Liquefaction of Cellulose for Production of Advanced Porous Carbon Materials
Abstract
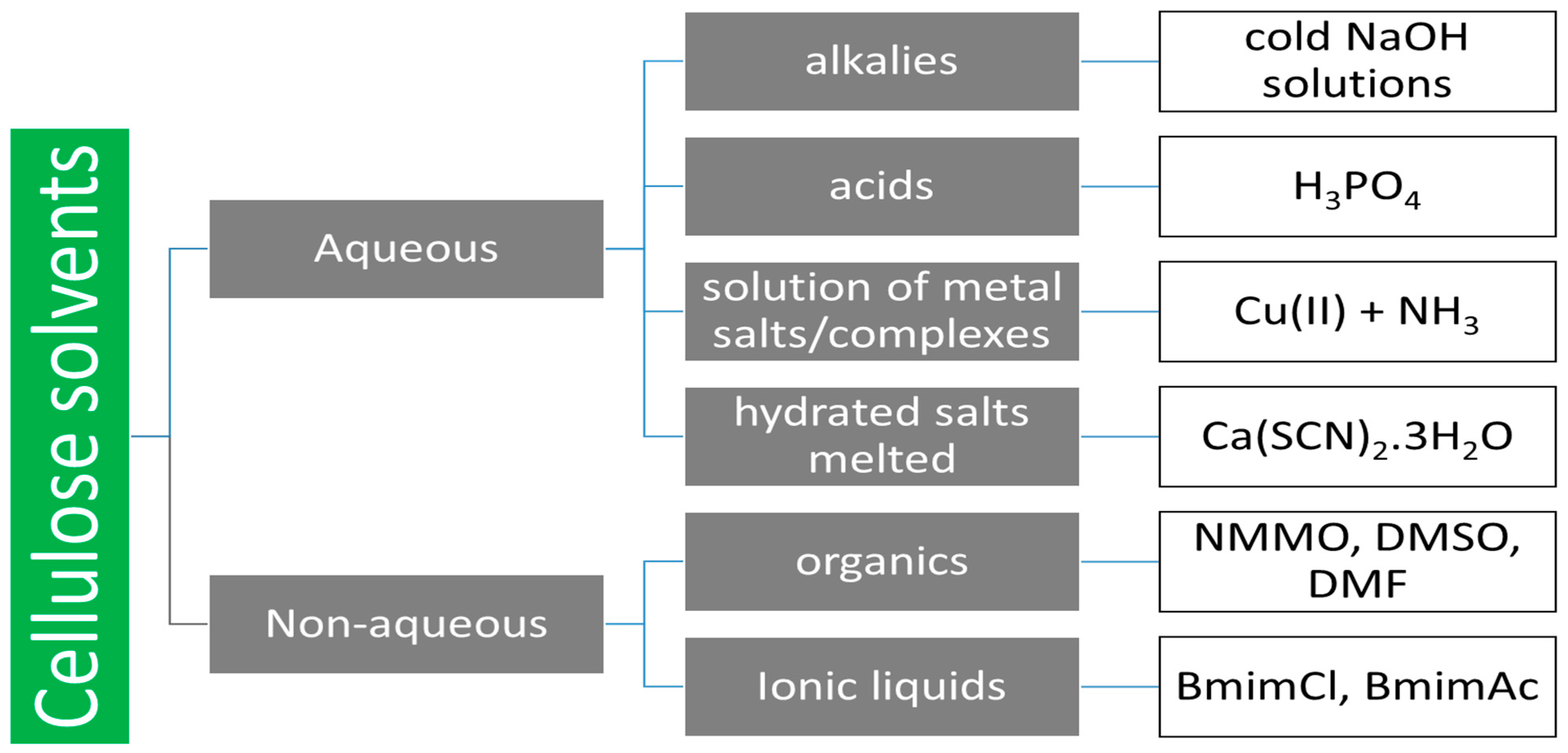
1. Introduction
2. Processes Based on Cellulose Decomposition
2.1. Biomass Pyrolysis
2.2. Hydrothermal Cellulose Liquefaction (HTL)
2.3. Effect of Processing Parameters on Bio-Oil Yield of HTL
2.4. Solvents in HTL
| Solvent | Conversion (%) | Temperature (°C) | Ref. |
|---|---|---|---|
| NaOH/H2O | 28–35 | 350 | [66] |
| NaOH/H2O | 77 | 120–250 | [67] |
| Urea/H2O | 100 | [68] | |
| H2SO4/H2O | 85 | 150 | [42] |
| H3PO4/H2O | 85 | 150 | [69] |
| Citric acid/H2O | 65 | 100 | [68] |
| Oxalic acid/H2O | 65 | 100 | [68] |
| Glycerol | 60 | 100 | [68] |
| Iso-propanol | 380 | [70] | |
| 2-propanol | 32–49 | 240–320 | [71] |
| 2-butanol | 27–53 | 240–320 | [71] |
| Ethanol | 100 | 100–250 | [60] |
| Methanol | 100 | 100–250 | [60] |
| 1-octanol | 270 | [72] | |
| Acetone | 60.5 | 299 | [73] |
| Polyethylene glycol | 150 | [74] | |
| Phenol | 130–150 | [74] | |
| Dimethylsulfoxide | 100 | [75] | |
| Ethylacetate | 100 | [76] | |
| [Alkylmethylimidazolium]Cl | 75–90 | 120 | [60] |
| [Bmim]Cl | 20 | 100 | [77] |
| [Bmim]Ac | 11.5 | 50 | [77] |
| [Amin]Cl | 3.5 | 100 | [78] |
| [Emim]Cl | 10 | 100 | [77] |
2.5. Other Decomposition Processes
3. Cellulose Dissolution
4. Porous Carbons Based on Liquefied/Dissolved Cellulose
5. Conclusions
- -
- Transformation of industrially available bio-oils towards their chemical stability and uniformity, enabling their conversion to nanostructured organic and consequently carbon matter.
