3. Results
FT-IR spectral analysis of Quetiapine Fumarate and formulations F2 were obtained. The observations concluded that there is no interaction between the drug and other excipients and the results are given below.
The FTIR spectral analysis of Quetiapine Fumarate alone showed the principal peaks at wave numbers of different Functional groups.
The FTIR spectral analysis of formulation F2 alone showed the principal peaks at wave numbers of different Functional groups.
The preliminary trial batches, composed of drug Quetiapine Fumarate, polymers PVP K 30, HPMC K 100, plasticizer glycerin, and solvent distilled water, are given in
Table 1.
Various physicochemical properties of Preliminary trial formulations such as WVTR, % Moisture uptake, % moisture content, thickness, folding endurance, and % drug content were evaluated for selection of the best formulation and the results are represented in
Table 2.
Based on the physicochemical properties of the preliminary trial formulations, T5 has been selected as an optimized formulation and selected for incorporation of permeation enhancers. The composition of permeation enhancers incorporated T5 formulation and was divided into three batches, namely F1, F2, and F3. The composition is given in
Table 3.
The physical properties such as weight, % moisture uptake, % moisture content, thickness, and % drug content were evaluated and the results are represented in
Table 4.
Hence, F2 showed better physical and in vitro permeation results, and was selected as the best formulation. The photo of formulation F2 is represented in
Figure 1.
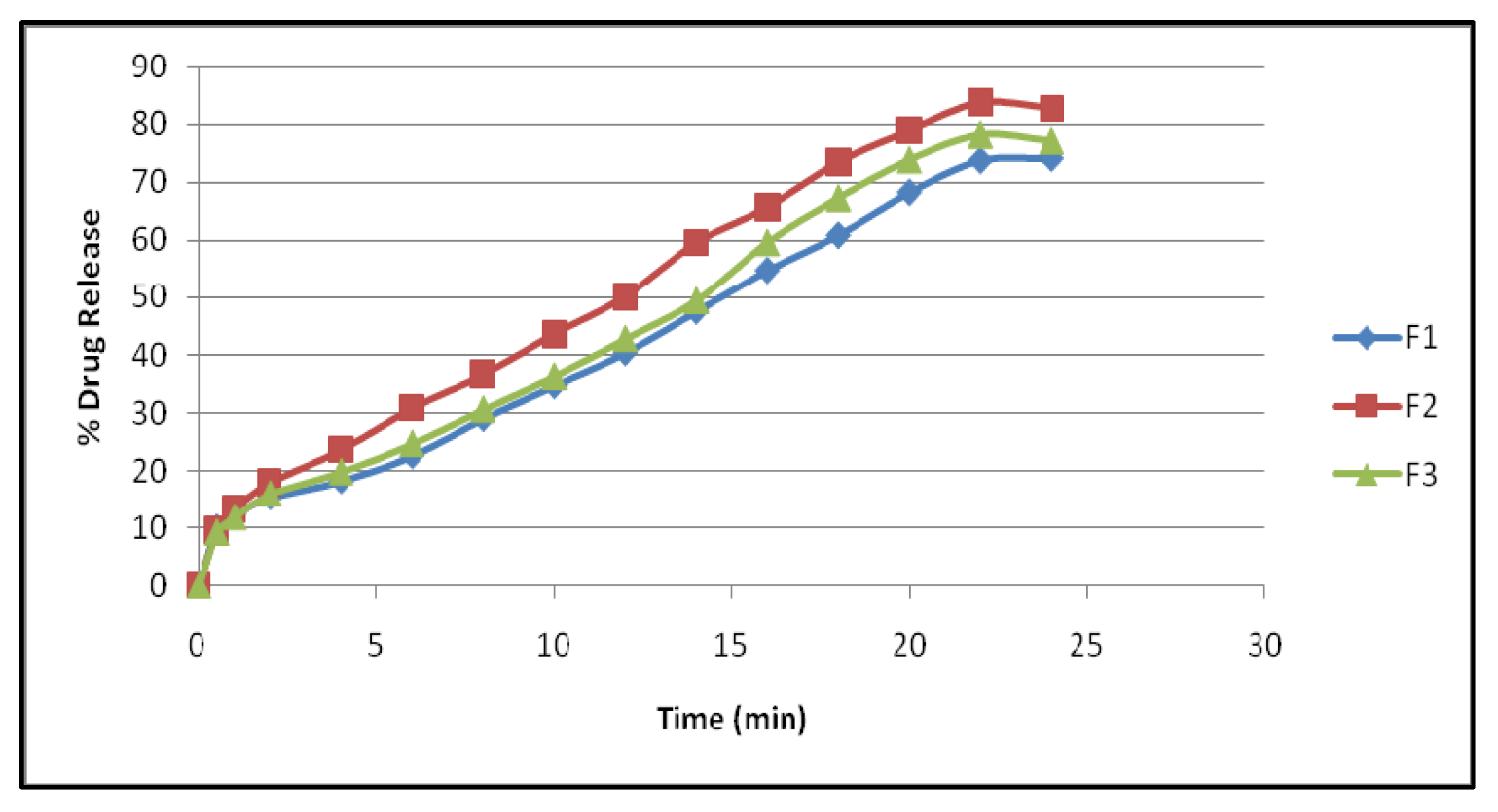
The comparative in vitro permeation study was carried out for F1, F2, and F3. The formulation F2 shows better release compared to F1 and F2 and the results are explored in
Table 5 and
Figure 2.
