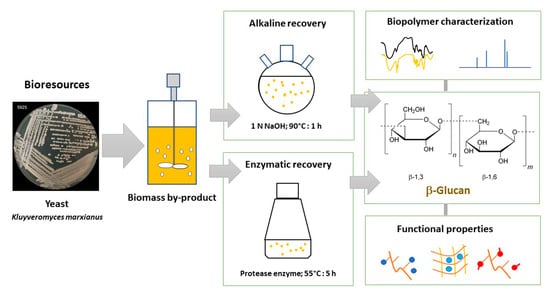
Functionality of Yeast β-Glucan Recovered from Kluyveromyces marxianus by Alkaline and Enzymatic Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Biomass Cultivation
2.3. Recovery of β-Glucan (BG) from Yeast Biomass
2.3.1. Alkaline Recovery of BG
2.3.2. Enzymatic Recovery of BG
2.4. Determination of BG Content
2.5. Composition Analysis of BG Extract
2.6. BG Recovery
2.7. Analysis of Residual Protein
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
2.9. Nuclear Magnetic Resonance Spectroscopy (NMR) of BG
2.10. In Vitro Functional Properties of BG
2.10.1. Water-Holding Capacity (WHC)
2.10.2. Water-Binding Capacity (WBC)
2.10.3. Swelling Capacity (SC)
2.10.4. Oil-Holding Capacity (OHC)
2.10.5. Glucose Adsorption Capacity (GAC)
2.11. Statistical Analysis
3. Results and Discussion
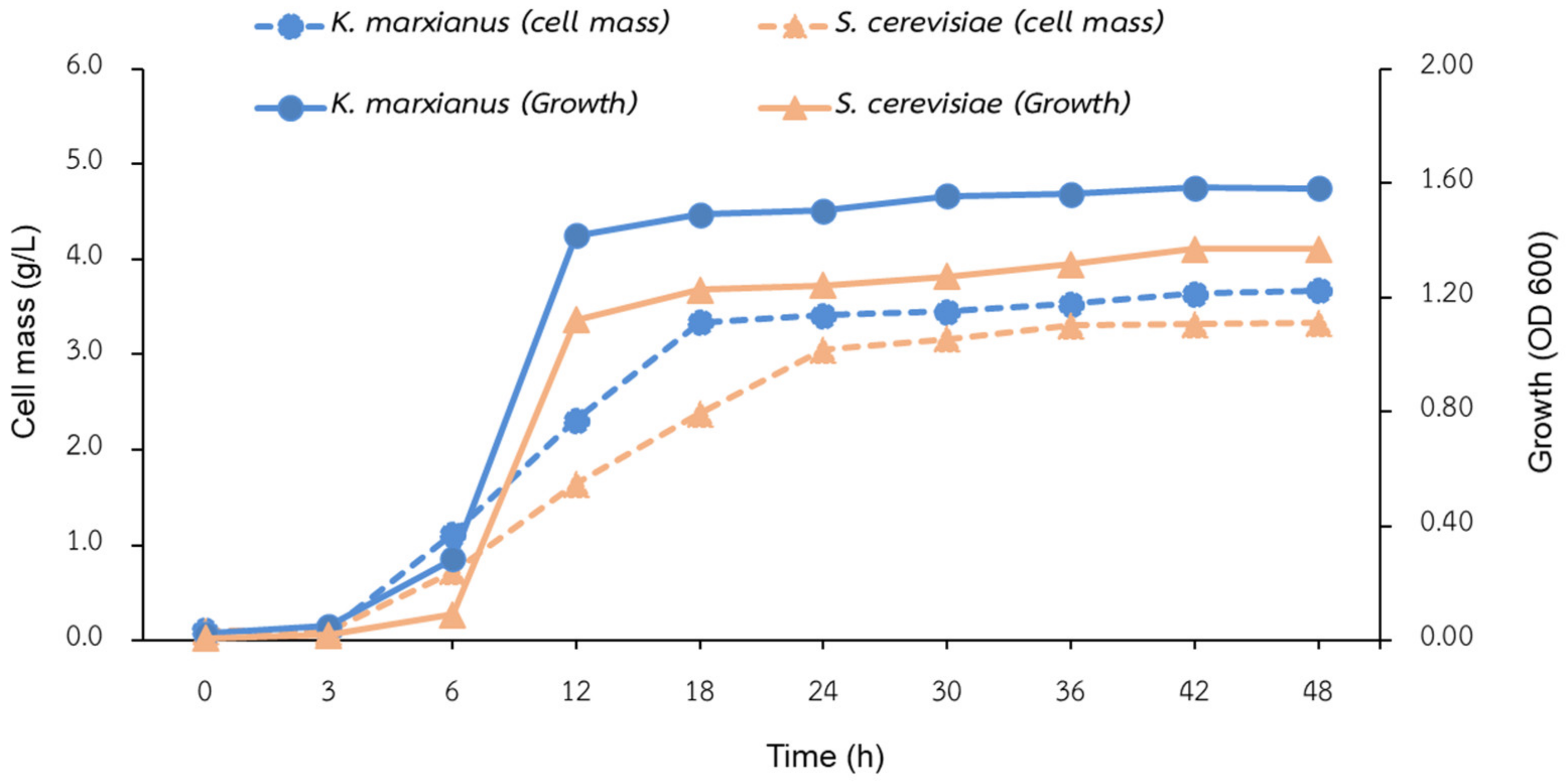
3.1. Biomass Cultivation
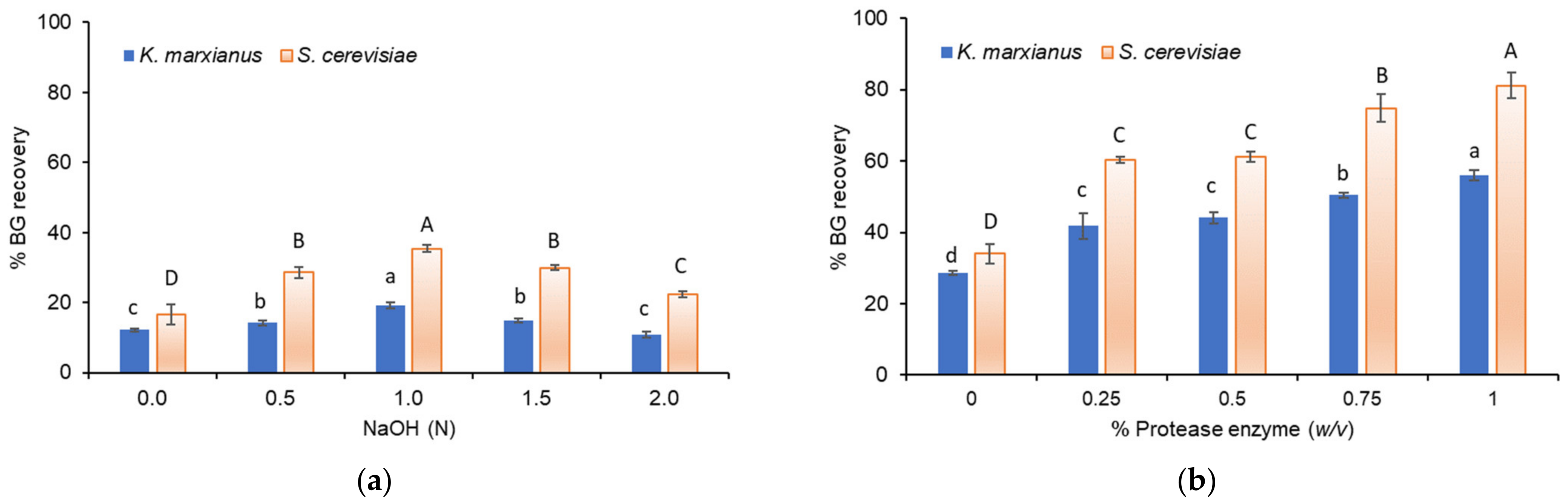
3.2. Recovery of BG from Yeast Biomass
3.3. Composition of K. marxianus, S. cerevisiae and Commercially Available BG
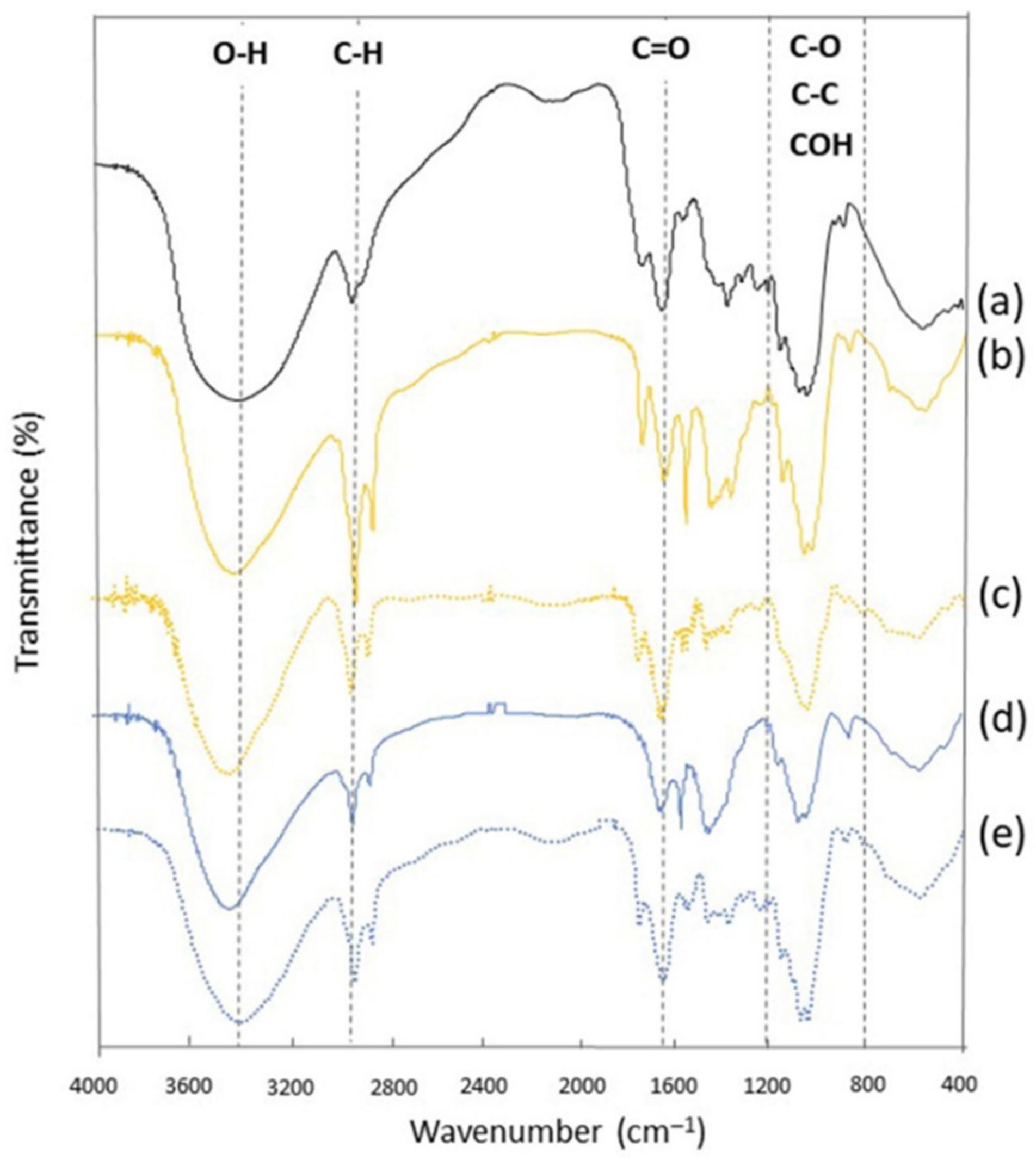
3.4. FTIR Spectra of BG Obtained by Alkaline and Enzymatic Recovery
3.5. Nuclear Magnetic Resonance Spectroscopy (NMR) of BG
3.6. In Vitro Functional Properties of BG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worrasinchai, S.; Suphantharika, M.; Pinjai, S.; Jamnong, P. Glucan prepared from spent brewer’s yeast as a fat replacer in mayonnaise. Food Hydrocoll. 2006, 20, 68–78. [Google Scholar] [CrossRef]
- Geller, A.; Shrestha, R.; Yan, J. Yeast-Derived β-glucan in cancer: Novel uses of a traditional therapeutic. Int. J. Mol. Sci. 2019, 20, 3618. [Google Scholar] [CrossRef] [PubMed]
- Bacha, U.; Nasir, M.; Iqbal, S.; Anjum, A.A. Nutraceutical, anti-inflammatory, and immune modulatory effects of β-glucan isolated from yeast. BioMed Res. Int. 2017, 2017, 8972678. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Y.; Zou, S.; Li, M.; Xu, X. Orally administered baker’s yeast β-glucan promotes glucose and lipid homeostasis in the livers of obesity and diabetes model mice. J. Agric. Food Chem. 2017, 65, 9665–9674. