapid shape adaptability, fast self—Healing, antioxidant and NIR/pH stimulus—Responsiveness for multidrug—Resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020, 30, 1910748. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full—Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Yan, Y.H.; Rong, L.H.; Ge, J.; Tiu, B.D.B.; Cao, P.F.; Advincula, R.C. Mussel—Inspired hydrogel composite with multi—Stimuli responsive behavior. Macromol. Mater. Eng. 2019, 304, 1800720. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, Q.; Zhang, Q.; Li, K.; Shi, X.; Liang, F.; Han, D. Temperature/near-infrared light-responsive conductive hydrogels for controlled drug release and real-time monitoring. Nanoscale 2020, 12, 8679–8686. [Google Scholar] [CrossRef] [PubMed]
- Blacklow, S.O.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 2019, 5, eaaw3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.C.; Tai, A.Z.; Tsai, N.Y.; Li, Y.C.E. An Injectable Hybrid Gelatin Methacryloyl (GelMA)/Phenyl Isothiocyanate-Modified Gelatin (Gel-Phe) Bioadhesive for Oral/Dental Hemostasis Applications. Polymers 2021, 13, 2386. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Zhao, X.; Li, X.; Wang, Q.; Zhong, W.; Mequanint, K.; Zhan, R.; Xing, M.; Luo, G. Snake extract–laden hemostatic bioadhesive gel cross-linked by visible light. Sci. Adv. 2021, 7, eabf9635. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, L.; Chen, Y.; Song, Y.; Lu, W.P.; Guo, Y. A self-healing, robust adhesion, multiple stimuli-response hydrogel for flexible sensors. Soft Matter 2020, 16, 2238–2248. [Google Scholar] [CrossRef]
- Schnabel, B.; Scharf, M.; Schwieger, K.; Windolf, M.; van der Pol, B.; Braunstein, V.; Appelt, A. Biomechanical comparison of a new staple technique with tension band wiring for transverse patella fractures. Clin. Biomech 2009, 24, 855–859. [Google Scholar] [CrossRef]
- Rathi, S.; Saka, R.; Domb, A.J.; Khan, W. Protein—Based bioadhesives and bioglues. Polym. Adv. Technol. 2019, 30, 217–234. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.S.; Newman, K.J.H.; Forward, D.P.; Hahn, D.M.; Ollivere, B.; Kojima, K.; Handley, R.; Rossiter, N.; Wixted, J.; Moran, C.G. A unified theory of bone healing and nonunion: BHN theory. Bone Jt. J. 2016, 98, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Shirvan, A.R.; Bashari, A.; Hemmatinejad, N. New insight into the fabrication of smart mucoadhesive buccal patches as a novel controlled-drug delivery system. Eur. Polym. J. 2019, 119, 541–550. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; Leporatti, S.; El-Kemary, M. Mucoadhesive hydrogel nanoparticles as smart biomedical drug delivery system. Appl. Sci. 2019, 9, 825. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Bai, B.; Zhao, H.; Xu, X.; Hu, N.; Wang, H. Stimuli-responsive Ca-alginate-based photothermal system with enhanced foliar adhesion for controlled pesticide release. Colloids Surf. B Biointerfaces 2021, 207, 112004. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e1901714. [Google Scholar] [CrossRef] [PubMed]
- Abdollahiyan, P.; Baradaran, B.; de la Guardia, M.; Oroojalian, F.; Mokhtarzadeh, A. Cutting-edge progress and challenges in stimuli responsive hydrogel microenvironment for success in tissue engineering today. J. Control. Release 2020, 328, 514–531. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N. Hemostasis and other benefits of fibrin sealants/glues in spine surgery beyond cerebrospinal fluid leak repairs. Surg. Neurol. Int. 2014, 5, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yuk, H.; Sarrafian, T.L.; Guo, C.F.; Griffiths, L.G.; Nabzdyk, C.S.; Zhao, X. An off-the-shelf bioadhesive patch for sutureless repair of gastrointestinal defects. Sci. Transl. Med. 2022, 14. [Google Scholar] [CrossRef]
- Kiratli, H.; Tarlan, B. Subconjunctival hemorrhage: Risk factors and potential indicators. Clin. Ophthalmol. 2013, 7, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.-H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 5, 149–165. [Google Scholar] [CrossRef]
