Sustainable Applications of Animal Waste Proteins
Abstract
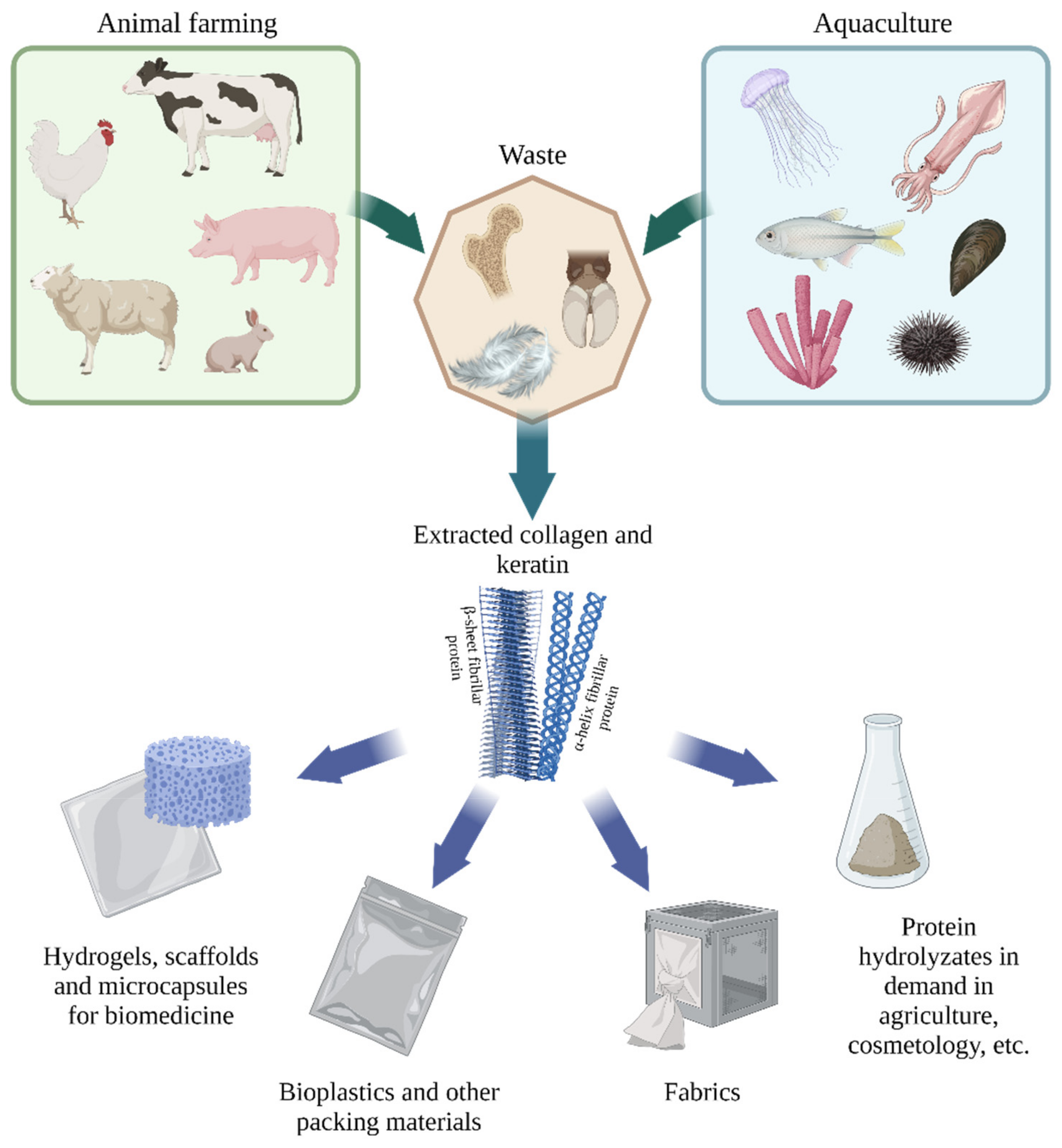
:1. Introduction
2. Main Proteins of Animal Waste
2.1. Keratin: Structure and Properties
2.1.1. Keratins of Wool
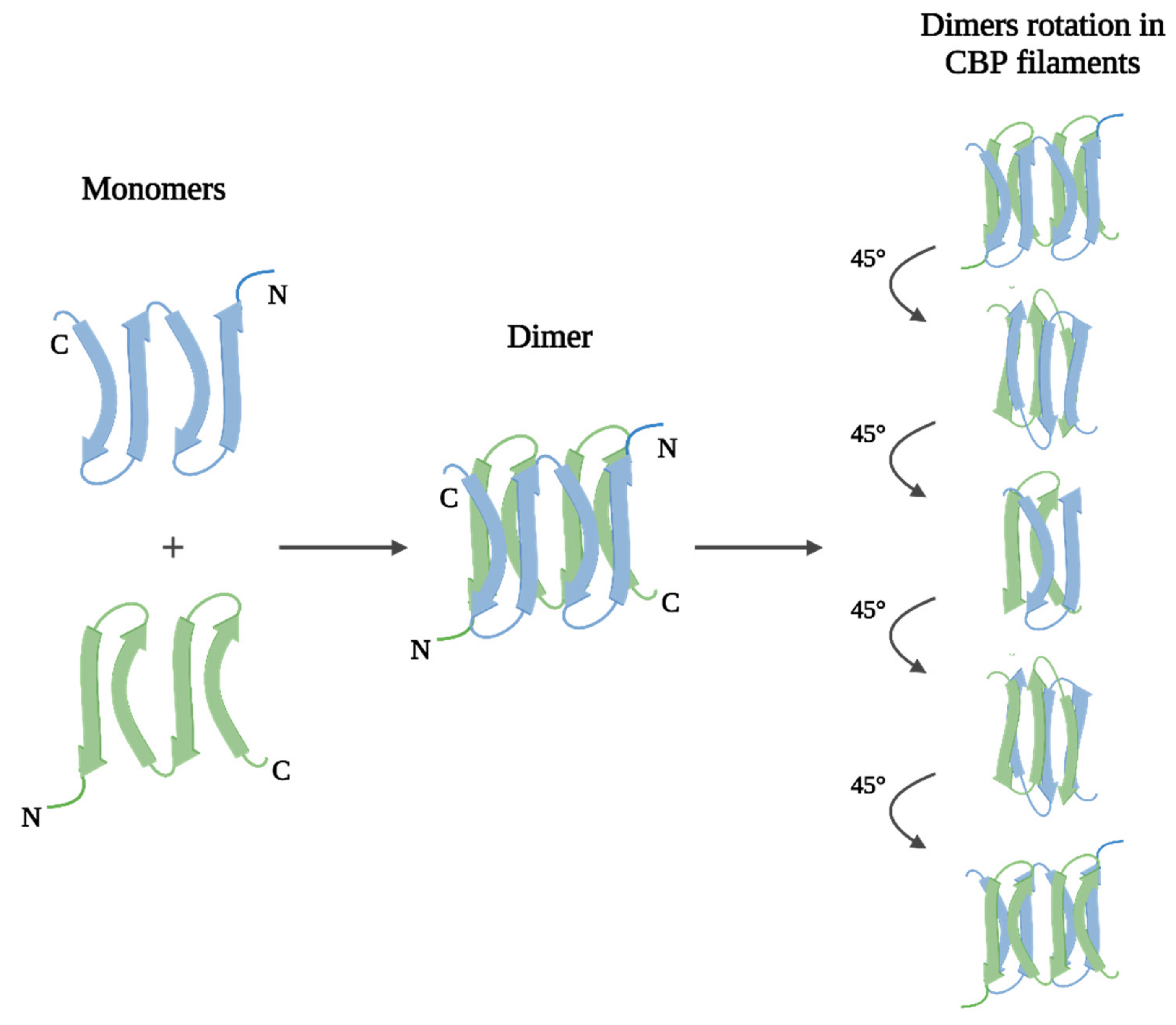
2.1.2. Keratins of Feathers
2.2. Collagen: Structure and Properties
3. Collagen and Keratin Applications
3.1. Wounds and Burns Treatment
3.2. Tissue Engineering
3.3. Drug Delivery Systems
3.4. Aesthetic Medicine and Cosmetics
3.5. Other Applications
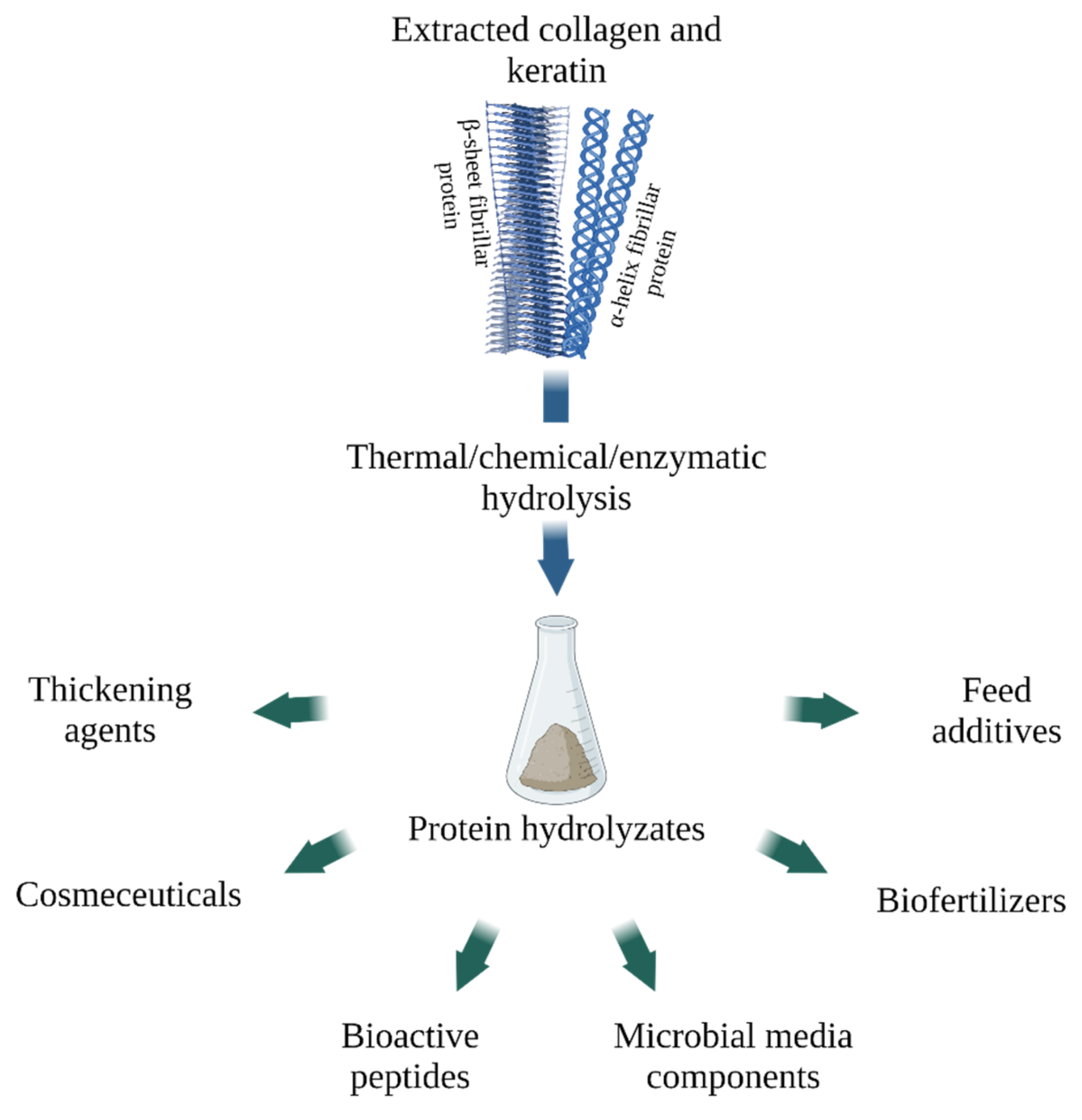
4. Animal Waste Protein Hydrolysates
4.1. Gelatin and Collagen Hydrolysate
4.2. Keratin Hydrolysate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO: Rome, Italy, 2021; pp. 14–18. [Google Scholar]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.-D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senadheera, T.R.; Dave, D.; Shahidi, F. Sea Cucumber Derived Type I Collagen: A Comprehensive Review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef] [PubMed]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; De Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [Green Version]
- Stingone, J.A.; Wing, S. Poultry Litter Incineration as a Source of Energy: Reviewing the Potential for Impacts on Environmental Health and Justice. New Solut. 2011, 21, 27–42. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D. Valorisation of chicken feathers: A review on recycling and recovery route—current status and future prospects. Clean Technol. Environ. Policy 2017, 19, 2363–2378. [Google Scholar] [CrossRef]
- Bouhamed, S.B.H.; Kechaou, N. Kinetic study of sulphuric acid hydrolysis of protein feathers. Bioprocess Biosyst. Eng. 2017, 40, 715–721. [Google Scholar] [CrossRef]
- Chiramba, R.; Charis, G.; Fungura, N.; Danha, G.; Mamvura, T. Production of activated carbon from poultry feathers for waste water treatment. Water Sci. Technol. 2019, 80, 1407–1412. [Google Scholar] [CrossRef]
- Pajarito, B.; Belarmino, A.J.; Calimbas, R.M.; Gonzales, J.R. Graphite Nanoplatelets from Waste Chicken Feathers. Materials 2020, 13, 2109. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Felix, M.; Bengoechea, C.; Guerrero, A. Proteins from Agri-Food Industrial Biowastes or Co-Products and Their Applications as Green Materials. Foods 2021, 10, 981. [Google Scholar] [CrossRef]
- Di Lullo, G.A.; Sweeney, S.M.; Körkkö, J.; Ala-Kokko, L.; Antonio, J.D.S. Mapping the Ligand-binding Sites and Disease-associated Mutations on the Most Abundant Protein in the Human, Type I Collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef] [Green Version]
- Müller, W.E.G. The Origin of Metazoan Complexity: Porifera as Integrated Animals. Integr. Comp. Biol. 2003, 43, 3–10. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef]
- Shestakova, A.; Timorshina, S.; Osmolovskiy, A. Biodegradation of Keratin-Rich Husbandry Waste as a Path to Sustainable Agriculture. Sustainability 2021, 13, 8691. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Molecular Structure, Assembly Mechanism, and Integration Into Functionally Distinct Intracellular Scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789. [Google Scholar] [CrossRef]
- Vaidya, M.M.; Kanojia, D. Keratins: Markers of cell differentiation or regulators of cell differentiation? J. Biosci. 2007, 32, 629–634. [Google Scholar] [CrossRef]
- Astbury, W.T.; Street, A. X-ray studies of the structure of hair, wool, and related fibres. I. General. Philos. Trans. R. Soc. Lond. A 1931, 230, 75–101. [Google Scholar] [CrossRef]
- Astbury, W.T.; Woods, H.J. X-ray studies of the structure of hair, wool, and related fibres. II.- the molecular structure and elastic properties of hair keratin. Philos. Trans. R. Soc. Lond. A 1933, 232, 333–394. [Google Scholar] [CrossRef]