- -
- Avoiding the loss of carbon, increasing the carbon yield during biomass treatment, e.g., by suitable cellulose derivatization, more extensive involvement of lignin constituent of biomass, etc.
- -
- Production of biomass- or cellulose-based carbon monoliths with hierarchical porosity is still a challenge even for carbons produced from petroleum-based chemicals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, S.; Gorton, L. Characterisation of the substituent distribution in starch and cellulose derivatives. Anal. Chim. Acta 2003, 497, 27–65. [Google Scholar] [CrossRef]
- Cao, J.-P.; Xiao, X.-B.; Zhang, S.-Y.; Zhao, X.-Y.; Sato, K.; Ogawa, Y.; Wei, X.-Y.; Takarada, T. Preparation and characterization of bio-oils from internally circulating fluidized-bed pyrolyses of municipal, livestock, and wood waste. Bioresour. Technol. 2011, 102, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of anionic structure on the dissolution of cellulose in ionic liquids revealed by molecular simulation. Carbohydr. Polym. 2013, 94, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ono, H. Characterization of the products resulting from ethylene glycol liquefaction of cellulose. J. Wood Sci. 2001, 47, 458–464. [Google Scholar] [CrossRef]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Hong, L.; di Bonito, M.; Pan, G. Decomposition of carboxymethyl cellulose based on nano-knife principle. J. Environ. Sci. 2019, 80, 93–98. [Google Scholar] [CrossRef]
- Yin, S.; Tan, Z. Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Appl. Energy 2012, 92, 234–239. [Google Scholar] [CrossRef]
- Hu, P.; Tan, B.; Long, M. Advanced nanoarchitectures of carbon aerogels for multifunctional environmental applications. Nanotechnol. Rev. 2016, 5, 23–29. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl. Catal. B Environ. 2015, 174–175, 225–243. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Acid Hydrolysis of Cellulose as the Entry Point into Biorefinery Schemes. ChemSusChem 2009, 2, 1096–1107. [Google Scholar] [CrossRef]
- McGath, M.; Jordan-Mowery, S.; Pollei, M.; Heslip, S.; Baty, J. Cellulose Acetate Lamination: A Literature Review and Survey of Paper-Based Collections in the United States. Restaurator. Int. J. Preserv. Libr. Arch. Mater. 2015, 36, 333–365. [Google Scholar] [CrossRef]
- Heinze, T.J.; Glasser, W.G. (Eds.) Cellulose Derivatives: Modification, Characterization, and Nanostructures; American Chemical Society: Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Burchard, W.; Habermann, N.; Klüfers, P.; Seger, B.; Wilhelm, U. Cellulose in Schweizer’s Reagent: A Stable, Polymeric Metal Complex with High Chain Stiffness. Angew. Chem. Int. Ed. 1994, 33, 884–887. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Gupta, D.; Deshmukh, N.A.; Mohite, L.V.; Pinjari, D.V. Influence of intensified cellulose dissolution process on spinning and properties of lyocell fibres. Chem. Eng. Process. Process Intensif. 2020, 155, 108063. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in)solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Qi, H.; Chang, C.; Zhang, L. Effects of temperature and molecular weight on dissolution of cellulose in NaOH/urea aqueous solution. Cellulose 2008, 15, 779–787. [Google Scholar] [CrossRef]
- Matthews, J.F.; Bergenstråhle, M.; Beckham, G.T.; Himmel, M.E.; Nimlos, M.R.; Brady, J.W.; Crowley, M.F. High-Temperature Behavior of Cellulose I. J. Phys. Chem. B 2011, 115, 2155–2166. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Mahmood, H.; Ibrahim, M.F.; Yusup, S.; Uemura, Y. Effects of Pressure and Temperature on the Dissolution of Cellulose in Ionic Liquids. Adv. Mater. Res. 2016, 1133, 588–592. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 2. Acidic solvents. Carbohydr. Polym. 2016, 151, 707–715. [Google Scholar] [CrossRef]
- Bioni, T.; Arêas, E.; Couto, L.; Favarin, G.; el Seoud, O. Dissolution of cellulose in mixtures of ionic liquid and molecular solvents: Relevance of solvent-solvent and cellulose-solvent interactions. Nord. Pulp Pap. Res. J. 2015, 30, 105–111. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Kunaver, M.; Jasiukaitytė, E.; Čuk, N. Ultrasonically assisted liquefaction of lignocellulosic materials. Bioresour. Technol. 2012, 103, 360–366. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis —A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Staš, M.; Auersvald, M.; Kejla, L.; Vrtiška, D.; Kroufek, J.; Kubička, D. Quantitative analysis of pyrolysis bio-oils: A review. TrAC Trends Anal. Chem. 2020, 126, 115857. [Google Scholar] [CrossRef]