The optimized formulation F2 has been subjected to a skin irritation study and the skin reactions were scored. The average score and PDI calculated for the patches are given in
Table 6 and
Table 7.
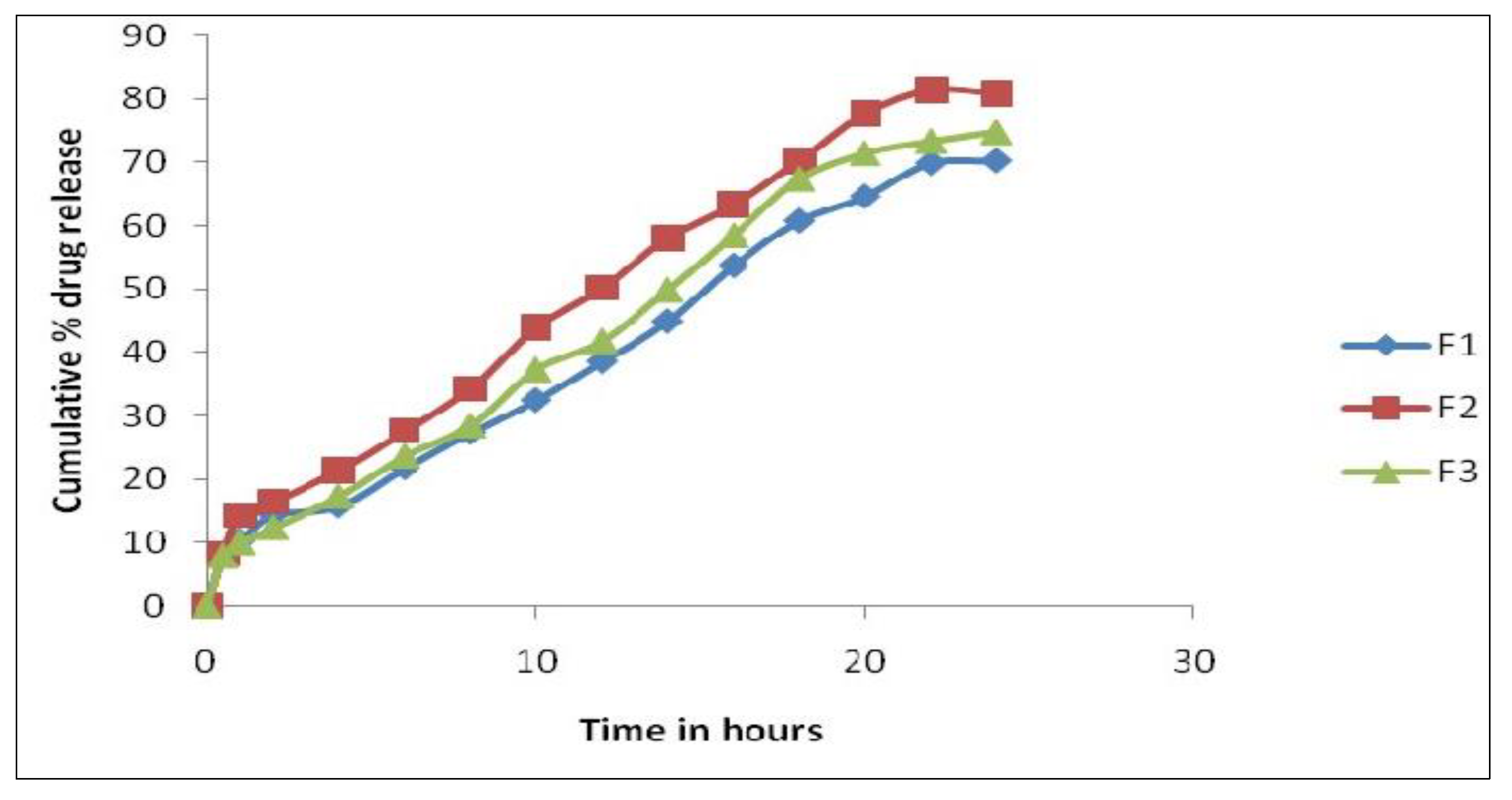
Further, formulation F2 was subjected to accelerated stability studies. The physicochemical parameters and cumulative in vitro permeability results at the end of the first, second, and third months are interpreted in
Table 8 and
Table 9 and
Figure 3.