- Parry, D.A.; Fraser, R.B.; Squire, J.M. Fifty years of coiled-coils and α-helical bundles: A close relationship between sequence and structure. J. Struct. Biol. 2008, 163, 258–269. [Google Scholar] [CrossRef]
- Parry, D.A.; Fraser, R.B.; Alibardi, L.; Rutherford, K.; Gemmell, N. Molecular structure of sauropsid β-keratins from tuatara (Sphenodon punctatus). J. Struct. Biol. 2019, 207, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreplak, L.; Doucet, J.; Dumas, P.; Briki, F. New Aspects of the α-Helix to β-Sheet Transition in Stretched Hard α-Keratin Fibers. Biophys. J. 2004, 87, 640–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alibardi, L. Cornification in reptilian epidermis occurs through the deposition of keratin-associated beta-proteins (beta-keratins) onto a scaffold of intermediate filament keratins. J. Morphol. 2013, 274, 175–193. [Google Scholar] [CrossRef]
- Alibardi, L. Sauropsids Cornification is Based on Corneous Beta-Proteins, a Special Type of Keratin-Associated Corneous Proteins of the Epidermis. J. Exp. Zool. Part B Mol. Dev. Evol. 2016, 326, 338–351. [Google Scholar] [CrossRef]
- Holthaus, K.B.; Eckhart, L.; Valle, L.D.; Alibardi, L. Review: Evolution and diversification of corneous beta-proteins, the characteristic epidermal proteins of reptiles and birds. J. Exp. Zool. Part B Mol. Dev. Evol. 2018, 330, 438–453. [Google Scholar] [CrossRef]
- Kadir, M.; Wang, X.; Zhu, B.; Liu, J.; Harland, D.; Popescu, C. The structure of the “amorphous” matrix of keratins. J. Struct. Biol. 2017, 198, 116–123. [Google Scholar] [CrossRef]
- Strasser, B.; Mlitz, V.; Hermann, M.; Tschachler, E.; Eckhart, L. Convergent evolution of cysteine-rich proteins in feathers and hair. BMC Evol. Biol. 2015, 15, 82. [Google Scholar] [CrossRef] [Green Version]
- Fraser, R.D.B.; Parry, D.A.D. Structural Hierarchy of Trichocyte Keratin Intermediate Filaments. In The Hair Fibre: Proteins, Structure and Development; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1054, pp. 57–70. [Google Scholar]
- Feroz, S.; Muhammad, N.; Ratnayake, J.; Dias, G. Keratin-Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Matsunaga, R.; Abe, R.; Ishii, D.; Watanabe, S.-I.; Kiyoshi, M.; Nöcker, B.; Tsuchiya, M.; Tsumoto, K. Bidirectional binding property of high glycine–tyrosine keratin-associated protein contributes to the mechanical strength and shape of hair. J. Struct. Biol. 2013, 183, 484–494. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Parry, D.A.D. Molecular packing in the feather keratin filament. J. Struct. Biol. 2008, 162, 1–13. [Google Scholar] [CrossRef]
- Calvaresi, M.; Eckhart, L.; Alibardi, L. The molecular organization of the beta-sheet region in Corneous beta-proteins (beta-keratins) of sauropsids explains its stability and polymerization into filaments. J. Struct. Biol. 2016, 194, 282–291. [Google Scholar] [CrossRef]
- Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A.S. Microbial enzymes catalyzing keratin degradation: Classification, structure, function. Biotechnol. Adv. 2020, 44, 107607. [Google Scholar] [CrossRef]
- Fraser, R.D.B.; Parry, D.A.D. The structural basis of the filament-matrix texture in the avian/reptilian group of hard β-keratins. J. Struct. Biol. 2011, 173, 391–405. [Google Scholar] [CrossRef]
- Sweeney, S.M.; Orgel, J.P.; Fertala, A.; McAuliffe, J.D.; Turner, K.R.; Di Lullo, G.A.; Chen, S.; Antipova, O.; Perumal, S.; Ala-Kokko, L.; et al. Candidate Cell and Matrix Interaction Domains on the Collagen Fibril, the Predominant Protein of Vertebrates. J. Biol. Chem. 2008, 283, 21187–21197. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, G.N.; Kartha, G. Structure of Collagen. Nature 1955, 176, 593–595. [Google Scholar] [CrossRef]
- Rich, A.; Crick, F.H.C. The Structure of Collagen. Nature 1955, 176, 915–916. [Google Scholar] [CrossRef]
- Protein Data Bank. Available online: https://www.rcsb.org/3d-view/ngl/1cag (accessed on 20 March 2022).
