Application of Polymers for Chemical Enhanced Oil Recovery: A Review
Abstract
:1. Introduction
2. Mechanisms of Polymer Applications for EOR
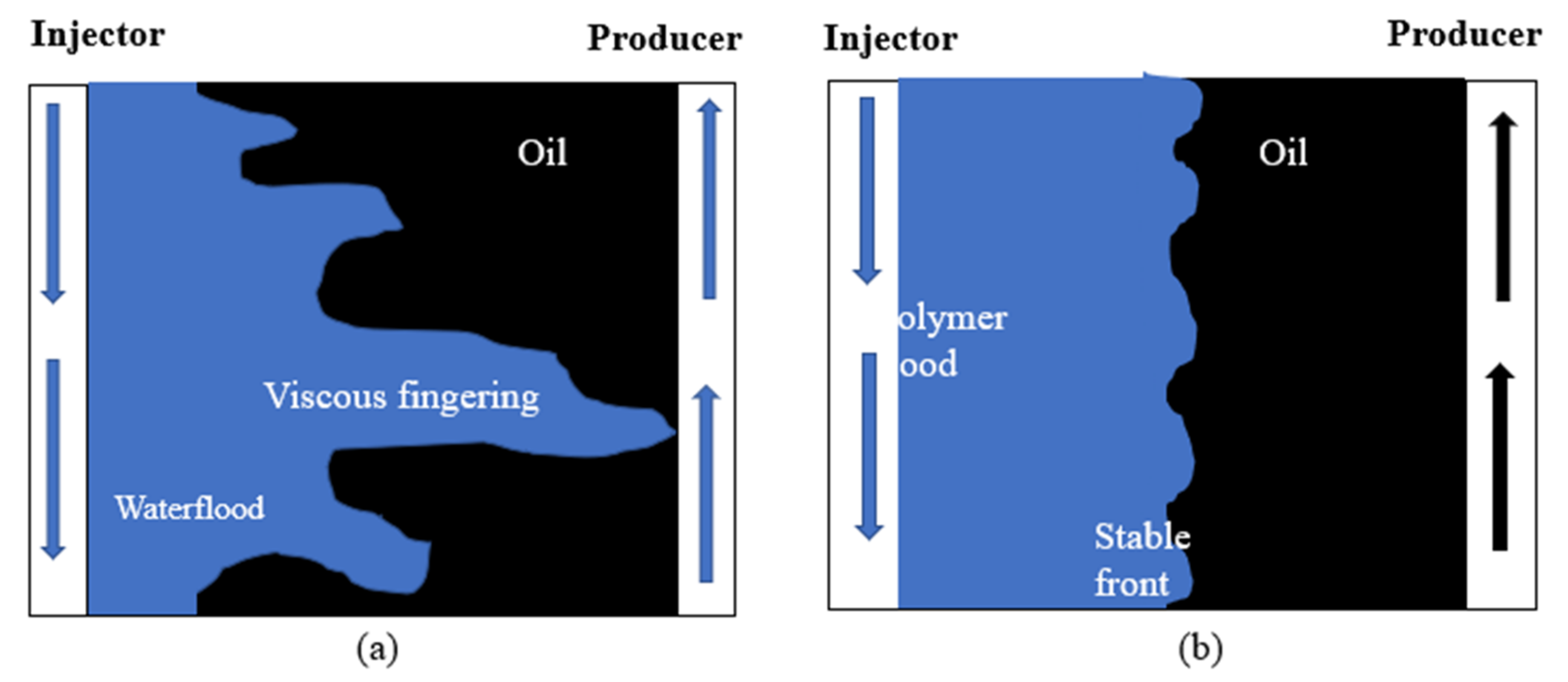
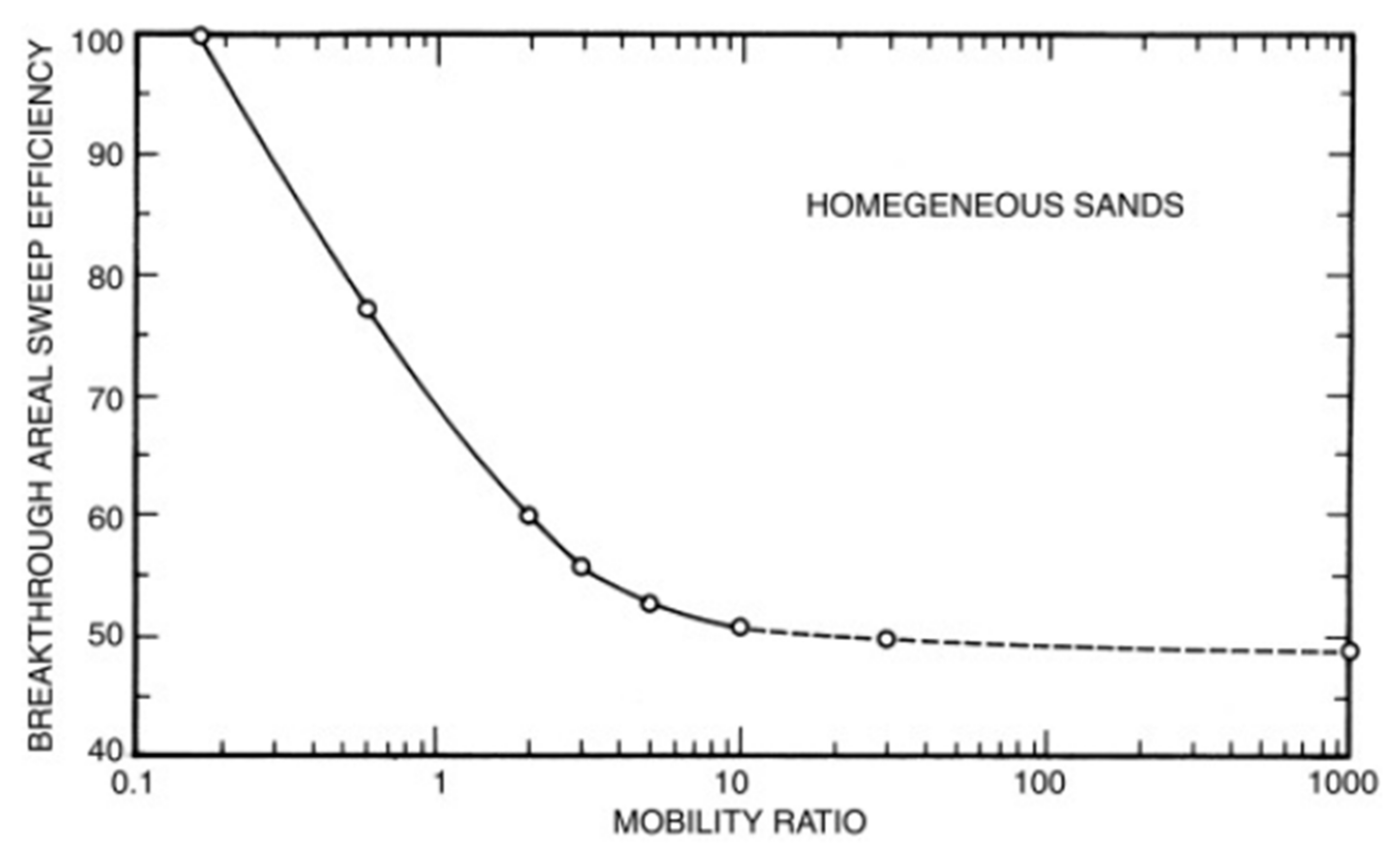
2.1. Mobility Ratio
2.2. Disproportionate Permeability Reduction
2.3. Viscoelasticity
3. Polymers Utilized for EOR
3.1. Natural Polymers (Biopolymers)
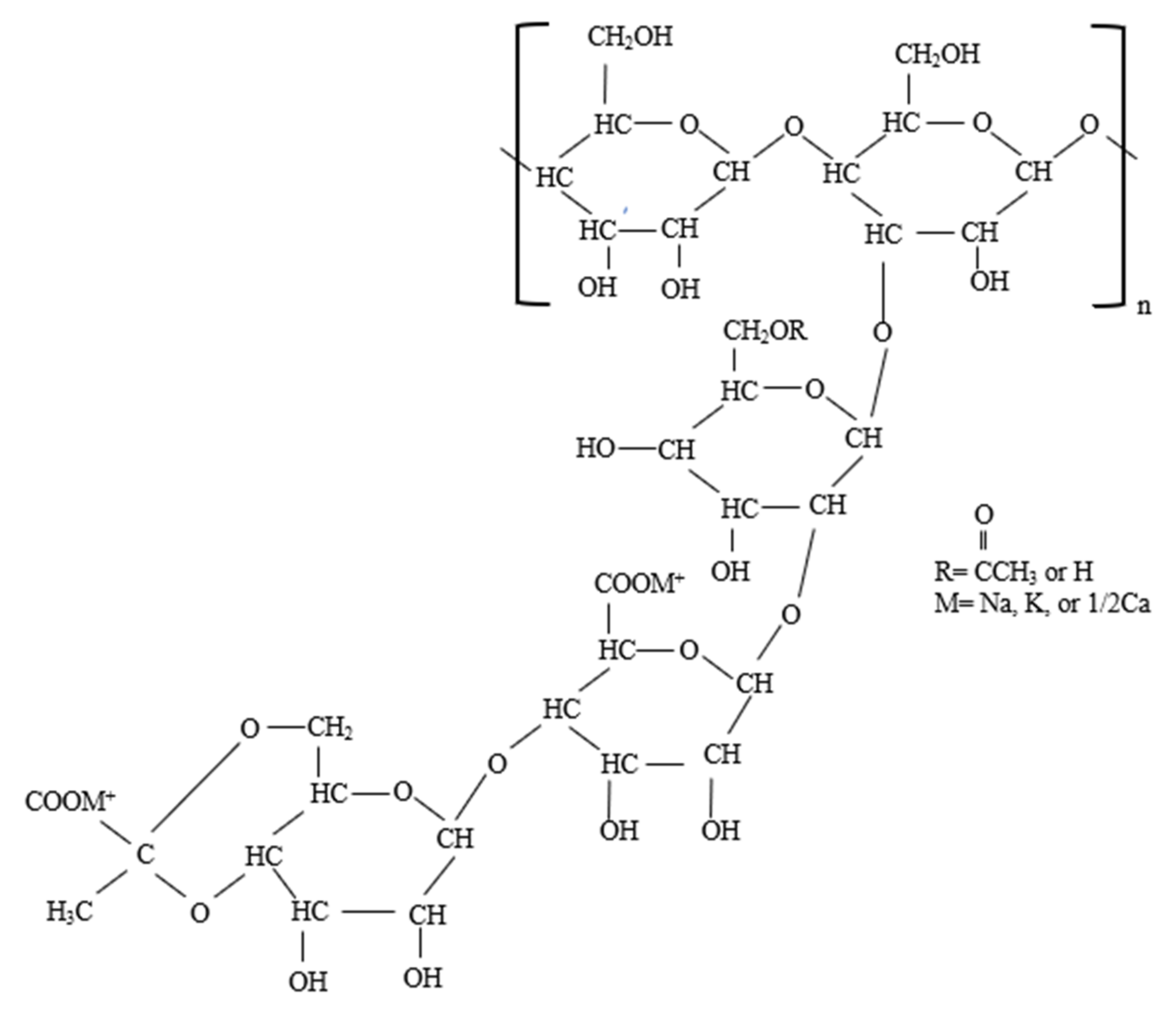
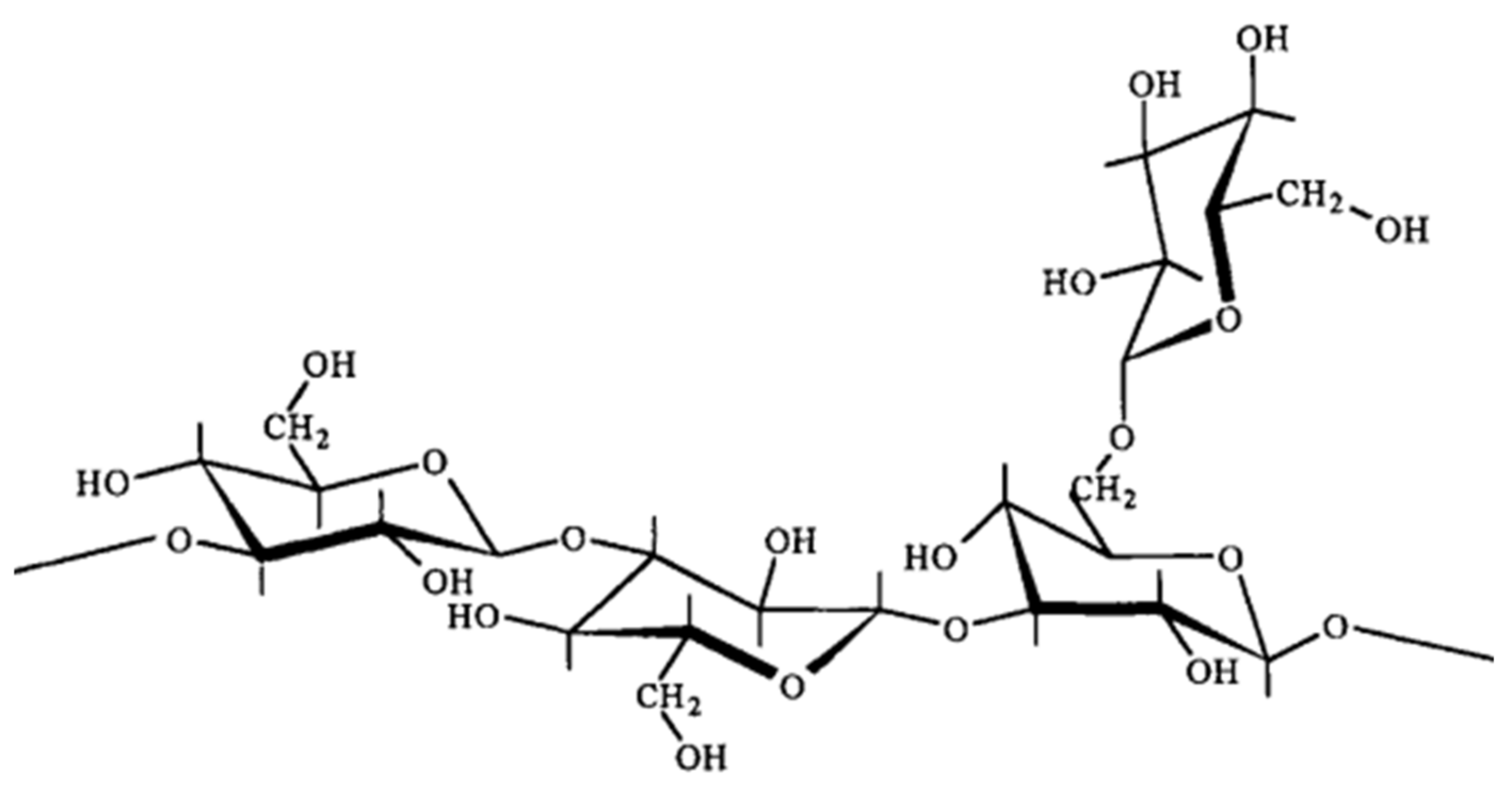
3.1.1. Xanthan Gum
3.1.2. Cellulose
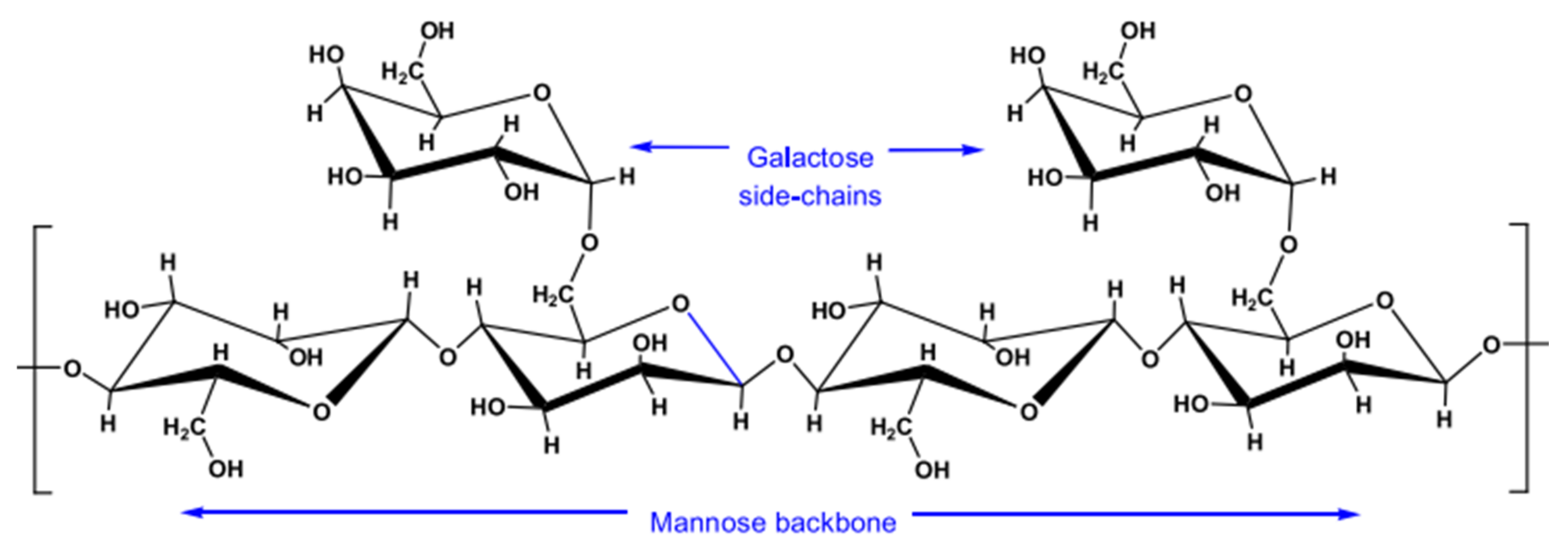
3.1.3. Guar Gum
3.1.4. Welan Gum
3.1.5. Schizophyllan
3.2. Synthetic Polymers
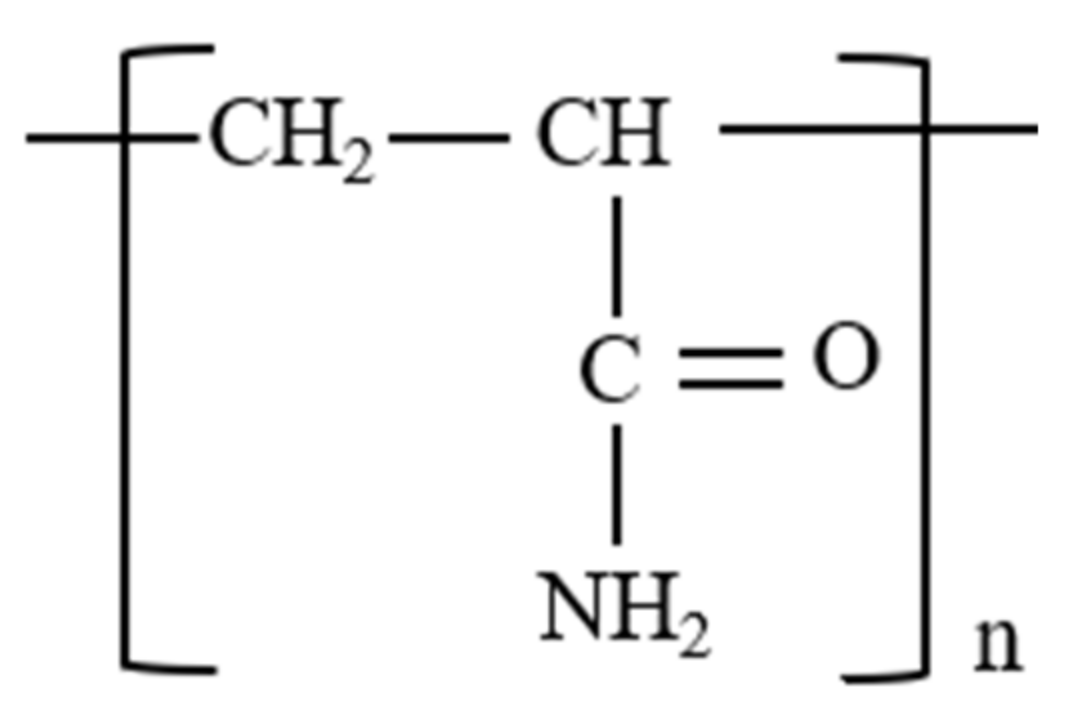
3.2.1. Polyacrylamide (PAM)
3.2.2. HPAM
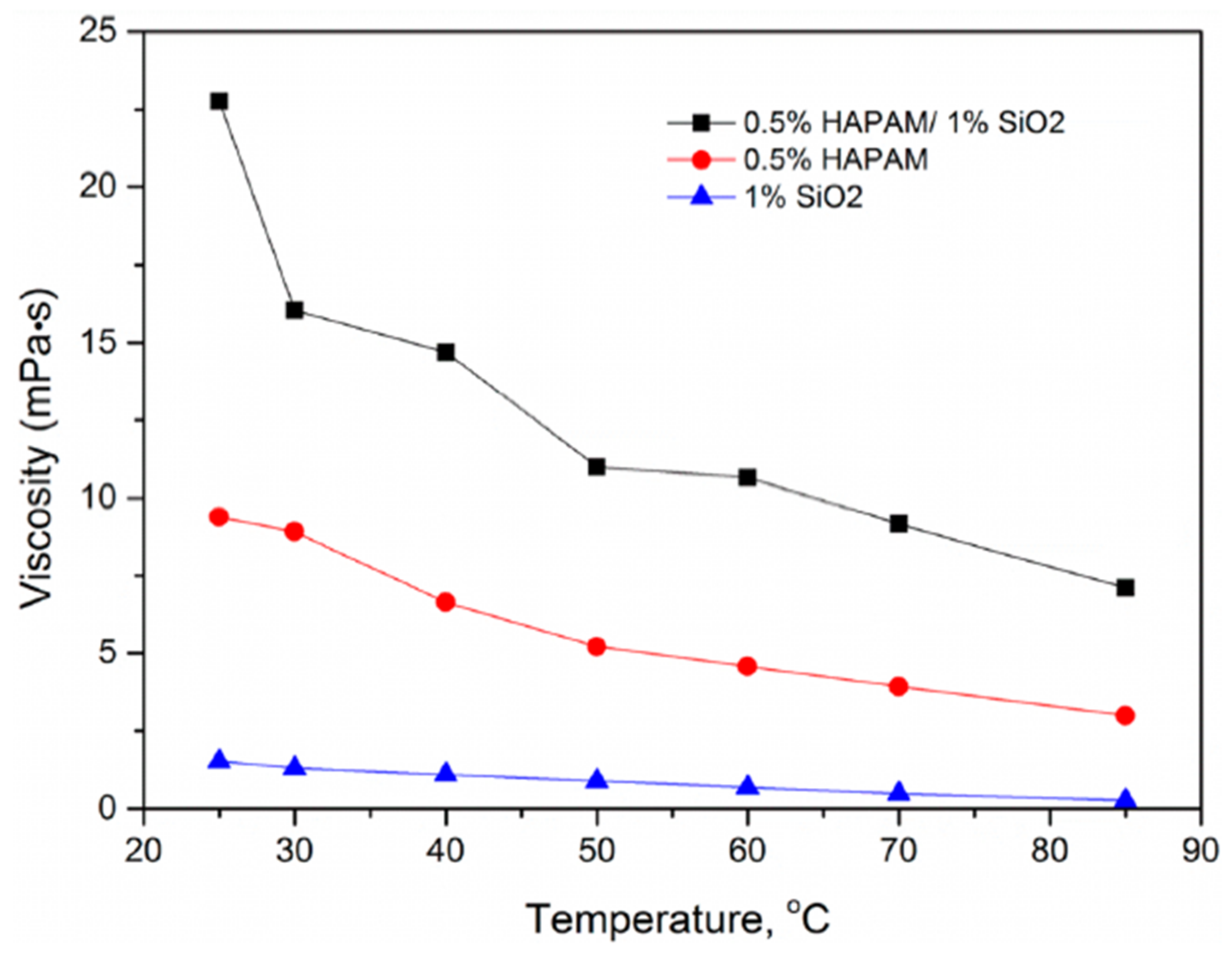
3.2.3. HAPAM
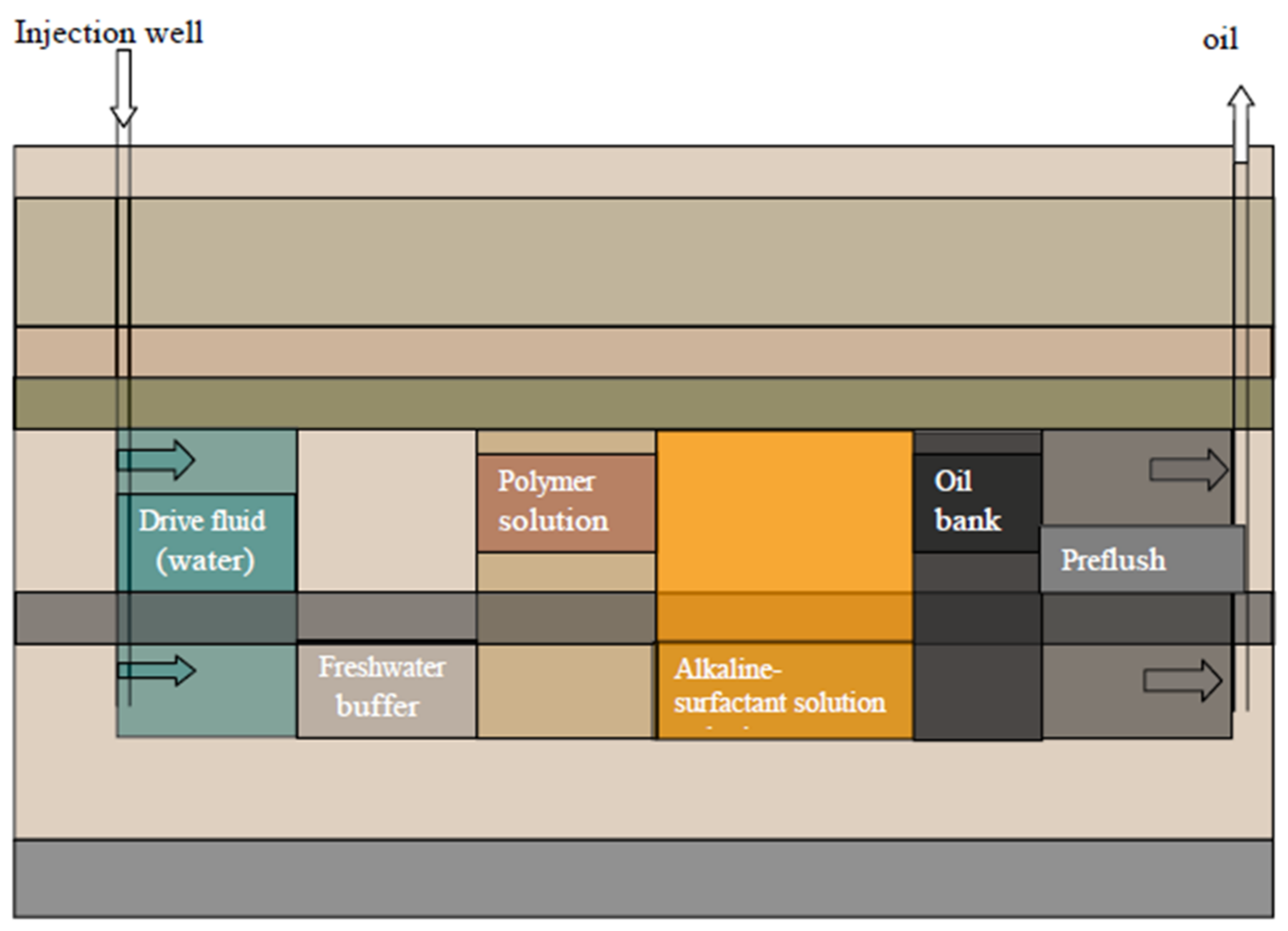
4. Polymer Flooding
5. Binary Combination of Polymers and Other Additives for EOR
5.1. Polymer Foam Flooding
5.2. Alkali–Polymer Flooding
5.3. Surfactant–Polymer Flooding
5.4. Alkali–Surfactant–Polymer (ASP) Flooding
5.5. Polymeric Nanofluid Flooding
6. Economic Perspectives of Polymer Application for Chemical EOR
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AA | Acrylic acid |
| AM | Acrylamide |
| AMC12S | 2-Acrylamido-dodecyl sulfonate |
| APTES | (3-Aminopropyl)triethoxysilane |
| ATBS | Sodium acrylamido terbutyl sulfonate |
| AN125 | Hydrolyzed acrylamido propyl sulfonated acid |
| HEC | Hydroxyethyl cellulose |
| HAHEC | Hydrophobically associating hydroxyethyl cellulose |
| HMPAM | Hydrophobically modified polyacrylamide |
| MeDiC8AM | 2-Methyl-N,N-dioctyl-acrylamide |
| NaBO2 | Sodium borate |
| Na2CO3 | Sodium carbonate |
| NH4OH | Ammonium hydroxide |
| OTES | Octyltriethoxysilane |
| PEG | Polyethylene glycol |
| TVP | Thermoviscosifying polymer |
References
- Gbadamosi, A.O.; Kiwalabye, J.; Junin, R.; Augustine, A. A review of gas enhanced oil recovery schemes used in the North Sea. J. Pet. Explor. Prod. Technol. 2018, 8, 1373–1387. [Google Scholar] [CrossRef] [Green Version]
- Agi, A.; Junin, R.; Jaafar, M.Z.; Sidek, M.A.; Yakasai, F.; Gbadamosi, A.; Oseh, J. Laboratory evaluation to field application of ultrasound: A state-of-the-art review on the effect of ultrasonication on enhanced oil recovery mechanisms. J. Ind. Eng. Chem. 2022, in press. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Jaafar, M.Z.; Amin, N.A.S.; Sidek, M.A.; Nyakuma, B.B.; Yakasai, F.; Gbadamosi, A.; Oseh, J.; Azli, N.B. Ultrasound-assisted nanofluid flooding to enhance heavy oil recovery in a simulated porous media. Arab. J. Chem. 2022, 15, 103784. [Google Scholar] [CrossRef]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: Half-century studies. J. Pet. Sci. Eng. 2016, 142, 85–100. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Abbas, A.H.; Elhag, H.H.; Sulaiman, W.R.W.; Gbadamosi, A.; Pourafshary, P.; Ebrahimi, S.S.; Alqohaly, O.Y.; Agi, A. Modelling of continuous surfactant flooding application for marginal oilfields: A case study of Bentiu reservoir. J. Pet. Explor. Prod. 2021, 11, 989–1006. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, M.B.; Al-Shehri, D.; Rahaman, M.M.; Keshavarz, A.; Hossain, S.M.Z. Comparative study of green and synthetic polymers for enhanced oil recovery. Polymers 2020, 12, 2429. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Jaafar, M.Z.; Mohsin, R.; Arsad, A.; Gbadamosi, A.; Fung, C.K.; Gbonhinbor, J. Synthesis and application of rice husk silica nanoparticles for chemical enhanced oil recovery. J. Mater. Res. Technol. 2020, 9, 13054–13066. [Google Scholar] [CrossRef]
- Ngouangna, E.N.; Manan, M.A.; Oseh, J.O.; Norddin, M.N.A.M.; Agi, A.; Gbadamosi, A.O. Influence of (3–Aminopropyl) triethoxysilane on silica nanoparticle for enhanced oil recovery. J. Mol. Liq. 2020, 315, 113740. [Google Scholar] [CrossRef]
- Delamaide, E.; Zaitoun, A.; Renard, G.; Tabary, R. Pelican lake field: First successful application of polymer flooding in a heavy-oil reservoir. SPE Reserv. Eval. Eng. 2014, 17, 340–354. [Google Scholar] [CrossRef]
- Pu, H.; Xu, Q. An update and perspective on field-scale chemical floods in Daqing oilfield, China. In Proceedings of the SPE-118746-MS, SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 15–18 March 2009; pp. 1–8. [Google Scholar]
- Mohsenatabar Firozjaii, A.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2020, 6, 115–122. [Google Scholar] [CrossRef]
- Sheng, J.J. Chapter 6—Polymer viscoelastic behavior and its effect on field facilities and operations. In Modern Chemical Enhanced Oil Recovery; Gulf Professional Publishing: Houston, TX, USA, 2011; pp. 207–238. ISBN 9781856177450. [Google Scholar]
- Wei, B.; Romero-Zerón, L.; Rodrigue, D. Oil displacement mechanisms of viscoelastic polymers in enhanced oil recovery (EOR): A review. J. Pet. Explor. Prod. Technol. 2014, 4, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for enhanced oil recovery technology. Procedia Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Pu, W.; Shen, C.; Wei, B.; Yang, Y.; Li, Y. A comprehensive review of polysaccharide biopolymers for enhanced oil recovery (EOR) from flask to field. J. Ind. Eng. Chem. 2018, 61, 1–11. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of polysaccharide biopolymer in petroleum recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Rock, A.; Hincapie, R.E.; Tahir, M.; Langanke, N.; Ganzer, L. On the role of polymer viscoelasticity in enhanced oil recovery: Extensive laboratory data and review. Polymers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: Prospects and challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Yekeen, N.; Augustine, A. Hybrid suspension of polymer and nanoparticles for enhanced oil recovery. Polym. Bull. 2019, 76, 6193–6230. [Google Scholar] [CrossRef]
- Sorbie, K.S. Polymer-Improved Oil Recovery; Springer: Berlin/Heidelberg, Germany, 1991; ISBN 9789401053549. [Google Scholar]
- Mishra, S.; Bera, A.; Mandal, A.; Mishra, S.; Bera, A.; Mandal, A. Effect of polymer adsorption on permeability reduction in enhanced oil recovery. J. Pet. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Al-Sharji, H.H.; Grattoni, C.A.; Dawe, R.A.; Zimmerman, R.W. Disproportionate permeability reduction due to polymer adsorption entanglement. In Proceedings of the SPE-68972-MS, SPE European Formation Damage Conference, The Hague, The Netherlands, 21–22 May 2001; pp. 1–11. [Google Scholar]
- Wang, D.; Cheng, J.; Xia, H.; Li, Q.; Shi, J. Viscous-elastic fluids can mobilize oil remaining after water-flood by force parallel to the oil-water interface. In Proceedings of the SPE-72123-MS, SPE Asia Pacific Improved Oil Recovery Conference, Kuala Lumpur, Malaysia, 6–9 October 2001; pp. 1–8. [Google Scholar]
- Azad, M.S.; Trivedi, J.J. Quantification of the viscoelastic effects during polymer flooding: A critical review. SPE J. 2019, 24, 2731–2757. [Google Scholar] [CrossRef]
- Wang, D.; Xia, H.; Liu, Z.; Yang, Q. Study of the Mechanism of Polymer Solution With Visco-Elastic Behavior Increasing Microscopic Oil Displacement Efficiency and the Forming of Steady “Oil Thread” Flow Channels. In Proceedings of the SPE-68723-MS, SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 April 2001; pp. 1–9. [Google Scholar]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244–245, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dong, M.; Gong, H.; Sun, M.; Li, Y. Effects of inorganic cations on the rheology of aqueous welan, xanthan, gellan solutions and their mixtures. Carbohydr. Polym. 2015, 121, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ghoumrassi-Barr, S.; Aliouche, D. A rheological study of xanthan polymer for enhanced oil recovery. J. Macromol. Sci. Part B 2016, 55, 793–809. [Google Scholar] [CrossRef]
- Song, K.-W.; Kim, Y.-S.; Chang, G.-S. Rheology of concentrated xanthan gum solutions: Steady shear flow behavior. Fibers Polym. 2006, 7, 129–138. [Google Scholar] [CrossRef]
