Recent Advances in Development of Natural Cellulosic Non-Woven Scaffolds for Tissue Engineering
Abstract
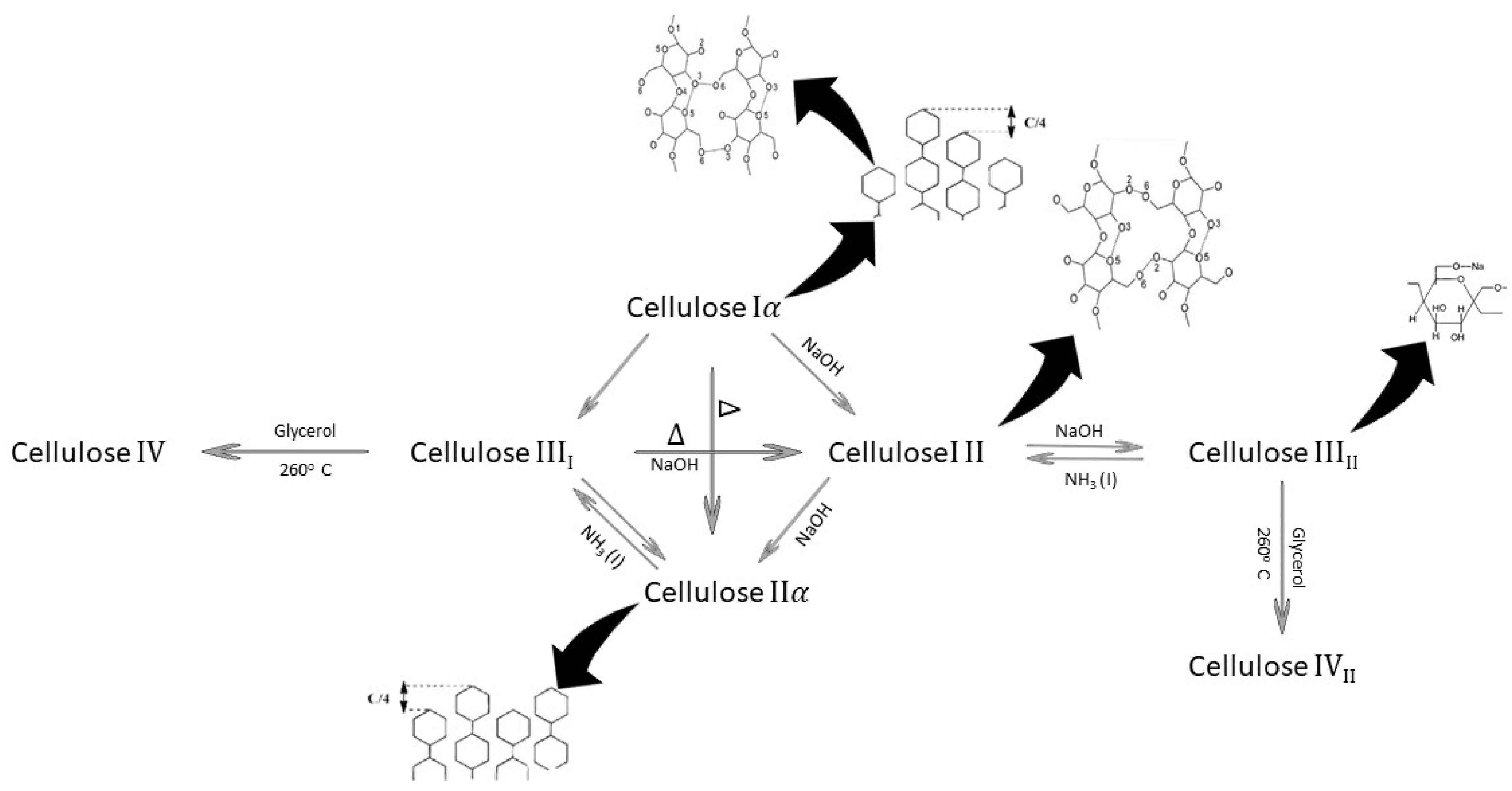
:1. Introduction
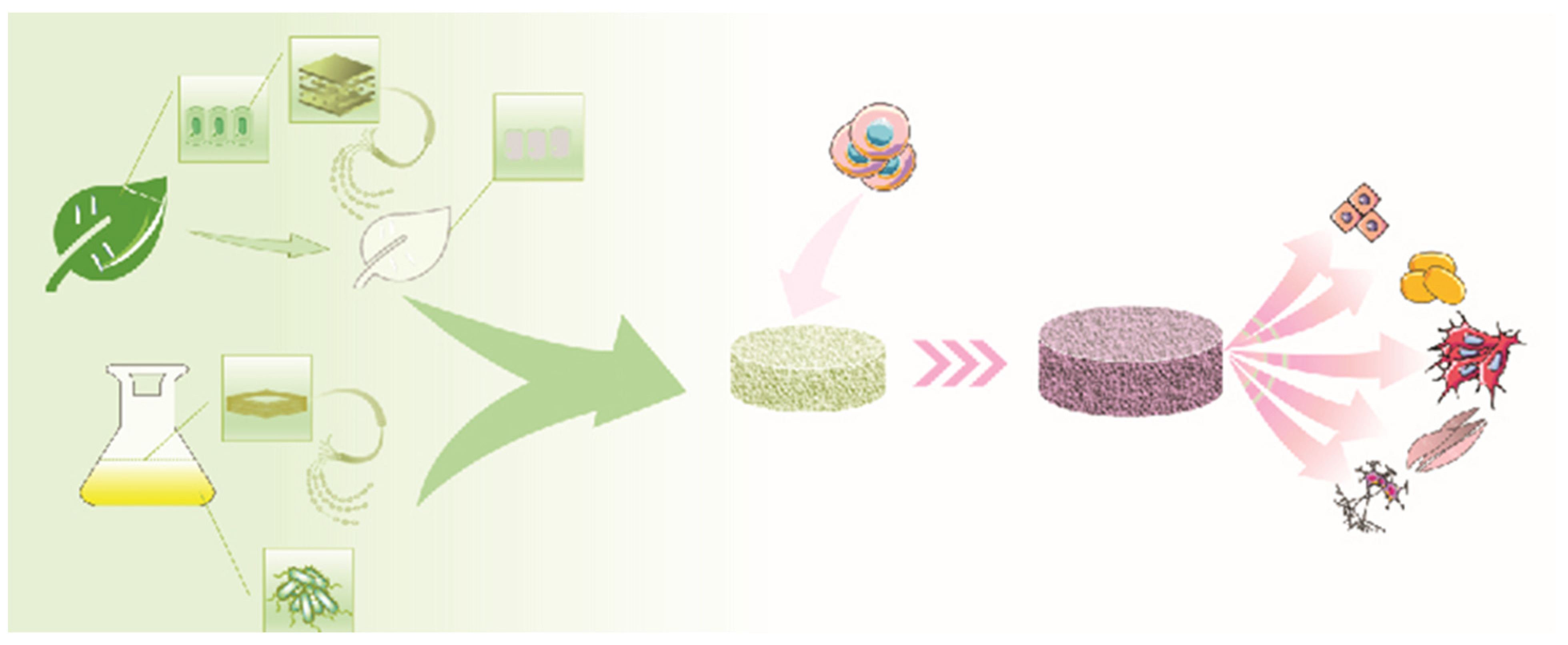
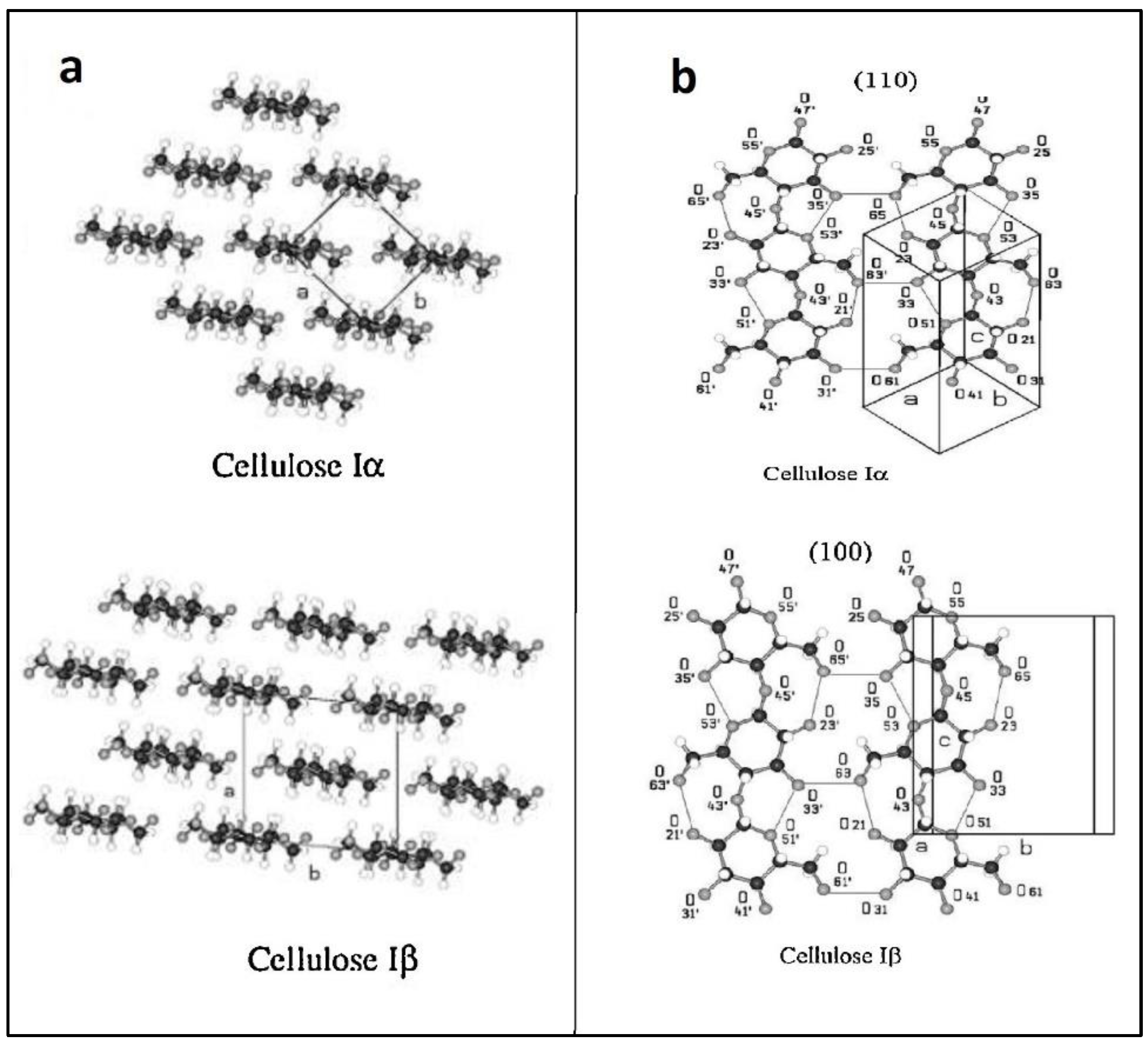
2. Cellulose Sources
3. Natural Cellulose for Bone Tissue Engineering
| Cellulose Source | Bioscaffold Platform | Achieved Results | Reference |
|---|---|---|---|
| BC | Three-dimensional macro/microporous-nanofibrous BC scaffold co-cultured with low-dose BMP-2-primed murine mesenchymal stem cells | Owing to the ECM-mimicking architecture, the scaffold provided an ideal environment for the proliferation, adhesion, and infiltration of osteoblast cells. | Dubey et al., 2021 [27] |
| BC | Three-dimensional printed porous composite scaffolds based on polycaprolactone/gelatin/BC/hydroxyapatite | The composite scaffolds induced promising osteoblast cell viability and adhesion. The pore size of the scaffolds was ideal for bone tissue substitution. | Cakmak et al., 2020 [28] |
| BC | Three-dimensional printed porous composite scaffolds composed of polyvinyl alcohol (PVA)/hexagonal boron nitride (hBN)/BC | The pore size and homogeneous structure of the scaffolds were desirable for bone tissue engineering. The addition of BC to the polymer blend resulted in a significant increase in human osteoblast cell viability. | Aki et al., 2020 [29] |
| BC | Multistage structural 3D porous microsphere composed of collagen/BC/BMP-2 | The porous microspheres promoted osteoblast differentiation and thus can be used to repair injured bone tissues. | Zhang et al., 2020 [30] |
| BC | BC-reinforced polyhydroxybutyrate (PHB) scaffolds | PHB/BC scaffolds implanted in mice with a calvarial defect enhanced in vivo osteoblast differentiation and bone formation. | Codreanu et al., 2020 [31] |
| BC | Antibacterial nanocomposite bioscaffolds based on BC/β-glucan incorporating hydroxyapatite nanoparticles (n-HAp) and graphene oxide (GO) | The antibacterial activity was proved by Gram staining. In vitro study using an osteoblast cell line revealed better biocompatibility and cell proliferation and adhesion due to the uniform distribution of the pore size, surface roughness, spongy morphology, and enhanced mechanical properties. | Khan, Haider et al., 2021 [37] |
| BC | Nanocomposite scaffolds composed of BC, magnetite (Fe3O4), and hydroxyapatite (HA) | The supermagnetic nanocomposite scaffold exhibited a high porosity of 81.1% and mechanical properties similar to those of human cancellous/trabecular bone. Moreover, it supports osteoblast cell attachment and proliferation, making it a candidate for bone tissue engineering. | Torgbo et al., 2019 [38] |