- Panchasara, H.; Ashwath, N. Effects of Pyrolysis Bio-Oils on Fuel Atomisation—A Review. Energies 2021, 14, 794. [Google Scholar] [CrossRef]
- Koriakin, A.; van Nguyen, H.; Kim, D.W.; Lee, C.-H. Direct thermochemical liquefaction of microcrystalline cellulose by sub- and supercritical organic solvents. J. Supercrit. Fluids 2014, 95, 175–186. [Google Scholar] [CrossRef]
- Jindal, M.K.; Jha, M.K. Catalytic Hydrothermal Liquefaction of Waste Furniture Sawdust to Bio-oil. Indian Chem. Eng. 2016, 58, 157–171. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, H.; Choi, J.W. Effects of transition metals on hydrothermal liquefaction of empty fruit bunches (EFB) for conversion to biofuel and valuable chemicals. Energy 2018, 162, 1–9. [Google Scholar] [CrossRef]
- Hardi, F.; Mäkelä, M.; Yoshikawa, K. Non-catalytic Hydrothermal Liquefaction of Biomass: An Experimental Design Approach. Energy Procedia 2017, 105, 75–81. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass, Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Panthapulakkal, S.; Raghunanan, L.; Sain, M.; Kc, B.; Tjong, J. Natural fiber and hybrid fiber thermoplastic composites. In Green Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 39–72. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Chen, W.; Wang, S.; Sun, R.-C. Hydrothermal conversion of xylose, glucose, and cellulose under the catalysis of transition metal sulfates. Carbohydr. Polym. 2015, 118, 44–51. [Google Scholar] [CrossRef]
- de Caprariis, B.; de Filippis, P.; Petrullo, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Liu, X.; Wang, Q.; Li, W.; Xie, H.; Zhao, Z.K. Kinetic study of acid-catalyzed cellulose hydrolysis in 1-butyl-3-methylimidazolium chloride. Bioresour. Technol. 2012, 112, 151–155. [Google Scholar] [CrossRef]
- Chauvette, G.; Heitz, M.; Rubio, M.; Khorami, J.; Chornet, E.; Ménard, H. TG/DTG as a rapid method for the characterization of solid residues derived from liquefaction of lignocellulosics. Thermochim. Acta 1985, 84, 1–5. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: A state of the art review. Renew. Sustain. Energy Rev. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Li, W.; Xie, X.; Tang, C.; Li, Y.; Li, L.; Wang, Y.; Wei, X.; Fan, D. Effects of hydroxyl and hydrogen free radicals on the liquefaction of cellulose in sub/supercritical ethanol. J. Fuel Chem. Technol. 2016, 44, 415–421. [Google Scholar] [CrossRef]
- Hernández, J.D.; Tran, K.-Q.; Trinh, T.T. Selective dissolution of woody biomass under hydrothermal conditions. Energy Procedia 2017, 142, 867–872. [Google Scholar] [CrossRef]
- Liu, S.; Tang, L.; Long, J.; Guan, J.; Li, X. Kinetic analysis and process modeling for cellulose valorization in cooperative ionic liquid pairs. Catal. Today 2016, 264, 75–82. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.W.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State-of-the-art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef]
- Isa, K.M.; Abdullah, T.A.T.; Ali, U.F.M. Hydrogen donor solvents in liquefaction of biomass: A review. Renew. Sustain. Energy Rev. 2018, 81, 1259–1268. [Google Scholar] [CrossRef]
- Danley, R.L. New heat flux DSC measurement technique. Thermochim. Acta 2002, 395, 201–208. [Google Scholar] [CrossRef]
- Miller, I.J.; Fellows, S.K. Catalytic effects during cellulose liquefaction. Fuel 1985, 64, 1246–1250. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef]
- Shan, X.; Shu, G.; Li, K.; Zhang, X.; Wang, H.; Cao, X.; Jiang, H.; Weng, H. Effect of hydrogenation of liquefied heavy oil on direct coal liquefaction. Fuel 2017, 194, 291–296. [Google Scholar] [CrossRef]
- Miller, I.J.; Saunders, E.R. Reactions of possible cellulose liquefaction intermediates under high pressure liquefaction conditions. Fuel 1987, 66, 123–129. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Microwave-assisted conversion of biomass and waste materials to biofuels. Renew. Sustain. Energy Rev. 2018, 82, 1149–1177. [Google Scholar] [CrossRef]
