Effect of Compatibilizer on the Persistent Luminescence of Polypropylene/Strontium Aluminate Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Composites
2.2.2. Characterization of Composites
Phosphorescence and Decay Measurements
Scanning Electron Microscope (SEM)
Fourier Transform Infrared Spectroscopy (FTIR)
Differential Scanning Calorimetry (DSC)
Thermo-Gravimetric Analysis (TGA)
Tensile Testing
3. Results
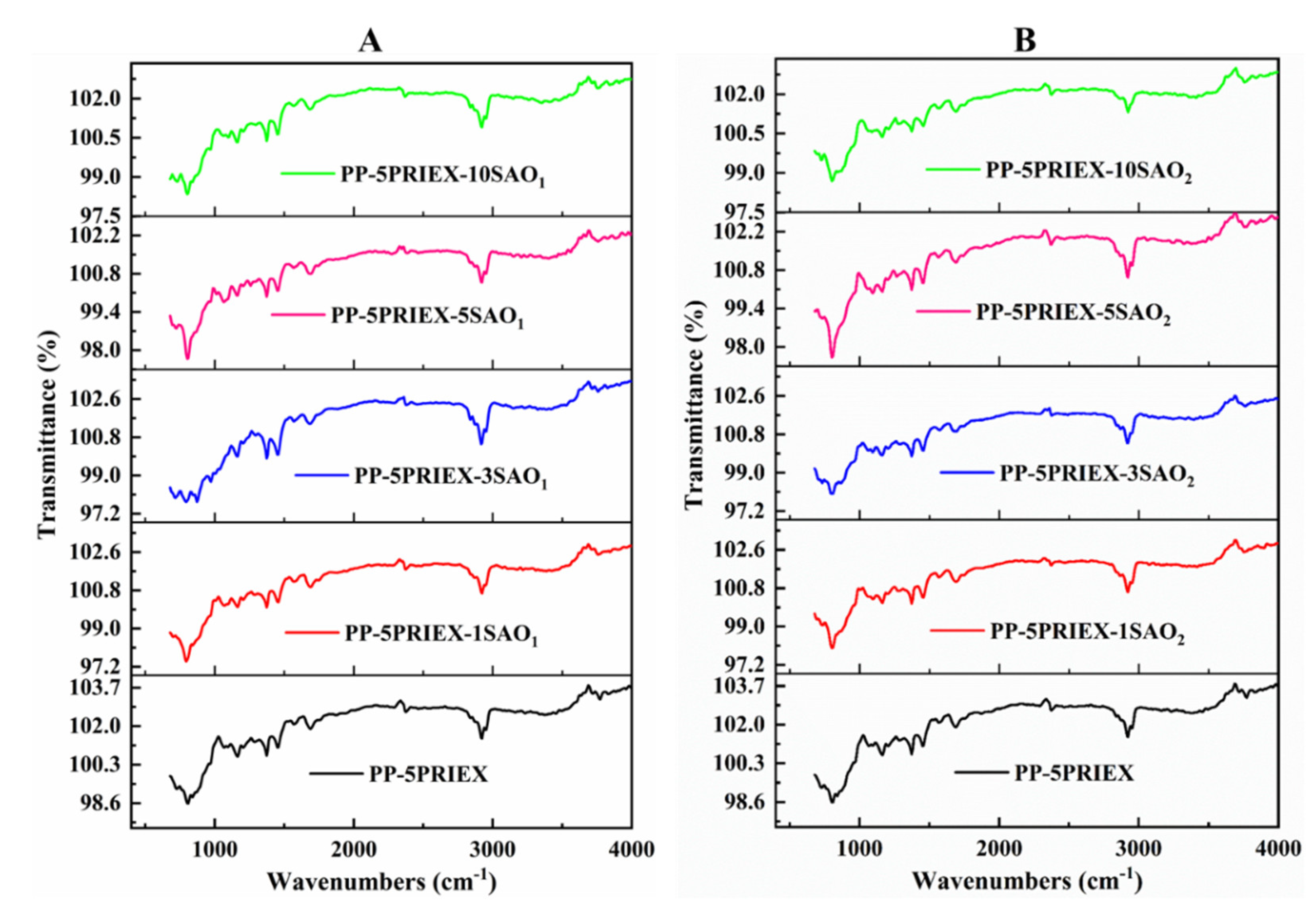
3.1. FTIR, DSC, and TGA Studies in PP/PRIEX/SAO1 and PP/PRIEX/SAO2 Composites
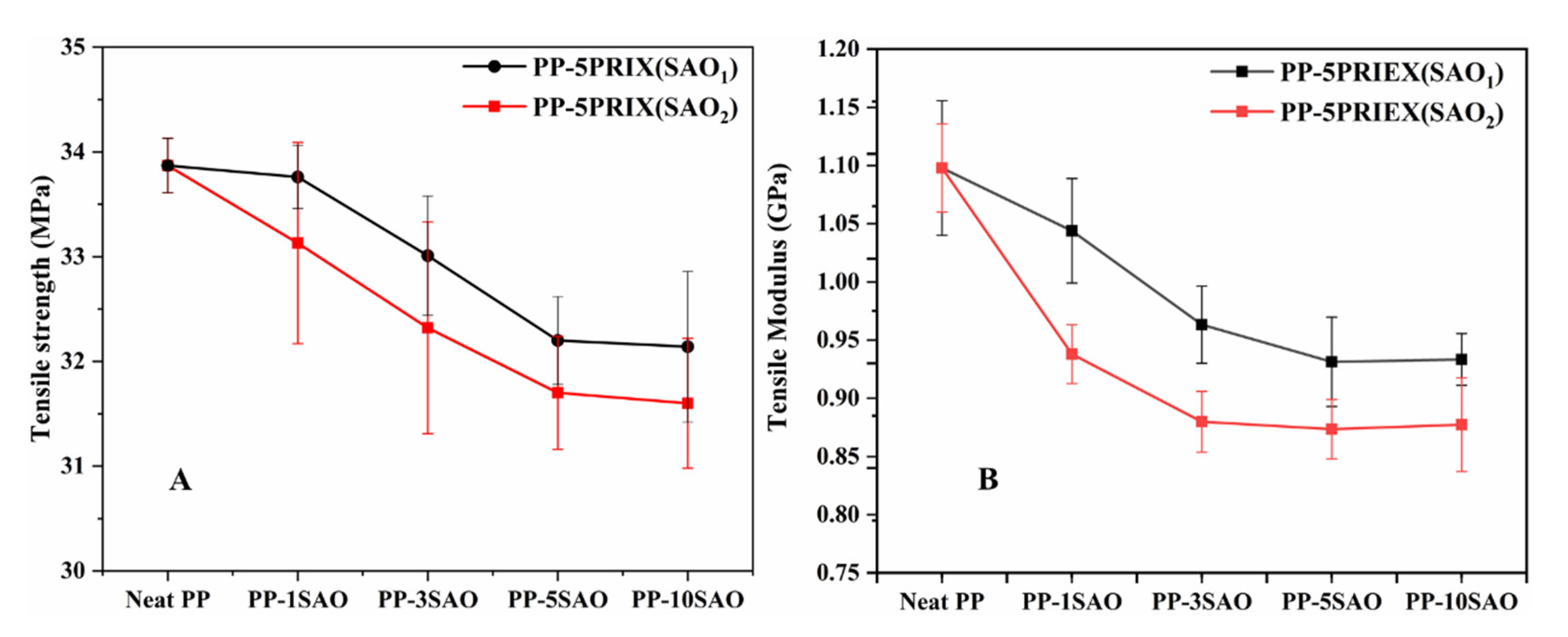
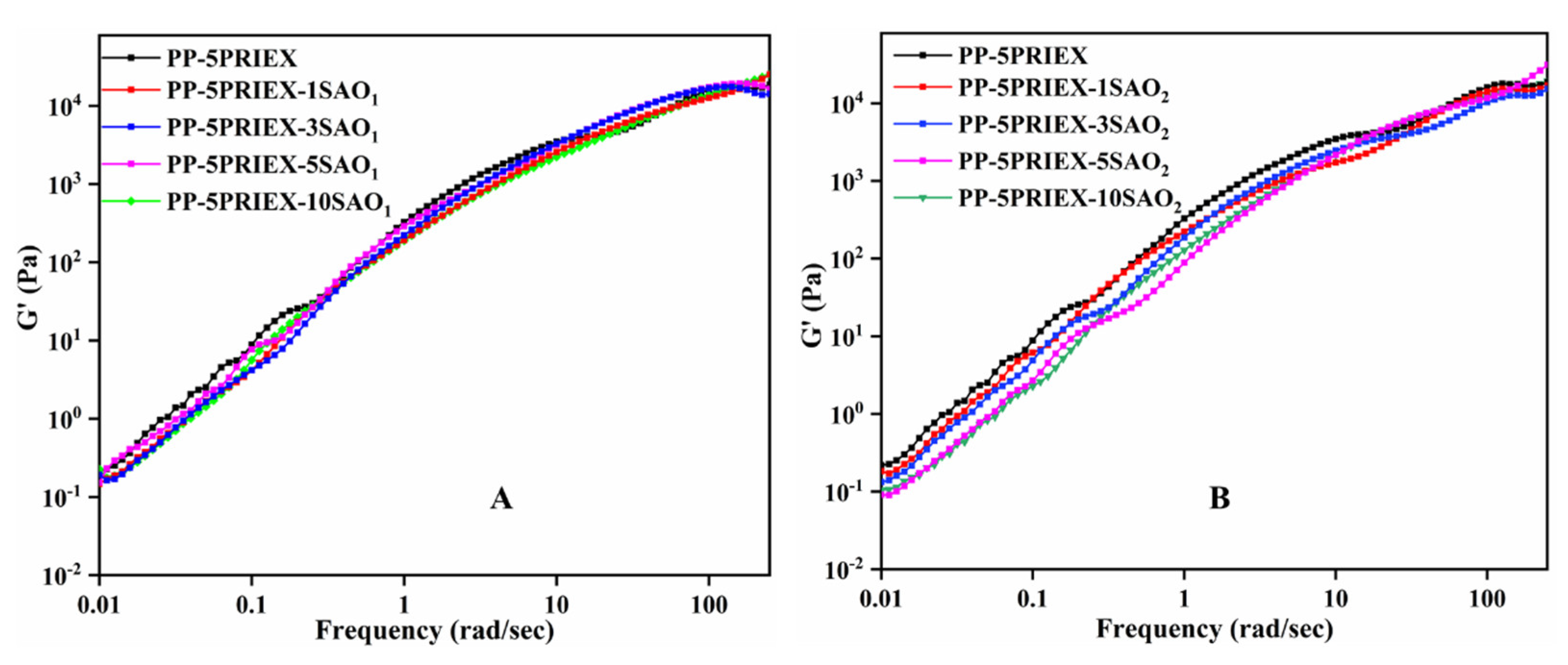
3.2. Tensile Strength, Tensile Modulus and Storage Modulus of PP/PRIEX/SAO1 and PP/PRIEX/SAO2 Composites
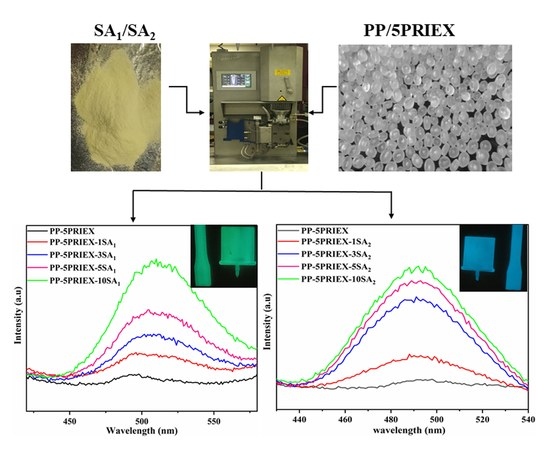
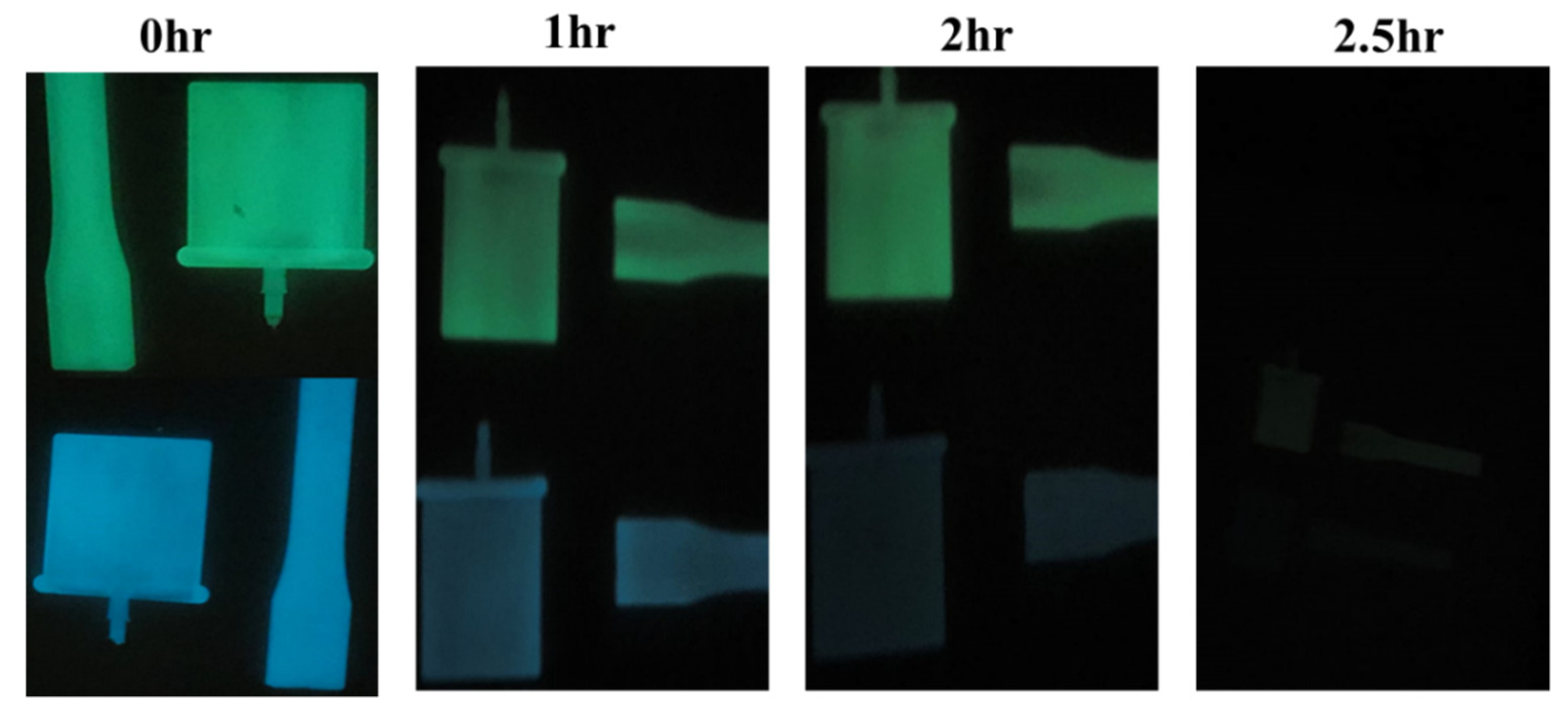
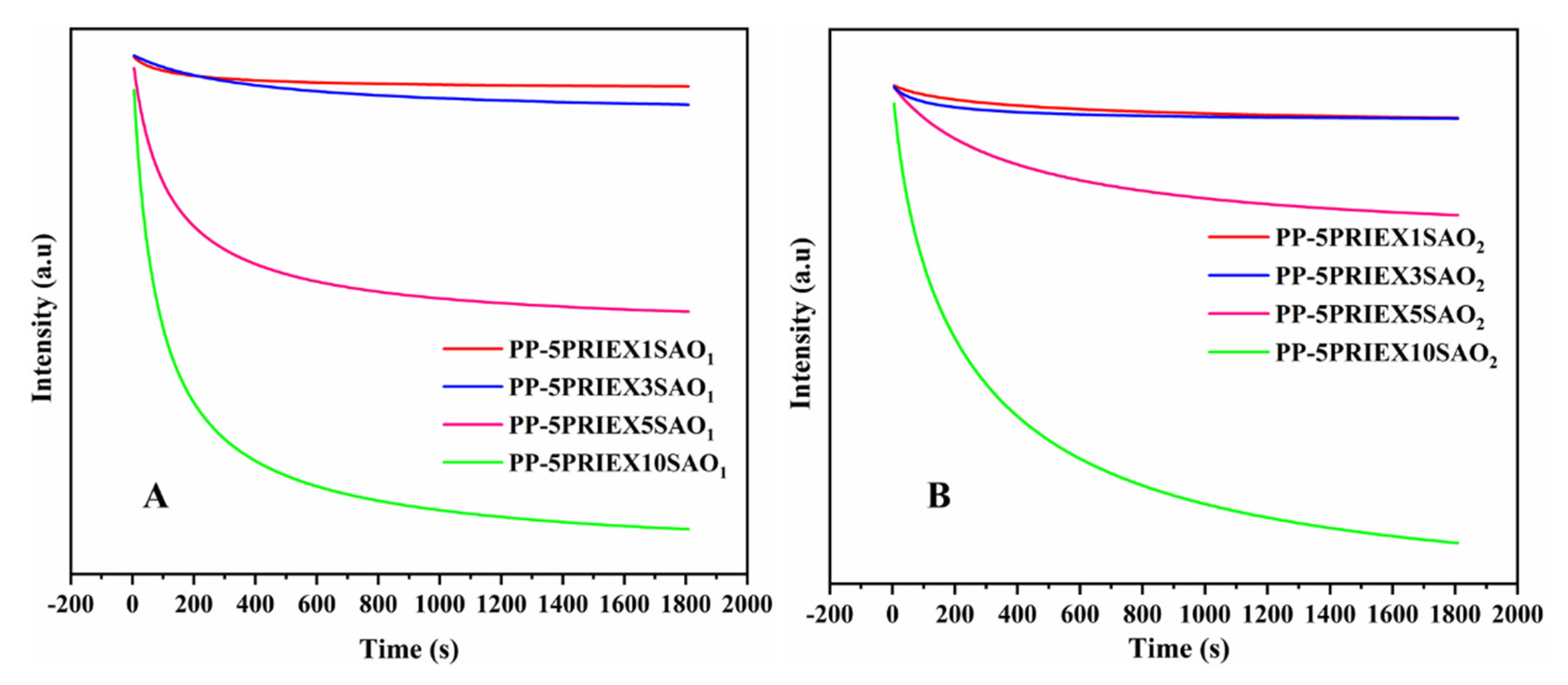
3.3. Phosphorescence Emission and Their Decay Studies in PP/PRIEX/SAO1 and PP/PRIEX/SAO2 Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, B.; Shi, M.; Pang, Z.; Zhu, Y.; Li, Y. Study on the optical performance of red-emitting phosphor: SrAl2O4: Eu2+, Dy3+/Sr2MgSi2O7: Eu2+, Dy3+/light conversion agent for long-lasting luminous fibers. J. Mater. Sci. Mater. Electron. 2021, 32, 17382–17394. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, C.-Y.; Lu, C.-H. Preparation and photoluminescence properties of Sr4Al14O25:Eu2+ phosphors synthesized via the microemulsion route. J. Mater. Sci. 2013, 24, 1458–1462. [Google Scholar] [CrossRef]