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Brazel, D.; Oberbaumer, I.; Dieringer, H.; Babel, W.; Glanville, R.W.; Deutzmann, R.; Kuhn, K. Completion of the amino acid sequence of the alpha1 chain of human basement membrane collagen (type IV) reveals 21 non-triplet interruptions located within the collagenous domain. JBIC J. Biol. Inorg. Chem. 1987, 168, 529–536. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Shah, N.K.; Brodskyb, B. Gly-X-Y Tripeptide Frequencies in Collagen: A Context for Host–Guest Triple-Helical Peptides. J. Struct. Biol. 1998, 122, 86–91. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Balakireva, A.V.; Kuznetsova, N.V.; Vukolova, M.N.; Litvitsky, P.F.; Zamyatnin, A.A., Jr. Collagenolytic Enzymes and their Applications in Biomedicine. Curr. Med. Chem. 2019, 26, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.A.B.A.; Sikarwar, A.S. Collagen: New Dimension in Cosmetic and Healthcare. Int. J. Biochem. Res. Rev. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Owczarzy, A.; Kurasiński, R.; Kulig, K.; Rogóż, W.; Szkudlarek, A.; Maciążek-Jurczyk, M. Collagen-structure, properties and application. Eng. Biomater. 2020, 23, 17–23. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Placone, J.K.; Navarro, J.; Laslo, G.W.; Lerman, M.J.; Gabard, A.R.; Herendeen, G.J.; Falco, E.E.; Tomblyn, S.; Burnett, L.; Fisher, J.P. Development and Characterization of a 3D Printed, Keratin-Based Hydrogel. Ann. Biomed. Eng. 2017, 45, 237–248. [Google Scholar] [CrossRef]
- Esparza, Y.; Ullah, A.; Wu, J. Molecular mechanism and characterization of self-assembly of feather keratin gelation. Int. J. Biol. Macromol. 2018, 107, 290–296. [Google Scholar] [CrossRef]
- Mori, H.; Hara, M. Transparent biocompatible wool keratin film prepared by mechanical compression of porous keratin hydrogel. Mater. Sci. Eng. C 2018, 91, 19–25. [Google Scholar] [CrossRef]
- Chen, M.; Ren, X.; Dong, L.; Li, X.; Cheng, H. Preparation of dynamic covalently crosslinking keratin hydrogels based on thiol/disulfide bonds exchange strategy. Int. J. Biol. Macromol. 2021, 182, 1259–1267. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Z.; Zhao, Q.; Yun, Y. Study on the Structure and Properties of Biofunctional Keratin from Rabbit Hair. Materials 2021, 14, 379. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Varga, M.; Sixta, B.; Bem, R.; Matia, I.; Jirkovska, A.; Adamec, M. Application of gentamicin-collagen sponge shortened wound healing time after minor amputations in diabetic patients—A prospective, randomised trial. Arch. Med Sci. 2014, 10, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhang, Q.; Li, Y.; Song, W.; Chen, A.; Liu, J.; Xuan, X. Collagen sponge prolongs taurine release for improved wound healing through inflammation inhibition and proliferation stimulation. Ann. Transl. Med. 2021, 9, 1010. [Google Scholar] [CrossRef]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef]
- Konop, M.; Laskowska, A.; Rybka, M.; Kłodzińska, E.; Sulejczak, D.; Schwartz, R.; Czuwara, J. Keratin Scaffolds Containing Casomorphin Stimulate Macrophage Infiltration and Accelerate Full-Thickness Cutaneous Wound Healing in Diabetic Mice. Molecules 2021, 26, 2554. [Google Scholar] [CrossRef]
- Tang, L.; Sierra, J.O.; Kelly, R.; Kirsner, R.S.; Li, J. Wool-derived keratin stimulates human keratinocyte migration and types IV and VII collagen expression. Exp. Dermatol. 2012, 21, 458–460. [Google Scholar] [CrossRef]
- Poranki, D.; Whitener, W.; Howse, S.; Mesen, T.; Howse, E.; Burnell, J.; Greengauz-Roberts, O.; Molnar, J.; Van Dyke, M. Evaluation of skin regeneration after burns in vivo and rescue of cells after thermal stress in vitro following treatment with a keratin biomaterial. J. Biomater. Appl. 2014, 29, 26–35. [Google Scholar] [CrossRef]
- Roy, D.C.; Tomblyn, S.; Isaac, K.M.; Kowalczewski, C.J.; Burmeister, D.M.; Burnett, L.R.; Christy, R.J. Ciprofloxacin-loaded keratin hydrogels reduce infection and support healing in a porcine partial-thickness thermal burn. Wound Repair Regen. 2016, 24, 657–668. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Orto, V.C.D.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, X.; Li, Z.; Liu, D.; Xue, X. Fabrication of pH-responsive TA-keratin bio-composited hydrogels encapsulated with photoluminescent GO quantum dots for improved bacterial inhibition and healing efficacy in wound care management: In vivo wound evaluations. J. Photochem. Photobiol. B Biol. 2020, 202, 111676. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Clohessy, R.M.; Holder, R.C.; Gabard, A.R.; Herendeen, G.J.; Christy, R.J.; Burnett, L.R.; Fisher, P.J.P. In Vivo Evaluation of Three-Dimensional Printed, Keratin-Based Hydrogels in a Porcine Thermal Burn Model. Tissue Eng. Part A 2020, 26, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.; Li, Y.; Yao, Y.; Yang, X.; Cao, Z.; Nie, H.; Yang, G. Injectable keratin hydrogels as hemostatic and wound dressing materials. Biomater. Sci. 2021, 9, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Yang, X.; Cao, Z.; Nie, H.; Bian, Y.; Yang, G. Glucose-triggered in situ forming keratin hydrogel for the treatment of diabetic wounds. Acta Biomater. 2021, 125, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, W.; Deng, J.; Kan, J.; Guo, T.; Wang, B.; Hao, S. Recombinant Human Hair Keratin Nanoparticles Accelerate Dermal Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 18681–18690. [Google Scholar] [CrossRef]
- Than, M.P.; Smith, R.A.; Cassidy, S.; Kelly, R.; Marsh, C.; Maderal, A.; Kirsner, R.S. Use of a keratin-based hydrogel in the management of recessive dystrophic epidermolysis bullosa. J. Dermatol. Treat. 2013, 24, 290–291. [Google Scholar] [CrossRef]
- Denyer, J.; Marsh, C.; Kirsner, R.S. Keratin gel in the management of Epidermolysis bullosa. J. Wound Care 2015, 24, 446–450. [Google Scholar] [CrossRef]
- Yesilova, Y.; Turan, E.; Aksoy, M.; Tanrikulu, O.; Eroglu, N.; Sürücü, H. Lack of effectiveness of keratin dressings in epidermolysis bullosa. Indian J. Dermatol. Venereol. Leprol. 2015, 81, 91–92. [Google Scholar] [CrossRef]
- Zhai, M.; Xu, Y.; Zhou, B.; Jing, W. Keratin-chitosan/n-ZnO nanocomposite hydrogel for antimicrobial treatment of burn wound healing: Characterization and biomedical application. J. Photochem. Photobiol. B Biol. 2018, 180, 253–258. [Google Scholar] [CrossRef]
- Azarniya, A.; Tamjid, E.; Eslahi, N.; Simchi, A. Modification of bacterial cellulose/keratin nanofibrous mats by a tragacanth gum-conjugated hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 134, 280–289. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. Fabrication and characterisation of melt-extruded chitosan/keratin/PCL/PEG drug-eluting sutures designed for wound healing. Mater. Sci. Eng. C 2021, 120, 111696. [Google Scholar] [CrossRef]
- Han, X.; Yang, R.; Wan, X.; Dou, J.; Yuan, J.; Chi, B.; Shen, J. Antioxidant and multi-sensitive PNIPAAm/keratin double network gels for self-stripping wound dressing application. J. Mater. Chem. B 2021, 9, 6212–6225. [Google Scholar] [CrossRef]
- Moay, Z.; Nguyen, L.; Hartrianti, P.; Lunny, D.; Leavesley, D.; Kok, Y.; Chong, S.; Chua, A.; Tee, S.-I.; Ng, K. Keratin-Alginate Sponges Support Healing of Partial-Thickness Burns. Int. J. Mol. Sci. 2021, 22, 8594. [Google Scholar] [CrossRef]