- Combariza, M.Y.; Martínez-Ramírez, A.P.; Blanco-Tirado, C. Perspectives in nanocellulose for crude oil recovery: A minireview. Energy Fuels 2021, 35, 15381–15397. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, S.; Yang, Z.; Li, X.; Chen, J.; Zhang, X.; Zheng, N. A review of recent advances and prospects on nanocellulose properties and its applications in oil and gas production. J. Nat. Gas Sci. Eng. 2021, 96, 104253. [Google Scholar] [CrossRef]
- Li, Q.; Wei, B.; Lu, L.; Li, Y.; Wen, Y.; Pu, W.; Li, H.; Wang, C. Investigation of physical properties and displacement mechanisms of surface-grafted nano-cellulose fluids for enhanced oil recovery. Fuel 2017, 207, 352–364. [Google Scholar] [CrossRef]
- Bai, J.; Wassmuth, F.R.; Jost, R.W.; Zhao, L. Hydrophobically-modified cellulosic polymers for heavy oil displacement in saline conditions. In Proceedings of the SPE Heavy Oil Conference Canada, Calgary, AB, Canada, 2–14 June 2012; pp. 1–9. [Google Scholar]
- Liu, P.; Mu, Z.; Wang, C.; Wang, Y. Experimental study of rheological properties and oil displacement efficiency in oilfields for a synthetic hydrophobically modified polymer. Sci. Rep. 2017, 7, 8791. [Google Scholar] [CrossRef]
- Li, M.-C.; Wu, Q.; Song, K.; Lee, S.; Qing, Y.; Wu, Y. Cellulose nanoparticles: Structure–morphology–rheology relationships. ACS Sustain. Chem. Eng. 2015, 3, 821–832. [Google Scholar] [CrossRef]
- Li, Q.; Wei, B.; Xue, Y.; Wen, Y.; Li, J. Improving the physical properties of nano- cellulose through chemical grafting for potential use in enhancing oil recovery. J. Bioresour. Bioprod. 2016, 1, 186–191. [Google Scholar]
- Nechyporchuk, O.; Belgacem, M.N.; Pignon, F. Current progress in rheology of cellulose nanofibril suspensions. Biomacromolecules 2016, 17, 2311–2320. [Google Scholar] [CrossRef]
- Adimule, V.; Kerur, S.S.; Chinnam, S.; Yallur, B.C.; Nandi, S.S. Guar gum and its nanocomposites as prospective materials for miscellaneous applications: A short review. Top. Catal. 2022, in press. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, G.; Yu, L.; Gong, H.; Dong, M.; Li, Y. The displacement efficiency and rheology of welan gum for enhanced heavy oil recovery. Polym. Adv. Technol. 2014, 25, 1122–1129. [Google Scholar] [CrossRef]
- Kaur, V.; Bera, M.B.; Panesar, P.S.; Kumar, H.; Kennedy, J.F. Welan gum: Microbial production, characterization, and applications. Int. J. Biol. Macromol. 2014, 65, 454–461. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Liu, T.; Chen, Y.; Gong, H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef]
- Ji, S.; Li, H.; Wang, G.; Lu, T.; Ma, W.; Wang, J.; Zhu, H.; Xu, H. Rheological behaviors of a novel exopolysaccharide produced by Sphingomonas WG and the potential application in enhanced oil recovery. Int. J. Biol. Macromol. 2020, 162, 1816–1824. [Google Scholar] [CrossRef]
- Gunaji, R.G.; Junin, R.; Bandyopadhyay, S.; Guttula, P. Production of biopolymer Schizophyllan using local sources in Malaysia. In Offshore Technology Conference Asia; OnePetro: Richardson, TX, USA, 2020; pp. 1–10. [Google Scholar]
- Grisel, M.; Muller, G. Rheological properties of schizophyllan in presence of borate ions. In Gels; Zrínyi, M., Ed.; Progress in Colloid & Polymer Science; Steinkopff: Darmstadt, Germany, 1996; Volume 102, pp. 32–37. ISBN 978-3-7985-1663-2. [Google Scholar]
- Skauge, A.; Zamani, N.; Gausdal Jacobsen, J.; Shaker Shiran, B.; Al-Shakry, B.; Skauge, T. Polymer Flow in Porous Media: Relevance to Enhanced Oil Recovery. Colloids Interfaces 2018, 2, 27. [Google Scholar] [CrossRef] [Green Version]
- Veerabhadrappa, S.K.; Doda, A.; Trivedi, J.J.; Kuru, E. On the effect of polymer elasticity on secondary and tertiary oil recovery. Ind. Eng. Chem. Res. 2013, 52, 18421–18428. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure–property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Chul, J.J.; Ke, Z.; Hyun, C.B.; Jin, C.H. Rheology and polymer flooding characteristics of partially hydrolyzed polyacrylamide for enhanced heavy oil recovery. J. Appl. Polym. Sci. 2012, 127, 4833–4839. [Google Scholar] [CrossRef]
- Afolabi, R.O. Effect of surfactant and hydrophobe content on the rheology of poly (acrylamide-co-N-dodecylacrylamide) for Potential enhanced oil recovery application. Am. J. Polym. Sci. 2015, 5, 41–46. [Google Scholar] [CrossRef]
- Afolabi, R.O.; Oluyemi, G.F.; Officer, S.; Ugwu, J.O. Hydrophobically associating polymers for enhanced oil recovery—Part A: A review on the effects of some key reservoir conditions. J. Pet. Sci. Eng. 2019, 180, 681–698. [Google Scholar] [CrossRef]
- Sarsenbekuly, B.; Kang, W.; Fan, H.; Yang, H.; Dai, C.; Zhao, B.; Aidarova, S.B. Study of salt tolerance and temperature resistance of a hydrophobically modified polyacrylamide based novel functional polymer for EOR. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 91–97. [Google Scholar] [CrossRef]
- Quan, H.; Li, Z.; Huang, Z. Self-assembly properties of a temperature- and salt-tolerant amphoteric hydrophobically associating polyacrylamide. RSC Adv. 2016, 6, 49281–49288. [Google Scholar] [CrossRef]
- Maia, A.M.S.; Costa, M.; Borsali, R.; Garcia, R.B. Rheological behavior and scattering studies of acrylamide-based copolymer solutions. Macromol. Symp. 2005, 229, 217–227. [Google Scholar] [CrossRef]
- Yang, B.; Mao, J.; Zhao, J.; Shao, Y.; Zhang, Y.; Zhang, Z.; Lu, Q. Improving the Thermal stability of hydrophobic associative polymer aqueous solution using a “triple-protection” strategy. Polymers 2019, 11, 949. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Xing, J.; Dong, Z.; Tang, Y.; Pu, W. Synthesis of polyacrylamide with superb salt-thickening performance. Ind. Eng. Chem. Res. 2015, 54, 10568–10574. [Google Scholar] [CrossRef]
- Shi, L.-T.; Li, C.; Zhu, S.-S.; Xu, J.; Sun, B.-Z.; Ye, Z.-B. Study on properties of branched hydrophobically modified polyacrylamide for polymer flooding. J. Chem. 2013, 2013, 675826. [Google Scholar] [CrossRef] [Green Version]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Half-century of heavy oil polymer flooding from laboratory core floods to pilot tests and field applications. In Proceedings of the SPE-174402-MS, SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 9 June 2015; pp. 1–26. [Google Scholar] [CrossRef]
- Standnes, D.C.; Skjevrak, I. Literature review of implemented polymer field projects. J. Pet. Sci. Eng. 2014, 122, 761–775. [Google Scholar] [CrossRef]
- Taber, J.J.; Martin, F.D.; Seright, R.S. EOR screening criteria revisited—Part 2: Applications and impact of oil prices. SPE Reserv. Eng. 1997, 12, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Aladasani, A.; Bai, B. Recent developments and updated screening criteria of enhanced oil recovery techniques. In Proceedings of the SPE-130726-MS, International Oil and Gas Conference and Exhibition in China, Beijing, China, 8 June 2010; pp. 1–24. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of polymer-flooding technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Yusuff, A.S. An overview of chemical enhanced oil recovery: Recent advances and prospects. Int. Nano Lett. 2019, 9, 171–202. [Google Scholar] [CrossRef] [Green Version]
- Abirov, R.; Ivakhnenko, A.P.; Abirov, Z.; Eremin, N.A. The associative polymer flooding: An experimental study. J. Pet. Explor. Prod. Technol. 2020, 10, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Shang, X.; Wang, Z.; Zhao, X. Experimental study on hydrophobically associating hydroxyethyl cellulose flooding system for enhanced oil recovery. Energy Fuels 2018, 32, 6713–6725. [Google Scholar] [CrossRef]
- Rellegadla, S.; Jain, S.; Agrawal, A. A holistic approach to determine the enhanced oil recovery potential of hydroxyethylcellulose, tragacanth gum and carboxymethylcellulose. J. Mol. Liq. 2021, 341, 117334. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Yin, H.; Feng, Y.; Quan, H. Comparative studies on enhanced oil recovery: Thermoviscosifying polymer versus polyacrylamide. Energy Fuels 2017, 31, 2479–2487. [Google Scholar] [CrossRef]