- Yeşilay Kaya, S.; Karacaoglu, E.; Karasu, B. Effect of Al/Sr ratio on the luminescence properties of SrAl2O4:Eu2+,Dy3+ phosphors. Ceram. Int. 2012, 38, 3701–3706. [Google Scholar] [CrossRef]
- Xue, Z.; Deng, S.; Liu, Y. Synthesis and luminescence properties of SrAl2O4:Eu2+,Dy3+ nanosheets. Phys. B Condens. Matter 2012, 407, 3808–3812. [Google Scholar] [CrossRef]
- Dutczak, D.; Milbrat, A.; Katelnikovas, A.; Meijerink, A.; Ronda, C.; Jüstel, T. Yellow persistent luminescence of Sr2SiO4:Eu2+,Dy3+. J. Lumin. 2012, 132, 2398–2403. [Google Scholar] [CrossRef]
- Katsumata, T.; Nabae, T.; Sasajima, K.; Komuro, S.; Morikawa, T. Effects of Composition on the Long Phosphorescent SrAl2O4: Eu2 +, Dy3+ Phosphor Crystals. J. Electrochem. Soc. 1997, 144, L243–L245. [Google Scholar] [CrossRef]
- Nance, J.; Sparks, T.D. Comparison of coatings for SrAl2O4:Eu2+,Dy3+ powder in waterborne road striping paint under wet conditions. Prog. Org. Coat. 2020, 144, 105637. [Google Scholar] [CrossRef]
- Nance, J.; Sparks, T.D. From streetlights to phosphors: A review on the visibility of roadway markings. Prog. Org. Coat. 2020, 148, 105749. [Google Scholar] [CrossRef]
