Melting Temperature Depression of Polymer Single Crystals: Application to the Eco-Design of Tie-Layers in Polyolefinic-Based Multilayered Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Morphology of the Single Crystals
2.4. Thermal Properties
2.5. Rheological Testing
2.6. Atomic Force Microscopy
3. Results and Discussion
3.1. Characterization of the Samples
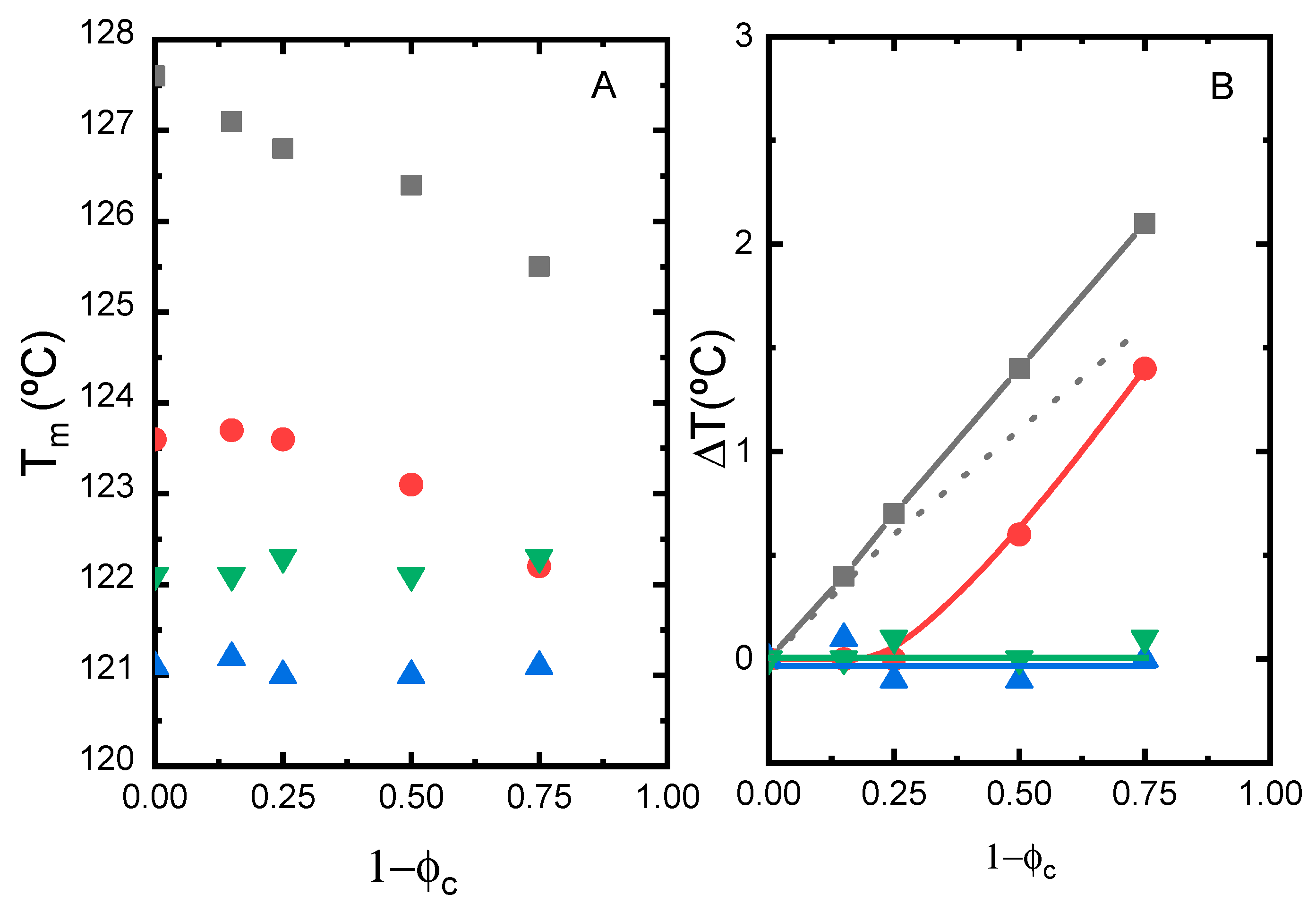
3.2. Melting Temperature Depression Analysis
3.3. Rheological Properties of Selected Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martín-Lara, M.A.; Godoy, V.; Quesada, L.; Lozano, E.J.; Calero, M. Environmental Status of Marine Plastic Pollution in Spain. Mar. Pollut. Bull. 2021, 170, 112677. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future Scenarios of Global Plastic Waste Generation and Disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Filipe, D.A.; Paço, A.; Duarte, A.C.; Rocha-Santos, T.; Silva, A.L.P. Are Biobased Plastics Green Alternatives?—A Critical Review. Int. J. Environ. Res. Public Health 2021, 18, 7729. [Google Scholar] [CrossRef] [PubMed]
- PlascticsEurope Plastics-the Facts 2021 an Analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 24 March 2022).