- Chen, X.; Yuk, H.; Wu, J.; Nabzdyk, C.S.; Zhao, X. Instant tough bioadhesive with triggerable benign detachment. Proc. Natl. Acad. Sci. USA 2020, 117, 15497–15503. [Google Scholar] [CrossRef]
- Ionov, L. Hydrogel-based actuators: Possibilities and limitations. Mater. Today 2014, 17, 494–503. [Google Scholar] [CrossRef]
- Wang, M.; Zhai, Y.; Ye, H.; Lv, Q.; Sun, B.; Luo, C.; Jiang, Q.; Zhang, H.; Xu, Y.; Jing, Y.; et al. High Co-loading Capacity and Stimuli-Responsive Release Based on Cascade Reaction of Self-Destructive Polymer for Improved Chemo-Photodynamic Therapy. ACS Nano 2019, 13, 7010–7023. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Xiong, W.; Sun, S.; Zhang, P.; Zhu, J.J. Recent advances in drug release monitoring. Nanophotonics 2019, 8, 391–413. [Google Scholar] [CrossRef]
- Naseem, F.; Shah, S.U.; Rashid, S.A.; Farid, A.; Almehmadi, M.; Alghamdi, S. Metronidazole Based Floating Bioadhesive Drug Delivery System for Potential Eradication of H. pylori: Preparation and In Vitro Characterization. Polymers 2022, 14, 519. [Google Scholar] [CrossRef]
- Céspedes-Valenzuela, D.N.; Sánchez-Rentería, S.; Cifuentes, J.; Gantiva-Diaz, M.; Serna, J.A.; Reyes, L.H.; Ostos, C.; la Portilla, C.C.-D.; Muñoz-Camargo, C.; Cruz, J.C. Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair. Polymers 2021, 14, 126. [Google Scholar] [CrossRef]
- Wong, Y.L.; Pandey, M.; Choudhury, H.; Lim, W.M.; Bhattamisra, S.K.; Gorain, B. Development of In-Situ Spray for Local Delivery of Antibacterial Drug for Hidradenitis Suppurativa: Investigation of Alternative Formulation. Polymers 2021, 13, 2770. [Google Scholar] [CrossRef]
- Spiridon, I.; Andrei, I.-M.; Anghel, N.; Dinu, M.; Ciubotaru, B.-I. Development and Characterization of Novel Cellulose Composites Obtained in 1-Ethyl-3-methylimidazolium Chloride Used as Drug Delivery Systems. Polymers 2021, 13, 2176. [Google Scholar] [CrossRef]
- Ageitos, J.; Robla, S.; Valverde-Fraga, L.; Garcia-Fuentes, M.; Csaba, N. Purification of Hollow Sporopollenin Microcapsules from Sunflower and Chamomile Pollen Grains. Polymers 2021, 13, 2094. [Google Scholar] [CrossRef]
- Anghel, N.; Dinu, V.; Verestiuc, L.; Spiridon, I. Transcutaneous Drug Delivery Systems Based on Collagen/Polyurethane Composites Reinforced with Cellulose. Polymers 2021, 13, 1845. [Google Scholar] [CrossRef]
- Antov, P.; Krišt’ák, L.; Réh, R.; Savov, V.; Papadopoulos, A.N. Eco-Friendly Fiberboard Panels from Recycled Fibers Bonded with Calcium Lignosulfonate. Polymers 2021, 13, 639. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Shlyakhtin, A.; Komarov, P.; Tavtorkin, A.; Kananykhina, E.; Elchaninov, A.; Vishnyakova, P.; Fatkhudinov, P.; Ivchenko, P. In Vitro and In Vivo Studies of Biodegradability and Biocompatibility of Poly (εCL)-b-Poly (EtOEP)-Based Films. Polymers 2020, 12, 3039. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Saha, N.; Sáha, T.; Öner, E.T.; Brodnjak, U.; Redl, H.; Von Byern, J.; Sáha, P. Polymer Based Bioadhesive Biomaterials for Medical Application—A Perspective of Redefining Healthcare System Management. Polymers 2020, 12, 3015. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.-C.; Ardelean, L.C.; Jitariu, A.-A.; Miu, C.A.; Streian, C.G. An Insight into the Structural Diversity and Clinical Applicability of Polyurethanes in Biomedicine. Polymers 2020, 12, 1197. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Anghel, N.; Dinu, M.V.; Vlad, S.; Bele, A.; Ciubotaru, B.-I.; Verestiuc, L.; Pamfil, D. Development and Performance of Bioactive Compounds-Loaded Cellulose/Collagen/Polyurethane Materials. Polymers 2020, 12, 1191. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Wei, S.-M.; Pei, M.-Y.; Pan, W.-L.; Thissen, H.; Tsai, S.-W. Gelatin Hydrogels Reinforced by Absorbable Nanoparticles and Fibrils Cured In Situ by Visible Light for Tissue Adhesive Applications. Polymers 2020, 12, 1113. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-dimensional printing constructs based on the chitosan for tissue regeneration: State of the art, developing directions and prospect trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Mokhtari, H.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Valiani, A.; Poursamar, S.A.