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Effects of yeast-derived β-glucans on blood cholesterol and macrophage functionality. J. Immunotoxicol. 2009, 6, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Liu, Y.; Shao, Y.; Ma, Y.; Wu, Y.; Guo, F.; Abbas, W.; Guo, Y.; Wang, Z. Yeast β-glucan altered intestinal microbiome and metabolome in older hens. Front. Microbiol. 2021, 12, 766878. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent brewer’s Yeast as a source of insoluble β-glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef] [PubMed]
- Apiwatanapiwat, W.; Rugthaworn, P.; Vaithanomsat, P.; Thanapase, W.; Kosugi, A.; Arai, T.; Mori, Y.; Murata, Y. Ethanol production at high temperature from cassava pulp by a newly isolated Kluyveromyces marxianus strain, TISTR5925. AIMS Energy 2013, 1, 3–16. [Google Scholar] [CrossRef][Green Version]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant activity of β-glucan. ISRN Pharm. 2012, 2012, 125864. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural features, modification, and functionalities of beta-glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef]
- Suphantharika, M.; Khunrae, P.; Thanardkit, P.; Verduyn, C. Preparation of spent brewer’s yeast β-glucans with a potential application as an immunostimulant for black tiger shrimp, Penaeus monodon. Bioresour. Technol. 2003, 88, 55–60. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Sukatta, U.; Choeyklin, R.; Boonpratuang, T.; Rukthaworn, P.; Apiwatanapiwat, W.; Boondaeng, A.; Janchai, P. Biological activities of the mycelial crude and β-glucan extracts of Auricularia cornea. Int. J. ChemTech Res. 2021, 14, 147–161. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Sangeethapriya, M.; Siddhuruju, P. Health related functional characteristics and antioxidant potential of mucilage (dietary fiber) from Zizyphus mauritiana fruits. Food Sci. Hum. Wellness 2014, 3, 79–88. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Physico-chemical properties and antioxidant activities of dietary fiber derived from defatted rice bran. Adv. J. Food Sci. Technol. 2011, 3, 339–347. [Google Scholar]