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer Packaging: Advances in Preparation Techniques and Emerging Food Applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.; Na, Y.; Choi, W.I.; Kim, S.; Kim, J.; Hong, J.; Sung, D. Functional Ferrocene Polymer Multilayer Coatings for Implantable Medical Devices: Biocompatible, Antifouling, and ROS-Sensitive Controlled Release of Therapeutic Drugs. Acta Biomater. 2021, 125, 242–252. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Wang, W.; Sheng, Y.; Zhang, R.; Bradley, M. Self-Healing Multilayer Films for Simultaneous Release of Hydrophilic and Hydrophobic Drugs. Soft Mater. 2021, 19, 254–262. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-Layer Assembled Polymeric Thin Films as Prospective Drug Delivery Carriers: Design and Applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Zhang, H.; Xu, Z.; Zhao, L.; Wang, J.; Qiu, Y.; Liu, B. Improvements on Biological and Antimicrobial Properties of Titanium Modified by AgNPs-Loaded Chitosan-Heparin Polyelectrolyte Multilayers. J. Mater. Sci. Mater. Med. 2019, 30, 52. [Google Scholar] [CrossRef]
- Baer, E.; Zhu, L. 50th Anniversary Perspective: Dielectric Phenomena in Polymers and Multilayered Dielectric Films. Macromolecules 2017, 50, 2239–2256. [Google Scholar] [CrossRef]
- Cho, C.; Son, J. Organic Thermoelectric Multilayers with High Stretchiness. Nanomaterials 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Alcouffe, P.; Sudre, G.; Pruvost, S.; Serghei, A.; Liu, C.; Maazouz, A.; Lamnawar, K. Unveiling the Effects of In Situ Layer–Layer Interfacial Reaction in Multilayer Polymer Films via Multilayered Assembly: From Microlayers to Nanolayers. Macromol. Mater. Eng. 2020, 305, 2000076. [Google Scholar] [CrossRef]
- Butler, T.I.; Morris, B.A. Chapter 15—PE Based Multilayer Film Structures. In Multilayer Flexible Packaging; Wagner, J.R., Ed.; Plastics Design Library; William Andrew Publishing: Boston, MA, USA, 2010; pp. 205–230. ISBN 978-0-8155-2021-4. [Google Scholar]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2018, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and Redesign Challenges in Multilayer Flexible Food Packaging—a Review. Foods 2021, 10, 2702. [Google Scholar] [CrossRef] [PubMed]
- van Belle, A.V.; Demets, R.; Mys, N.; van Kets, K.V.; Dewulf, J.; van Geem, K.V.; de Meester, S.D.; Ragaert, K. Microstructural Contributions of Different Polyolefins to the Deformation Mechanisms of Their Binary Blends. Polymers 2020, 12, 1171. [Google Scholar] [CrossRef]
- Chen, S.-C.; Zhang, L.-H.; Zhang, G.; Zhong, G.-C.; Li, J.; Zhang, X.-M.; Chen, W.-X. An Investigation and Comparison of the Blending of LDPE and PP with Different Intrinsic Viscosities of PET. Polymers 2018, 10, 147. [Google Scholar] [CrossRef] [Green Version]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Crist, B.; Hill, M.J. Recent Developments in Phase Separation of Polyolefin Melt Blends. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 2329–2353. [Google Scholar] [CrossRef]
- Zhao, L.; Choi, P. A Review of the Miscibility of Polyethylene Blends. Mater. Manuf. Processes 2006, 21, 135–142. [Google Scholar] [CrossRef]