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Recent trends in three-dimensional bioinks based on alginate for biomedical applications. Materials 2020, 13, 3980. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Kharaziha, M.; Kasiri-Asgarani, M.; Omidi, M.; Razzaghi, M.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. CNT and rGO reinforced PMMA based bone cement for fixation of load bearing implants: Mechanical property and biological response. J. Mech. Behav. Biomed. Mater. 2021, 116, 104320. [Google Scholar] [CrossRef] [PubMed]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Brabazon, D.; Kharaziha, M.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Berto, F. Additive Manufacturing of Polymer Matrix Composites; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Mokhtari, H.; Tavakoli, S.; Safarpour, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. Recent advances in chemically-modified and hybrid carrageenan-based platforms for drug delivery, wound healing, and tissue engineering. Polymers 2021, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, N.; Rezaei, A.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Luo, H.; RamaKrishna, S.; Berto, F. Recent advances on bioprinted gelatin methacrylate-based hydrogels for tissue repair. Tissue Eng. Part A 2021, 27, 679–702. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, X.; Shi, S.Q.; Li, J. A Tough and Mildew-Proof Soybean-Based Adhesive Inspired by Mussel and Algae. Polymers 2020, 12, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]

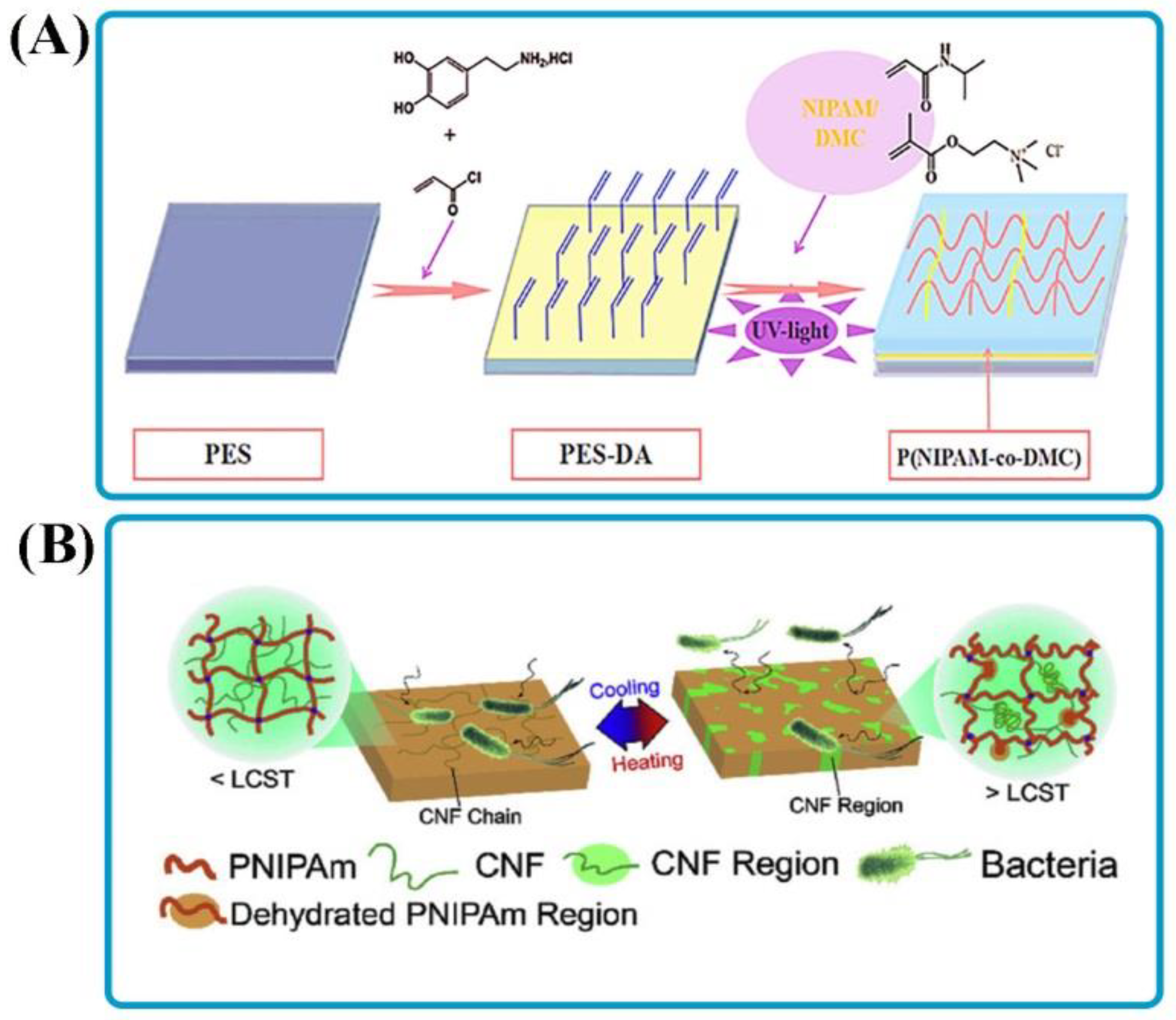
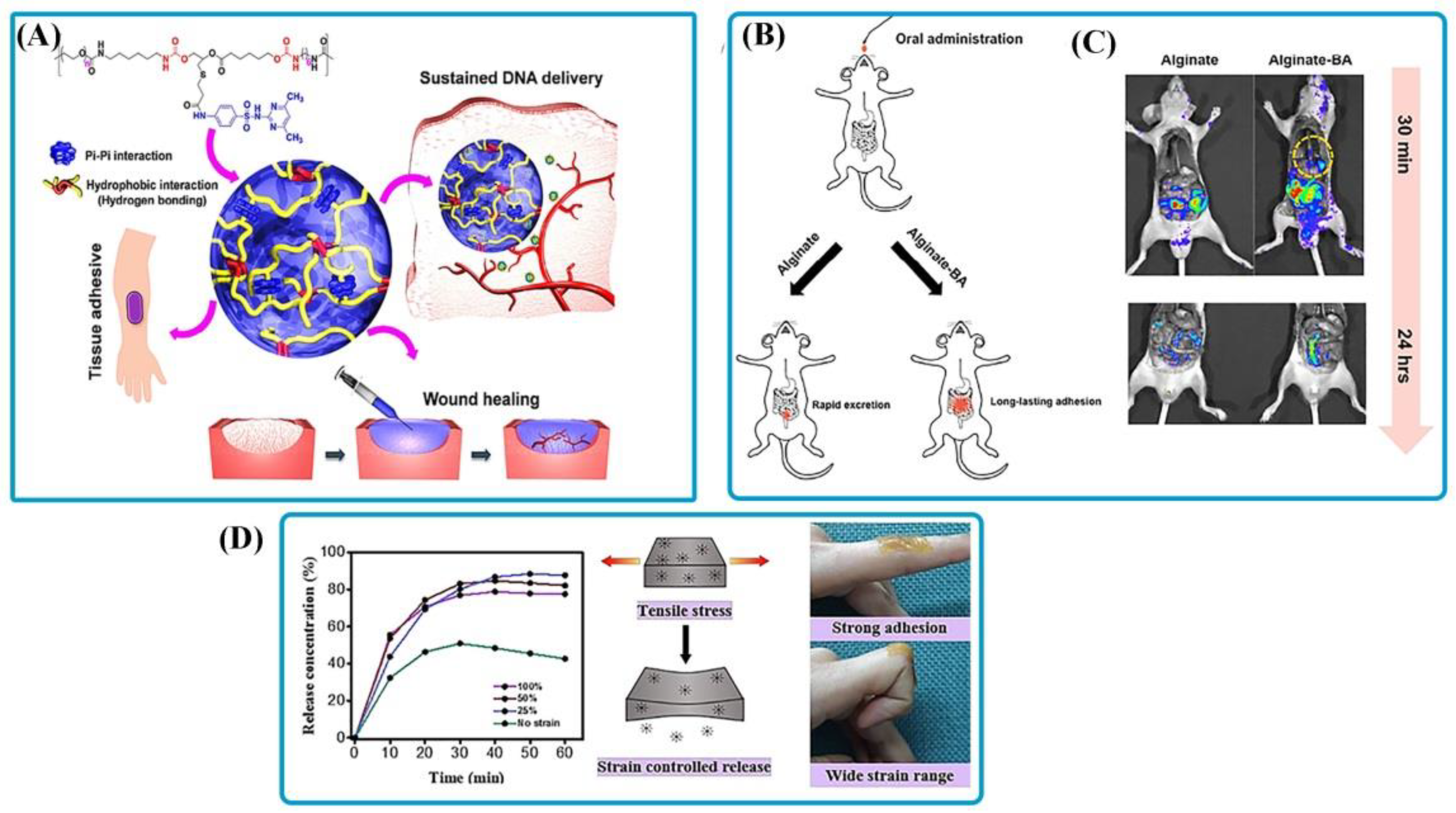
| Compounds | Stimulus-Response Agents | Application | Summary | Role of Stimuli | Ref. |
|---|---|---|---|---|---|
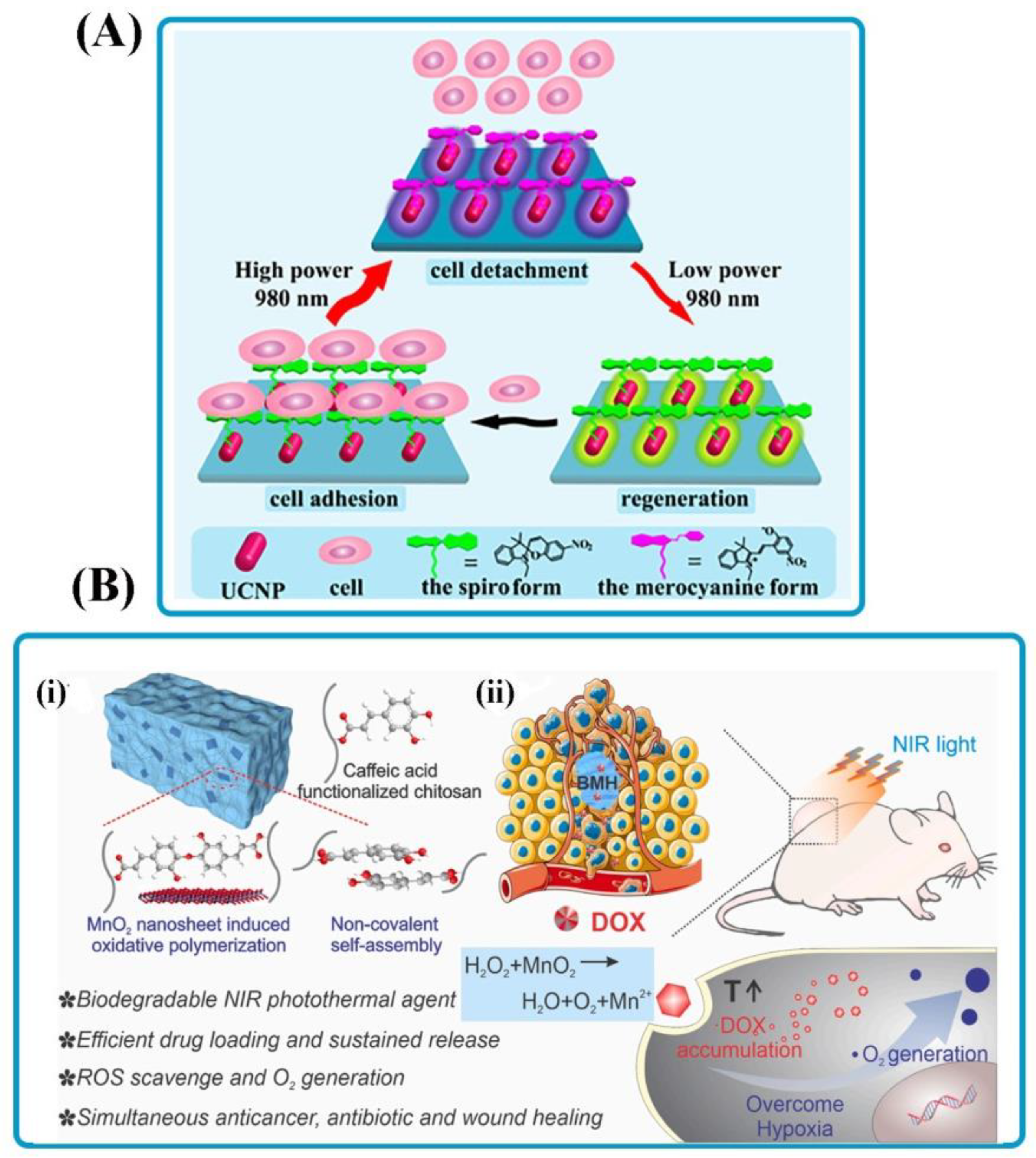
| Spiropyran, multishellupconversion nanoparticles | Multishell Upconversion nanoparticles | – | The interactions between spiropyran and cell surface protein fibronectin were switchable even after 10 cycles. | By simply decreasing/increasing the excitation power density of the same 980 nm laser, cell adhesion/detachment can be switched quickly. | [51] |
| Catechol functionalized chitosan | MnO2 nanosheets | – | BMH hydrogel successfully eliminated cancer cells in vitro giant solid tumors in vivo and had effective antibacterial properties without antibiotics. | By NIR irradiation, BMH hydrogel reduced the hypoxic tumor microenvironment by degrading internal hydrogen peroxide into oxygen and simultaneously releasing the anticancer doxorubicin hydrochloride. | [53] |
| Chitosan–polyvinyl alcohol-loaded tannic acid-TiO2 | – | Artificial electronic skin | Irradiation causes a change in surface wettability from hydrophobic to hydrophilic, leading to increases in electrical characteristics, mechanical strength, and adhesive properties. | Controllable swelling ratio upon irradiation with UV and visible light. | [54] |
| Thiol–PEG/ maleimide | Upconverting nanoparticles | Tissue engineering | Preparing light-sensitive adhesive hydrogels with spatiotemporally regulated biological functions for cell culture without causing significant photodamage to the cells | Photochemical processes are activated by converting NIR light (974 nm) into local UV emission. | [55] |
| PNIPAM/graphene oxide (GO) | Graphene (808 nm) | Cell capture | The bioadhesives efficiently captured cells via the adhesive oligopeptide and released a NIR light stimulus, suitable for cell preservation and therapeutic cell delivery. | NIR light efficiently triggered cell release; continuous NIR irradiation efficiently released the cells from adhesive hydrogel. | [56] |
| Dodecyl, chitosan | WS2 nanosheets | Wound healing | Bioadhesive hydrogels with a positive charge, macropores, and alkyl chains could catch and limit microorganisms. | WS2 nanosheets produced heat when exposed to NIR, and the antibiotic was triggered to release at the wound site. | [57] |
| PDA and PNIPAM | PDA | Wound healing | The coating of PDA–NPs onto hydrogel surfaces was effective in cell affinity, tissue adhesiveness, and growth factor/protein immobilization ability. | Pulsatile release of drugs and quick healing (1 min) after unfavorable damage with the assistance of NIR laserirradiation. | [58] |