- Sowunmi, A.; Efeovbokhan, V.E.; Orodu, O.D.; Ojo, T. Comparative study on the effects of guar gum nanocomposite and guar gum polymer solutions on enhanced oil recovery Comparative study on the effects of guar gum nanocomposite and guar gum polymer solutions on enhanced oil recovery. In Proceedings of the 2nd International Conference on Sustainable Infrastructural Development (ICSID 2020), Ota, Nigeria, 27–28 July 2020; pp. 1–13. [Google Scholar]
- Olabode, O.; Ojo, T.; Oguntade, T.; Oduwole, D. Recovery potential of biopolymer (B-P) formulation from Solanum tuberosum (waste) starch for enhancing recovery from oil reservoirs. Energy Rep. 2020, 6, 1448–1455. [Google Scholar] [CrossRef]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Gao, C. Application of a novel biopolymer to enhance oil recovery. J. Pet. Explor. Prod. Technol. 2016, 6, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Castro, R.H.; Llanos, S.; Rodríguez, J.; Quintero, H.I.; Manrique, E. Polymers for EOR application in high temperature and high viscosity oils: Rock–fluid behavior. Energies 2020, 13, 5944. [Google Scholar] [CrossRef]
- Rafati, R.; Oludara, O.K.; Haddad, A.S.; Hamidi, H. Experimental investigation of emulsified oil dispersion on bulk foam stability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Johns, R.T.; Dindoruk, B. Chapter 1—Gas flooding. In Enhanced Oil Recovery Field Case Studies; Sheng, J.J., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2013; pp. 1–22. ISBN 978-0-12-386545-8. [Google Scholar]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Padmanabhan, E.; Junin, R.; Samin, A.M.; Gbadamosi, A.O.; Oguamah, I. A comprehensive review of experimental studies of nanoparticles-stabilized foam for enhanced oil recovery. J. Pet. Sci. Eng. 2018, 164, 43–74. [Google Scholar] [CrossRef]
- Adebayo, A.R. Foam flow in different pore systems—Part 2: The roles of pore attributes on the limiting capillary pressure, trapping coefficient, and relative permeability of foamed gas. SPE J. 2021, 26, 3926–3948. [Google Scholar] [CrossRef]
- Majeed, T.; Kamal, M.S.; Zhou, X.; Solling, T. A review on foam stabilizers for enhanced oil recovery. Energy Fuels 2021, 35, 5594–5612. [Google Scholar] [CrossRef]
- Adebayo, A.R. Foam flow in different pore systems—Part 1: The roles of pore attributes and their variation on trapping and apparent viscosity of foam. SPE J. 2021, 26, 3908–3925. [Google Scholar] [CrossRef]
- Yu, W.; Kanj, M.Y. Review of foam stability in porous media: The effect of coarsening. J. Pet. Sci. Eng. 2022, 208, 109698. [Google Scholar] [CrossRef]
- Abdelgawad, K.Z.; Adebayo, A.R.; Isah, A.; Muhammed, N.S. A literature review of strength and stability of foam and their relationship with the absolute permeability of porous media. J. Pet. Sci. Eng. 2022, 211, 110195. [Google Scholar] [CrossRef]
- Samin, A.M.; Manan, M.A.; Idris, A.K.; Yekeen, N.; Said, M.; Alghol, A. Protein foam application for enhanced oil recovery. J. Dispers. Sci. Technol. 2017, 38, 604–609. [Google Scholar] [CrossRef]
- Imuetinyan, H.; Agi, A.; Gbadamosi, A.; Junin, R.; Oseh, J. Oil-water interfacial tension, wettability alteration and foaming studies of natural surfactant extracted from Vernonia Amygdalina. Pet. Res. 2021, in press. [Google Scholar] [CrossRef]
- Said, M.S.; Jaafar, M.Z.; Omar, S.; Sharbini, S.N. Influence of whey protein isolate on CO2 foams stability in three different types of crude oil. Case Stud. Chem. Environ. Eng. 2022, 5, 100191. [Google Scholar] [CrossRef]
- Azdarpour, A.; Junin, R.B.; Manan, M.; Hamidi, H.; Rafati, R. The effects of controlling parameters on polymer enhanced foam (PEF) stability. J. Teknol. 2015, 73, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Raghav Chaturvedi, K.; Kumar, R.; Trivedi, J.; Sheng, J.J.; Sharma, T. Stable silica nanofluids of an oilfield polymer for enhanced CO2 absorption for oilfield applications. Energy Fuels 2018, 32, 12730–12741. [Google Scholar] [CrossRef]
- Pandey, A.; Sinha, A.S.K.; Chaturvedi, K.R.; Sharma, T. Experimental investigation on effect of reservoir conditions on stability and rheology of carbon dioxide foams of nonionic surfactant and polymer: Implications of carbon geo-storage. Energy 2021, 235, 121445. [Google Scholar] [CrossRef]
- Dehdari, B.; Parsaei, R.; Riazi, M.; Rezaei, N.; Zendehboudi, S. New insight into foam stability enhancement mechanism, using polyvinyl alcohol (PVA) and nanoparticles. J. Mol. Liq. 2020, 307, 112755. [Google Scholar] [CrossRef]
- Wang, D.; Han, D.; Xu, G.; Yang, L. Influence of partially hydrolyzed polyacrylamide on the foam capability of α-Olefin Sulfonate surfactant. Pet. Explor. Dev. 2008, 35, 335–338. [Google Scholar] [CrossRef]
- Hernando, L.; Satken, B.; Omari, A.; Bertin, H. Transport of polymer stabilized foams in porous media: Associative polymer versus PAM. J. Pet. Sci. Eng. 2018, 169, 602–609. [Google Scholar] [CrossRef]
- Ahmed, S.; Elraies, K.A.; Tan, I.M.; Hashmet, M.R. Experimental investigation of associative polymer performance for CO2 foam enhanced oil recovery. J. Pet. Sci. Eng. 2017, 157, 971–979. [Google Scholar] [CrossRef]
- Hanamertani, A.S.; Ahmed, S. Probing the role of associative polymer on scCO2-Foam strength and rheology enhancement in bulk and porous media for improving oil displacement efficiency. Energy 2021, 228, 120531. [Google Scholar] [CrossRef]
- Hernando, L.; Bertin, H.J.; Omari, A.; Dupuis, G.; Zaitoun, A. Polymer-enhanced foams for water profile control. In SPE Improved Oil Recovery Conference; OnePetro: Richardson, TX, USA, 2016. [Google Scholar] [CrossRef]
- Bashir, A.; Sharifi Haddad, A.; Rafati, R. Nanoparticle/polymer-enhanced alpha olefin sulfonate solution for foam generation in the presence of oil phase at high temperature conditions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123875. [Google Scholar] [CrossRef]
- Bashir, A.; Sharifi Haddad, A.; Rafati, R. Experimental investigation of nanoparticles/polymer enhanced CO2-foam in the presence of hydrocarbon at high-temperature conditions. In Proceedings of the SPE-193802-MS, SPE International Heavy Oil Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2018; Society of Petroleum Engineers: Kuwait City, Kuwait, 2018; pp. 1–13. [Google Scholar]
- Wei, P.; Guo, K.; Xie, Y. Polysaccharide-stabilized oil-laden foam for enhancing oil recovery. J. Pet. Sci. Eng. 2020, 195, 107597. [Google Scholar] [CrossRef]
- Wei, B.; Li, H.; Li, Q.; Lu, L.; Li, Y.; Pu, W.; Wen, Y. Investigation of synergism between surface-grafted nano-cellulose and surfactants in stabilized foam injection process. Fuel 2018, 211, 223–232. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, Y.; Huang, D.; Wei, B. Design and Fabrication of anionic/cationic surfactant foams stabilized by lignin–cellulose nanofibrils for enhanced oil recovery. Energy Fuels 2020, 34, 16493–16501. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M. Influence of surfactant and electrolyte concentrations on surfactant Adsorption and foaming characteristics. J. Pet. Sci. Eng. 2017, 149, 612–622. [Google Scholar] [CrossRef]
- Fu, C.; Liu, N. Study of the synergistic effect of the nanoparticle-surfactant-polymer system on CO2 foam apparent viscosity and stability at high pressure and temperature. Energy Fuels 2020, 34, 13707–13716. [Google Scholar] [CrossRef]
- Telmadarreie, A.; Trivedi, J.J. CO2 Foam and CO2 polymer enhanced foam for heavy oil recovery and CO2 storage. Energies 2020, 13, 5735. [Google Scholar] [CrossRef]
- Sheng, J.J. A comprehensive review of alkaline–surfactant–polymer (ASP) flooding. Asia-Pac. J. Chem. Eng. 2014, 9, 471–489. [Google Scholar] [CrossRef]
- Sheng, J.J. Critical review of alkaline-polymer flooding. J. Pet. Explor. Prod. Technol. 2017, 7, 147–153. [Google Scholar] [CrossRef]
- Kazempour, M.; Sundstrom, E.; Alvarado, V. Effect of alkalinity on oil recovery during polymer floods in sandstone. SPE Reserv. Eval. Eng. 2012, 15, 195–209. [Google Scholar] [CrossRef]