- Zhai, J.; Li, W.; Zha, J.; Cheng, Q.; Bian, X.; Dang, Z. Space Charge Suppression of Polyethylene Induced by Blending with Ethylene-Butyl Acrylate Copolymer. CSEE J. Power Energy Syst. 2020, 6, 152–159. [Google Scholar] [CrossRef]
- Gururajan, G.; Froude, V.; Riutta, S.; Thomas, A.; Gao, I.; Samuels, S.L.; Massouda, D.F.; Weinberg, M.; Ogale, A.A. Effect of Poly(Ethylene Methyl Acrylate) Copolymer on Thermal, Morphological, and Mechanical Properties of Polypropylene Copolymer Blown Films. J. Appl. Polym. Sci. 2008, 107, 2500–2508. [Google Scholar] [CrossRef]
- Jana, R.N.; Nando, G.B. In Situ Compatibilization of the Blends of Low-Density Polyethylene (LDPE) and Polydimethylsiloxane (PDMS) Rubber by Ethylene Methyl Acrylate (EMA) Copolymer. J. Thermoplast. Compos. Mater. 2007, 20, 75–92. [Google Scholar] [CrossRef]
- Peón, J.; Vega, J.F.; del Amo, B.; Martínez-Salazar, J. Phase Morphology and Melt Viscoelastic Properties in Blends of Ethylene/Vinyl Acetate Copolymer and Metallocene-Catalysed Linear Polyethylene. Polymers 2003, 44, 2911–2918. [Google Scholar] [CrossRef]
- Khonakdar, H.A.; Wagenknecht, U.; Jafari, S.H.; Hässler, R.; Eslami, H. Dynamic Mechanical Properties and Morphology of Polyethylene/Ethylene Vinyl Acetate Copolymer Blends. Adv. Polym. Technol. 2004, 23, 307–315. [Google Scholar] [CrossRef]
- Faker, M.; Razavi Aghjeh, M.K.; Ghaffari, M.; Seyyedi, S.A. Rheology, Morphology and Mechanical Properties of Polyethylene/Ethylene Vinyl Acetate Copolymer (PE/EVA) Blends. Eur. Polym. J. 2008, 44, 1834–1842. [Google Scholar] [CrossRef]
- Alothman, O.Y. Processing and Characterization of High Density Polyethylene/Ethylene Vinyl Acetate Blends with Different VA Contents. Adv. Mater. Sci. Eng. 2012, 2012, 635693. [Google Scholar] [CrossRef] [Green Version]
- Iannaccone, A.; Amitrano, S.; Pantani, R. Rheological and Mechanical Behavior of Ethyl Vinyl Acetate/Low Density Polyethylene Blends for Injection Molding. J. Appl. Polym. Sci. 2013, 127, 1157–1163. [Google Scholar] [CrossRef]
- Pham, N.T.-H. Characterization of Low-Density Polyethylene and LDPE-Based/Ethylene-Vinyl Acetate with Medium Content of Vinyl Acetate. Polymers 2021, 13, 2352. [Google Scholar] [CrossRef]
- Cho, K.; Jeon, H.K.; Moon, T.J. Thermal and Mechanical Properties of HDPE/Ionomer Blends. J. Mater. Sci. 1993, 28, 6650–6656. [Google Scholar] [CrossRef]
- Li, T.; Yan, N. Mechanical Properties of Wood Flour/HDPE/Ionomer Composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1–12. [Google Scholar] [CrossRef]
- Ali, S.; Ji, Y.; Zhang, Q.; Zhao, H.; Chen, W.; Wang, D.; Meng, L.; Li, L. Preparation of Polyethylene and Ethylene/Methacrylic Acid Copolymer Blend Films with Tunable Surface Properties through Manipulating Processing Parameters during Film Blowing. Polymers 2019, 11, 1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blázquez-Blázquez, E.; Pérez, E.; Lorenzo, V.; Cerrada, M.L. Crystalline Characteristics and Their Influence in the Mechanical Performance in Poly(ε-Caprolactone)/High Density Polyethylene Blends. Polymers 2019, 11, 1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishi, T.; Wang, T.T. Melting Point Depression and Kinetic Effects of Cooling on Crystallization in Poly(Vinylidene Fluoride)-Poly(Methyl Methacrylate) Mixtures. Macromolecules 1975, 8, 909–915. [Google Scholar] [CrossRef]