4. Discussion
Solvent evaporation was used to develop a transdermal patch containing quetiapine fumarate. These thin, translucent, smooth, stable, and very permeable patches were created by combining many formulation parameters. Each batch appears to be identical and contains no apparent fractures.
It was evident from the FTIR graphs that the major functional groups of pure Quetiapine Fumarate were not affected by polymer interaction or preparation methods. The drug’s partition coefficient was 2.6 in n-octane/phosphate buffer pH 7.4, indicating lipophilicity. Higher valued compounds are extremely lipophilic and stay dissolved in the stratum corneum.
A mixture of PVP K 30 and HPMC K 100 was shown to be the most effective patch-producing polymer. The overall polymer concentration was kept between 0.2 and 0.4 g per patch to provide folding durability, slimness, and an appropriate thickness.
The critical analysis of the plasticizer includes the plasticizer glycerol, which was applied at a concentration of 30% w/w of the total polymer weight, and was able to generate a flexible patch without having a significant impact on their releasing property.
The patch loses its flexibility and becomes stiff if the amount of plasticizer, glycerol, is larger than 0.1 mL (i.e., 30% w/w of polymer), therefore the above two factors were adjusted to make a proper patch. This plasticizer penetrates the polymer particles and softens them. Latex coalescence and patch formation are aided by this softening.
It becomes thick and breaks when the polymer concentration surpasses 0.2–0.4 g/mL. At 50 mg PVP, the patch becomes too thin and no longer fulfills the physicochemical requirements.
The slope of the Korsmeyer–Peppas equation indicates that ‘n’ is smaller than 0.5. This indicates that the transdermal patch released the medicine via a fickian (diffusion-controlled) transport mechanism, presumably as a result of a polymer that regulates drug diffusion across the polymeric matrix.
The patch’s medication release followed the zero-order equation. The correlation between the Hixson–Crowell and motored cube root equations is poor.
The improved formulation’s skin irritation was also tested. The mice in the F2 skin irritation investigation showed no erythema or edema after 1 h, 24 h, 48 h, and 72 h. So, it is safe to use.
The formulation T5 had the greatest WVTR, % moisture absorption, % moisture content, thickness, folding endurance, and % drug content of the 10 testing formulations. This may be a result of both polymers’ hydrophilicity.
There was no discernible change in the drug content between the patches of formulation T1 to T8. The results suggested that the approach utilized to manufacture a patch with a homogeneous medication content and a small number of patch variables was effective. This refers to the uniform distribution of the medicine throughout the patch production process.
The chosen formulation was compared to a commercial transdermal matrix diffusion patch. Three-month stability tests were conducted. Visual appearance, color, texture, drug content, and drug release were investigated. The results demonstrated no significant change in visual appearance, color, or texture during the period of three months. Accelerated heating lowered the drug content to 95.34%. The accelerated temperatures have no effect on the release kinetics.
Data analysis of the work includes the following:
A natural and synthetic permeation enhancer, linalool 1,8-cineole, was added to the T5 formulation to test its capacity to penetrate the dialysis membrane (three formulations were developed: namely, F1, F2, and F3). Physical factors such as weight fluctuation, thickness, moisture content, moisture absorption, folding endurance, and medication content are also evaluated.
This F1, F2, and F3 weighed 345, 380, and 350 mg. The three formulations were 0.48 mm, 0.53 mm, and 0.47 mm thick. Like the commercial version, this F2 formulation was optimally thick.
F2 had the highest % moisture content and absorption, resulting in a more flexible, easy-to-handle, and smooth patch.
F1–F3 showed a drug content of 98.86%, 99.48%, and 99.24%. There was no change. The 24 h in vitro release profile of F1–F3 exhibited a maximum release of 74.29%, 82.73%, and 77.27%, correspondingly.
The 90% release data were fitted to multiple kinetic models [
29,
30,
31,
32,
33] to determine the mechanism and rate of drug release. There was an initial burst of the drug followed by a longer sustained release in the F2 formulation. After 24 h, F1 and F3 released little, however, F4 released in an erratic pattern.
This study helped to determine the polymer’s efficacy in controlled release testing.
We determined the efficacy of the dialysis membrane permeation enhancers to investigate them further in ex vivo and in vivo in an animal model investigation.
Future studies on the pharmacokinetic characteristics of the novel topical formulation of Quetiapine Fumarate can be done in conjunction with this investigation.