- Peerajit, P.; Chiewchan, N.; Devahastin, S. Effects of pretreatment methods on health-related functional properties of high dietary fibre powder from lime residues. Food Chem. 2012, 132, 1891–1898. [Google Scholar] [CrossRef]
- Chotigavin, N.; Sriphochanart, W.; Yaiyen, S.; Kudan, S. Increasing the Production of β-Glucan from Saccharomyces carlsbergensis RU01 by Using Tannic Acid. Appl. Biochem. Biotechnol. 2021, 193, 2591–2601. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, Q.; Cui, S.W.; Liu, H.Z. A new isolation method of β-d-glucans from spent yeast Saccharomyces cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Cho, K.C.; White, P.J. Enzymatic analysis of β-glucan content in different oat genotypes. Cereal Chem. 1993, 70, 539–542. [Google Scholar]
- Ragaee, S.; Campbell, G.; Scoles, G.; McLeod, J.; Tyler, R. Studies on rye (Secale cereale L.) lines exhibiting a range of extract viscosities. 1. Composition, molecular weight distribution of water extracts, and biochemical characteristics of purified water-extractable arabinoxylan. J. Agric. Food Chem. 2001, 49, 2437–2445. [Google Scholar] [CrossRef]
- Barsanti, L.; Vismara, R.; Passarelli, V.; Gualtieri, P. Paramylon (β-1,3-glucan) content in wild type and WZSL mutant of Euglena gracilis. Effects of growth conditions. J. Appl. Phycol. 2001, 13, 59–65. [Google Scholar] [CrossRef]
- Bobadilla, F.; Rodriguez-Tirado, C.; Imarai, M.; Galotto, M.J.; Andersson, R. Soluble β-1,3/1, 6-glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Carbohydr. Polym. 2013, 92, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Kim, Y.S. Water-solubility of β-glucans in various edible mushrooms-research note. Cereal Chem. 2005, 10, 294–297. [Google Scholar] [CrossRef]