- Martínez-Salazar, J.; Alizadeh, A.; Jiménez, J.J.; Plans, J. On the Melting Behaviour of Polymer Single Crystals in a Mixture with a Compatible Oligomer: 2. Polyethylene/Paraffin. Polymers 1996, 37, 2367–2371. [Google Scholar] [CrossRef]
- Martínez-Salazar, J.; Cuesta, M.S.; Plans, J. On Phase Separation in High- and Low-Density Polyethylene Blends: 1. Melting-Point Depression Analysis. Polymers 1991, 32, 2984–2988. [Google Scholar] [CrossRef]
- Martín, S.; Expósito, M.T.; Vega, J.F.; Martínez-Salazar, J. Microstructure and Properties of Branched Polyethylene: Application of a Three-Phase Structural Model. J. Appl. Polym. Sci. 2013, 128, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Niaounakis, M. Recycling of Flexible Plastic Packaging; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Reynolds, P.J. A Surlyn® Ionomer as a Self-Healing and Self-Sensing Composite. Master’s Thesis, University of Birmingham, Birmingham, UK, 2012. [Google Scholar]
- Gómez, G.H.; Gasparini, T.M.; Canevarolo, S.V. Solid-State Morphology Evolution of Sodium Neutralized Poly(Ethylene-Ran-Methacrylic Acid) Ionomer Under Dry and Wet Thermal Annealing. Mater. Res. 2019, 22, e20180592. [Google Scholar] [CrossRef] [Green Version]
- Wunderlich, B.; Czornyj, G. A Study of Equilibrium Melting of Polyethylene. Macromolecules 1977, 10, 906–913. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of Fusion of Poly-β-Propiolactone and Poly-ϵ-Caprolactone. Comparative Analysis of the Melting of Aliphatic Polylactone and Polyester Chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Palierne, J.F. Linear Rheology of Viscoelastic Emulsions with Interfacial Tension. Rheol. Acta 1990, 29, 204–214. [Google Scholar] [CrossRef]
- Groves, D.J.; McLeish, T.C.B.; Chohan, R.K.; Coates, P.D. Predicting the Rheology of Linear with Branched Polyethylene Blends. Rheol. Acta 1996, 35, 481–491. [Google Scholar] [CrossRef]
- Lee, H.S.; Denn, M.M. Blends of Linear and Branched Polyethylenes. Polym. Eng. Sci. 2000, 40, 1132–1142. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Vega, J.F.; Jargour, N.; Núñez-Ramírez, R.; Liu, G.; Wang, D.; Trujillo, M.; Müller, A.J.; Martínez-Salazar, J. The Influence of Short-Chain Branching on the Morphology and Structure of Polyethylene Single Crystals. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1751–1762. [Google Scholar] [CrossRef]
- Hirai, N. A Source of Screw Dislocation on Polyethylene Single Crystals. J. Polym. Sci. 1962, 59, 321–328. [Google Scholar] [CrossRef]
- Reneker, D.H.; Geil, P.H. Morphology of Polymer Single Crystals. J. Appl. Phys. 1960, 31, 1916–1925. [Google Scholar] [CrossRef]
- Keller, A. Crystal Configurations and Their Relevance to the Crystalline Texture and Crystallization Mechanism in Polymers. Kolloid-Z 1967, 197, 98–115. [Google Scholar] [CrossRef]
- Kawai, R.U.; Ujihara, K.; Maeda, H. Fold Structure of Solution Grown Crystals of Alkyl-Branched of Polyethylene. Die Makromol. Chem. 1970, 132, 87–111. [Google Scholar] [CrossRef]
- Bair, H.E.; Salovey, R.; Huseby, T.W. Melting and Annealing of Polyethylene Single Crystals. Polymers 1967, 8, 9–20. [Google Scholar] [CrossRef]
