Environmental Consequences of Rubber Crumb Application: Soil and Water Pollution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Heavy Metals Concentration
2.3. PAHs Analysis
2.4. Ecotoxicological Tests
3. Results and Discussion
3.1. Heavy Metals Concentration
3.2. PAHs
3.3. Ecotoxicity
4. Policy Implications and Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bianco, I.; Panepinto, D.; Zanetti, M. End-of-Life Tyres: Comparative Life Cycle Assessment of Treatment Scenarios. Appl. Sci. 2021, 11, 3599. [Google Scholar] [CrossRef]
- Myhre, M.; MacKillop, D.A. Rubber recycling. Rubber Chem. Technol. 2002, 75, 429–474. [Google Scholar] [CrossRef]
- Ferdous, W.; Manalo, A.; Siddique, R.; Mendis, P.; Yan, Z.G.; Wong, H.S.; Lokuge, W.; Aravinthan, T.; Schubel, P. Recycling of landfill wastes (tyres, plastics and glass) in construction—A review on global waste generation, performance, application and future opportunities. Resour. Conserv. Recycl. 2021, 173, 13. [Google Scholar] [CrossRef]
- Sibeko, M.A.; Adeniji, A.O.; Okoh, O.O.; Hlangothi, S.P. Trends in the management of waste tyres and recent experimental approaches in the analysis of polycyclic aromatic hydrocarbons (PAHs) from rubber crumbs. Environ. Sci. Pollut. Res. 2020, 27, 43553–43568. [Google Scholar] [CrossRef] [PubMed]
- Halsband, C.; Sorensen, L.; Booth, A.M.; Herzke, D. Car Tire Crumb Rubber: Does Leaching Produce a Toxic Chemical Cocktail in Coastal Marine Systems? Front. Environ. Sci. 2020, 8, 15. [Google Scholar] [CrossRef]
- Landi, D.; Gigli, S.; Germani, M.; Marconi, M. Investigating the feasibility of a reuse scenario for textile fibres recovered from end-of-life tyres. Waste Manag. 2018, 75, 187–204. [Google Scholar] [CrossRef]
- Strukar, K.; Sipos, T.K.; Milicevic, I.; Busic, R. Potential use of rubber as aggregate in structural reinforced concrete element—A review. Eng. Struct. 2019, 188, 452–468. [Google Scholar] [CrossRef]
- Wang, J.Q.; Du, B. Experimental studies of thermal and acoustic properties of recycled aggregate crumb rubber concrete. J. Build. Eng. 2020, 32, 7. [Google Scholar] [CrossRef]
- Wisniewska, P.; Zedler, L.; Formela, K. Processing, Performance Properties, and Storage Stability of Ground Tire Rubber Modified by Dicumyl Peroxide and Ethylene-Vinyl Acetate Copolymers. Polymers 2021, 13, 4014. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.O.; Rocha, M.R.; Alves, A.; Ratola, N. A review of potentially harmful chemicals in crumb rubber used in synthetic football pitches. J. Hazard. Mater. 2021, 409, 32. [Google Scholar] [CrossRef] [PubMed]
- Celeiro, M.; Armada, D.; Dagnac, T.; de Boer, J.; Llompart, M. Hazardous compounds in recreational and urban recycled surfaces made from crumb rubber. Compliance with current regulation and future perspectives. Sci. Total Environ. 2021, 755, 11. [Google Scholar] [CrossRef] [PubMed]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Simultaneous removal of multiple polycyclic aromatic hydrocarbons (PAHs) from urban stormwater using low-cost agricultural/industrial byproducts as sorbents. Chemosphere 2021, 274, 12. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Rice, L. Toxicity of tire wear particle leachate to the marine macroalga, Ulva lactuca. Environ. Pollut. 2010, 158, 3650–3654. [Google Scholar] [CrossRef]
- Khellaf, N.; Zerdaoui, M. Phytoaccumulation of zinc by the aquatic plant, Lemna gibba L. Bioresour. Technol. 2009, 100, 6137–6140. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.; Claassen, L.; Mennen, M.; Timen, A.; te Wierik, M.J.M.; Timmermans, D.R.M. Public Perceptions of Contentious Risk: The Case of Rubber Granulate in the Netherlands. Int. J. Environ. Res. Public Health 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoczynska, E.; Leonards, P.E.G.; Llompart, M.; de Boer, J. Analysis of recycled rubber: Development of an analytical method and determination of polycyclic aromatic hydrocarbons and heterocyclic aromatic compounds in rubber matrices. Chemosphere 2021, 276, 10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Karedal, M.; Nielsen, J.; Adlercreutz, M.; Bergendorf, U.; Strandberg, B.; Antonsson, A.B.; Tinnerberg, H.; Albin, M. Exposure, respiratory symptoms, lung function and inflammation response of road-paving asphalt workers. Occup. Environ. Med. 2018, 75, 494–500. [Google Scholar] [CrossRef]
- Diekmann, A.; Giese, U.; Schaumann, I. Polycyclic aromatic hydrocarbons in consumer goods made from recycled rubber material: A review. Chemosphere 2019, 220, 1163–1178. [Google Scholar] [CrossRef]