- Tan, H.; Wang, T.; Shao, Y.; Yu, C.; Hu, L. Crucial Breakthrough of Functional Persistent Luminescence Materials for Biomedical and Information Technological Applications. Front. Chem 2019, 7, 387. [Google Scholar] [CrossRef]
- Soni, A.K.; Singh, B.P. Luminescent Materials in Lighting, Display, Solar Cell, Sensing, and Biomedical Applications. In Luminescence-OLED Technology and Applications; Pyshkin, S., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Mokhtar, O.M.; Attia, Y.A.; Wassel, A.R.; Khattab, T.A. Production of photochromic nanocomposite film via spray-coating of rare-earth strontium aluminate for anti-counterfeit applications. Luminescence 2021, 36, 1933–1944. [Google Scholar] [CrossRef]
- Liu, J.; Lécuyer, T.; Seguin, J.; Mignet, N.; Scherman, D.; Viana, B.; Richard, C. Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv. Drug Deliv. Rev. 2019, 138, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4: Eu2 +, Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Zeng, W.; Han, S.; Li, G.; Guo, H.; Li, Y.; Qiang, Q. Long persistent composite phosphor CaAl2O4:Eu2+,Nd3+/Y3Al5O12:Ce3+: A novel strategy to tune the colors of persistent luminescence. New J. Chem. 2016, 40, 485–491. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y. The synthesis and afterglow luminescence properties of a novel red afterglow phosphor: ZrO2:Sm3+,Sn4+. J. Lumin. 2012, 132, 2842–2846. [Google Scholar] [CrossRef]