| Compounds | Stimulus-Response Agents | Application | Summary | Role of Stimuli | Ref. |
|---|---|---|---|---|---|
| Pluronic® 127 hydroxypropyl methylcellulose (HPMC) | Pluronic® F127 | Wound infections | Ex vivo and in vivo studies showed bioadhesives with suitable antibacterial therapy of burn wound infections and anti-inflammatory activities. HPMC adhesive increased gel and bioadhesive strength | Formation of a stiff gel by increasing temperature from 4 to 32–37 °C. | [65] |
| Poly(acrylic acid)(PAA)/PNIPAM-co-dopamine methacrylamide (PDA) | PNIPAM | Epidermal sensors | The hydrogel with adhesive strength and self-healing ability demonstrated unusual fatigue and crack resistance properties. | Temperature-sensitive hydrogels, the lowest adhesion strength of hydrogel was at 25 °C. | [66] |
| Gelatin and chondroitin sulfate | Chondroitin sulfate | Surgical adhesive for sealing | In vivo and ex vivo, the injectable self-healing bioadhesive is used as a multifunctional tissue adhesive/sealant for closing bleeding wounds. | Exceptional tissue adherence at 37 °C diminished at low temperatures (20 °C), allowing it to detach from tissue easily. | [67] |
| Polydopamine-coated Tetronics–tyramine | Tetronic, tyramine (37 to 4 °C) | Tissue engineering | Adhesive hydrogels promoted human dermal fibroblast attachment, controlled by serum protein adsorption, creating a cell sheet after growth. | Cell sheet translocation process by changing temperature from 37 °C to 4 °C. | [68] |
| Hyaluronic acid (HA), methylcellulose, polyethylene glycol (PEG) | Methylcellulose | Surgical adhesive for sealing | Free-flowing, injectable at ambient temperature, gelation point about 40 ± 2 s, and lack of cellular toxicity | The transition of bioadhesive from sol at four °C to gel state at 37 °C. | [69] |
| Catechol modified quaternized chitosan, poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide) (PLEL) | PLEL | Wound healing | The injectable thermo-sensitive adhesive hydrogel offered excellent properties as a wound dressing for promoting wound healing (only in 7 days), biocompatibility, and bioactivity through in vivo degradation, stimulated endothelial cells migration, and angiogenesis. | The temperature-triggered reversible sol (25 °C)–gel (37 °C) transition of PLEL solution. | [70] |
| Galactose modified xyloglucan (mXG) and hydroxybutyl chitosan | Galactose modified xyloglucan | Wound healing | According to in vivo findings, bioadhesive was an excellent anti-adhesion system for avoiding repeated adhesion following adhesiolysis, promoting wound healing and reducing scar formation. | Gelation temperature and time depended on the total solid content of bioadhesive hydrogels. | [71] |
| PIPAAm, butyl methacrylate (BMA) | PNIPAAm | Regenerative medicine and tissue engineering | Increasing BMA concentration improved the cell adhesion, owing to increased cellular protein adsorption. | Celladhesion and detachment from hydrophobized thermos-responsive brushes. | [72] |
| PNIPAAm-g-chitosan | PNIPAAm | Tissue engineering | Hydrogels showed outstanding biocompatibility to MSCs, fibroblasts, and osteoblasts, allowing cell encapsulation without toxicity. | LCST at around 30.71–32.02 °C indicated hydrogels had potential for in situ injection. | [73] |
| Pluronics, hyaluronic acid, corn silk extract, and nanosilver | Pluronics | Wound healing | From a biological point of view, hydrogels had good biocompatibility and exhibited antibacterial activity toward gram-positive and gram-negative bacteria. | Viscoelastic parameters changed in the temperature ranging from 25 to 40 °C. | [74] |
| Collagen, chitosan, and bioactive glass | Chitosan | Bone tissue engineering | The addition of collagen to the system resulted in larger pore size and enough interconnectivity, making it suitable for use as biomaterials for bone tissue engineering. | Gelation temperature at 37 °C. | [75] |
| Compounds | Stimulus-Response Agents | Application | Summary | Role of Stimuli | Ref. |