- Luo, T.; Hao, S.; Chen, X.; Wang, J.; Yang, Q.; Wang, Y.; Weng, Y.; Wei, H.; Zhou, J.; Wang, B. Development and assessment of kerateine nanoparticles for use as a hemostatic agent. Mater. Sci. Eng. C 2016, 63, 352–358. [Google Scholar] [CrossRef]
- Wang, J.; Hao, S.; Luo, T.; Yang, Q.; Wang, B. Development of feather keratin nanoparticles and investigation of their hemostatic efficacy. Mater. Sci. Eng. C 2016, 68, 768–773. [Google Scholar] [CrossRef]
- Cheng, Z.; Qing, R.; Hao, S.; Ding, Y.; Yin, H.; Zha, G.; Chen, X.; Ji, J.; Wang, B. Fabrication of ulcer-adhesive oral keratin hydrogel for gastric ulcer healing in a rat. Regen. Biomater. 2021, 8, rbab008. [Google Scholar] [CrossRef]
- Kafi, A.; Aktar, M.K.; Phanny, Y.; Todo, M. Adhesion, proliferation and differentiation of human mesenchymal stem cell on chitosan/collagen composite scaffold. J. Mater. Sci. Mater. Med. 2019, 30, 131. [Google Scholar] [CrossRef]
- Oh, G.-W.; Nguyen, V.-T.; Heo, S.-Y.; Ko, S.-C.; Kim, C.S.; Park, W.S.; Choi, I.-W.; Jung, W.-K. 3D PCL/fish collagen composite scaffolds incorporating osteogenic abalone protein hydrolysates for bone regeneration application: In Vitro and In Vivo studies. J. Biomater. Sci. Polym. Ed. 2021, 32, 355–371. [Google Scholar] [CrossRef]
- Hu, K.; Hu, M.; Xiao, Y.; Cui, Y.; Yan, J.; Yang, G.; Zhang, F.; Lin, G.; Yi, H.; Han, L.; et al. Preparation recombination human-like collagen/fibroin scaffold and promoting the cell compatibility with osteoblasts. J. Biomed. Mater. Res. Part A 2021, 109, 346–353. [Google Scholar] [CrossRef]
- Hwangbo, H.; Lee, H.; Roh, E.J.; Kim, W.; Joshi, H.P.; Kwon, S.Y.; Choi, U.Y.; Han, I.-B.; Kim, G.H. Bone tissue engineering via application of a collagen/hydroxyapatite 4D-printed biomimetic scaffold for spinal fusion. Appl. Phys. Rev. 2021, 8, 21403. [Google Scholar] [CrossRef]
- Ryan, E.J.; Ryan, A.J.; Vázquez, A.G.G.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, E.R.; Nourbakhsh, N.; Kenari, M.A.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.J.; Garrett, J.P.; Sierpinski, P.; Ma, J.; Atala, A.; Smith, T.L.; Koman, L.A.; Van Dyke, M.E. Peripheral Nerve Regeneration Using a Keratin-Based Scaffold: Long-Term Functional and Histological Outcomes in a Mouse Model. J. Hand Surg. 2008, 33, 1541–1547. [Google Scholar] [CrossRef]
- Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008, 29, 118–128. [Google Scholar] [CrossRef]
- Hill, P.S.; Apel, P.J.; Barnwell, J.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M. Repair of Peripheral Nerve Defects in Rabbits Using Keratin Hydrogel Scaffolds. Tissue Eng. Part A 2011, 17, 1499–1505. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Ramadan, M.; Van Dyke, M.; Kokai, L.E.; Philips, B.J.; Rubin, J.P.; Marra, K.G. Keratin Gel Filler for Peripheral Nerve Repair in a Rodent Sciatic Nerve Injury Model. Plast. Reconstr. Surg. 2012, 129, 67–78. [Google Scholar] [CrossRef]
- Pace, L.A.; Plate, J.F.; Mannava, S.; Barnwell, J.C.; Koman, L.A.; Li, Z.; Smith, T.L.; Van Dyke, M. A Human Hair Keratin Hydrogel Scaffold Enhances Median Nerve Regeneration in Nonhuman Primates: An Electrophysiological and Histological Study. Tissue Eng. Part A 2014, 20, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhang, L.; Wei, Y.; Chen, T.; Ji, X.; Ye, K.; Yu, J.; Tang, B.; Sun, X.; Hu, J. Human hair keratins promote the regeneration of peripheral nerves in a rat sciatic nerve crush model. J. Mater. Sci. Mater. Med. 2019, 30, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.R.; Costa, J.B.; Costa, L.; Silva-Correia, J.; Moay, Z.K.; Ng, K.W.; Reis, R.L.; Oliveira, J.M. Enhanced performance of chitosan/keratin membranes with potential application in peripheral nerve repair. Biomater. Sci. 2019, 7, 5451–5466. [Google Scholar] [CrossRef]
- Shen, D.; Wang, X.; Zhang, L.; Zhao, X.; Li, J.; Cheng, K.; Zhang, J. The amelioration of cardiac dysfunction after myocardial infarction by the injection of keratin biomaterials derived from human hair. Biomaterials 2011, 32, 9290–9299. [Google Scholar] [CrossRef]
- Tomblyn, S.; Kneller, E.L.P.; Walker, S.J.; Ellenburg, M.D.; Kowalczewski, C.J.; Van Dyke, M.; Burnett, L.; Saul, J.M. Keratin hydrogel carrier system for simultaneous delivery of exogenous growth factors and muscle progenitor cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 864–879. [Google Scholar] [CrossRef] [Green Version]
- Passipieri, J.; Baker, H.; Siriwardane, M.; Ellenburg, M.D.; Vadhavkar, M.; Saul, J.M.; Tomblyn, S.; Burnett, L.; Christ, G.J. Keratin Hydrogel Enhances In Vivo Skeletal Muscle Function in a Rat Model of Volumetric Muscle Loss. Tissue Eng. Part A 2017, 23, 556–571. [Google Scholar] [CrossRef]
- Baker, H.; Passipieri, J.; Siriwardane, M.; Ellenburg, M.D.; Vadhavkar, M.; Bergman, C.R.; Saul, J.M.; Tomblyn, S.; Burnett, L.; Christ, G.J. Cell and Growth Factor-Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss in a Mouse Model. Tissue Eng. Part A 2017, 23, 572–584. [Google Scholar] [CrossRef]
- Barati, D.; Kader, S.; Shariati, S.R.P.; Moeinzadeh, S.; Sawyer, R.H.; Jabbari, E. Synthesis and Characterization of Photo-Cross-Linkable Keratin Hydrogels for Stem Cell Encapsulation. Biomacromolecules 2017, 18, 398–412. [Google Scholar] [CrossRef]
- Sharma, L.A.; Love, R.; Ali, M.A.; Sharma, A.; Macari, S.; Avadhani, A.; Dias, G.J. Healing Response of Rat pulp Treated with an Injectable Keratin Hydrogel. J. Appl. Biomater. Funct. Mater. 2017, 15, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Duncan, W.J.; Greer, P.F.C.; Lee, M.-H.; Loch, C.; Gay, J.H.A. Wool-derived keratin hydrogel enhances implant osseointegration in cancellous bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2447–2454. [Google Scholar] [CrossRef]
- Cohen, D.J.; Hyzy, S.L.; Haque, S.; Olson, L.C.; Boyan, B.D.; Saul, J.M.; Schwartz, Z. Effects of Tunable Keratin Hydrogel Erosion on Recombinant Human Bone Morphogenetic Protein 2 Release, Bioactivity, and Bone Induction. Tissue Eng. Part A 2018, 24, 1616–1630. [Google Scholar] [CrossRef]
- Bloise, N.; Patrucco, A.; Bruni, G.; Montagna, G.; Caringella, R.; Fassina, L.; Tonin, C.; Visai, L. In Vitro Production of Calcified Bone Matrix onto Wool Keratin Scaffolds via Osteogenic Factors and Electromagnetic Stimulus. Materials 2020, 13, 3052. [Google Scholar] [CrossRef]
- Zhao, X.; Lui, Y.S.; Choo, C.K.C.; Sow, W.T.; Huang, C.L.; Ng, K.W.; Tan, L.P.; Loo, J.S.C. Calcium phosphate coated Keratin–PCL scaffolds for potential bone tissue regeneration. Mater. Sci. Eng. C 2015, 49, 746–753. [Google Scholar] [CrossRef]
- Arslan, Y.E.; Arslan, T.S.; Derkus, B.; Emregul, E.; Emregul, K.C. Fabrication of human hair keratin/jellyfish collagen/eggshell-derived hydroxyapatite osteoinductive biocomposite scaffolds for bone tissue engineering: From waste to regenerative medicine products. Colloids Surf. B Biointerfaces 2017, 154, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Dias, G. Hydroxypropylmethyl cellulose (HPMC) crosslinked keratin/hydroxyapatite (HA) scaffold fabrication, characterization and in vitro biocompatibility assessment as a bone graft for alveolar bone regeneration. Heliyon 2021, 7, e08294. [Google Scholar] [CrossRef] [PubMed]