- Medica, K.; Maharaj, R.; Alexander, D.; Soroush, M. Evaluation of an alkali-polymer flooding technique for enhanced oil recovery in Trinidad and Tobago. J. Pet. Explor. Prod. Technol. 2020, 10, 3947–3959. [Google Scholar] [CrossRef]
- Ding, M.; Wang, Y.; Yuan, F.; Zhao, H.; Li, Z. A comparative study of the mechanism and performance of surfactant- and alkali-polymer flooding in heavy-oil recovery. Chem. Eng. Sci. 2020, 219, 115603. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, M.; Shirif, E. Study of alkaline/polymer flooding for heavy-oil recovery using channeled sandpacks. SPE Reserv. Eval. Eng. 2011, 14, 310–319. [Google Scholar] [CrossRef]
- Bashir, A.; Sharifi Haddad, A.; Rafati, R. A review of fluid displacement mechanisms in surfactant-based chemical enhanced oil recovery processes: Analyses of key influencing factors. Pet. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Hongyan, W.; Xulong, C.; Jichao, Z.; Aimei, Z. Development and application of dilute surfactant–polymer flooding system for Shengli oilfield. J. Pet. Sci. Eng. 2009, 65, 45–50. [Google Scholar] [CrossRef]
- Druetta, P.; Picchioni, F. Surfactant—Polymer flooding: Influence of the injection scheme. Energy Fuels 2018, 32, 12231–12246. [Google Scholar] [CrossRef]
- Yusuf, S.M.; Junin, R.; Muhamad Sidek, M.A.; Agi, A.; Ahmad Fuad, M.F.I.; Rosli, N.R.; Rahman, N.A.; Yahya, E.; Muhamad Soffian Wong, N.A.; Mustaza, M.H. Screening the synergy of sodium dodecylbenzenesulfonate and carboxymethyl cellulose for surfactant-polymer flooding. Pet. Res. 2021, in press. [Google Scholar] [CrossRef]
- Kalam, S.; Kamal, M.S.; Patil, S.; Hussain, S.M.S. Impact of spacer nature and counter ions on rheological behavior of novel polymer-cationic gemini surfactant systems at high temperature. Polymers 2020, 12, 1027. [Google Scholar] [CrossRef]
- Ge, J.-J.; Zhang, T.-C.; Pan, Y.-P.; Zhang, X. The effect of betaine surfactants on the association behavior of associating polymer. Pet. Sci. 2021, 18, 1441–1449. [Google Scholar] [CrossRef]
- Afolabi, F.; Mahmood, S.M.; Yekeen, N.; Akbari, S.; Sharifigaliuk, H. Polymeric surfactants for enhanced oil recovery: A review of recent progress. J. Pet. Sci. Eng. 2022, 208, 109358. [Google Scholar] [CrossRef]
- Raffa, P.; Broekhuis, A.A.; Picchioni, F. Polymeric surfactants for enhanced oil recovery: A review. J. Pet. Sci. Eng. 2016, 145, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Saxena, N.; Mandal, A. Synthesis and evaluation of physicochemical properties of anionic polymeric surfactant derived from Jatropha oil for application in enhanced oil recovery. J. Ind. Eng. Chem. 2016, 43, 106–116. [Google Scholar] [CrossRef]
- Babu, K.; Pal, N.; Bera, A.; Saxena, V.K.; Mandal, A. Studies on interfacial tension and contact angle of synthesized surfactant and polymeric from castor oil for enhanced oil recovery. Appl. Surf. Sci. 2015, 353, 1126–1136. [Google Scholar] [CrossRef]
- Pal, N.; Babu, K.; Mandal, A. Surface tension, dynamic light scattering and rheological studies of a new polymeric surfactant for application in enhanced oil recovery. J. Pet. Sci. Eng. 2016, 146, 591–600. [Google Scholar] [CrossRef]
- Mehrabianfar, P.; Bahraminejad, H.; Manshad, A.K. An introductory investigation of a polymeric surfactant from a new natural source in chemical enhanced oil recovery (CEOR). J. Pet. Sci. Eng. 2021, 198, 108172. [Google Scholar] [CrossRef]
- Co, L.; Zhang, Z.; Ma, Q.; Watts, G.; Zhao, L.; Shuler, P.J.; Tang, Y. Evaluation of functionalized polymeric surfactants for EOR applications in the Illinois Basin. J. Pet. Sci. Eng. 2015, 134, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Nowrouzi, I.; Khaksar Manshad, A.; Mohammadi, A.H. Effects of Tragacanth Gum as a natural polymeric surfactant and soluble ions on chemical smart water injection into oil reservoirs. J. Mol. Struct. 2020, 1200, 127078. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Liu, Z.; Liu, R.; Liu, W.; Zhang, H. Effects of molecular structure of polymeric surfactant on its physico-chemical properties, percolation and enhanced oil recovery. J. Ind. Eng. Chem. 2021, 101, 165–177. [Google Scholar] [CrossRef]
- Han, X.; Chen, Z.; Zhang, G.; Yu, J. Surfactant-polymer flooding formulated with commercial surfactants and enhanced by negative salinity gradient. Fuel 2020, 274, 117874. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.; Zhao, F. Successful polymer flooding and surfactant-polymer flooding projects at Shengli Oilfield from 1992 to 2012. J. Pet. Explor. Prod. Technol. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Jian, G.; Liu, W.; Cheng, L.; Hou, Q.; Li, J. Recent progress and effects analysis of surfactant-polymer flooding field tests in China. In Proceedings of the SPE-165213-MS, SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2 July 2013; pp. 1–8. [Google Scholar]
- Hamouma, M.; Delbos, A.; Dalmazzone, C.; Colin, A. Polymer surfactant interactions in oil enhanced recovery processes. Energy Fuels 2021, 35, 9312–9321. [Google Scholar] [CrossRef]
- Klimenko, A.; Molinier, V.; Passade-Boupat, N.; Bourrel, M. Effect of surfactants at ultra-low concentration in oil/brine diphasic displacement: The way to unlock EOR polymer field projects. J. Pet. Sci. Eng. 2022, 211, 110190. [Google Scholar] [CrossRef]
- Saxena, N.; Goswami, A.; Dhodapkar, P.K.; Nihalani, M.C.; Mandal, A. Bio-based surfactant for enhanced oil recovery: Interfacial properties, emulsification and rock-fluid interactions. J. Pet. Sci. Eng. 2019, 176, 299–311. [Google Scholar] [CrossRef]
- Yu, F.; Jiang, H.; Xu, F.; Zhen, F.; Wang, J.; Cheng, B.; Su, H.; Li, J. A multi-scale experimental study of hydrophobically-modified polyacrylamide flood and surfactant-polymer flood on enhanced heavy oil recovery. J. Pet. Sci. Eng. 2019, 182, 106258. [Google Scholar] [CrossRef]
- Wibowo, A.D.K.; Yoshi, L.A.; Handayani, A.S. Joelianingsih Synthesis of polymeric surfactant from palm oil methyl ester for enhanced oil recovery application. Colloid Polym. Sci. 2021, 299, 81–92. [Google Scholar] [CrossRef]
- Pogaku, R.; Mohd Fuat, N.H.; Sakar, S.; Cha, Z.W.; Musa, N.; Awang Tajudin, D.N.A.; Morris, L.O. Polymer flooding and its combinations with other chemical injection methods in enhanced oil recovery. Polym. Bull. 2018, 75, 1753–1774. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Arsad, A.; Abbas, A.; Gbadamosi, A.; Azli, N.B.; Oseh, J. Synergy of the flow behaviour and disperse phase of cellulose nanoparticles in enhancing oil recovery at reservoir condition. PLoS ONE 2019, 14, e0220778. [Google Scholar] [CrossRef]
- Vargo, J.; Turner, J.; Bob, V.; Pitts, M.J.; Wyatt, K.; Surkalo, H.; Patterson, D. Alkaline-surfactant-polymer flooding of the cambridge minnelusa field. SPE Reserv. Eval. Eng. 2000, 3, 552–558. [Google Scholar] [CrossRef]
- Sui, X.; Chen, Z.; Kurnia, I.; Han, X.; Yu, J.; Zhang, G. Alkaline-surfactant-polymer flooding of active oil under reservoir conditions. Fuel 2020, 262, 116647. [Google Scholar] [CrossRef]
- Zhapbasbayev, U.K.; Kudaibergenov, S.E.; Mankhanova, A.E.; Sadykov, R.M. Experimental study of alkaline-surfactant-polymer compositions for ASP-flooding of cores from highly viscous oil reservoirs. Thermophys. Aeromech. 2018, 25, 909–916. [Google Scholar] [CrossRef]
- Panthi, K.; Sharma, H.; Mohanty, K.K. ASP flood of a viscous oil in a carbonate rock. Fuel 2016, 164, 18–27. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, G.; Ge, J.; Liao, K.; Pei, H.; Jiang, P.; Li, X. Study on organic alkali-surfactant-polymer flooding for enhanced ordinary heavy oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 230–239. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, D.; Yan, W.; Puerto, M.; Hirasaki, G.J.; Miller, C.A. Favorable attributes of alkaline-surfactant-polymer flooding. SPE J. 2008, 13, 5–16. [Google Scholar] [CrossRef]
- Ghosh, P.; Sharma, H.; Mohanty, K.K. ASP flooding in tight carbonate rocks. Fuel 2019, 241, 653–668. [Google Scholar] [CrossRef]