|---|---|---|---|---|---|
| PAA/Zinc (II) ion | PAA and dopamine | - | Coacervate bioadhesive with good mechanical and self-healing properties. | Oxidation of catechol groups at basic pH favored the formation of strong adhesion. | [95] |
| Carbon nanotubes and GO/tectomers | Tectomer | Tissue engineering | The hybrid materials can be used as pH-switchable bioadhesive coatings and scaffolds for tumor models in ex vivo studying. | Controlled release from a pH-dependent peptidic coating. | [96] |
| Chitosan-grafted-dihydrocaffeicacid/oxidized pullulan | Chitosan-g-dihydrocaffeic acid | Drug delivery | Good injectability, a decent gelation duration, and pH-dependent equilibrated swelling ratios, morphologies, and rheological properties were observed by bioadhesive hydrogels. | At acidic conditions, the hydrogels had a larger swelling ratio and pore size than at pH 7.4. | [97] |
| D-α-tocopheryl PEG 1000 succinate conjugated chitosan. | Chitosan | Drug delivery | Invivo pharmacokinetic results demonstrated the relative bioavailability of bioadhesive micelles was effective beneficial for brain cancer therapies with the prolonged release. | A pH decrease triggered the drug release. | [98] |
| Dopamine-conjugated HA/mesoporous silica | Dopamine | Drug Delivery | In vivo studies confirmed the injection of bioadhesives could achieve high therapeutic efficiency against tumor growth while avoiding significant damage to healthy organs. | The faster release rate of the drug at pH 5.0 than at pH 7.4. | [99] |
| Collagen and PEG | Collagen | Diabetic wound repair | Bioadhesive loaded stem cell factor as an anti-inflammatory and biocompatibility dressing was used for tissue regeneration. | Effective in drug release rate. | [100] |
| Chitosan and pectin | Chitosan | Drug delivery and tissue regeneration | Based on ex vivo testing, membranes loaded with antimicrobial peptides had simultaneous antibacterial effectiveness against oral streptococci as well as cytocompatibility with both soft and hard tissue. | Temporary preventive and therapeutic distribution in the oral cavity with a ‘supply on demand’ release behavior in a pH-controlled manner | [101] |
| PAA and PAAm | PAA and PAAm | Drug delivery | In vitro findings showed dual pH-responsive bioadhesive hydrogel can release lipophilic or hydrophilic pharmaceuticals based on the pH of the environment while preventing drug metabolism, degradation, and excretion. | In alkaline or acid conditions, the bioadhesive can conduct programmable and bidirectional bending by shrinking anionic and cationic networks and asymmetric swelling. | [102] |
| PAA | PAA | Sensor | Bacterial detachment is caused by increasing brush thickness, disparity, and solution pH. | Tuning the attachment and detachment of bacteria in various pH values. | [103] |
| Compounds | Stimulus-Response Agents | Application | Summary | Role of Stimuli | Ref. |
|---|---|---|---|---|---|
| Poly [anilineTetramermethacrylamide]-co-[dopamine methacrylamide]-co-[poly(ethylene glycol) methyl ether methacrylate]} | - | Bone tissue engineering | A conductive bioadhesive with biocompatibility and strong adhesion was prepared for regeneration of comminuted bone fracture; the adhesive strength of hydrogel was less than that of the cortical bone and showed in in vivo cytotoxicity. | Electrical conductivity of bioadhesive enhanced with the increase of AT, which improved cellular activities. | [112] |
| AA and PEG dimethacrylate/GO/gelatin | Graphene oxide | Wound healing | Adhesive hydrogel with good thermal and mechanical stability indicated viability of more than 94% for human fibroblasts, while curcumin-loaded samples showed a reduction of bacteria of 90%. | At 0 and V, the slow and fast release was achieved, while intermediate kinetics was found at 12 and V. | [113] |
| Xanthan gum, chitosan, and iron oxide magnetic | - | Muscle, skin, cartilage, and connective tissue engineering | In vitro studies showed that bioadhesive hydrogels improved fibroblasts’ growth and adherence in an external magnetic field compared to the pristine hydrogel. | In a magnetic field, adhesion and proliferation of fibroblasts were enhanced in hydrogels containing magnetic nanoparticles. | [114] |