- Zheng, R.; Xu, L.; Qin, W.; Chen, J.; Dong, B.; Zhang, L.; Song, H. Electrospinning preparation and photoluminescence properties of SrAl2O4:Ce3+ nanowires. J. Mater. Sci. 2011, 46, 7517–7524. [Google Scholar] [CrossRef]
- Lephoto, M.A.; Ntwaeaborwa, O.M.; Pitale, S.S.; Swart, H.C.; Botha, J.R.; Mothudi, B.M. Synthesis and characterization of BaAl2O4:Eu2+ co-doped with different rare earth ions. Phys. B Condens. Matter 2012, 407, 1603–1606. [Google Scholar] [CrossRef]
- Poulose, A.M.; Shaikh, H.; Anis, A.; Alhamidi, A.; Kumar, N.S.; Elnour, A.Y.; Al-Zahrani, S.M. Long Persistent Luminescent HDPE Composites with Strontium Aluminate and Their Phosphorescence, Thermal, Mechanical, and Rheological Characteristics. Materials 2022, 15, 1142. [Google Scholar] [CrossRef]
- Cheng, L.-X.; Liu, T.; Li, L.; Yang, L.; He, H.-W.; Zhang, J.-C. Self-repairing inorganic phosphors/polymer composite film for restructuring luminescent patterns. Mater. Res. Express 2021, 8, 065302. [Google Scholar] [CrossRef]