| BC | Fisetin-loaded BC scaffold | In vitro studies based on BM-MSCs showed no cytotoxicity and an increase in cell viability. The gene expression assay indicated the osteogenic potential of the fisetin-loaded BC scaffold. | Kheiry et al., 2018 [39] |
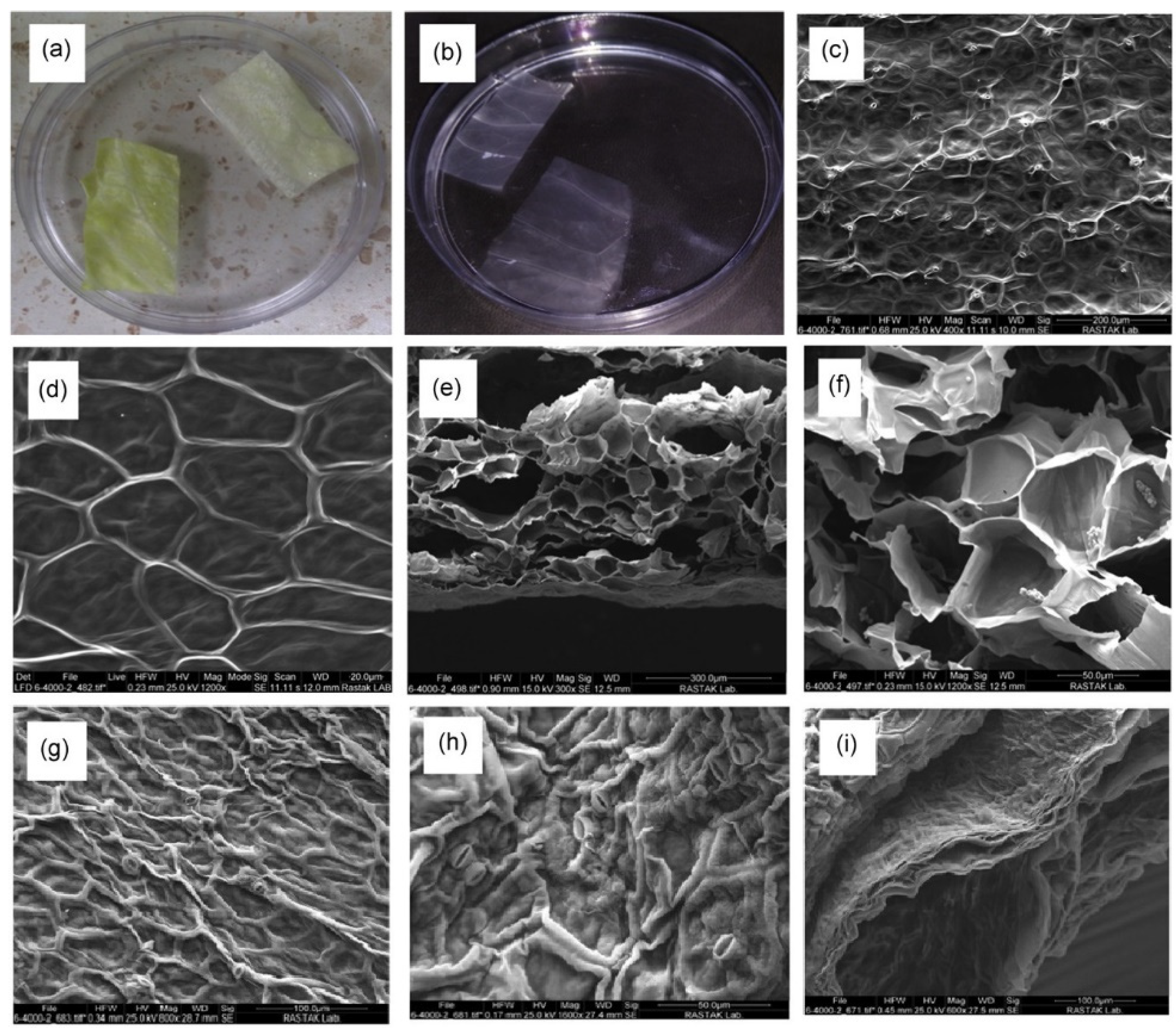
| PC | Decellularized cabbage | On co-culturing BM-MSCs and the decellularized scaffold, the bone-related genes were significantly expressed, which is due to the rough surface and high specific surface area. | Salehi et al., 2021 [32] |
| PC | Decellularized and oxidized bamboo stem | The hydrophilicity of the scaffold was increased by the oxidation process. Plus, in vitro studies validated the improved MSC viability, adhesion, and osteogenic differentiation with the oxidized decellularized plant scaffold compared to the control groups. | Mohan et al., 2021 [33] |
| PC | Outermost skin of onion | The decellularized scaffold maintains a porous structure, moderate roughness, and a high tensile strength. The in vitro assessments proved the pro-osteogenic potential of the scaffold. | Salehi et al., 2021 [32] |
| PC | Collagen-coated decellularized red apple | The decellularized scaffold possesses a high porosity. In vitro studies indicated a higher bone formation potential for the scaffold. | Latour et al., 2020 [40] |
| PC | Decellularized spinach leaf | The surface topography and vasculature of the scaffold supported the attachment and proliferation of cultured BM-MSCs. The genes expressed during in vitro studies showed the pro-osteogenic nature of the scaffold due to its optimum surface composition, hydrophilicity, and high specific surface area. | Salehi et al., 2020 [36] |
| PC | Decellularized apple, broccoli, sweet pepper, and carrot | In vivo studies on a rat calvarial defect model showed facilitated bone mineralization in the presence of the decellularized plant scaffold. | Lee et al., 2019 [41] |
| PC | Poly-L-lysine-coated decellularized carrot | After plant decellularization, no cytotoxicity was shown in vitro. The decellularized plant-based scaffold supported MC3T3-E1 pre-osteoblast cells’ adhesion, proliferation, and osteogenic differentiation. | Contessi Negrini et al., 2020 [42] |
4. Natural Cellulose for Skin Tissue Engineering
5. Natural Cellulose for Cardiac Tissue Engineering
6. Natural Cellulose for Vascular Tissue Engineering
7. Natural Cellulose for Dental Tissue Engineering