- Cal, F.; Arslan, T.S.; Derkus, B.; Kiran, F.; Cengiz, U.; Arslan, Y.E. Synthesis of Silica-Based Boron-Incorporated Collagen/Human Hair Keratin Hybrid Cryogels with the Potential Bone Formation Capability. ACS Appl. Bio Mater. 2021, 4, 7266–7279. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, Y.-K.; Tang, K.-C.; Yang, K.-C.; Cheng, N.-C.; Yu, J. Keratin scaffolds with human adipose stem cells: Physical and biological effects toward wound healing. J. Tissue Eng. Regen. Med. 2019, 13, 1044–1058. [Google Scholar] [CrossRef]
- Parker, R.N.; Trent, A.; Stefaniak, K.L.R.; Van Dyke, M.E.; Grove, T.Z. A comparative study of materials assembled from recombinant K31 and K81 and extracted human hair keratins. Biomed. Mater. 2020, 15, 65006. [Google Scholar] [CrossRef]
- Suarato, G.; Contardi, M.; Perotto, G.; Heredia-Guerrero, J.A.; Fiorentini, F.; Ceseracciu, L.; Pignatelli, C.; Debellis, D.; Bertorelli, R.; Athanassiou, A. From fabric to tissue: Recovered wool keratin/polyvinylpyrrolidone biocomposite fibers as artificial scaffold platform. Mater. Sci. Eng. C 2020, 116, 111151. [Google Scholar] [CrossRef]
- Das, A.; Das, A.; Basu, A.; Datta, P.; Gupta, M.; Mukherjee, A. Newer guar gum ester/chicken feather keratin interact films for tissue engineering. Int. J. Biol. Macromol. 2021, 180, 339–354. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Foo, S.E.M.; Tan, N.S.; Yuan, Y.; Lin, W.; Zhang, Z.; Ng, K.W. Culturing Fibroblasts in 3D Human Hair Keratin Hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 5187–5198. [Google Scholar] [CrossRef]
- Kakkar, P.; Madhan, B. Fabrication of keratin-silica hydrogel for biomedical applications. Mater. Sci. Eng. C 2016, 66, 178–184. [Google Scholar] [CrossRef]
- Wu, P.; Dai, X.; Chen, K.; Li, R.; Xing, Y. Fabrication of regenerated wool keratin/polycaprolactone nanofiber membranes for cell culture. Int. J. Biol. Macromol. 2018, 114, 1168–1173. [Google Scholar] [CrossRef]
- Bajestani, M.I.; Kader, S.; Monavarian, M.; Mousavi, S.M.; Jabbari, E.; Jafari, A. Material properties and cell compatibility of poly(γ-glutamic acid)-keratin hydrogels. Int. J. Biol. Macromol. 2020, 142, 790–802. [Google Scholar] [CrossRef]
- Thompson, M.; Giuffre, A.; McClenny, C.; Van Dyke, M. A keratin-based microparticle for cell delivery. J. Biomater. Appl. 2021, 35, 579–591. [Google Scholar] [CrossRef]
- Esparza, Y.; Bandara, N.; Ullah, A.; Wu, J. Hydrogels from feather keratin show higher viscoelastic properties and cell proliferation than those from hair and wool keratins. Mater. Sci. Eng. C 2018, 90, 446–453. [Google Scholar] [CrossRef]
- Fujioka, K.; Takada, Y.; Sato, S.; Miyata, T. Novel delivery system for proteins using collagen as a carrier material: The minipellet. J. Control. Release 1995, 33, 307–315. [Google Scholar] [CrossRef]
- Higaki, M.; Azechi, Y.; Takase, T.; Igarashi, R.; Nagahara, S.; Sano, A.; Fujioka, K.; Nakagawa, N.; Aizawa, C.; Mizushima, Y. Collagen minipellet as a controlled release delivery system for tetanus and diphtheria toxoid. Vaccine 2001, 19, 3091–3096. [Google Scholar] [CrossRef]
- Le, V.-M.; Lang, M.-D.; Shi, W.-B.; Liu, J.-W. A collagen-based multicellular tumor spheroid model for evaluation of the efficiency of nanoparticle drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 540–544. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Phan, T.T.V.; Kim, H.H.; Nguyen, T.P.; Oh, J. Rapid microwave-assisted synthesis of gold loaded hydroxyapatite collagen nano-bio materials for drug delivery and tissue engineering application. Ceram. Int. 2019, 45, 2977–2988. [Google Scholar] [CrossRef]
- Di Martino, A.; Drannikov, A.; Surgutskaia, N.S.; Ozaltin, K.; Postnikov, P.S.; Marina, T.E.; Sedlarik, V. Chitosan-collagen based film for controlled delivery of a combination of short life anesthetics. Int. J. Biol. Macromol. 2019, 140, 1183–1193. [Google Scholar] [CrossRef]
- Leng, Q.; Li, Y.; Pang, X.; Wang, B.; Wu, Z.; Lu, Y.; Xiong, K.; Zhao, L.; Zhou, P.; Fu, S. Curcumin nanoparticles incorporated in PVA/collagen composite films promote wound healing. Drug Deliv. 2020, 27, 1676–1685. [Google Scholar] [CrossRef]
- Geanaliu-Nicolae, R.-E.; Andronescu, E. Blended Natural Support Materials—Collagen Based Hydrogels Used in Biomedicine. Materials 2020, 13, 5641. [Google Scholar] [CrossRef]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A porous collagen-based hydrogel and implantation method for corneal stromal regeneration and sustained local drug delivery. Sci. Rep. 2020, 10, 16936. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ronsin, O.; Gravez, B.; Farman, N.; Baumberger, T.; Jaisser, F.; Coradin, T.; Hélary, C. Nanostructured Dense Collagen-Polyester Composite Hydrogels as Amphiphilic Platforms for Drug Delivery. Adv. Sci. 2021, 8, 2004213. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ham, T.R.; Haque, S.; Sparks, J.L.; Saul, J.M. Alkylation of human hair keratin for tunable hydrogel erosion and drug delivery in tissue engineering applications. Acta Biomater. 2015, 23, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.L.P.; González, J.A.; Fabian, L.; Perez, C.J.; Villanueva, M.E.; Copello, G.J. Sustainable and smart keratin hydrogel with pH-sensitive swelling and enhanced mechanical properties. Mater. Sci. Eng. C 2017, 78, 619–626. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, Y.; Li, Y.; Yang, X.; Cao, Z.; Yang, G. Tunable keratin hydrogel based on disulfide shuffling strategy for drug delivery and tissue engineering. J. Colloid Interface Sci. 2019, 544, 121–129. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Z.; Zhang, H.; Ma, X.; Su, W.; Sun, X.; Li, X. Bio-responsive alginate-keratin composite nanogels with enhanced drug loading efficiency for cancer therapy. Carbohydr. Polym. 2017, 175, 159–169. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Z.; Cui, X.; Chen, X.; Su, W.; Ren, X.; Li, X. Tumor-targeted and nitric oxide-generated nanogels of keratin and hyaluronan for enhanced cancer therapy. Nanoscale 2018, 10, 12109–12122. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Zhi, X.; Li, P.; Jiang, X.; Yuan, J. Triple stimuli-responsive keratin nanoparticles as carriers for drug and potential nitric oxide release. Mater. Sci. Eng. C 2018, 91, 606–614. [Google Scholar] [CrossRef]
- Aluigi, A.; Ballestri, M.; Guerrini, A.; Sotgiu, G.; Ferroni, C.; Corticelli, F.; Gariboldi, M.B.; Monti, E.; Varchi, G. Organic solvent-free preparation of keratin nanoparticles as doxorubicin carriers for antitumour activity. Mater. Sci. Eng. C 2018, 90, 476–484. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, P. One-Pot Synthesis of Chicken-Feather-Keratin-Based Prodrug Nanoparticles with High Drug Content for Tumor Intracellular DOX Delivery. Langmuir 2019, 35, 8007–8014. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, P. Bio-inspired keratin-based core-crosslinked micelles for pH and reduction dual-responsive triggered DOX delivery. Int. J. Biol. Macromol. 2019, 123, 1150–1156. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, M.; Liu, P. pH-Activated surface charge-reversal double-crosslinked hyaluronic acid nanogels with feather keratin as multifunctional crosslinker for tumor-targeting DOX delivery. Int. J. Biol. Macromol. 2020, 150, 1104–1112. [Google Scholar] [CrossRef]
- Gaio, E.; Guerrini, A.; Ballestri, M.; Varchi, G.; Ferroni, C.; Martella, E.; Columbaro, M.; Moret, F.; Reddi, E. Keratin nanoparticles co-delivering Docetaxel and Chlorin e6 promote synergic interaction between chemo- and photo-dynamic therapies. J. Photochem. Photobiol. B Biol. 2019, 199, 111598. [Google Scholar] [CrossRef]