- Kesarwani, H.; Saxena, A.; Saxena, N.; Sharma, S. Oil mobilization potential of a novel anionic Karanj oil surfactant: Interfacial, wetting characteristic, adsorption, and oil recovery studies. Energy Fuels 2021, 35, 10597–10610. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Characterization and evaluation of a natural surfactant extracted from Soapwort plant for alkali-surfactant-polymer (ASP) slug injection into sandstone oil reservoirs. J. Mol. Liq. 2020, 318, 114369. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Khaksar Manshad, A. Characterization and likelihood application of extracted mucilage from Hollyhocks plant as a natural polymer in enhanced oil recovery process by alkali-surfactant-polymer (ASP) slug injection into sandstone oil reservoirs. J. Mol. Liq. 2020, 320, 114445. [Google Scholar] [CrossRef]
- Al Kalbani, M.; Jordan, M.; Mackay, E.; Sorbie, K.; Nghiem, L. Modelling the impact of Alkaline-surfactant and Alkaline-surfactant-polymer flooding processes on scale precipitation and management. J. Pet. Sci. Eng. 2021, 205, 108777. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, S.; Dong, M.; Yue, X.; Yang, J. Effect of viscosity of alkaline/surfactant/polymer (ASP) solution on enhanced oil recovery in heterogeneous reservoirs. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 8 June 2004; pp. 1–14. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, A. Characterization of rock-fluid and fluid-fluid interactions in presence of a family of synthesized zwitterionic surfactants for application in enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 1–12. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Primary evaluation of a synthesized surfactant from waste chicken fat as a renewable source for chemical slug injection into carbonate oil reservoirs. J. Mol. Liq. 2020, 306, 112843. [Google Scholar] [CrossRef]
- Khan, M.Y.; Samanta, A.; Ojha, K.; Mandal, A. Design of alkaline/surfactant/polymer (ASP) slug and its use in enhanced oil recovery. Pet. Sci. Technol. 2009, 27, 1926–1942. [Google Scholar] [CrossRef]
- Abalkhail, N.; Liyanage, P.J.; Upamali, K.A.N.; Pope, G.A.; Mohanty, K.K. Alkaline-surfactant-polymer formulation development for a HTHS carbonate reservoir. J. Pet. Sci. Eng. 2020, 191, 107236. [Google Scholar] [CrossRef]
- Sharma, H.; Dufour, S.; Arachchilage, G.W.P.P.; Weerasooriya, U.; Pope, G.A.; Mohanty, K. Alternative alkalis for ASP flooding in anhydrite containing oil reservoirs. Fuel 2015, 140, 407–420. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Divya Laxmi, K.V.; Mandal, A. Interfacial behaviour, wettability alteration and emulsification characteristics of a novel surfactant: Implications for enhanced oil recovery. Chem. Eng. Sci. 2018, 187, 200–212. [Google Scholar] [CrossRef]
- Li, J.; Niu, L.; Lu, X. Performance of ASP compound systems and effects on flooding efficiency. J. Pet. Sci. Eng. 2019, 178, 1178–1193. [Google Scholar] [CrossRef]
- Gao, C. Viscosity of partially hydrolyzed polyacrylamide under shearing and heat. J. Pet. Explor. Prod. Technol. 2013, 3, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Yekeen, N.; Agi, A.; Oseh, J.O. Recent advances and prospects in polymeric nanofluids application for enhanced oil recovery. J. Ind. Eng. Chem. 2018, 66, 1–19. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Abdullah, M.O.; Jaafar, M.Z.; Arsad, A.; Wan Sulaiman, W.R.; Norddin, M.N.A.M.; Abdurrahman, M.; Abbas, A.; Gbadamosi, A.; et al. Application of polymeric nanofluid in enhancing oil recovery at reservoir condition. J. Pet. Sci. Eng. 2020, 194, 107476. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Gbadamosi, A.; Abbas, A.; Azli, N.B.; Oseh, J. Influence of nanoprecipitation on crystalline starch nanoparticle formed by ultrasonic assisted weak-acid hydrolysis of cassava starch and the rheology of their solutions. Chem. Eng. Process.–Process Intensif. 2019, 142, 107556. [Google Scholar] [CrossRef]
- Rezaei, A.; Abdi-Khangah, M.; Mohebbi, A.; Tatar, A.; Mohammadi, A.H. Using surface modified clay nanoparticles to improve rheological behavior of Hydrolized Polyacrylamid (HPAM) solution for enhanced oil recovery with polymer flooding. J. Mol. Liq. 2016, 222, 1148–1156. [Google Scholar] [CrossRef]
- Maurya, N.K.; Mandal, A. Studies on behavior of suspension of silica nanoparticle in aqueous polyacrylamide solution for application in enhanced oil recovery. Pet. Sci. Technol. 2016, 34, 429–436. [Google Scholar] [CrossRef]
- Hu, Z.; Haruna, M.; Gao, H.; Nourafkan, E.; Wen, D. Rheological Properties of Partially Hydrolyzed Polyacrylamide Seeded by Nanoparticles. Ind. Eng. Chem. Res. 2017, 56, 3456–3463. [Google Scholar] [CrossRef]
- Corredor-Rojas, L.M.; Hemmati-Sarapardeh, A.; Husein, M.M.; Dong, M.; Maini, B.B. Rheological behavior of surface modified silica nanoparticles dispersed in partially hydrolyzed polyacrylamide and xanthan gum solutions: Experimental measurements, mechanistic understanding, and model development. Energy Fuels 2018, 32, 10628–10638. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. Effect of hydrophobic and hydrophilic metal oxide nanoparticles on the performance of xanthan gum solutions for heavy oil recovery. Nanomaterials 2019, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakkoli, O.; Kamyab, H.; Shariati, M.; Mustafa Mohamed, A.; Junin, R. Effect of nanoparticles on the performance of polymer/surfactant flooding for enhanced oil recovery: A review. Fuel 2022, 312, 122867. [Google Scholar] [CrossRef]
- Bagaria, H.G.; Xue, Z.; Neilson, B.M.; Worthen, A.J.; Yoon, K.Y.; Nayak, S.; Cheng, V.; Lee, J.H.; Bielawski, C.W.; Johnston, K.P. Iron oxide nanoparticles grafted with sulfonated copolymers are stable in concentrated brine at elevated temperatures and weakly adsorb on silica. ACS Appl. Mater. Interfaces 2013, 5, 3329–3339. [Google Scholar] [CrossRef]
- Bagaria, H.G.; Neilson, B.M.; Worthen, A.J.; Xue, Z.; Yoon, K.Y.; Cheng, V.; Lee, J.H.; Velagala, S.; Huh, C.; Bryant, S.L.; et al. Adsorption of iron oxide nanoclusters stabilized with sulfonated copolymers on silica in concentrated NaCl and CaCl2 brine. J. Colloid Interface Sci. 2013, 398, 217–226. [Google Scholar] [CrossRef]
- Cheraghian, G.; Khalili Nezhad, S.S.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Adsorption polymer on reservoir rock and role of the nanoparticles, clay and SiO2. Int. Nano Lett. 2014, 4, 114. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Foster, E.; Wang, Y.; Nayak, S.; Cheng, V.; Ngo, V.W.; Pennell, K.D.; Bielawski, C.W.; Johnston, K.P. Effect of grafted copolymer composition on iron oxide nanoparticle stability and transport in porous media at high salinity. Energy Fuels 2014, 28, 3655–3665. [Google Scholar] [CrossRef]
- Iqbal, M.; Lyon, B.A.; Ureña-Benavides, E.E.; Moaseri, E.; Fei, Y.; McFadden, C.; Javier, K.J.; Ellison, C.J.; Pennell, K.D.; Johnston, K.P. High temperature stability and low adsorption of sub-100 nm magnetite nanoparticles grafted with sulfonated copolymers on Berea sandstone in high salinity brine. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Sugunan, A.; Gillgren, T.; Larsson, J.A.; Zhang, Z.-B.; Zhang, S.-L.; Nordgren, N.; Sommertune, J.; Ahniyaz, A. Surfactant-free stabilization of aqueous graphene dispersions using starch as a dispersing agent. ACS Omega 2021, 6, 12050–12062. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, C.K.B.; Medeiros, F.S.; Diniz, B.R.S.; Viana, M.M.; Caliman, V.; Silva, G.G. Nanofluids based on hydrolyzed polyacrylamide and aminated graphene oxide for enhanced oil recovery in different reservoir conditions. Fuel 2022, 310, 122299. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Gbadamosi, A.; Manan, M.; Jaafar, M.Z.; Abdullah, M.O.; Arsad, A.; Azli, N.B.; Abdurrahman, M.; Yakasai, F. Comparing natural and synthetic polymeric nanofluids in a mid-permeability sandstone reservoir condition. J. Mol. Liq. 2020, 317, 113947. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Abbas, A.; Gbadamosi, A.; Azli, N.B. Influence of ultrasonic on the flow behavior and disperse phase of cellulose nano-particles at fluid–fluid interface. Nat. Resour. Res. 2020, 29, 1427–1446. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. Impact of PAM-grafted nanoparticles on the performance of hydrolyzed polyacrylamide solutions for heavy oil recovery at different salinities. Ind. Eng. Chem. Res. 2019, 58, 9888–9899. [Google Scholar] [CrossRef]