8. Natural Cellulose for Other Tissue Engineering Applications
9. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, X.; Zhao, H.; Li, Y.; Lee, A.L.; Li, Z.; Fu, M.; Li, C.; Yang, Y.Y.; Yuan, P. Synthetic peptide hydrogels as 3D scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2020, 160, 78–104. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, P.; Fu, Z.; Meng, S.; Dai, L.; Yang, H. Applications of nanomaterials in tissue engineering. RSC Adv. 2021, 11, 19041–19058. [Google Scholar] [CrossRef]
- Einchhorn, S.; Dufresne, A.; Aranguren, M.; Capadona, J.; Rowan, S.; Weder, C.; Veigel, S. Review: Current international research into cellulose nanofibres and composites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystal Based Composites: A Review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Li, Q.; Ma, L.; Gao, C. Biomaterials for in situ tissue regeneration: Development and perspectives. J. Mater. Chem. B 2015, 3, 8921–8938. [Google Scholar] [CrossRef] [PubMed]
- Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Mohd Yusof, N.; Idris, A. Review on nanocrystalline cellulose in bone tissue engineering applications. Polymers 2020, 12, 2818. [Google Scholar] [CrossRef]
- Bouhlouli, M.; Pourhadi, M.; Karami, F.; Talebi, Z.; Ranjbari, J.; Khojasteh, A. Applications of Bacterial Cellulose as a Natural Polymer in Tissue Engineering. ASAIO J. 2021, 67, 709–720. [Google Scholar] [CrossRef]
- Luo, H.; Cha, R.; Li, J.; Hao, W.; Zhang, Y.; Zhou, F. Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydr. Polym. 2019, 224, 115144. [Google Scholar] [CrossRef]
- Chinta, M.L.; Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Parcha, S.R. Assessment of properties, applications and limitations of scaffolds based on cellulose and its derivatives for cartilage tissue engineering: A review. Int. J. Biol. Macromol. 2021, 175, 495–515. [Google Scholar] [CrossRef]
- Bacakova, L.; Novotná, K.; Parizek, M. Polysaccharides as cell carriers for tissue engineering: The use of cellulose in vascular wall reconstruction. Physiol. Res. 2014, 63, S29. [Google Scholar] [CrossRef]
- Bar-Shai, N.; Sharabani-Yosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant-vs. Bacterial-derived cellulose for wound healing: A review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef] [PubMed]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-based scaffolds in tissue engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Mężykowska, O.; Piątek, J. Bacterial nanocelullose in biomedical applications: A review. Polym. Int. 2019, 68, 1841–1847. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingues, R.M.; Gomes, M.E.; Reis, R.L. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Ouyang, H.; Hu, X.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. State-of-art review on preparation, surface functionalization and biomedical applications of cellulose nanocrystals-based materials. Int. J. Biol. Macromol. 2021, 186, 591–615. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef]
- Lei, L.; Li, S.; Gu, Y. Cellulose synthase complexes: Composition and regulation. Front. Plant Sci. 2012, 3, 75. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.W.; Roberts, E. Evolution of the cellulose synthase (CesA) gene family: Insights from green algae and seedless plants. In Cellulose: Molecular and Structural Biology; Springer: Dordrecht, The Netherlands, 2007; pp. 17–34. [Google Scholar]
- Zhong, C. Industrial-Scale production and applications of bacterial cellulose. Front. Bioeng. Biotechnol. 2020, 8, 8. [Google Scholar] [CrossRef]
- Peter, Z. Order in cellulosics: Historical review of crystal structure research on cellulose. Carbohydr. Polym. 2020, 254, 117417. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Bao, F.; Zhang, X.; Song, Z.; Liao, Y.; Fei, Y.; Bunpetch, V.; Heng, B.C.; Shen, W.; Liu, H. Nanomaterial-based scaffolds for bone tissue engineering and regeneration. Nanomedicine 2020, 15, 1995–2017. [Google Scholar] [CrossRef]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L. Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Dubey, S.; Mishra, R.; Roy, P.; Singh, R. 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with BMP-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 2021, 167, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D printed polycaprolactone/gelatin/bacterial cellulose/hydroxyapatite composite scaffold for bone tissue engineering. Polymers 2020, 12, 1962. [Google Scholar] [CrossRef]
- Aki, D.; Ulag, S.; Unal, S.; Sengor, M.; Ekren, N.; Lin, C.-C.; Yılmazer, H.; Ustundag, C.B.; Kalaskar, D.M.; Gunduz, O. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 2020, 196, 109094. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.-c.; Li, X.-y.; Jiang, F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020, 236, 116043. [Google Scholar] [CrossRef]