- Gong, X.; Dang, G.; Guo, J.; Liu, Y.; Gong, Y. A sodium alginate/feather keratin composite fiber with skin-core structure as the carrier for sustained drug release. Int. J. Biol. Macromol. 2020, 155, 386–392. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, X.; Zhai, D.; Gao, F.; Guo, T.; Li, W.; Hao, S.; Ji, J.; Wang, B. Development of keratin nanoparticles for controlled gastric mucoadhesion and drug release. J. Nanobiotechnol. 2018, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- Peyton, C.C.; Keys, T.; Tomblyn, S.; Burmeister, D.; Beumer, J.H.; Holleran, J.L.; Sirintrapun, J.; Washburn, S.; Hodges, S.J. Halofuginone infused keratin hydrogel attenuates adhesions in a rodent cecal abrasion model. J. Surg. Res. 2012, 178, 545–552. [Google Scholar] [CrossRef]
- Zhu, Q.; Gong, Y.; Guo, T.; Deng, J.; Ji, J.; Wang, B.; Hao, S. Thermo-sensitive keratin hydrogel against iron-induced brain injury after experimental intracerebral hemorrhage. Int. J. Pharm. 2019, 566, 342–351. [Google Scholar] [CrossRef]
- Lusiana; Reichl, S.; Müller-Goymann, C.C. Keratin film made of human hair as a nail plate model for studying drug permeation. Eur. J. Pharm. Biopharm. 2011, 78, 432–440. [Google Scholar] [CrossRef]
- Lusiana; Reichl, S.; Müller-Goymann, C.C. Infected nail plate model made of human hair keratin for evaluating the efficacy of different topical antifungal formulations against Trichophyton rubrum in vitro. Eur. J. Pharm. Biopharm. 2013, 84, 599–605. [Google Scholar] [CrossRef]
- Valkov, A.; Zinigrad, M.; Sobolev, A.; Nisnevitch, M. Keratin Biomembranes as a Model for Studying Onychomycosis. Int. J. Mol. Sci. 2020, 21, 3512. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A.K. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Zhang, Q.; Ma, J. New soft tissue filler derived from autologous keratin and fibroblast for neck wrinkles. J. Cosmet. Dermatol. 2018, 17, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Leichner, C.; Steinbring, C.; Baus, R.A.; Baecker, D.; Gust, R.; Bernkop-Schnürch, A. Reactive keratin derivatives: A promising strategy for covalent binding to hair. J. Colloid Interface Sci. 2019, 534, 533–541. [Google Scholar] [CrossRef]
- Baus, R.A.; Leichner, C.; Steinbring, C.; Bernkop-Schnürch, A. Strategies for improved hair binding: Keratin fractions and the impact of cationic substructures. Int. J. Biol. Macromol. 2020, 160, 201–211. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Miller, A.T.; Bridgewater, N.J. Collagen Sausage Casing. U.S. Patent 4,388,331, 14 June 1983. [Google Scholar]
- Lionetto, F.; Corcione, C.E. Recent Applications of Biopolymers Derived from Fish Industry Waste in Food Packaging. Polymers 2021, 13, 2337. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Sharma, S.; Gupta, A.; Alashwal, B.Y. Keratin based bioplastic film from chicken feathers and its characterization. Int. J. Biol. Macromol. 2018, 111, 352–358. [Google Scholar] [CrossRef]
- Fernández-D’Arlas, B. Tough and Functional Cross-linked Bioplastics from Sheep Wool Keratin. Sci. Rep. 2019, 9, 14810. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers 2020, 12, 158. [Google Scholar] [CrossRef] [Green Version]
- Pulidori, E.; Micalizzi, S.; Bramanti, E.; Bernazzani, L.; Duce, C.; De Maria, C.; Montemurro, F.; Pelosi, C.; De Acutis, A.; Vozzi, G.; et al. One-Pot Process: Microwave-Assisted Keratin Extraction and Direct Electrospinning to Obtain Keratin-Based Bioplastic. Int. J. Mol. Sci. 2021, 22, 9597. [Google Scholar] [CrossRef]
- Yang, W.; Shan, Z. Application of wool keratin: An anti-ultraviolet wall material in spray drying. J. Food Sci. Technol. 2021, 58, 4235–4244. [Google Scholar] [CrossRef]
- Brenner, M.; Weichold, O. Autogenous Cross-Linking of Recycled Keratin from Poultry-Feather Waste to Hydrogels for Plant-Growth Media. Polymers 2021, 13, 3581. [Google Scholar] [CrossRef]
- Soydan, A.S.; Günaydin, G.K.; Ergezer, H.; Palamutcu, S. Moisture management and antimicrobial performance of collagen peptide enriched knitted fabrics. J. Text. Inst. 2021, 112, 1023–1036. [Google Scholar] [CrossRef]
- Hou, E.-J.; Huang, C.-S.; Lee, Y.-C.; Chu, H.-T. Upcycled aquaculture waste as textile ingredient for promoting circular economy. Sustain. Mater. Technol. 2022, 31, e00336. [Google Scholar] [CrossRef]
- Song, K.; Xu, H.; Xu, L.; Xie, K.; Yang, Y. Cellulose nanocrystal-reinforced keratin bioadsorbent for effective removal of dyes from aqueous solution. Bioresour. Technol. 2017, 232, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Qian, X.; Li, X.; Zhao, Y.; Yu, Z. Fabrication of a novel functional CNC cross-linked and reinforced adsorbent from feather biomass for efficient metal removal. Carbohydr. Polym. 2019, 222, 115016. [Google Scholar] [CrossRef]
- Zhu, W.; Qian, X.; Yu, H.; Li, X.; Song, K. Fabrication of mechanical robust keratin adsorbent by induced molecular network transition and its dye adsorption performance. Environ. Sci. Pollut. Res. 2020, 27, 41577–41584. [Google Scholar] [CrossRef]
- Song, K.; Qian, X.; Zhu, X.; Li, X.; Hong, X. Fabrication of mechanical robust keratin film by mesoscopic molecular network reconstruction and its performance for dye removal. J. Colloid Interface Sci. 2020, 579, 28–36. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, F.; Zhu, S.; Lin, Y.; Meng, Z.; Yu, R.; Liu, X.Y. Meso-Reconstruction of Wool Keratin 3D “Molecular Springs” for Tunable Ultra-Sensitive and Highly Recovery Strain Sensors. Small 2020, 16, e2000128. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sypka, M.; Jodłowska, I.; Białkowska, A.M. Keratinases as Versatile Enzymatic Tools for Sustainable Development. Biomolecules 2021, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Larichev, T.; Kozlova, O.; Prosekov, A.; Babich, O. Comparative Analysis of Collagen-Containing Waste Biodegradation, Amino Acid, Peptide and Carbohydrate Composition of Hydrolysis Products. Appl. Sci. 2021, 11, 11511. [Google Scholar] [CrossRef]
- Baehaki, A.; Suhartono, M.T.; Sukarno; Syah, D.; Setyahadi, S. Antioxidant activity of collagen hydrolysates from fish skin with a microbial collagenase. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1677–1682. [Google Scholar]
- Harris, M.; Potgieter, J.; Ishfaq, K.; Shahzad, M. Developments for Collagen Hydrolysate in Biological, Biochemical, and Biomedical Domains: A Comprehensive Review. Materials 2021, 14, 2806. [Google Scholar] [CrossRef]
- Keenan, T.R. Polymer Science: A Comprehensive Reference; Elsevier Science: Amsterdam, The Netherlands, 2012; 7760p. [Google Scholar]
- Jin, H.-X.; Xu, H.-P.; Li, Y.; Zhang, Q.-W.; Xie, H. Preparation and Evaluation of Peptides with Potential Antioxidant Activity by Microwave Assisted Enzymatic Hydrolysis of Collagen from Sea Cucumber Acaudina Molpadioides Obtained from Zhejiang Province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.-H.; Zhang, X.-Y.; Wang, Z.; Zhang, X.; Liu, S.-C.; Song, X.-Y.; Zhang, Y.-Z.; Ding, J.-M.; Chen, X.-L.; Xu, F. Potential of Thermolysin-like Protease A69 in Preparation of Bovine Collagen Peptides with Moisture-Retention Ability and Antioxidative Activity. Mar. Drugs 2021, 19, 676. [Google Scholar] [CrossRef]