- Pan, Z.; Huang, H.; Zhou, C.; Xiao, X.; He, X.; Lai, F.; Xiong, J. Highly efficient conversion of camphor tree sawdust into bio-oil and biochar products by liquefaction in ethanol-water cosolvent. J. Anal. Appl. Pyrolysis 2018, 136, 186–198. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.; Piszczyk, Ł. The Study on Application of Biopolyols Obtained by Cellulose Biomass Liquefaction Performed with Crude Glycerol for the Synthesis of Rigid Polyurethane Foams. J. Polym. Environ. 2018, 26, 2546–2554. [Google Scholar] [CrossRef]
- Haverly, M.R.; Schulz, T.C.; Whitmer, L.E.; Friend, A.J.; Funkhouser, J.M.; Smith, R.G.; Young, M.K.; Brown, R.C. Continuous solvent liquefaction of biomass in a hydrocarbon solvent. Fuel 2018, 211, 291–300. [Google Scholar] [CrossRef]
- Barnés, M.C.; de Visser, M.M.; van Rossum, G.; Kersten, S.R.A.; Lange, J.-P. Liquefaction of wood and its model components. J. Anal. Appl. Pyrolysis 2017, 125, 136–143. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, X. Recent progress in the direct liquefaction of typical biomass. Prog. Energy Combust. Sci. 2015, 49, 59–80. [Google Scholar] [CrossRef]
- Li, Q.; Liu, D.; Hou, X.; Wu, P.; Song, L.; Yan, Z. Hydro-liquefaction of microcrystalline cellulose, xylan and industrial lignin in different supercritical solvents. Bioresour. Technol. 2016, 219, 281–288. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Montoya, S. Production of Bioethanol from Biomass: An Overview. In Biofuel Technologies; Gupta, V.K., Tuohy, M.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 397–441. [Google Scholar] [CrossRef]
- Moretti, M.M.D.; Perrone, O.M.; Nunes, C.D.C.; Taboga, S.; Boscolo, M.; da Silva, R.; Gomes, E. Effect of pretreatment and enzymatic hydrolysis on the physical-chemical composition and morphologic structure of sugarcane bagasse and sugarcane straw. Bioresour. Technol. 2016, 219, 773–777. [Google Scholar] [CrossRef]
- Deng, H.; Meredith, W.; Uguna, C.N.; Snape, C.E. Impact of solvent type and condition on biomass liquefaction to produce heavy oils in high yield with low oxygen contents. J. Anal. Appl. Pyrolysis 2015, 113, 340–348. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and valorization of cellulose, lignin and lignocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Lacerda, V.D.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Bascones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Pérez-Lebeña, E.; Martín-Gil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulosic waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 2015, 180, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Badiei, M.; Asim, N.; Jahim, J.M.; Sopian, K. Comparison of Chemical Pretreatment Methods for Cellulosic Biomass. APCBEE Procedia 2014, 9, 170–174. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, T.; Shi, Y.; Wang, J.; Li, J.; Yang, H. H2SO4 and NaOH Pretreatment to Enhance Bio-Oil Yield of Pine Wood Liquefaction in Methanol. BioResources 2017, 12, 3801–3815. [Google Scholar] [CrossRef][Green Version]
- Hu, T.; Gong, M.; Xu, C.C.; Bassi, A. Investigation of an alternative cell disruption approach for improving hydrothermal liquefaction of microalgae. Fuel 2017, 197, 138–144. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, J.; Lan, P.; Yang, H.; Lu, J.; Wang, Z. Study on the Dissolution Mechanism of Cellulose by ChCl-Based Deep Eutectic Solvents. Materials 2020, 13, 278. [Google Scholar] [CrossRef]
- Cheng, S.; D’cruz, I.; Wang, M.; Leitch, M.; Xu, C.C. Highly Efficient Liquefaction of Woody Biomass in Hot-Compressed Alcohol−Water Co-solvents. Energy Fuels 2010, 24, 4659–4667. [Google Scholar] [CrossRef]
- Kleinert, M.; Gasson, J.R.; Barth, T. Optimizing solvolysis conditions for integrated depolymerisation and hydrodeoxygenation of lignin to produce liquid biofuel. J. Anal. Appl. Pyrolysis 2009, 85, 108–117. [Google Scholar] [CrossRef]
- Aysu, T.; Küçük, M.M. Liquefaction of giant fennel (Ferula orientalis L.) in supercritical organic solvents: Effects of liquefaction parameters on product yields and character. J. Supercrit. Fluids 2013, 83, 104–123. [Google Scholar] [CrossRef]