| PAA grafted gum ghatti (GGH) | Gum ghatti | Drugs delivery by the skin | A histopathology examination demonstrated reversible changes in skin structure. | The release was observed over a two-fold increase in the drug after applying an electric stimulus. | [115] |
| Nanoclay (laponite), multiwalled carbon nanotubes (CNTs), and NIPAM | CNTs | Human motion sensing | Multifunctional conductive flexible hydrogels with self-healing, sticky, and 3D printable properties without any toxicity for the L929 cells. | Conductive bioadhesive hydrogels for wearable electronic devices revealed good electrical stability and multifunctional stretchability. | [116] |
| Chitosan-aniline oligomer/polyvinyl alcohol | Polyaniline | Tissue engineering | Biocompatibility testing demonstrated the conductive substrate offered the platform with more cellular activity than non-conductive materials. | Rising in drug release after electrical stimulation in comparison with non-stimulated webs. | [117] |
| GO-PAA | Graphene oxide | Artificial muscle and tissue engineering scaffold | Bioadhesive hydrogel showed good compatibility with bone marrow-derived mesenchymal stem cells. | Under the circumstance of electrical stimulation, the morphology of adherent cells was changed, and the differentiation of neural stem cells was promoted. | [118] |
| Compounds | Stimulus-Response Agents | Application | Summary | Role of Stimuli | Ref. |
|---|---|---|---|---|---|
| Thioglycolic acid, chitosan, gold nanoparticle | Thioglycolic acid | - | Ultra-low concentrations of thrombin, as well as low molecular weight anatoxin, are detected selectively and reproducibly. | Detect early biomarkers in complex body fluid. | [122] |
| Phenylboronic acid and cis -diol modified PEG | Modified PEG | Drug delivery | The injectable, self-healing and adhesive hydrogel could have applications in 3D cell culture substrates for tissue engineering and controlled macromolecule release. | Size-dependent controlled release of proteins encapsulated within the network and the glucose-responsive release of larger proteins. | [123] |
| Hyaluronic acid cross-linked with divinyl sulfone. | Hyaluronic acid | Diabetic patients | The released insulin from glucose-responsive nanocarriers displayed a practical hypoglycemic effect for a longer time after oral administration to diabetic rats than insulin-loaded nanocarriers. | Regulation of insulin. | [124] |
| 2-nitroimidazole–l-cysteine–alginate | 2-nitroimidazole | Diabetic patients | Invivo experiments on type I diabetic rats showed that the hyperglycemia risk was reduced following oral administration, and a standard glucose range was maintained for a long time. | Blood glucose regulation via glucose catalysis by glucose-responsive adhesives. | [120] |
| Compounds | Stimulus/Stimulus-Response Agents | Application | Summary | Role of Stimuli | Ref. |
|---|---|---|---|---|---|
| PEG, PSMEU | pH and thermal/PSMEU | Wound healing | Bioadhesive hydrogels were used in vivo to seal cutaneous wounds, absorb wound exudates, and promote tissue regeneration in the injured area. | Free-flowing PEG–PSMEU copolymer sols (pH 8.5, 23 °C) were converted into stable gels in the body (pH 7.4, 37 °C). | [128] |
| Alginate–boronic acid conjugate | pH- and glucose/boronic acid-diol complexation | Drug delivery systems | Alginate-BA hydrogels showed great promise in various applications, including pressure-sensitive biological glues to biomedical substrates requiring stretchability, self-healing, and multiresponsiveness. | Effect on the viscoelastic and mechanical properties of bioadhesive hydrogels. | [129] |
| Dopamine functionalized 4-armed PEG (4-arm-PEG-DA) and phenylboronic acid | pH, glucose, and dopamine triple-responsive/Dopamine and modified PEG | Drug delivery, Tissue engineering | Bioadhesive showed good adherence to tissues, and in vitro cytotoxicity experiments showed hydrogels were very cytocompatible. | The disintegration rate of hydrogel increased by decreasing pH value from 9 to 3. | [132] |