- Xiao, M.; Jiang, M.; Wu, K.; Yang, H.; Ni, X.; Yan, W.; Jiang, F. Investigation on curdlan dissociation by heating in water. Food Hydrocoll. 2017, 70, 57–64. [Google Scholar] [CrossRef]
- Jamas, S.; Easson, D.D.; Ostroff, G.R. Glucan Preparation. U.S. Patent 5,622,939, 22 April 1997. [Google Scholar]
- Thanardkit, P.; Khunrae, P.; Suphantharika, M.; Verduyn, C. Glucan from spent brewer’s yeast: Preparation, analysis and use as a potential immunostimulant in shrimp feed. World J. Microbiol. Biotechnol. 2002, 18, 527–539. [Google Scholar] [CrossRef]
- Zechner-Krpan, V.; Petravic-Tominac, V.; Gospodaric, I.; Sajli, L.; Dakovic, S.; Filipovic-Grcic, J. Characterization of β-glucans isolated from brewer’s yeast and dried by different methods. Food Technol. Biotechnol. 2010, 48, 189–197. [Google Scholar]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar]
- Wang, Y.J.; Yao, S.J.; Guan, Y.X.; Wu, T.X.; Kennedy, J.F. A novel process for preparation of (1 → 3)-β-D-glucan sulphate by a heterogeneous reaction and its structural elucidation. Carbohydr. Polym. 2005, 59, 1. [Google Scholar] [CrossRef]
- Mangolim, C.S.; da Silva, T.T.; Fenelon, V.C.; do Nascimento, A.; Sato, F.; Matioli, G. Use of FT-IR, FT-Raman and thermal analysis to evaluate the gel formation of curdlan produced by Agrobacterium sp. IFO 13140 and determination of its rheological properties with food applicability. Food Chem. 2017, 232, 369–378. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Kawarska, A.; Stasiak-Różańska, L.; Gientka, I.; Majewska, E. Evaluation of the efficiency of different disruption methods on yeast cell wall preparation for β-glucan isolation. Molecules 2014, 19, 20941–20961. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Xiong, Z.; Wang, G.; Xia, Y.; Lai, P.; Ai, L. Structural characterization and rheological properties of β-D-glucan from hull-less barley (Hordeum vulgare L. var. nudum Hook. f.). Phytochemistry 2018, 155, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Mıckova, K.; Jablonský, A.S.; Ivan, J.; Jiří, S.; Vladimír, E.; Eliška, K.; Jana, C. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Størseth, T.R.; Kirkvold, S.; Skjermo, J.; Reitan, K.I. A branched β-d-(1 → 3, 1 → 6)-glucan from the marine diatom Chaetoceros debilis (Bacillariophyceae) characterized by NMR. Carbohydr. Res. 2006, 341, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.M.; Steluti, R.M.; Dekker, R.F.H.; Cardoso, M.S.; da Silva, M.L.C. Structural characterization of botryosphaeran: A (1 → 3; 1 → 6)-β-D-glucan produced by the ascomyceteous fungus, Botryosphaeria sp. Carbohydr. Res. 2003, 338, 1691–1698. [Google Scholar] [CrossRef]
- Dong, Q.; Yao, J.; Yang, X.; Fang, J. Structural characterization of a water-soluble β-D-glucan from fruiting bodies of Agaricus blazei Murr. Carbohydr. Res. 2002, 337, 1417–1421. [Google Scholar] [CrossRef]
- Saito, H.; Yokoi, M.; Yoshioka, Y. Effect of hydration on conformational change or stabilization of (1 → 3)-β-D-glucans of various chain lengths in the solid state as studied by high resolution solid-state 13CNMR spectroscopy. Macromolecules 1989, 22, 3892–3898. [Google Scholar] [CrossRef]
- Fairweather, J.K.; Him, J.L.K.; Heux, L.; Driguez, H.; Bulone, V. Structural characterization by 13C-NMR spectroscopy of products synthesized in vitro by polysaccharide synthases using 13C-enriched glycosyl donors: Application to a UDP glucose: (1 → 3) β-D-glucan synthase from blackberry (Rubus fruticosus). Glycobiology 2004, 14, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.L.; Ferreira, M.C.; Marv˜ao, M.R.; Rocha, J. Determination of the degree of acetylation of chitin materials by 13C CP/MAS NMR spectroscopy. Int. J. Biol. Macromol. 2001, 28, 359–363. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13CNMR. Carbohydr. Polym. 2004, 58, 409–416. [Google Scholar] [CrossRef]
- López, G.; Ros, G.; Rincón, F. Relationship between physical and hydration properties of soluble and insoluble fiber of artichoke. J. Agric. Food Chem. 1996, 44, 2773–2778. [Google Scholar] [CrossRef]