- Yamazaki, J.; Minami, E.; Saka, S. Liquefaction of beech wood in various supercritical alcohols. J. Wood Sci. 2006, 52, 527–532. [Google Scholar] [CrossRef]
- Cemek, M. Liquid products from Verbascum stalk by supercritical fluid extraction. Energy Convers. Manag. 2001, 42, 6. [Google Scholar] [CrossRef]
- Pierson, Y.; Bobbink, F.; Yan, N. Alcohol Mediated Liquefaction of Lignocellulosic Materials: A Mini Review. Chem. Eng. Process Tech. 2013, 5, 1014. [Google Scholar]
- Yan, H.; Yang, Y.; Tong, D.; Xiang, X.; Hu, C. Catalytic conversion of glucose to 5-hydroxymethylfurfural over SO42−/ZrO2 and SO42−/ZrO2–Al2O3 solid acid catalysts. Catal. Commun. 2009, 10, 1558–1563. [Google Scholar] [CrossRef]
- Aellig, C.; Hermans, I. Continuous D-Fructose Dehydration to 5- Hydroxymethylfurfural Under Mild Conditions. ChemSusChem 2012, 5, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Rodríguez, H.; Rahman, M.; Rogers, R.D. Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem. Commun. 2011, 47, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xia, K.; Cai, W.; Yang, R.; Wang, L.; Wang, B. Investigations about dissolution of cellulose in the 1-allyl-3-alkylimidazolium chloride ionic liquids. Carbohydr. Polym. 2012, 87, 1058–1064. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Dogan, H.; Hilmioglu, N.D. Dissolution of cellulose with NMMO by microwave heating. Carbohydr. Polym. 2009, 75, 90–94. [Google Scholar] [CrossRef]
- Xiao, W.; Han, L.; Zhao, Y. Comparative study of conventional and microwave-assisted liquefaction of corn stover in ethylene glycol. Ind. Crops Prod. 2011, 34, 1602–1606. [Google Scholar] [CrossRef]
- Zhang, J.; An, Y.; Borrion, A.; He, W.; Wang, N.; Chen, Y.; Li, G. Process characteristics for microwave assisted hydrothermal carbonization of cellulose. Bioresour. Technol. 2018, 259, 91–98. [Google Scholar] [CrossRef]
- Jewena, N.; Miyanomae, R.; Sasaki, M.; Mashimo, T. Hydrothermal decomposition of cellulose using strong gravitational field. J. Supercrit. Fluids 2017, 120, 379–383. [Google Scholar] [CrossRef]
- Budarin, V.L.; Clark, J.H.; Lanigan, B.A.; Shuttleworth, P.; Macquarrie, D.J. Microwave assisted decomposition of cellulose: A new thermochemical route for biomass exploitation. Bioresour. Technol. 2010, 101, 3776–3779. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bao, X.; Zhou, C.; Zhang, L.; Yagoub, A.E.-G.A.; Yang, H.; Ma, H. Ultrasound-ionic liquid enhanced enzymatic and acid hydrolysis of biomass cellulose. Ultrason. Sonochem. 2018, 41, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Gräsvik, J.; Eliasson, B.; Mikkola, J.-P. Halogen-free ionic liquids and their utilization as cellulose solvents. J. Mol. Struct. 2012, 1028, 156–163. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508. [Google Scholar] [CrossRef]
- Stolarska, O.; Pawlowska-Zygarowicz, A.; Soto, A.; Rodríguez, H.; Smiglak, M. Mixtures of ionic liquids as more efficient media for cellulose dissolution. Carbohydr. Polym. 2017, 178, 277–285. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.-X.; Yu, J.; Hsieh, Y.-L. Dissolution behaviour and solubility of cellulose in NaOH complex solution. Carbohydr. Polym. 2010, 81, 668–674. [Google Scholar] [CrossRef]
- Bergenstråhle, M.; Wohlert, J.; Himmel, M.E.; Brady, J.W. Simulation studies of the insolubility of cellulose. Carbohydr. Res. 2010, 345, 2060–2066. [Google Scholar] [CrossRef]
- Jiang, X.; Bai, Y.; Chen, X.; Liu, W. A review on raw materials, commercial production and properties of lyocell fiber. J. Bioresour. Bioprod. 2020, 5, 16–25. [Google Scholar] [CrossRef]
- el Seoud, O.; Nawaz, H.; Arêas, E. Chemistry and Applications of Polysaccharide Solutions in Strong Electrolytes/Dipolar Aprotic Solvents: An Overview. Molecules 2013, 18, 1270–1313. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, F.; Pan, C.; Fang, Y. Cellulose-Based Thermoplastics and Elastomers via Controlled Radical Polymerization. In Thermosoftening Plastics; Evingür, G.A., Pekcan, Ö., Achilias, D.S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zampano, G.; Bertoldo, M.; Bronco, S. Poly(ethyl acrylate) surface-initiated ATRP grafting from wood pulp cellulose fibers. Carbohydr. Polym. 2009, 75, 22–31. [Google Scholar] [CrossRef]