| PNIPAM/PDA/clay | Light-and thermos/PDA, PNIPAM | Electronic skin | In vitro cytotoxicity results indicated that hydrogel with high adhesiveness and biocompatibility suggested good cell affinity and biocompatibility. | Locally controllable deformation of the hydrogel by remote NIR irradiation. | [133] |
| Thiolated chitosan and thiolated chondroitin sulfate | pH and redox/Amino groups, carboxyl and sulfate groups | Wound healing and tissue engineering | Multilayer systems with disulfide bonds aided tuning cell contact, film degradation, and controlled release of bioactive compounds. | Cross-linking in alkaline pH or reduction of disulfide bonds changed mechanical and surface properties and cell function. | [134] |
| Collagen (COL), guar gum (GG), PNIPAM, GO | Light and thermal/PNIPAM and GO | Wound healing, wearable electronic devices, and sensors. | A bioadhesive hydrogel with many functions was synthesized, including quick wound healing, super-ductility, injectability, remoldability, conductive, thermo-sensitive, NIR-responsive, and accelerated wound healing. | Phase change occurs shortly after touches the human body. | [135] |
| PAA, oligo(ethylene glycol) methacrylate, 2-(2-methoxyethoxy) ethyl methacrylate, chitosan | pH and thermal/PAA (pH-sensitive) and oligo(ethylene glycol) methacrylate and 2-(2-methoxyethoxy) ethyl methacrylate (Thermal sensitive) | Drug delivery | In vitro cytotoxicity studies confirmed that hydrogels had excellent cell compatibility, with 5-Fu-loaded hydrogels having a lower cell growth inhibition efficiency for normal LO2 cells but a higher cell growth inhibition efficiency for cancer HepG2 cells than pure 5-Fu at the same drug concentration. | The value of medication released was low in an acidic environment (pH 1.2) but high in a neutral environment. | [136] |
| Poly (1-butyl-3-vinylimidazolium bis(trifluoromethanesulfonyl)imide) ([PBVIm] [TFSI]) | Strain and electric | Utilized in clothing to monitor various body movements | Membranes possessed washable, comfortable, good mechanical properties and satisfactory moisture proof sensing performance. | - | [137] |
| 1-vinyl-3-butylimidazolium bromide ([VBIM+] Br-) ionic liquid, vinyl-modified lignin (v-lignin), acrylamide (AM), borax, ammonium persulfate | Strain and thermoresponsive | Electronic skin, human–machine interface, and remote medical healthcare | Hydrogel showed high stretchability, excellent toughness, and impressive stress loading-unloading cyclic stability. | Motion capture and gesture identification by the hydrogel strain sensor. | [138] |
| Lignin/poly(ionic liquids)/ 3-butyl-1-isopropyl-1H-imidazol-3-ium bromide/1-vinylimidazole and bromobutane | pH and temperature responsive | Drug delivery | The hybrid hydrogel was more successful at killing malignant cells in an invitro cytotoxicity and drug release testing. | Drug release occurred at intracellular acidic pH. | [139] |
| 1-vinyl-3-butylimidazolium tetrafluoroborate/1-butyl-3-methylimidazolium tetrafluoroborate | Strain and light | Reusable wearable electronics | Ionogel integrated excellent mechanical properties, ultra-strong adhesive, self- healing ability, and recyclability. | Detection of physical motion and physiological signals of human body. | [140] |
| Cetylpyridinium salicylate/cetpylpyridinium chloride | pH and temperature | Drug delivery | The preparation of hybrid pharmaceutical ionogels through encapsulation of the chemotherapeutic drug imatinib mesylate within the ionogel matrix. | The maximum release drug was conducted at an acidic pH at 37 °C. | [141] |
| Dual-cross-linked ionohydrogel | Temperature and strain | Wearable ionotronic devices | The bioadhesives possessed excellent mechanical properties, transparency, high ionic conductivity, and robust adhesion, along with the advantages of superior antifreezing and long-term antidehydration properties. | [142] | |
| 1-methyl-3-(oxiran-2-ylmethyl)-1H-imidazol-3-ium chloride/methoxy polyethylenglycol-aldehyde/chitosan | Magnetic, pH responsive | Drug delivery | The findings of the cytotoxicity assay demonstrated that medications loaded nanocarriers have a higher cytotoxicity effect than free drugs. | pH-responsive branched nanocarrier for co-delivery of DOX and MTX. | [143] |