- Tian, S.; Wen, J.; Fan, H.; Chen, Y.; Yan, J.; Zhang, P. Sunlight-activated long persistent luminescent polyurethane incorporated with amino-functionalized SrAl2O4:Eu2+,Dy3+phosphor. Polym. Int. 2016, 65, 1238–1244. [Google Scholar] [CrossRef]
- Bem, D.B.; Swart, H.C.; Luyt, A.S.; Coetzee, E.; Dejene, F.B. Properties of green SrAl2O4 phosphor in LDPE and PMMA polymers. J. Appl. Polym. Sci. 2010, 117, 2635–2640. [Google Scholar] [CrossRef]
- Wan, M.; Jiang, X.; Nie, J.; Cao, Q.; Zheng, W.; Dong, X.; Fan, Z.H.; Zhou, W. Phosphor powders-incorporated polylactic acid polymeric composite used as 3D printing filaments with green luminescence properties. J. Appl. Polym. Sci. 2019, 137, 48644. [Google Scholar] [CrossRef]
- Oguzlar, S.; Ongun, M.Z.; Keskin, O.Y.; Delice, T.K.; Azem, F.A.; Birlik, I.; Ertekin, K. Investigation of Spectral Interactions between a SrAl2O4:Eu(2+), Dy(3+) Phosphor and Nano-Scale TiO2. J. Fluoresc. 2020, 30, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.M.; Anis, A.; Shaikh, H.; Alhamidi, A.; Siva Kumar, N.; Elnour, A.Y.; Al-Zahrani, S.M. Strontium Aluminate-Based Long Afterglow PP Composites: Phosphorescence, Thermal, and Mechanical Characteristics. Polymers 2021, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Paukkeri, R.; Lehtinen, A. Thermal behaviour of polypropylene fractions: 1. Influence of tacticity and molecular weight on crystallization and melting behaviour. Polymers 1993, 34, 4075–4082. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, J.; Park, M.; Lim, S.; Choe, C.R. Transesterification Reaction of the BaSO4-Filled PBT/Poly(ethylene terephthalate) Blend. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2589–2597. [Google Scholar]
- Zhu, J.; Abeykoon, C.; Karim, N. Investigation into the effects of fillers in polymer processing. Int. J. Lightweight Mater. Manuf. 2021, 4, 370–382. [Google Scholar] [CrossRef]
- Clabau, F.; Rocquefelte, X.; Jobic, S.; Deniard, P.; Whangbo, M.H.; Garcia, A.; Le Mercier, T. Mechanism of Phosphorescence Appropriate for the Long-Lasting Phosphors Eu2+-Doped SrAl2O4 with Codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. [Google Scholar] [CrossRef]
- Tuomas Aitasalo, J.H. Hogne Jungner, Mika Lastusaari, Janne Niittykoski, Mechanisms of persistent luminescence in Eu2+, RE3+ doped alkaline earth aluminates. J. Lumin. 2001, 94–95, 59–63. [Google Scholar] [CrossRef]
- Huang, Y.M.; Ma, Q.-l. Long afterglow of trivalent dysprosium doped strontium aluminate. J. Lumin. 2015, 160, 271–275. [Google Scholar] [CrossRef]
- Xu, J.; Tanabe, S. Persistent luminescence instead of phosphorescence: History, mechanism, and perspective. J. Lumin. 2019, 205, 581–620. [Google Scholar] [CrossRef]