- Medronho, B.; Lindman, B. Competing forces during cellulose dissolution: From solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Kraessig, H. Cellulose chemistry and its applications, T. P. Nevell and S. H. Zeronian, Eds., Halsted Press, John Wiley, New York, 1985, 552 pp. J. Polym. Sci. Part C Polym. Lett. 1987, 25, 87–88. [Google Scholar] [CrossRef]
- Liebert, T.F.; Heinze, T.J.; Edgar, K.J. (Eds.) Cellulose Solvents: For Analysis, Shaping and Chemical Modification; American Chemical Society: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Boerstoel, H.; Maatman, H.; Westerink, J.B.; Koenders, B.M. Liquid crystalline solutions of cellulose in phosphoric acid. Polymer 2001, 42, 7371–7379. [Google Scholar] [CrossRef]
- Keshk, S.M.A.S. Effect of different alkaline solutions on crystalline structure of cellulose at different temperatures. Carbohydr. Polym. 2015, 115, 658–662. [Google Scholar] [CrossRef]
- Yan, L.; Gao, Z. Dissolving of cellulose in PEG/NaOH aqueous solution. Cellulose 2008, 15, 789–796. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Gao, S. Dissolution and regeneration of cellulose in NaOH/thiourea aqueous solution. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1521–1529. [Google Scholar] [CrossRef]
- Piltonen, P.; Hildebrandt, N.C.; Westerlind, B.; Valkama, J.-P.; Tervahartiala, T.; Illikainen, M. Green and efficient method for preparing all-cellulose composites with NaOH/urea solvent. Compos. Sci. Technol. 2016, 135, 153–158. [Google Scholar] [CrossRef]
- Sarko, A.; Southwick, J.; Hayashi, J. Packing Analysis of Carbohydrates and Polysaccharides. 7. Crystal Structure of Cellulose IIII and Its Relationship to Other Cellulose Polymorphs. Macromolecules 1976, 9, 857–863. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, H.; Lue, A.; Hu, K.; Cheng, G.; Zhang, L. Role of sodium zincate on cellulose dissolution in NaOH/urea aqueous solution at low temperature. Carbohydr. Polym. 2011, 83, 1185–1191. [Google Scholar] [CrossRef]
- Xiong, B.; Zhao, P.; Hu, K.; Zhang, L.; Cheng, G. Dissolution of cellulose in aqueous NaOH/urea solution: Role of urea. Cellulose 2014, 21, 1183–1192. [Google Scholar] [CrossRef]
- Egal, M.; Budtova, T.; Navard, P. The dissolution of microcrystalline cellulose in sodium hydroxide-urea aqueous solutions. Cellulose 2008, 15, 361–370. [Google Scholar] [CrossRef]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.M.; Perveen, A.; Jahan, R.A.; Matin, M.A.; Wong, S.Y.; Li, X.; Arafat, M.T. Preparation of different polymorphs of cellulose from different acid hydrolysis medium. Int. J. Biol. Macromol. 2019, 130, 969–976. [Google Scholar] [CrossRef]
- Mittal, A.; Katahira, R.; Himmel, M.E.; Johnson, D.K. Effects of alkaline or liquid-ammonia treatment on crystalline cellulose: Changes in crystalline structure and effects on enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 41. [Google Scholar] [CrossRef]
- Wei, W.; Wu, S. Depolymerization of cellulose into high-value chemicals by using synergy of zinc chloride hydrate and sulfate ion promoted titania catalyst. Bioresour. Technol. 2017, 241, 760–766. [Google Scholar] [CrossRef]
- Fischer, S.; Leipner, H.; Thümmler, K.; Brendler, E.; Peters, J. Inorganic Molten Salts as Solvents for Cellulose. Cellulose 2003, 10, 227–236. [Google Scholar] [CrossRef]
- Hudson, S.M.; Cuculo, J.A. The Solubility of Unmodified Cellulose: A Critique of the Literature. J. Macromol. Sci. Part C 1980, 18, 1–82. [Google Scholar] [CrossRef]
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A. Solvents applied in the field of cellulose chemistry: A mini review. Polymers 2005, 15, 84–90. [Google Scholar] [CrossRef]
- Ting, S.R.S.; Davis, T.P.; Zetterlund, P.B. Retardation in RAFT Polymerization: Does Cross-Termination Occur with Short Radicals Only? Macromolecules 2011, 44, 4187–4193. [Google Scholar] [CrossRef]
- Heinze, T.; Dicke, R.; Koschella, A.; Kull, A.H.; Klohr, E.-A.; Koch, W. Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol. Chem. Phys. 2000, 201, 627–631. [Google Scholar] [CrossRef]