- Eckert, T.; Jährling-Butkus, M.; Louton, H.; Burg-Roderfeld, M.; Zhang, R.; Zhang, N.; Hesse, K.; Petridis, A.K.; Kožár, T.; Steinmeyer, J.; et al. Efficacy of Chondroprotective Food Supplements Based on Collagen Hydrolysate and Compounds Isolated from Marine Organisms. Mar. Drugs 2021, 19, 542. [Google Scholar] [CrossRef]
- Khatri, M.; Naughton, R.J.; Clifford, T.; Harper, L.D.; Corr, L. The effects of collagen peptide supplementation on body composition, collagen synthesis, and recovery from joint injury and exercise: A systematic review. Amino Acids 2021, 53, 1493–1506. [Google Scholar] [CrossRef]
- Jendricke, P.; Centner, C.; Zdzieblik, D.; Gollhofer, A.; König, D. Specific Collagen Peptides in Combination with Resistance Training Improve Body Composition and Regional Muscle Strength in Premenopausal Women: A Randomized Controlled Trial. Nutrients 2019, 11, 892. [Google Scholar] [CrossRef] [Green Version]
- Kirmse, M.; Oertzen-Hagemann, V.; De Marées, M.; Bloch, W.; Platen, P. Prolonged Collagen Peptide Supplementation and Resistance Exercise Training Affects Body Composition in Recreationally Active Men. Nutrients 2019, 11, 1154. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.; Sanz, N.; Sánzhez, A.C.; Correa, B.; Pérez-Martín, R.I.; Sotelo, C.G. Molecular Weight Analysis of Blue Shark (Prionace glauca) Collagen Hydrolysates by GPC-LS.; Effect of High Molecular Weight Hydrolysates on Fibroblast Cultures: mRNA Collagen Type I Expression and Synthesis. Int. J. Mol. Sci. 2021, 23, 32. [Google Scholar] [CrossRef]
- González-Serrano, D.J.; Hadidi, M.; Varcheh, M.; Jelyani, A.Z.; Moreno, A.; Lorenzo, J.M. Bioactive Peptide Fractions from Collagen Hydrolysate of Common Carp Fish Byproduct: Antioxidant and Functional Properties. Antioxidants 2022, 11, 509. [Google Scholar] [CrossRef]
- Brook, M.; Scaife, P.; Bass, J.; Cegielski, J.; Watanabe, S.; Wilkinson, D.; Smith, K.; Phillips, B.; Atherton, P. A collagen hydrolysate/milk protein-blend stimulates muscle anabolism equivalently to an isoenergetic milk protein-blend containing a greater quantity of essential amino acids in older men. Clin. Nutr. 2021, 40, 4456–4464. [Google Scholar] [CrossRef]
- Prokopová, A.; Pavlačková, J.; Mokrejš, P.; Gál, R. Collagen Hydrolysate Prepared from Chicken By-Product as a Functional Polymer in Cosmetic Formulation. Molecules 2021, 26, 2021. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Jiménez-Alvarado, R.; Aguirre-Álvarez, G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- De Miranda, R.B.; Weimer, P.; Rossi, R.C. Effects of hydrolyzed collagen supplementation on skin aging: A systematic review and meta-analysis. Int. J. Dermatol. 2021, 60, 1449–1461. [Google Scholar] [CrossRef]
- Taskin, M.; Kurbanoglu, E. Evaluation of waste chicken feathers as peptone source for bacterial growth. J. Appl. Microbiol. 2011, 111, 826–834. [Google Scholar] [CrossRef]
- Stiborova, H.; Branska, B.; Vesela, T.; Lovecka, P.; Stranska, M.; Hajslova, J.; Jiru, M.; Patakova, P.; Demnerova, K. Transformation of raw feather waste into digestible peptides and amino acids. J. Chem. Technol. Biotechnol. 2016, 91, 1629–1637. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Nakhshiniev, B.; Zaini, I.N.; Saidov, N.; Takahashi, F.; Yoshikawa, K. Characterization of potential liquid fertilizers obtained by hydrothermal treatment of chicken feathers. Environ. Prog. Sustain. Energy 2018, 37, 375–382. [Google Scholar] [CrossRef]
- Tasaki, K. A novel thermal hydrolysis process for extraction of keratin from hog hair for commercial applications. Waste Manag. 2020, 104, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Skopinska-Wiśniewska, J.; Kozlowska, J.; Płanecka, A.; Kurzawa, M. Photochemical behaviour of hydrolysed keratin. Int. J. Cosmet. Sci. 2011, 33, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Holkar, C.R.; Jadhav, A.J.; Bhavsar, P.S.; Kannan, S.; Pinjari, D.V.; Pandit, A.B. Acoustic Cavitation Assisted Alkaline Hydrolysis of Wool Based Keratins To Produce Organic Amendment Fertilizers. ACS Sustain. Chem. Eng. 2016, 4, 2789–2796. [Google Scholar] [CrossRef]
- Mokrejš, P.; Svoboda, P.; Hrnčiřík, J.; Janacova, D.; Vasek, V. Processing poultry feathers into keratin hydrolysate through alkaline-enzymatic hydrolysis. Waste Manag. Res. J. A Sustain. Circ. Econ. 2011, 29, 260–267. [Google Scholar] [CrossRef]
- Callegaro, K.; Brandelli, A.; Daroit, D.J. Beyond plucking: Feathers bioprocessing into valuable protein hydrolysates. Waste Manag. 2019, 95, 399–415. [Google Scholar] [CrossRef]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Biotransformation of keratin waste to amino acids and active peptides based on cell-free catalysis. Biotechnol. Biofuels 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Mokrejš, P.; Huťťa, M.; Pavlačková, J.; Egner, P. Preparation of Keratin Hydrolysate from Chicken Feathers and Its Application in Cosmetics. J. Vis. Exp. 2017, 129, e56254. [Google Scholar] [CrossRef]
- Jin, H.-S.; Song, K.; Baek, J.-H.; Lee, J.-E.; Kim, D.J.; Nam, G.-W.; Kang, N.J.; Lee, D.-W. Identification of Matrix Metalloproteinase-1-Suppressive Peptides in Feather Keratin Hydrolysate. J. Agric. Food Chem. 2018, 66, 12719–12729. [Google Scholar] [CrossRef]
- Kshetri, P.; Roy, S.S.; Chanu, S.B.; Singh, T.S.; Tamreihao, K.; Sharma, S.K.; Ansari, M.; Prakash, N. Valorization of chicken feather waste into bioactive keratin hydrolysate by a newly purified keratinase from Bacillus sp. RCM-SSR-102. J. Environ. Manag. 2020, 273, 111195. [Google Scholar] [CrossRef]
- Tamreihao, K.; Mukherjee, S.; Khunjamayum, R.; Devi, L.J.; Asem, R.S.; Ningthoujam, D.S. Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J. Basic Microbiol. 2019, 59, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Gaidau, C.; Stanca, M.; Niculescu, M.-D.; Alexe, C.-A.; Becheritu, M.; Horoias, R.; Cioineag, C.; Râpă, M.; Stanculescu, I.R. Wool Keratin Hydrolysates for Bioactive Additives Preparation. Materials 2021, 14, 4696. [Google Scholar] [CrossRef]
- Kshetri, P.; Roy, S.S.; Sharma, S.; Singh, T.S.; Alam Ansari, M.; Prakash, N.; Ngachan, S.V. Transforming Chicken Feather Waste into Feather Protein Hydrolysate Using a Newly Isolated Multifaceted Keratinolytic Bacterium Chryseobacterium sediminis RCM-SSR-7. Waste Biomass Valorization 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Hassan, M.A.; Taha, T.H.; Hamad, G.M.; Hashem, M.; Alamri, S.; Mostafa, Y.S. Biochemical characterisation and application of keratinase from Bacillus thuringiensis MT1 to enable valorisation of hair wastes through biosynthesis of vitamin B-complex. Int. J. Biol. Macromol. 2020, 153, 561–572. [Google Scholar] [CrossRef]
- Mokrejs, P.; Hutta, M.; Pavlackova, J.; Egner, P.; Benicek, L. The cosmetic and dermatological potential of keratin hydrolysate. J. Cosmet. Dermatol. 2017, 16, e21–e27. [Google Scholar] [CrossRef]
- Wan, M.-Y.; Dong, G.; Yang, B.-Q.; Feng, H. Identification and characterization of a novel antioxidant peptide from feather keratin hydrolysate. Biotechnol. Lett. 2016, 38, 643–649. [Google Scholar] [CrossRef]
- Cavello, I.; Bezus, B.; Cavalitto, S. The keratinolytic bacteria Bacillus cytotoxicus as a source of novel proteases and feather protein hydrolysates with antioxidant activities. J. Genet. Eng. Biotechnol. 2021, 19, 107. [Google Scholar] [CrossRef]