- Ni, Z.; Fan, T.; Bai, S.; Zhou, S.; Lv, Y.; Ni, Y.; Xu, B. Effect of the Concentration of SrAl2O4: Eu2+and Dy3+ (SAO) on Characteristics and Properties of Environment-Friendly Long-Persistent Luminescence Composites from Polylactic Acid and SAO. Scanning 2021, 2021, 6337768. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.H.C.; Moreira, R.P.; Felinto, M.C.F.C.; Teixeira, V.C.; Brito, H.F.; Malta, O.L. SrAl2O4: Eu2+, Dy3+ persistent luminescent materials functionalized with the Eu3+(TTA)-complex by microwave-assisted method. J. Alloy. Compd. 2021, 882, 160608. [Google Scholar] [CrossRef]
- Eftimov, T.; Kostova, I.; Arapova, A.; Patronov, G. Rise and decay time responses of Sr aluminate phosphorescent materials. J. Lumin. 2021, 235, 117985. [Google Scholar] [CrossRef]



| Material | Tc (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| PP-5PRIEX | 116.1 | 158.8 | 99.4 | 48.0 |
| PP-5PRIEX/1SAO1 | 115.1 | 160.2 | 87.9 | 42.5 |
| PP-5PRIEX/3SAO1 | 114.0 | 160.8 | 70.5 | 34.1 |
| PP-5PRIEX/5SAO1 | 114.8 | 159.2 | 67.6 | 32.7 |
| PP-5PRIEX/10SAO1 | 114.4 | 160.1 | 62.1 | 30.0 |
| Material | Tc (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| PP-5PRIEX | 116.1 | 158.8 | 99.4 | 48.0 |
| PP-5PRIEX/1SAO2 | 114.5 | 160.2 | 81.2 | 39.2 |
| PP-5PRIEX/3SAO2 | 115.4 | 159.8 | 79.9 | 38.6 |