- Sharma, T.; Iglauer, S.; Sangwai, J.S. Silica nanofluids in an oilfield polymer polyacrylamide: Interfacial properties, wettability alteration, and applications for chemical enhanced oil recovery. Ind. Eng. Chem. Res. 2016, 55, 12387–12397. [Google Scholar] [CrossRef]
- Bera, A.; Shah, S.; Shah, M.; Agarwal, J.; Vij, R.K. Mechanistic study on silica nanoparticles-assisted guar gum polymer flooding for enhanced oil recovery in sandstone reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124833. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Junin, R.; Manan, M.A.; Agi, A.; Oseh, J.O.; Usman, J. Effect of aluminium oxide nanoparticles on oilfield polyacrylamide: Rheology, interfacial tension, wettability and oil displacement studies. J. Mol. Liq. 2019, 296, 111863. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.V.S.; Tiwari, P. Silica nanoparticle assisted polymer flooding of heavy crude oil: Emulsification, rheology, and wettability alteration characteristics. Ind. Eng. Chem. Res. 2018, 57, 6364–6376. [Google Scholar] [CrossRef]
- Pal, N.; Kumar, N.; Mandal, A. Stabilization of dispersed oil droplets in nanoemulsions by synergistic effects of the gemini surfactant, PHPA polymer, and silica nanoparticle. Langmuir 2019, 35, 2655–2667. [Google Scholar] [CrossRef]
- Kumar, N.; Gaur, T.; Mandal, A. Characterization of SPN Pickering emulsions for application in enhanced oil recovery. J. Ind. Eng. Chem. 2017, 54, 304–315. [Google Scholar] [CrossRef]
- Keykhosravi, A.; Vanani, M.B.; Aghayari, C. TiO2 nanoparticle-induced Xanthan Gum Polymer for EOR: Assessing the underlying mechanisms in oil-wet carbonates. J. Pet. Sci. Eng. 2021, 204, 108756. [Google Scholar] [CrossRef]
- Khalilinezhad, S.S.; Mohammadi, A.H.; Hashemi, A.; Ghasemi, M. Rheological characteristics and flow dynamics of polymer nanohybrids in enhancing oil recovery from low permeable carbonate oil reservoirs. J. Pet. Sci. Eng. 2021, 197, 107959. [Google Scholar] [CrossRef]
- Gbadamosi, A.; Yusuff, A.; Agi, A.; Muruga, P.; Junin, R.; Jeffrey, O. Mechanistic study of nanoparticles-assisted xanthan gum polymer flooding for enhanced oil recovery: A comparative study. J. Pet. Explor. Prod. Technol. 2022, 12, 207–213. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Z.; Dong, H.; Vladimirovna Mikhailova, M.; Davarpanah, A. Hybrid application of nanoparticles and polymer in enhanced oil recovery processes. Polymers 2021, 13, 1414. [Google Scholar] [CrossRef]
- Ponnapati, R.; Karazincir, O.; Dao, E.; Ng, R.; Mohanty, K.K.; Krishnamoorti, R. Polymer-functionalized nanoparticles for improving waterflood sweep efficiency: Characterization and transport properties. Ind. Eng. Chem. Res. 2011, 50, 13030–13036. [Google Scholar] [CrossRef]
- Cao, J.; Song, T.; Wang, X.; Zhu, Y.; Wang, S.; Zhao, M.; Miao, Y.; Zhang, J. Studies on the rheological properties of amphiphilic nanosilica and a partially hydrolyzed polyacrylamide hybrid for enhanced oil recovery. Chem. Eng. Sci. 2019, 206, 146–155. [Google Scholar] [CrossRef]
- Pu, W.-F.; Liu, R.; Wang, K.-Y.; Li, K.-X.; Yan, Z.-P.; Li, B.; Zhao, L. Water-soluble core–shell hyperbranched polymers for enhanced oil recovery. Ind. Eng. Chem. Res. 2015, 54, 798–807. [Google Scholar] [CrossRef]
- Hamdi, S.S.; Al-Kayiem, H.H.; Alsabah, M.S.; Muhsan, A.S. A comparative study of dispersed and grafted nanofluids of graphene nanoplatelets with natural polymer in high salinity brine for enhanced oil recovery. J. Pet. Sci. Eng. 2022, 210, 110004. [Google Scholar] [CrossRef]
- Maurya, N.K.; Kushwaha, P.; Mandal, A. Studies on interfacial and rheological properties of water soluble polymer grafted nanoparticle for application in enhanced oil recovery. J. Taiwan Inst. Chem. Eng. 2017, 70, 319–330. [Google Scholar] [CrossRef]
- Aliabadian, E.; Sadeghi, S.; Rezvani Moghaddam, A.; Maini, B.; Chen, Z.; Sundararaj, U. Application of graphene oxide nanosheets and HPAM aqueous dispersion for improving heavy oil recovery: Effect of localized functionalization. Fuel 2020, 265, 116918. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, M.; Zheng, C.; Guo, S.; Li, X.; Zhang, Z. Water-dispersible reactive nanosilica and poly(2-acrylamido-2-methyl-1-propanesulfonic acid sodium) nanohybrid as potential oil displacement agent for enhanced oil recovery. Energy Fuels 2017, 31, 6345–6351. [Google Scholar] [CrossRef]
- Orodu, K.B.; Afolabi, R.O.; Oluwasijuwomi, T.D.; Orodu, O.D. Effect of aluminum oxide nanoparticles on the rheology and stability of a biopolymer for enhanced oil recovery. J. Mol. Liq. 2019, 288, 110864. [Google Scholar] [CrossRef]
- Behzadi, A.; Mohammadi, A. Environmentally responsive surface-modified silica nanoparticles for enhanced oil recovery. J. Nanopart. Res. 2016, 18, 266. [Google Scholar] [CrossRef]
- Liu, R.; Pu, W.; Sheng, J.J.; Du, D. Star-like hydrophobically associative polyacrylamide for enhanced oil recovery: Comprehensive properties in harsh reservoir conditions. J. Taiwan Inst. Chem. Eng. 2017, 80, 639–649. [Google Scholar] [CrossRef]
- Liu, R.; Pu, W.-F.; Du, D.-J. Synthesis and characterization of core–shell associative polymer that prepared by oilfield formation water for chemical flooding. J. Ind. Eng. Chem. 2017, 46, 80–90. [Google Scholar] [CrossRef]
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Stephen, K.D. Potential application of low-salinity polymeric-nanofluid in carbonate oil reservoirs: IFT reduction, wettability alteration, rheology and emulsification characteristics. J. Mol. Liq. 2019, 284, 735–747. [Google Scholar] [CrossRef]
- Ye, Z.; Qin, X.; Lai, N.; Peng, Q.; Li, X.; Li, C. Synthesis and performance of an acrylamide copolymer containing nano-SiO2 as enhanced oil recovery chemical. J. Chem. 2013, 2013, 1–33. [Google Scholar]
- Yang-Chuan, K.; Guang-Yao, W.; Yi, W. Preparation, morphology and properties of nanocomposites of polyacrylamide copolymers with monodisperse silica. Eur. Polym. J. 2008, 44, 2448–2457. [Google Scholar] [CrossRef]
- Lai, N.; Wu, T.; Ye, Z.; Zhang, Y.; Zhou, N.; Zeng, F. Hybrid hyperbranched polymer based on modified nano-SiO2 for enhanced oil recovery. Chem. Lett. 2016, 45, 1189–1191. [Google Scholar] [CrossRef]
- Maghzi, A.; Kharrat, R.; Mohebbi, A.; Ghazanfari, M.H. The impact of silica nanoparticles on the performance of polymer solution in presence of salts in polymer flooding for heavy oil recovery. Fuel 2014, 123, 123–132. [Google Scholar] [CrossRef]
- Yousefvand, H.; Jafari, A. Enhanced oil recovery using polymer/nanosilica. Procedia Mater. Sci. 2015, 11, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Cheraghian, G. Effect of nano titanium dioxide on heavy oil recovery during polymer flooding. Pet. Sci. Technol. 2016, 34, 633–641. [Google Scholar] [CrossRef]
- Khalilinezhad, S.S.; Cheraghian, G.; Karambeigi, M.S.; Tabatabaee, H.; Roayaei, E. Characterizing the role of clay and silica nanoparticles in enhanced heavy oil recovery during polymer flooding. Arab. J. Sci. Eng. 2016, 41, 2731–2750. [Google Scholar] [CrossRef]
- Zheng, C.; Cheng, Y.; Wei, Q.; Li, X.; Zhang, Z. Suspension of surface-modified nano-SiO2 in partially hydrolyzed aqueous solution of polyacrylamide for enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 169–177. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Cheng, J.; Wu, J. Actual field data show that production costs of polymer flooding can be lower than waterflooding. In SPE International Improved Oil Recovery Conference in Asia Pacific; OnePetro: Richardson, TX, USA, 2003; pp. 1–3. [Google Scholar]