- Lan, G.S.; Chen, H.X.; Chen, S.H.; Tian, J.G. Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food Res. Int. 2012, 49, 406–410. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Lukondeh, T.; Ashbolt, N.J.; Rogers, P.L.; Hook, J.M. NMR confirmation of an alkali-insoluble glucan from Kluyveromyces marxianus cultivated on a lactose-based medium. World J. Microbiol. Biotechnol. 2003, 19, 349–355. [Google Scholar] [CrossRef]
- Petravić-Tominac, V.; Zechner-Krpan, V.; Grba, S.; Srečec, S.; Panjkota-Krbavčić, I.; Vidović, L. Biological effects of yeast β-glucans. Agric. Conspec. Sci. 2010, 75, 149–158. [Google Scholar]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [PubMed]


| BG Sources | Original BG in Yeast Biomass (g/100 g) | Recovery Method | Yield (%) | BG in Extract (g/100 g) |
|---|---|---|---|---|
| K. marxianus TISTR 5925 | 9.05 ± 0.42 b | Alkaline method | 6.48 ± 0.58 c | 43.40 ± 0.87 b |
| Enzymatic method | 20.22 ± 0.31 b | 44.91 ± 0.60 b | ||
| S. cerevisiae Kyokai NO. 9 | 11.87 ± 0.51 a | Alkaline method | 8.88 ± 0.39 c | 43.55 ± 0.41 b |
| Enzymatic method | 29.87 ± 0.47 a | 43.84 ± 0.79 b | ||
| Commercial (S. cerevisiae) | - | NA | - | 48.69 ± 0.63 a |
| Composition | BG Sources | ||||
|---|---|---|---|---|---|
| K. marxianus TISTR 5925 | S. cerevisiae Kyokai NO. 9 | Commercial (S. cerevisiae) | |||
| Alkaline Method | Enzymatic Method | Alkaline Method | Enzymatic Method | ||
| Moisture (%) | 5.72 ± 0.01 b | 5.62 ± 0.01 b | 6.89 ± 0.01 a | 6.75 ± 0.01 a | 5.30 ± 0.01 c |
| Carbohydrate (%) | 67.21 ± 0.31 a | 52.05 ± 0.33 e | 65.16 ± 0.11 b | 54.59 ± 0.36 d | 59.29 ± 0.23 c |
| Fiber (%) | 18.58 ± 0.04 e | 21.64 ± 0.09 a | 19.93 ± 0.04 d | 21.34 ± 0.07 b | 20.76 ± 0.02 c |
| Protein (%) | 4.96 ± 0.13 d | 14.80 ± 0.25 a | 3.79 ± 0.09 e | 11.96 ± 0.39 b | 11.61 ± 0.12 c |
| Fat (%) | 2.98 ± 0.06 d | 4.78 ± 0.03 a | 3.91 ± 0.11 c | 4.53 ± 0.06 b | 2.61 ± 0.01 e |
| Ash (%) | 0.55 ± 0.25 bc | 1.11 ± 0.06 a | 0.33 ± 0.01 c | 0.83 ± 0.06 ab | 0.43 ± 0.21 c |
| Functional Properties | BG Sources | ||||
|---|---|---|---|---|---|