- Frey, M.W.; Li, L.; Xiao, M.; Gould, T. Dissolution of cellulose in ethylene diamine/salt solvent systems. Cellulose 2006, 13, 147–155. [Google Scholar] [CrossRef]
- Gupta, K.M.; Jiang, J. Cellulose dissolution and regeneration in ionic liquids: A computational perspective. Chem. Eng. Sci. 2015, 121, 180–189. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Anugwom, I.; Virtanen, P.; Sjöholm, R.; Mikkola, J.P. Dissolution of lignocellulosic materials and its constituents using ionic liquids—A review. Ind. Crops Prod. 2010, 32, 175–201. [Google Scholar] [CrossRef]
- Shibata, M.; Teramoto, N.; Nakamura, T.; Saitoh, Y. All-cellulose and all-wood composites by partial dissolution of cotton fabric and wood in ionic liquid. Carbohydr. Polym. 2013, 98, 1532–1539. [Google Scholar] [CrossRef]
- Casarano, R.; Nawaz, H.; Possidonio, S.; da Silva, V.C.; el Seoud, O.A. A convenient solvent system for cellulose dissolution and derivatization: Mechanistic aspects of the acylation of the biopolymer in tetraallylammonium fluoride/dimethyl sulfoxide. Carbohydr. Polym. 2011, 86, 1395–1402. [Google Scholar] [CrossRef]
- Hu, Y.; Acharya, S.; Abidi, N. Cellulose porosity improves its dissolution by facilitating solvent diffusion. Int. J. Biol. Macromol. 2019, 123, 1289–1296. [Google Scholar] [CrossRef]
- Ha, S.H.; Mai, N.L.; An, G.; Koo, Y.-M. Microwave-assisted pretreatment of cellulose in ionic liquid for accelerated enzymatic hydrolysis. Bioresour. Technol. 2011, 102, 1214–1219. [Google Scholar] [CrossRef]
- Iguchi, M.; Aida, T.M.; Watanabe, M.; Smith, R.L. Dissolution and recovery of cellulose from 1-butyl-3-methylimidazolium chloride in presence of water. Carbohydr. Polym. 2013, 92, 651–658. [Google Scholar] [CrossRef]
- Cuissinat, C.; Navard, P.; Heinze, T. Swelling and dissolution of cellulose. Part IV: Free floating cotton and wood fibres in ionic liquids. Carbohydr. Polym. 2008, 72, 590–596. [Google Scholar] [CrossRef]
- Jiang, G.; Senthil, R.A.; Sun, Y.; Kumar, T.R.; Pan, J. Recent progress on porous carbon and its derivatives from plants as advanced electrode materials for supercapacitors. J. Power Sources 2022, 520, 230886. [Google Scholar] [CrossRef]
- John, K.I.; Omorogie, M.O. Biomass-based hydrothermal carbons for catalysis and environmental cleanup: A review. Green Chem. Lett. Rev. 2022, 15, 162–186. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, L.; Zhang, S.; Li, Q.; Hu, X. Hydrothermal carbonization of cellulose in aqueous phase of bio-oil: The significant impacts on properties of hydrochar. Fuel 2022, 315, 123132. [Google Scholar] [CrossRef]
- Wang, C.; Liu, T. Activated carbon materials derived from liquefied bark-phenol formaldehyde resins for high performance supercapacitors. RSC Adv. 2016, 6, 105540–105549. [Google Scholar] [CrossRef]
- Ozbay, N.; Yargic, A.S. Carbon foam production from bio-based polyols of liquefied spruce tree sawdust: Effects of biomass/solvent mass ratio and pyrolytic oi laddition. J. Appl. Polym. Sci. 2019, 136, 47185. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Tao, Y.; Zhou, J.; Zhang, H. Hierarchical porous carbon materials produced from heavy bio-oil for high-performance supercapacitor electrodes. J. Energy Storage 2022, 47, 103624. [Google Scholar] [CrossRef]
- Luo, Z.; Lin, N.; Sun, M.; Wang, Y.; Zhu, X. Synthesis of 3D-interconnected hierarchical porous carbon from heavy fraction of bio-oil using crayfish shell as the biological template for high-performance supercapacitors. Carbon 2021, 173, 910–917. [Google Scholar] [CrossRef]
- Li, J.; Xiao, R.; Li, M.; Zhang, H.; Wu, S.; Xia, C. Template-synthesized hierarchical porous carbons from bio-oil with high performance for supercapacitor electrodes. Fuel Process. Technol. 2019, 192, 239–249. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Zhao, B.; Zhang, Y. Preparation of hydrogen storage carbon materials using bio-oil heavy components as carbon-containing precursor. Fuel Process. Technol. 2020, 203, 106386. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, B.; Li, H.-X.; Zhang, X.-H.; Tian, X.; Jin, L.; Cao, Q. Honeycomb-like carbon with tunable pore size from bio-oil for supercapacitor. Microporous Mesoporous Mater. 2020, 309, 110551. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Wu, Q.; Kuang, Y. Acetylated cellulose nanocrystals with high-crystallinity obtained by one-step reaction from the traditional acetylation of cellulose. Carbohydr. Polym. 2020, 229, 115553. [Google Scholar] [CrossRef] [PubMed]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End. Polymers 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Zuppolini, S.; Salama, A.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Cellulose Amphiphilic Materials: Chemistry, Process and Applications. Pharmaceutics 2022, 14, 386. [Google Scholar] [CrossRef]