- Wang, D.; Cheng, J.; Wu, J.; Wang, G. Experiences learned after production of more than 300 million barrels of oil by polymer flooding in daqing oil field. In Proceedings of the SPE-77693-MS, SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 29 September 2002; pp. 1–10. [Google Scholar]







| Reservoir Depth, ft | <9000 | 700–9460 | NC | <5250 |
|---|---|---|---|---|
| Porosity, % | NA | NA | NA | ≥21 |
| Permeability, mD | >10 | 1.8–5500 | 50 | >1000 |
| Oil viscosity, cP | 10–100 | 0.4–4000 | <150 | <5400 |
| Oil gravity, °API | >15 | 13–42.5 | NC | >11 |
| Oil saturation, % | >50 | 34–82 | NA | >50 |
| Temperature, °F | <200 | <237 | <200 | <149 |
| Salinity, ppm | NA | NA | <50,000 | <46,000 |
| Reference | [62] | [63] | [64] | [4] |
| Polymer Type | Advantages | Disadvantages |
|---|---|---|
| HPAM |
|
|
| HAPAM |
|
|
| Xanthan gum |
|
|
| Welan gum |
|
|
| Guar gum |
|
|
| Cellulose |
|
|
| Carboxymethylcellulose |
|
|
| Hydroxyethylcellulose |
|
|
| Schizophyllan |
|
|
| Scleroglucan |
|
|
| Polymer Type and Conc. | Experimental Condition(s) | Core Type | Rock Condition | Remarks | Ref. |
|---|---|---|---|---|---|
| HPAM HAPAM 4000 ppm | Brine salinity = 92,000 ppm, T = 82 °C, μo= 1.6 mPa.s (@ 60 °C), Flow rate = 0.1 cc/min | Sandpack | ϕ = 24–27%, k = 2549 mD | The associative polymer recorded 6.52% incremental oil recovery over waterflooding as compared to 1.67% recorded by HPAM flooding. Hence, the associative polymer was recommended for pilot-scale test of South Turgay Basin. | [66] |
| HEC HAHEC 6000 ppm | μo = 72 mPa.s (@ 50 °C), salinity = 15,296 mg/L, flow rate = 0.5 mL/min, temperature = 60 °C | Sandpack | ϕ = 32% | HAHEC displayed better viscosifying properties compared to HEC. Moreover, HAHEC lowered the IFT at the oil–water interface and caused emulsification of crude oil, which led to better oil recovery after waterflooding process. | [67] |
| HECT ragacanth gum HPAM | TDS = 5.567 g/L, oil viscosity = 0.31–0.48 (@ 45 °C) | Sandpack | ϕ = 35–36% | Incremental oil recovery of 7.38%, 6.71%, and 5.83% was recorded for HEC, tragacanth gum, and HPAM, respectively. | [68] |
| TVP PAM | TDS = 101,000 mg/L, Flow rate = 2 mL/min, temperature = 45 and 85 °C | Sandstone | ϕ = 20%, k = 200 mD | As compared to PAM which showed a monotonic decrease in viscosity, the thermoviscosifying polymer exhibited better thermothickening ability and salt tolerance. Oil displacement tests showed that TVP recorded higher oil recovery of 16.4% and 15.5% at 45 and 85 °C, respectively. PAM recorded 12.0% and 9.2% under the same conditions. | [69] |
| Guar gum | Temperature = 28 °C, oil viscosity = 24.8° API | Sandstone | ϕ = 15–39%, k = 206–248 mD | As compared to waterflooding, the use of guar gum resulted in an additional 20–26% incremental oil recovery. | [70] |
| Starch | Sandstone | ϕ = ~23–27%, k = ~291–293 mD | The application of starch biopolymers derived from waste material yielded 52–74% recovery from the sandstone cores. | [71] | |
| Xanthan | Brine = 3.0 wt.% | Glassbead pack | ϕ = 36.9%, k = 3.79 darcys | The polymer exhibited good stability in high-salinity brine. Moreover, 3 wt.% concentration of the polymer yielded 30% incremental oil recovery over waterflooding. | [72] |
| HPAM | Brine = 3.0 wt.%, temperature = 25 °C oil viscosity = 450 cP, flow rate = 4 mL/min | Glassbead pack | ϕ = 37%, k = 3.4 darcys | Oil displacement results revealed that the application of HPAM resulted in approximately 22% incremental oil recovery over waterflooding process. | [51] |
| Welan gum Xanthan gum | Temperature = 50 °C, flow rate = 0.5 mL/min, salinity = 9374 mg/L, oil viscosity = 458 cP (@ 50 °C) | Sandpack | ϕ = 38%, k = 0.18–1.51 μm2 | At the same concentration, the elastic and viscous modulus of welan gum were higher than xanthan gum. Moreover, the core flooding results showed that welan gum recorded 7.3% and 25.4% additional oil recovery over xanthan gum and waterflooding, respectively. | [42] |
| Schizophyllan | Oil viscosity = 35 cP, salinity = 180 g/L, temperature = 55 °C) | Sandstone | ϕ = 24%, k = 1900 mD | The injection of schizophylan yielded good oil recovery and residual resistance factor. | [73] |
| Scleroglucan ATBS | TDS = 3800 mg/L, oil viscosity = 390 cP (@ 100 °C). | Sandstone | ϕ = 18.8–20.4% | As compared to the sulfonated polyacrylamide (2500 mg/L), scleroglucan (935 mg/L) recorded approximately 10% incremental oil recovery. | [74] |
| Surfactant Type | Polymer Type | System Type | Rock Type | Exp Conditions | Findings | Ref. |
|---|---|---|---|---|---|---|
| Sodium dodecylbenzenesulfonate (SDBS) | Carboxymethyl cellulose | SP | Sandpack | Flowrate = 0.5 mL/min | The injection of SP slug resulted in 14–20% incremental oil recovery. The incremental oil recovery was attributed to factors such as emulsion generation, IFT reduction, and optimum viscosity of the SP slug. | [112] |
| SDBS | HPAM | SP | Sandstone | Flow rate = ~1 ft/day, brine = 40 g/L NaCl | The use of polymers in SP flooding reduced the PV of the injectant. Homogeneous formulation of SP flooding recovered 66% OOIP. Moreover, the use of a homogeneous SP system reduced the adsorption of the surfactant on rock pores. | [127] |
| Alkoxysulfate | HPAM | SP | Sandpack | Oil viscosity = 6.6 cP (@ 55 °C), formation water salinity = 107.83 g/L | A low concentration (500 ppm) of surfactant was found to enhance the oil recovery efficiency of the polymer flood by 13% OOIP. Moreover, the authors suggested that the optimal salinity of the surfactant show be greater than that of the injected water. Ultralow surfactant concentration was recommended to avoid issues associated with high surfactant concentration, such as persistent emulsions and aqueous solubility. | [128] |
| Anionic surfactant Nonionic surfactant | HPAM | SP | Sandpack | Oil viscosity = 1300 cP, Oil density = 970.1 kg/m3, flow rate = 0.001 mL/min, temperature = 70 °C | SP demonstrated good emulsion stability. The injection of 0.5 PV of SP flood resulted in 30.7–32.7% incremental oil recovery. | [107] |
| Soap-nut surfactant 8000–10,000 ppm | HPAM 1000 ppm | SP | Sandpack | Oil viscosity = 18.9 ° API, 2 wt.% brine solution | The IFT of the solution decreases with an increase in the surfactant concentration. Moreover, the presence of the surfactant altered the wettability of the sandstone rock surface from 83.5° to 20.8°. The adsorption of the natural surfactant on quartz surface was low due to electrostatic repulsion. SP flooding process recorded approximately 30% incremental oil recovery with different slug injections. | [129] |
| Polyether carboxylate anionic nonionic surfactant | HMPAM, HPAM | SP | Sandstone | Oil viscosity = 562.4 cP (@ 65 °C), oil density = 0.963 g/cm3, pressure = 10 MPa, temperature = 65 °C | The application of SP flooding yielded 15.54% incremental heavy oil recovery. The synergic combination of polymer flooding and SP flooding yielded 40.64% incremental oil recovery. | [130] |
| Soldium allyl-sulfonate, acrylic ester, allyl glycidyl ether | Acrylamide | Polymeric surfactant | Sandstone | Flow rate = 0.8 mL/min, temperature = 55 °C | As compared to polymer (HPAM) flooding that resulted in 11.5% incremental oil recovery after waterflooding process, the use of polymeric surfactant flooding achieved 17.5% incremental oil recovery. | [123] |
| Sodium methyl ester sulfonate | Acrylamide | Polymeric surfactant | Sandpack | Oil viscosity = 23.11 °API, 40 cP (@ 30 °C) | The polymeric surfactant reduced IFT at oil–water interface to 0.37 mN/m at the optimum salinity. Besides, the polymeric surfactant exhibited shear thinning behavior. Finally, 26% incremental oil recovery over conventional waterflooding was recorded during the flooding of sandpack. | [117] |
| Sodium methyl ester sulfonate | Acrylamide | Polymeric surfactant | Sandstone | Flow rate = 1.83 mL/s | The synthesized polymeric surfactant reduced the contact angle of oil-wet quartz surface to 25.47° after 10 min contact time. The IFT of the oil–water interface was also reduced to 2.3 mN/m. Finally, a total recovery of 77.98% was achieved by the injection of the polymeric surfactant. | [131] |