- Codreanu, A.; Balta, C.; Herman, H.; Cotoraci, C.; Mihali, C.V.; Zurbau, N.; Zaharia, C.; Rapa, M.; Stanescu, P.; Radu, I.-C. Bacterial cellulose-modified polyhydroxyalkanoates scaffolds promotes bone formation in critical size calvarial defects in mice. Materials 2020, 13, 1433. [Google Scholar] [CrossRef] [Green Version]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Cabbage-derived three-dimensional cellulose scaffold-induced osteogenic differentiation of stem cells. J. Cell. Physiol. 2021, 236, 5306–5316. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.C.; Unnikrishnan, P.; Krishnan, A.G.; Nair, M.B. Decellularization and oxidation process of bamboo stem enhance biodegradation and osteogenic differentiation. Mater. Sci. Eng. C 2021, 119, 111500. [Google Scholar]
- Carter, D.R.; Hayes, W.C. The compressive behavior of bone as a two-phase porous structure. J. Bone Jt. Surgery Am. Vol. 1977, 59, 954–962. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Natural cellulose-based scaffold for improvement of stem cell osteogenic differentiation. J. Drug Deliv. Sci. Technol. 2021, 63, 102453. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Efficient mineralization and osteogenic gene overexpression of mesenchymal stem cells on decellularized spinach leaf scaffold. Gene 2020, 757, 144852. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Haider, A.; Abd Razak, S.I.; Kadir, M.R.A.; Shah, S.A.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/β-glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 2021, 14, 102924. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019, 237, 121868. [Google Scholar] [CrossRef]
- Kheiry, E.V.; Parivar, K.; Baharara, J.; Bazzaz, B.S.F.; Iranbakhsh, A. The osteogenesis of bacterial cellulose scaffold loaded with fisetin. Iran. J. Basic Med. Sci. 2018, 21, 965. [Google Scholar]
- Latour, M.L.; Tarar, M.; Hickey, R.J.; Cuerrier, C.M.; Catelas, I.; Pelling, A.E. Plant-derived Cellulose Scaffolds for Bone Tissue Engineering. BiorXiv 2020. [Google Scholar]
- Lee, J.; Jung, H.; Park, N.; Park, S.-H.; Ju, J.H. Induced osteogenesis in plants decellularized scaffolds. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Toffoletto, N.; Farè, S.; Altomare, L. Plant tissues as 3D natural scaffolds for adipose, bone and tendon tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. Part A 2021, 109, 1751–1764. [Google Scholar] [CrossRef]
- Graça, M.F.; Miguel, S.P.; Cabral, C.S.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.-J.; Zhao, X.-Q.; Ma, X.-F.; Xue, N.; Liu, X.-Z.; Wang, F.-P.; Jia, S.-R.; Zhong, C. Fabrication of Bacterial Cellulose-Based Dressings for Promoting Infected Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 32716–32728. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Zhang, M.; Wu, Z.; Wei, J.; Jiang, Y.; Sheng, N.; Liang, Q.; Zhang, D.; Chen, S. All-natural injectable hydrogel with self-healing and antibacterial properties for wound dressing. Cellulose 2020, 27, 2637–2650. [Google Scholar] [CrossRef]