| K. marxianus TISTR 5925 | S. cerevisiae Kyokai NO. 9 | Commercial (S. cerevisiae) | |||
| Alkaline Method | Enzymatic Method | Alkaline Method | Enzymatic Method | ||
| WHC (g/g) | 1.96 ± 0.01 | 1.96 ± 0.03 | 1.98 ± 0.01 | 1.97 ± 0.01 | 1.97 ± 0.01 |
| WBC (g/g) | 0.14 ± 0.01 b | 0.18 ± 0.01 a | 0.19 ± 0.01 a | 0.09 ± 0.01 c | 0.13 ± 0.01 b |
| SC (mL/g) | 41.50 ± 0.01 | 41.49 ± 0.14 | 41.53 ± 0.59 | 41.72 ± 0.28 | 41.79 ± 0.27 |
| OHC (g/g) | 2.80 ± 0.01 ab | 2.94 ± 0.20 ab | 3.00 ± 0.05 a | 2.65 ± 0.05 b | 3.00 ± 0.32 a |
| GAC (mmol/g) | 0.12 ± 0.01 c | 0.17 ± 0.01 c | 0.30 ± 0.01 b | 0.14 ± 0.01 c | 0.39 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaithanomsat, P.; Boonlum, N.; Trakunjae, C.; Apiwatanapiwat, W.; Janchai, P.; Boondaeng, A.; Phalinphattharakit, K.; Nimitkeatkai, H.; Jarerat, A. Functionality of Yeast β-Glucan Recovered from Kluyveromyces marxianus by Alkaline and Enzymatic Processes. Polymers 2022, 14, 1582. https://doi.org/10.3390/polym14081582
Vaithanomsat P, Boonlum N, Trakunjae C, Apiwatanapiwat W, Janchai P, Boondaeng A, Phalinphattharakit K, Nimitkeatkai H, Jarerat A. Functionality of Yeast β-Glucan Recovered from Kluyveromyces marxianus by Alkaline and Enzymatic Processes. Polymers. 2022; 14(8):1582. https://doi.org/10.3390/polym14081582
Chicago/Turabian StyleVaithanomsat, Pilanee, Nutthamon Boonlum, Chanaporn Trakunjae, Waraporn Apiwatanapiwat, Phornphimon Janchai, Antika Boondaeng, Kanokwan Phalinphattharakit, Hataitip Nimitkeatkai, and Amnat Jarerat. 2022. "Functionality of Yeast β-Glucan Recovered from Kluyveromyces marxianus by Alkaline and Enzymatic Processes" Polymers 14, no. 8: 1582. https://doi.org/10.3390/polym14081582
APA StyleVaithanomsat, P., Boonlum, N., Trakunjae, C., Apiwatanapiwat, W., Janchai, P., Boondaeng, A., Phalinphattharakit, K., Nimitkeatkai, H., & Jarerat, A. (2022). Functionality of Yeast β-Glucan Recovered from Kluyveromyces marxianus by Alkaline and Enzymatic Processes. Polymers, 14(8), 1582. https://doi.org/10.3390/polym14081582