- Koch, W. Properties and Uses of Ethylcellulose. Ind. Eng. Chem. 1937, 29, 687–690. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, F. Hydroxyethyl Cellulose-Based Hydrogels with Various Pore Sizes Prepared by Freeze-Drying. J. Macromol. Sci. Part B 2010, 50, 340–349. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Hydroxypropyl Cellulose Based Non-Volatile Gel Polymer Electrolytes for Dye-Sensitized Solar Cell Applications using 1-methyl-3-propylimidazolium iodide ionic liquid. Sci. Rep. 2015, 5, 18056. [Google Scholar] [CrossRef]
- Klepel, O.; Danneberg, N. Porous Carbon Monoliths Made from Cellulose and Starch. C—J. Carbon Res. 2020, 6, 32. [Google Scholar] [CrossRef]
- Blankenship, L.S.; Mokaya, R. Modulating the porosity of carbons for improved adsorption of hydrogen, carbon dioxide, and methane: A review. Mater. Adv. 2022, 3, 1905–1930. [Google Scholar] [CrossRef]
- Lee, B.-M.; Jeong, C.-U.; Hong, S.-K.; Yun, J.-M.; Choi, J.-H. Eco-friendly fabrication of porous carbon monoliths from water-soluble carboxymethyl cellulose for supercapacitor applications. J. Ind. Eng. Chem. 2020, 82, 367–373. [Google Scholar] [CrossRef]
- Jeong, M.-J.; Lee, S.; Yang, B.S.; Potthast, A.; Kang, K.-Y. Cellulose Degradation by Calcium Thiocyanate. Polymers 2019, 11, 1494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, R.; Xiang, J.; Kang, H.; Liu, Z.; Huang, Y. Dissolution Mechanism of Cellulose in N,N-Dimethylacetamide/Lithium Chloride: Revisiting through Molecular Interactions. J. Phys. Chem. B 2014, 118, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lu, A.; Zhang, L. Gelation behavior of cellulose in NaOH/urea aqueous system via cross-linking. Cellulose 2013, 20, 1669–1677. [Google Scholar] [CrossRef]
- Korhonen, O.; Budtova, T. Gelation of cellulose-NaOH solutions in the presence of cellulose fibers. Carbohydr. Polym. 2019, 224, 115152. [Google Scholar] [CrossRef]
- Xin, Y.; Xiong, Q.; Bai, Q.; Miyamoto, M.; Li, C.; Shen, Y.; Uyama, H. A hierarchically porous cellulose monolith: A template-free fabricated, morphology-tunable, and easily functionalizable platform. Carbohydr. Polym. 2017, 157, 429–437. [Google Scholar] [CrossRef]
- Parajuli, P.; Acharya, S.; Hu, Y.; Abidi, N. Cellulose-based monoliths with enhanced surface area and porosity. J. Appl. Polym. Sci. 2020, 137, 48975. [Google Scholar] [CrossRef]
- Ferry, M.A.; Maruyama, J.; Asoh, T.-A.; Uyama, H. Fused sphere carbon monoliths with honeycomb-like porosity from cellulose nanofibers for oil and water separation. RSC Adv. 2021, 11, 2202–2212. [Google Scholar] [CrossRef]
- Hina, K.; Zou, H.; Qian, W.; Zuo, D.; Yi, C. Preparation and performance comparison of cellulose-based activated carbon fibres. Cellulose 2018, 25, 607–617. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryeziu, A.; Slovák, V.; Parchaňská, A. Liquefaction of Cellulose for Production of Advanced Porous Carbon Materials. Polymers 2022, 14, 1621. https://doi.org/10.3390/polym14081621
Kryeziu A, Slovák V, Parchaňská A. Liquefaction of Cellulose for Production of Advanced Porous Carbon Materials. Polymers. 2022; 14(8):1621. https://doi.org/10.3390/polym14081621
Chicago/Turabian StyleKryeziu, Arjeta, Václav Slovák, and Alžběta Parchaňská. 2022. "Liquefaction of Cellulose for Production of Advanced Porous Carbon Materials" Polymers 14, no. 8: 1621. https://doi.org/10.3390/polym14081621
APA StyleKryeziu, A., Slovák, V., & Parchaňská, A. (2022). Liquefaction of Cellulose for Production of Advanced Porous Carbon Materials. Polymers, 14(8), 1621. https://doi.org/10.3390/polym14081621