- Pillai, M.M.; Tran, H.N.; Sathishkumar, G.; Manimekalai, K.; Yoon, J.; Lim, D.; Noh, I.; Bhattacharyya, A. Symbiotic culture of nanocellulose pellicle: A potential matrix for 3D bioprinting. Mater. Sci. Eng. C 2021, 119, 111552. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; Goodarzi, V.; Mousavi, S.M.; Khonakdar, H.A.; Zamanlui, S. Lightweight aerogels based on bacterial cellulose/silver nanoparticles/polyaniline with tuning morphology of polyaniline and application in soft tissue engineering. Int. J. Biol. Macromol. 2020, 152, 57–67. [Google Scholar] [CrossRef]
- Sajjad, W.; Khan, T.; Ul-Islam, M.; Khan, R.; Hussain, Z.; Khalid, A.; Wahid, F. Development of modified montmorillonite-bacterial cellulose nanocomposites as a novel substitute for burn skin and tissue regeneration. Carbohydr. Polym. 2019, 206, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.V.; Wright, T.; Rahman, M.M.; Xu, J.; Coburn, J.M. In vitro biocompatibility of decellularized cultured plant cell-derived matrices. ACS Biomater. Sci. Eng. 2020, 6, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, B.; Muthusamy, S.; Selvakumar, R.; Rajeswaran, N.; Sampath, S.; Jaisankar, S.; Krishnakumar, G.S. Decellularized natural 3D cellulose scaffold derived from Borassus flabellifer (Linn.) as extracellular matrix for tissue engineering applications. Carbohydr. Polym. 2021, 272, 118494. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.F.; Serpooshan, V.; Morris, V.B.; Sayed, N.; Pardon, G.; Abilez, O.J.; Nakayama, K.H.; Pruitt, B.L.; Wu, S.M.; Yoon, Y.-s. Big bottlenecks in cardiovascular tissue engineering. Commun. Biol. 2018, 1, 1–4. [Google Scholar] [CrossRef]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; Zhuang, D.; Jiang, H.; Chen, X.; He, Y. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2021, 6, 1388–1401. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Robbins, E.R.; Pins, G.D.; Laflamme, M.A.; Gaudette, G.R. Creation of a contractile biomaterial from a decellularized spinach leaf without ECM protein coating: An in vitro study. J. Biomed. Mater. Res. Part A 2020, 108, 2123–2132. [Google Scholar] [CrossRef]
- Simeoni, R.B.; Mogharbel, B.F.; Francisco, J.C.; Miyague, N.I.; Irioda, A.C.; Souza, C.M.; Souza, D.; Stricker, P.E.F.; da Rosa, N.N.; Souza, C.F. Beneficial Roles of Cellulose Patch-Mediated Cell Therapy in Myocardial Infarction: A Preclinical Study. Cells 2021, 10, 424. [Google Scholar] [CrossRef]