| Compounds | Stimuli | Application | Summary | Ref |
|---|---|---|---|---|
| Poly(glycerol sebacate)-co-poly(ethylene glycol)-g-catechol | Photothermal | Wound closure | Bioadhesives perform superior wound closure and healing of skin incisions than medical glue and surgical suture, with good hemostasis and a high killing ratio of bacteria. | [157] |
| Ferric ion, protocatechualdehyde containing catechol and aldehyde groups and quaternized chitosan | NIR responsiveness | Wound closure | Bioadhesives presents good biocompatibility, hemostasis, antibacterial activity, injectability, and multifunctional adhesiveness. | [158] |
| Hyaluronic acid-graft-dopamine andreduced graphene oxide | NIR responsiveness | Drug delivery | Bioadhesive hemostatic antioxidative conductive hydrogels with sustained drug release properties are an ideal wound dressing for promoting full-thickness skin regeneration. | [159] |
| Poly(N-isopropylacrylamide) terminated with catechols/ polypyrrole nanoparticles | pH, temperature, and NIR light–responsive | Drug delivery | Bioadhesive with multi-responsive behavior, especially NIR light response, can be profitable in removable sealant materials and remotely controlled release systems. | [160] |
| Graphene aerogel/poly(N-isopropylacrylamide)hydrogel/polydopamine nanoparticles | Thermo- and NIR responsiveness | Drug delivery | Correlation between the drug release and the resistance allowed the drug-release behavior of the bioadhesive hydrogels to be monitored using electrical signals | [161] |
| Alginate/PNIPAm | Thermoresponsive | Sealing leakage and wound healing | Inspired by embryonic wound contraction, bioadhesive can support skin wound healing with stretchability, toughness, tissue adhesion, and antimicrobial function. | [162] |
| Gelatin Methacryloyl (GelMA)/PhenylIsothiocyanate-Modified Gelatin | Light-responsive | Hemostasis | The produced bioadhesive with injectability and immediate hemostatic effect can be used as a fast cross-linkable hemostatic agent for irregular wounds in oral/dental surgical procedures. | [163] |
| Hemocoagulase/GelMA | Visible light-responsive | Hemostasis | The bioadhesives resulted in fast hemostasis and tissue sealing through the activation and aggregation of platelets as well as the effective transformation of fibrinogen into fibrin. | [164] |
| GO/poly(vinylalcohol)/PAA grafted with N-hydroxysuccinimide ester | Electro-responsive | Bioelectronic | The obtained bioadhesive with biocompatibility, applicability, mechanical and electrical stability, and recording and stimulation functionalities can be used to improve tissue–device integration and enhance the performance of biointegrated electronic devices. | [155] |
| Gelatin/PAAm/Clay hydrogel | Salt ions, pH, and stress | BioSensor | A capacitive pressure sensor with ability of high conductivity, high self-healing efficiency, and robust adhesion has been designed for monitoring human motions. | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadem, E.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Das, O.; Berto, F. Cutting-Edge Progress in Stimuli-Responsive Bioadhesives: From Synthesis to Clinical Applications. Polymers 2022, 14, 1709. https://doi.org/10.3390/polym14091709
Khadem E, Kharaziha M, Bakhsheshi-Rad HR, Das O, Berto F. Cutting-Edge Progress in Stimuli-Responsive Bioadhesives: From Synthesis to Clinical Applications. Polymers. 2022; 14(9):1709. https://doi.org/10.3390/polym14091709
Chicago/Turabian StyleKhadem, Elham, Mahshid Kharaziha, Hamid Reza Bakhsheshi-Rad, Oisik Das, and Filippo Berto. 2022. "Cutting-Edge Progress in Stimuli-Responsive Bioadhesives: From Synthesis to Clinical Applications" Polymers 14, no. 9: 1709. https://doi.org/10.3390/polym14091709
APA StyleKhadem, E., Kharaziha, M., Bakhsheshi-Rad, H. R., Das, O., & Berto, F. (2022). Cutting-Edge Progress in Stimuli-Responsive Bioadhesives: From Synthesis to Clinical Applications. Polymers, 14(9), 1709. https://doi.org/10.3390/polym14091709