- Callegaro, K.; Welter, N.; Daroit, D.J. Feathers as bioresource: Microbial conversion into bioactive protein hydrolysates. Process Biochem. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Kaur, M.; Bhari, R.; Singh, R.S. Chicken feather waste-derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia 2021, 76, 1807–1815. [Google Scholar] [CrossRef]
- Bálint, B.; Bagi, Z.; Tóth, A.; Rákhely, G.; Perei, K.; Kovács, K.L. Utilization of keratin-containing biowaste to produce biohydrogen. Appl. Microbiol. Biotechnol. 2005, 69, 404–410. [Google Scholar] [CrossRef]
- Serna-Cock, L.; Rengifo-Guerrero, C.A.; Rojas-Restrepo, M.A. Use of Earthworm (Eisenia foetida) Flour and Hydrolyzed Chicken Feathers as Sources of Nitrogen and Minerals for Ethanol Production. Waste Biomass Valorization 2018, 9, 1513–1522. [Google Scholar] [CrossRef]


| Type | Location | Class |
|---|---|---|
| I | tendons, skin, artery walls, cornea, bones | Fibrillar |
| II | cartilage, vitreous body | Fibrillar |
| III | skin, vessel wall, reticular fibres, intestines, uterus | Fibrillar |
| IV | basal membrane, capillaries | Network-forming |
| V | skin, placenta, cornea, bones | Fibrillar |
| VI | bones, vessels, skin, cornea, cartilage | Network-forming |
| VII | skin, mucous membranes, bladder, umbilical cord, amniotic fluid | FACIT 1 |
| VIII | skin, heart, brain, kidneys, vessels, cartilage, bones | Network-forming |
| IX | cornea, vitreous body, cartilage | FACIT |
| X | cartilage | Network-forming |
| XI | cartilage, intervertebral discs | Fibrillar |
| XII | tendons, skin, cartilage | FACIT |
| XIII | skeletal muscles, heart, eye, skin, endothelial cells | MACIT 2 |
| XIV | skin, nerves, tendons, bones, cartilage | FACIT |
| XV | skin, capillary vessels, heart, ovaries, testicles, placenta, kidneys | MULTIPLEXINs 3 |
| XVI | skin, heart, kidneys, smooth muscle | FACIT |
| XVII | hemi desmosomes in epitelia | MACIT |
| XVIII | liver, kidneys, lungs | MULTIPLEXINs |
| XIX | skin, kidneys, liver, placenta, spleen, prostate gland | FACIT |
| XX | corneal epithelium | FACIT |
| XXI | stomach, skeletal muscles, kidneys, vessels, heart, placenta | FACIT |
| XXII | tissue junctions | FACIT |
| XXIII | metastatic carcinogenic cells | MACIT |
| XXIV | brain, muscle, kidneys, liver, lungs, ovaries, testicles, bones | Fibrillar |
| XXV | brain, eye, heart, testicles | MACIT |
| XXVI | testicles, ovaries | FACIT |
| XXVII | cartilage | Fibrillar |
| XXVIII | skin, nerves | Network-forming |
| XXIX | skin | Network-forming |
| Tissue Engineering Area | Biocomposite Type | References |
|---|---|---|
| Nerve regeneration | Keratin hydrogels and sponges | [87,88,89,90,91,92] |
| Chitosan/keratin membranes | [93] | |
| Muscle regeneration, including cardiac ones | Keratin hydrogels | [94,95,96,97] |
| Bone and joint regeneration, including dental implantation | Keratin hydrogels | [98,99,100,101,102] |
| Keratin-polycaprolactone composites coating calcium phosphate | [103] | |
| Keratin/collagen/hydroxyapatite scaffolds | [104] | |
| Hydroxypropyl methylcellulose crosslinked keratin scaffold, containing hydroxyapatite | [105] | |
| Boron- and silicon-incorporated collagen/keratin cryogels | [106] | |
| Skin regeneration | Keratin materials | [107,108] |
| Keratin/polyvinylpyrrolidone scaffold | [109] | |
| Guar gum ester/keratin films | [110] |
| Sources | Extraction Method | Claimed Properties | Recommended or Current Applications | Ref. 1 |
|---|---|---|---|---|
| Sea cucumber Acaudina Molpadioides | Enzymatic (neutrase) | Antioxidant activity | Biomedicines and functional foods | [172] |
| Bovine bones | Enzymatic (thermolysin-like protease A69) | Moisture-retention ability and antioxidant activity | Cosmetics, biomedicines and functional foods | [173] |
| Marine (commercial drug Fortigel® by Gelita AG, Eberbach, Germany) | Combined chemical and enzymatic | Chondroprotective properties | Osteoarthritis treatment | [174] |
| Not specified (commercial drug Bodybalance® by Gelita AG, Eberbach, Germany) | Combined chemical and enzymatic | Improving body composition and regional muscle strength | Biologically active additives (functional foods) | [175,176,177] |
| Blue shark Prionace glauca | Enzymatic (papain, alcalase) | Increasing effect on mRNA collagen type I expression and pro-collagen I production | Cosmetics, biomedicines and functional foods | [178] |
| Common carp fish Cyprinus carpio byproduct | Enzymatic (alcalase) | Antioxidant activity | Biomedicines and functional foods | [179] |
| Not specified (combined food supplement containing collagen hydrolyzate Fresubin® 3.2 kcal DRINK) | Not specified | Stimulation of muscle anabolism | Functional foods | [180] |
| Chicken stomachs (cosmetic gel formulation) | Enzymatic (Protamex® (Novozymes, Copenhagen, Denmark)) | Increasing skin elasticity, decreasing skin roughness, reduction of wrinkles | Cosmetics | [181] |
| Sources | Extraction Method | Claimed Properties | Recommended or Current Applications | Ref. 1 |
|---|---|---|---|---|
| Chicken feathers | Alkaline-enzymatic | Skin moisturizing ability | Cosmetics | [193] |
| Poultry feathers | Enzymatic (extremophilic bacteria keratinase) | Matrix metalloproteinase-1-suppressive activity | Biomedicines, cosmetics | [194] |
| Chicken feathers | Enzymatic (Bacillus sp. RCM-SSR-102 keratinase) | Antioxidant and antityrosinase activity | Biomedicines, cosmetics and functional foods | [195] |
| Chicken feathers | Enzymatic (bacterial keratinase) | Increasing the water holding capacity, N, carbon (C) and mineral content of the soil, enhancing seed germination and growth of plant | Biofertilizers | [196] |
| Sheep wool | Alkaline-enzymatic (Protamex, Esperase (Novozymes, Copenhagen, Denmark), and Valkerase (BioResource International, Durham, NC, USA) | Antimicrobial activity, plant growth stimulation | Biofertilizers | [197] |
| Chicken feathers | Enzymatic (Chryseobacterium sediminis RCM-SSR-7 keratinase) | High digestibility due to the high concentration in the composition of essential amino acids and trace elements (phosphorus, potassium, calcium and iron) | Functional foods, biofertilizers | [198] |
| Donkey hairs | Enzymatic (Bacillus thuringiensis keratinase) | Suitable amino acids composition for Saccharomyces cerevisiae strain ATCC 64712 to produce vitamin B-complex | Microbial media preparation | [199] |
| Poultry feathers | Alkaline and enzymatic (Pseudomonas sp. keratinase) | Complete, low-cost alternative to other microorganism culture media | Microbial media preparation | [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timorshina, S.; Popova, E.; Osmolovskiy, A. Sustainable Applications of Animal Waste Proteins. Polymers 2022, 14, 1601. https://doi.org/10.3390/polym14081601
Timorshina S, Popova E, Osmolovskiy A. Sustainable Applications of Animal Waste Proteins. Polymers. 2022; 14(8):1601. https://doi.org/10.3390/polym14081601
Chicago/Turabian StyleTimorshina, Svetlana, Elizaveta Popova, and Alexander Osmolovskiy. 2022. "Sustainable Applications of Animal Waste Proteins" Polymers 14, no. 8: 1601. https://doi.org/10.3390/polym14081601
APA StyleTimorshina, S., Popova, E., & Osmolovskiy, A. (2022). Sustainable Applications of Animal Waste Proteins. Polymers, 14(8), 1601. https://doi.org/10.3390/polym14081601