- Bai, H.; Xie, B.; Wang, Z.; Li, M.; Sun, P.; Wei, S.; Wang, W.; Wu, H.; Bai, L.; Li, J. Application of the Tissue-Engineered Plant Scaffold as a Vascular Patch. ACS Omega 2021, 6, 11595–11601. [Google Scholar] [CrossRef]
- Liu, L.; Ji, X.; Mao, L.; Wang, L.; Chen, K.; Shi, Z.; Ahmed, A.A.Q.; Thomas, S.; Vasilievich, R.V.; Xiao, L. Hierarchical-structured bacterial cellulose/potato starch tubes as potential small-diameter vascular grafts. Carbohydr. Polym. 2021, 6, 119034. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, K.; Feng, J.; Liu, J.; Huang, R.; Chen, Z.; Yang, J.; Dai, Z.; Chen, Y.; Wang, N. Construction of Small-Diameter Vascular Graft by Shape-Memory and Self-Rolling Bacterial Cellulose Membrane. Adv. Healthc. Mater. 2017, 6, 1601343. [Google Scholar] [CrossRef] [PubMed]
- Scherner, M.; Reutter, S.; Klemm, D.; Sterner-Kock, A.; Guschlbauer, M.; Richter, T.; Langebartels, G.; Madershahian, N.; Wahlers, T.; Wippermann, J. In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: Proof of concept? J. Surg. Res. 2014, 189, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Arefi, A.H.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Ranjbar, R.; Seifalian, A.; Rahbar, M. Decellularized and biological scaffolds in dental and craniofacial tissue engineering: A comprehensive overview. J. Mater. Res. Technol. 2021, 15, 1217–1251. [Google Scholar] [CrossRef]
- Voicu, G.; Jinga, S.-I.; Drosu, B.-G.; Busuioc, C. Improvement of silicate cement properties with bacterial cellulose powder addition for applications in dentistry. Carbohydr. Polym. 2017, 174, 160–170. [Google Scholar] [CrossRef]
- An, S.-J.; Lee, S.-H.; Huh, J.-B.; Jeong, S.I.; Park, J.-S.; Gwon, H.-J.; Kang, E.-S.; Jeong, C.-M.; Lim, Y.-M. Preparation and characterization of resorbable bacterial cellulose membranes treated by electron beam irradiation for guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 2236. [Google Scholar] [CrossRef]
- Guo, R.; Li, J.; Chen, C.; Xiao, M.; Liao, M.; Hu, Y.; Liu, Y.; Li, D.; Zou, J.; Sun, D. Biomimetic 3D bacterial cellulose-graphene foam hybrid scaffold regulates neural stem cell proliferation and differentiation. Colloids Surf. B Biointerfaces 2021, 200, 111590. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, F.; Bai, Q.; Song, D.; Zheng, Z.; Wang, Y.; Liu, X.; Abdulrahman, A.