| PP-5PRIEX/5SAO2 | 115.3 | 159.6 | 75.6 | 36.5 |
| PP-5PRIEX/10SAO2 | 115.8 | 159.1 | 72.1 | 34.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulose, A.M.; Shaikh, H.; Anis, A.; Alhamidi, A.; Kumar, N.S.; Elnour, A.Y.; Al-Zahrani, S.M. Effect of Compatibilizer on the Persistent Luminescence of Polypropylene/Strontium Aluminate Composites. Polymers 2022, 14, 1711. https://doi.org/10.3390/polym14091711
Poulose AM, Shaikh H, Anis A, Alhamidi A, Kumar NS, Elnour AY, Al-Zahrani SM. Effect of Compatibilizer on the Persistent Luminescence of Polypropylene/Strontium Aluminate Composites. Polymers. 2022; 14(9):1711. https://doi.org/10.3390/polym14091711
Chicago/Turabian StylePoulose, Anesh Manjaly, Hamid Shaikh, Arfat Anis, Abdullah Alhamidi, Nadavala Siva Kumar, Ahmed Yagoub Elnour, and Saeed M. Al-Zahrani. 2022. "Effect of Compatibilizer on the Persistent Luminescence of Polypropylene/Strontium Aluminate Composites" Polymers 14, no. 9: 1711. https://doi.org/10.3390/polym14091711
APA StylePoulose, A. M., Shaikh, H., Anis, A., Alhamidi, A., Kumar, N. S., Elnour, A. Y., & Al-Zahrani, S. M. (2022). Effect of Compatibilizer on the Persistent Luminescence of Polypropylene/Strontium Aluminate Composites. Polymers, 14(9), 1711. https://doi.org/10.3390/polym14091711