- Leung, W.M.; John Manley, R.; Panaras, A.R. Isothermal Growth of Low Molecular Weight Polyethylene Single Crystals from Solution. 2. Melting and Dissolution Behavior. Macromolecules 1985, 18, 753–759. [Google Scholar] [CrossRef]
- Hocquet, S.; Dosière, M.; Thierry, A.; Lotz, B.; Koch, M.H.J.; Dubreuil, N.; Ivanov, D.A. Morphology and Melting of Truncated Single Crystals of Linear Polyethylene. Macromolecules 2003, 36, 8376–8384. [Google Scholar] [CrossRef]
- Organ, S.J.; Hobbs, J.K.; Miles, M.J. Reorganization and Melting of Polyethylene Single Crystals: Complementary TEM, DSC, and Real-Time AFM Studies. Macromolecules 2004, 37, 4562–4572. [Google Scholar] [CrossRef]
- Ramos, J.; Vega, J.F.; Sanmartín, S.; Martínez-Salazar, J. Coarse-Grained Simulations on the Crystallization, Melting and Annealing Processes of Short Chain Branched Polyolefins. Eur. Polym. J. 2016, 85, 478–488. [Google Scholar] [CrossRef]
- Peón, J.; Domínguez, C.; Vega, J.F.; Aroca, M.; Martínez-Salazar, J. Viscoelastic Behaviour of Metallocene-Catalysed Polyethylene and Low Density Polyethylene Blends: Use of the Double Reptation and Palierne Viscoelastic Models. J. Mater. Sci. 2003, 38, 4757–4764. [Google Scholar] [CrossRef]







| Sample | ε (%) | Mw (kg·mol−1) | Mw/Mn | MFI (g/10 min) | ρ (g·cm−3) | Tm (°C) d |
|---|---|---|---|---|---|---|
| PE0.0 | 0.00 | 158 | 2.0 | 0.3 | 0.952 | 134.2 |
| PE0.3 | 0.28 | 124 | 2.0 | 0.8 | 0.938 | 125.9 |
| PE0.5 | 0.53 | 115 | 2.0 | 0.9 | 0.931 | 121.4 |
| PE_ZN | 1.20 a/0.60 b | 104 c | 4.0 c | 1.0 | 0.918 | 121.0 |
| Sample | mol-% Comonomer | MFI (g/10 min) | Mw (kg·mol−1) d | ρ (g·cm−3) d | Tm (°C) e | α e |
|---|---|---|---|---|---|---|
| PCL | - | 1.8 b | 50.0 | 1.14 | 58.0 | 0.50 g |
| EEA | 5.0% | 20.0 c | n.d. | 0.93 | 54.5/94.9 | 0.23 f |
| EVA | 5.5% | 8.0 c | n.d. | 0.94 | 52.6/84.9 | 0.25 f |
| EMAANa a | 5.4% | 4.5 c | 95.0 | 0.97 | 62.5/81.2 | 0.16 f |
| Sample | Tci (°C) | Ts (°C) | Tc (°C) | Tm (°C) | ΔHm (J·g−1) | α |
|---|---|---|---|---|---|---|
| PE0.0 | 72 | 98 | 80 | 127.6 | 235.6 | 0.80 |
| PE0.3 | 70 | 95 | 77 | 123.7 | 212.6 | 0.73 |
| PE0.5 | 65 | 91 | 73 | 121.0 | 194.0 | 0.66 |
| PE_ZN | 67 | 93 | 60 | 122.0 | 170.3 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega, J.F.; Souza-Egipsy, V.; Expósito, M.T.; Ramos, J. Melting Temperature Depression of Polymer Single Crystals: Application to the Eco-Design of Tie-Layers in Polyolefinic-Based Multilayered Films. Polymers 2022, 14, 1622. https://doi.org/10.3390/polym14081622
Vega JF, Souza-Egipsy V, Expósito MT, Ramos J. Melting Temperature Depression of Polymer Single Crystals: Application to the Eco-Design of Tie-Layers in Polyolefinic-Based Multilayered Films. Polymers. 2022; 14(8):1622. https://doi.org/10.3390/polym14081622
Chicago/Turabian StyleVega, Juan F., Virginia Souza-Egipsy, M. Teresa Expósito, and Javier Ramos. 2022. "Melting Temperature Depression of Polymer Single Crystals: Application to the Eco-Design of Tie-Layers in Polyolefinic-Based Multilayered Films" Polymers 14, no. 8: 1622. https://doi.org/10.3390/polym14081622
APA StyleVega, J. F., Souza-Egipsy, V., Expósito, M. T., & Ramos, J. (2022). Melting Temperature Depression of Polymer Single Crystals: Application to the Eco-Design of Tie-Layers in Polyolefinic-Based Multilayered Films. Polymers, 14(8), 1622. https://doi.org/10.3390/polym14081622