-A.; Bian, Y.; Xu, X. Oscillating Magnetic Field Regulates Cell Adherence and Endothelialization Based on Magnetic Nanoparticle-Modified Bacterial Cellulose. ACS Appl. Mater. Interfaces 2020, 12, 52467–52478. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, J.; Zhao, S.; Luo, H.; Yang, Z.; Gama, M.; Zhang, Q.; Su, D.; Wan, Y. Biocompatibility evaluation of bacterial cellulose as a scaffold material for tissue-engineered corneal stroma. Cellulose 2020, 27, 2775–2784. [Google Scholar] [CrossRef] [Green Version]
- Binnetoglu, A.; Demir, B.; Akakin, D.; Demirci, E.K.; Batman, C. Bacterial cellulose tubes as a nerve conduit for repairing complete facial nerve transection in a rat model. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 277–283. [Google Scholar] [CrossRef]
- Salehi, M.H.; Golbaten-Mofrad, H.; Jafari, S.H.; Goodarzi, V.; Entezari, M.; Hashemi, M.; Zamanlui, S. Electrically conductive biocompatible composite aerogel based on nanofibrillated template of bacterial cellulose/polyaniline/nano-clay. Int. J. Biol. Macromol. 2021, 173, 467–480. [Google Scholar] [CrossRef]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The application status of nanoscale cellulose-based hydrogels in tissue engineering and regenerative biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Torgbo, S.; Sukyai, P. Biodegradation and thermal stability of bacterial cellulose as biomaterial: The relevance in biomedical applications. Polym. Degrad. Stab. 2020, 179, 109232. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghazadeh, M.R.; Delfanian, S.; Aghakhani, P.; Homaeigohar, S.; Alipour, A.; Shahsavarani, H. Recent Advances in Development of Natural Cellulosic Non-Woven Scaffolds for Tissue Engineering. Polymers 2022, 14, 1531. https://doi.org/10.3390/polym14081531
Aghazadeh MR, Delfanian S, Aghakhani P, Homaeigohar S, Alipour A, Shahsavarani H. Recent Advances in Development of Natural Cellulosic Non-Woven Scaffolds for Tissue Engineering. Polymers. 2022; 14(8):1531. https://doi.org/10.3390/polym14081531
Chicago/Turabian StyleAghazadeh, Mohammad Reza, Sheyda Delfanian, Pouria Aghakhani, Shahin Homaeigohar, Atefeh Alipour, and Hosein Shahsavarani. 2022. "Recent Advances in Development of Natural Cellulosic Non-Woven Scaffolds for Tissue Engineering" Polymers 14, no. 8: 1531. https://doi.org/10.3390/polym14081531
APA StyleAghazadeh, M. R., Delfanian, S., Aghakhani, P., Homaeigohar, S., Alipour, A., & Shahsavarani, H. (2022). Recent Advances in Development of Natural Cellulosic Non-Woven Scaffolds for Tissue Engineering. Polymers, 14(8), 1531. https://doi.org/10.3390/polym14081531