| Alkali Type | Surfactant Type | Polymer Type | Experimental Condition(s) | Rock Type | Finding | Ref. |
|---|---|---|---|---|---|---|
| Na2CO3 | Alkylbenzene sulfonate, fatty alcohol propoxylated sulfate, cocamidopropyl hydroxysultaine | HPAM (MW = 20 × 106) | Formation brine (7500 ppm TDS), injection brine (5300 ppm) oil viscosity = 60 cp (@ 62 °C) | N/A | Injection of 0.3 PV of ASP slug resulted in incremental oil recovery of 44.5% over waterflooding. | [135] |
| NaOH (2500 ppm) | Anionic surfactant from waste chicken fat (5500 ppm) | Hollyhocks (2000 ppm) | Oil viscosity = 41.34 cP (@ 15.56 °C), temperature = 80 °C, flow rate = 0.2 mL/min, salinity = 62,000 TDS | Sandstone | The novel polymer solution non-Newtonian behavior. Moreover, 27.9% incremental oil recovery was achieved with the use of ASP slug injection into sandstone. | [143] |
| Na2CO3 | Carboxybetaine zwitterionic surfactant | HPAM | Oil viscosity = 30 °API | Sandpack | The surfactant altered the permeability of the oil-wet quartz sample. The experimental result from sandpack flooding indicates the ASP slug injection recovered 30.82% OOIP. | [146] |
| NaOH | Anionic surfactant from waste chicken fat (5500 ppm) | HPAM (1000 ppm) | Flow rate = 0.2 mL/min, temperature = 75 °C | Carbonate | The alkali–surfactant mixture reduced the IFT and altered the wettability of the carbonate from oil-wet to water-wetting condition. For the application of ASP in carbonate, 17.8% incremental oil recovery was recorded. | [147] |
| NaOH | SDBS | HPAM | – | Sandstone | Ultralow interfacial tension was generated using a very low concentration of alkali and surfactant while the injected polymer enhances the mobility control. An additional 20% OOIP over conventional waterflooding was found. | [148] |
| Ethoxylated diisopropylamine | Carboxylate and sulfonate surfactant | HPAM (3330S) | Salinity = 60,000 ppm, temperature = 100 °C | Carbonate | ASP yielded ultralow IFT, low surfactant retention, and high recovery in carbonate cores characterized by high permeability, nonfracture, and HTHS condition. Cumulative oil recovery using ASP slug ranges from 85.2 to 93.6%. | [149] |
| NaBO2 NH4OH | Isobutyl alcohol-3-ethoxylate, internal olefin sulfonate | HPAM 3630S, 3330S, AN 125 | Formation brine = 147,507 ppm, hardness = 2144 ppm (Ca2+, Mg2+), injection brine = 1–3 wt.% NaCl | Carbonate and Sandstone | The use of sodium metaborate and ammonium hydroxide as alkalis in the ASP corefloods yielded low surfactant retention and high oil recoveries. | [150] |
| Na2CO3 NaOH | PS, IOS, IBA-EO, TSPC, EPS | HPAM (FP 3330S) 3500 ppm | Oil viscosity = 8 cP, NaCl = 22,390 ppm, Na2SO4 = 2464 ppm, CaCl2.2H2O = 983 ppm, and MgCl2.6H2O = 2340 ppm | Limestone cores | The study revealed that the pore throat radii of the rock must be bigger than the polymer hydrodynamic radius for successful polymer transport. Moreover, the secondary application of ASP yielded 77–87% cumulative OOIP in low-permeability rocks. | [137] |
| Triethylamine | Sodium ethyl ester sulfonate (SEES) | HPAM | Oil viscosity = 23.55 °API (30 °C), Brine = 1 wt.% | Sandpack | Alkali and surfactant played a crucial role in the IFT reduction to ultralow values. Besides, 34.79% incremental oil recovery was achieved with 0.2 wt.% HPAM and 0.8 wt.% SEES. | [151] |
| Ethanolamine | Sulfonate-based surfactants | HPAM (1000 ppm) | Salinity = 13,659.9 ppm (NaCl, MgCl2, and CaCl2) | Sandpack | The use of organic alkali resulted in ultralow IFT, stable oil-in-water emulsion, and enhanced oil displacement efficiency. Moreover, approximately 20% incremental oil recovery was recorded during sandpack flooding. | [138] |
| Monoethylamine NaOH | Tragacanth gum | Viscosity = 31.14 ° API, formation water salinity = 74,000 TDS, temperature = 75 °C | Carbonates | The synthesized polymeric surfactant increased the viscosity of water and reduced the mobility ratio of the injectant. Moreover, IFT was reduced to 2.329 mN/m at the optimum salinity conditions. For the ASP flooding, 21.4% incremental oil recovery was recorded. | [122] | |
| NaOH Na2CO3 | Alkylbenzene sulfonate | HPAM (MW = 25 × 106) | Oil viscosity = 9.8 cP (@ 45 °C) | Sandstone | The study showed that the viscosity, IFT, and hydrodynamic diameter of ASP containing weak alkali surpassed those of strong alkali at the same concentration. ASP containing weak alkali had 22% incremental oil recovery. | [152] |
| NP Type | Polymer/Copolymer Type | PNF Conc. | Brine/Conc. | Temp | Porous Medium Type | Incremental Oil Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| SiO2, Al2O3 | HPAM | 100–2500 ppm | 0.6 wt% KCl | – | Sandpack | 5.0–9.0 | [182] |
| SiO2 | PEOMA | 10,000 ppm | 1.0 wt.% NaCl | 30 °C | Berea sandstone | 19.5 | [183] |
| APTES-SiO2 OTES-SiO2 | HPAM | 625 ppm NP 2500 ppm HPAM | 2000–10,000 ppm | 90 °C | Sandstone core | 4.6–12.3 | [184] |
| SiO2 | PAMAM | 1500 ppm | 10 wt.% NaCl, 0.15 wt.% MgCl2 0.10 wt.% CaCl2 | 90 °C | Berea Sandstone | 16.3 | [185] |
| Graphene | Gum arabic | 50 ppm | 3.0 wt.% NaCl | 90 °C | Berea sandstone | 17.12 | [186] |
| SiO2 | Prop-2-enamide/AM | 8000 ppm | – | 80 °C | Quartz sand | 21.0 | [187] |
| GO | HPAM | 0.2 wt.% NP 0.05 wt.% HPAM | 25 °C | Sandpack | 7.8 | [188] | |
| SiO2 | AMPS | 50,000 ppm | – | 80 °C | Quartz sand | 23.22 | [189] |
| Al2O3 | Potato starch Gum arabic | 1.3 wt% 3–5 wt.% | 3.0 wt.% NaCl | 25 °C | Sandstone | 5.16–7.18 | [190] |
| SiO2 | PEG | 10,000 ppm | – | 80 °C | Glass micromodel | 20.0 | [191] |
| SiO2 Al2O3 TiO2 | Xanthan gum | 5000 ppm | 3.0 wt.% NaCl | 80 °C | Sandstone | 7.2–11.2 | [181] |
| SiO2 | MeDiC8AM | 1500 ppm | 12 wt.% (NaCl & CaCl2) | 82.3 °C | Sandstone | 20.0 | [192] |
| SiO2 | AMC12S | 1100 ppm | 18 wt.% | 110 °C | Sandstone | 24.0 | [193] |
| ZnO/SiO2 | Xanthan | 2000 ppm | 1660 ppm | 75 °C | Carbonate | 19.28 | [194] |
| SiO2 | AA/AM | 2000 ppm | 2 wt.% NaCl, 0.18 wt.% CaCl2 | 65°C | Sandstone | 20.1 | [195] |
| SiO2 | PA–S | 3000 ppm | 5 wt.% NaCl, 2 wt.% CaCl2 | 25 °C | – | 12.77 | [196] |
| SiO2 | AM/AA | 1500 ppm | – | – | – | 18.84 | [197] |
| SiO2 | HPAM | 1000 ppm | 2.4 wt.% (NaCl, CaCl2, MgCl2) | 25 °C | Glass micromodel | 10.0 | [198] |
| SiO2 | HPAM | 800 ppm | 3 wt.% NaCl | – | Glass micromodel | 10.0 | [199] |
| TiO2 | HPAM | – | 2 wt.% (NaCl, CaCl2, MgCl2.6H2O, Na2HCO3) | – | Sandstone | 4.0 * | [200] |
| MMT Clay | HPAM | 1000 ppm | 10 wt.% (NaCl, CaCl2, MgCl2) | 90 °C | Quartz sand | 33.0 | [157] |
| SiO2 | Guar gum | 0.2 wt.% NP 4.0 wt.% guar gum | – | 50 °C | Sandstone | 12.95 | [174] |
| SiO2 | Xanthan | 0.3 wt.% NP 5000 ppm XG | 4445 ppm | 30 °C | Sandstone | 20.82 | [176] |
| SiO2 Clay | HPAM | 1500 ppm | 2.0 wt.% (NaCl, CaCl2, MgCl2.6H2O) | – | Sandstone | 13.0 | [201] |
| SiO2 | HPAM | 600 ppm | 6.0 wt.% (NaCl, CaCl2, MgCl2.6H2O Na2SO4 Na2HCO3) | 80 °C | Quartz sand | 10.54 | [202] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gbadamosi, A.; Patil, S.; Kamal, M.S.; Adewunmi, A.A.; Yusuff, A.S.; Agi, A.; Oseh, J. Application of Polymers for Chemical Enhanced Oil Recovery: A Review. Polymers 2022, 14, 1433. https://doi.org/10.3390/polym14071433
Gbadamosi A, Patil S, Kamal MS, Adewunmi AA, Yusuff AS, Agi A, Oseh J. Application of Polymers for Chemical Enhanced Oil Recovery: A Review. Polymers. 2022; 14(7):1433. https://doi.org/10.3390/polym14071433
Chicago/Turabian StyleGbadamosi, Afeez, Shirish Patil, Muhammad Shahzad Kamal, Ahmad A. Adewunmi, Adeyinka S. Yusuff, Augustine Agi, and Jeffrey Oseh. 2022. "Application of Polymers for Chemical Enhanced Oil Recovery: A Review" Polymers 14, no. 7: 1433. https://doi.org/10.3390/polym14071433
APA StyleGbadamosi, A., Patil, S., Kamal, M. S., Adewunmi, A. A., Yusuff, A. S., Agi, A., & Oseh, J. (2022). Application of Polymers for Chemical Enhanced Oil Recovery: A Review. Polymers, 14(7), 1433. https://doi.org/10.3390/polym14071433