- Perkins, A.N.; Inayat-Hussain, S.H.; Deziel, N.C.; Johnson, C.H.; Ferguson, S.S.; Garcia-Milian, R.; Thompson, D.C.; Vasiliou, V. Evaluation of potential carcinogenicity of organic chemicals in synthetic turf crumb rubber. Environ. Res. 2019, 169, 163–172. [Google Scholar] [CrossRef]
- Canepari, S.; Castellano, P.; Astolfi, M.L.; Materazzi, S.; Ferrante, R.; Fiorini, D.; Curini, R. Release of particles, organic compounds, and metals from crumb rubber used in synthetic turf under chemical and physical stress. Environ. Sci. Pollut. Res. 2018, 25, 1448–1459. [Google Scholar] [CrossRef]
- Brandsma, S.H.; Brits, M.; Groenewoud, Q.R.; van Velzen, M.J.M.; Leonards, P.E.G.; de Boert, J. Chlorinated Paraffins in Car Tires Recycled to Rubber Granulates and Playground Tiles. Environ. Sci. Technol. 2019, 53, 7595–7603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, M.K.; Lemay, J.C.; Shubin, S.P.; Prueitt, R.L. Comprehensive multipathway risk assessment of chemicals associated with recycled (“crumb”) rubber in synthetic turf fields. Environ. Res. 2018, 160, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Bierwisch, A.; Kaiser, E. ERASSTRI—European risk assessment study on synthetic turf rubber infill—Part 3: Exposure and risk characterisation. Sci. Total Environ. 2020, 718, 9. [Google Scholar] [CrossRef]
- Kruger, O.; Kalbe, U.; Richter, E.; Egeler, P.; Rombke, J.; Berger, W. New approach to the ecotoxicological risk assessment of artificial outdoor sporting grounds. Environ. Pollut. 2013, 175, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Menichini, E.; Abate, V.; Attias, L.; De Luca, S.; Di Domenico, A.; Fochi, I.; Forte, G.; Iacovella, N.; Iamiceli, A.L.; Izzo, P.; et al. Artificial-turf playing fields: Contents of metals, PAHs, PCBs, PCDDs and PCDFs, inhalation exposure to PAHs and related preliminary risk assessment. Sci. Total Environ. 2011, 409, 4950–4957. [Google Scholar] [CrossRef]
- LaPlaca, S.B.; van den Hurk, P. Toxicological effects of micronized tire crumb rubber on mummichog (Fundulus heteroclitus) and fathead minnow (Pimephales promelas). Ecotoxicology 2020, 29, 524–534. [Google Scholar] [CrossRef]
- Li, X.L.; Berger, W.; Musante, C.; Mattina, M.I. Characterization of substances released from crumb rubber material used on artificial turf fields. Chemosphere 2010, 80, 279–285. [Google Scholar] [CrossRef]
- Halle, L.L.; Palmqvist, A.; Kampmann, K.; Khan, F.R. Ecotoxicology of micronized tire rubber: Past, present and future considerations. Sci. Total Environ. 2020, 706, 13. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Markovski, S.; Rodwell, G.; Rahman, M.T.; Kurmus, H.; Mirzababaei, M.; Arulrajah, A.; Horpibulsuk, S.; et al. Recycling waste rubber tyres in construction materials and associated environmental considerations: A review. Resour. Conserv. Recycl. 2020, 155, 17. [Google Scholar] [CrossRef]
- Bridger, S.; Knowles, M. A Complete Method for Environmental Samples by Simultaneous Axially Viewed ICP-OES Following US EPA Guidelines (Agilent Technologies). 2010. Available online: https://www.agilent.com/cs/library/applications/ICPES-29.pdf (accessed on 18 January 2022).
- Depaolini, A.R.; Bianchi, G.; Fornai, D.; Cardelli, A.; Badalassi, M.; Cardelli, C.; Davoli, E. Physical and chemical characterization of representative samples of recycled rubber from end-of-life tires. Chemosphere 2017, 184, 1320–1326. [Google Scholar] [CrossRef]
- Llompart, M.; Sanchez-Prado, L.; Lamas, J.P.; Garcia-Jares, C.; Roca, E.; Dagnac, T. Hazardous organic chemicals in rubber recycled tire playgrounds and pavers. Chemosphere 2013, 90, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kobeticova, K.; Simek, Z.; Brezovsky, J.; Hofman, J. Toxic effects of nine polycyclic aromatic compounds on Enchytraeus crypticus in artificial soil in relation to their properties. Ecotoxicol. Environ. Saf. 2011, 74, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Lock, K.; Janssen, C.R. Ecotoxicity of zinc in spiked artificial soils versus contaminated field soils. Environ. Sci. Technol. 2001, 35, 4295–4300. [Google Scholar] [CrossRef]
- Mao, L.G.; Zhang, L.; Zhang, Y.N.; Jiang, H.Y. Ecotoxicity of 1,3-dichloropropene, metam sodium, and dazomet on the earthworm Eisenia fetida with modified artificial soil test and natural soil test. Environ. Sci. Pollut. Res. 2017, 24, 18692–18698. [Google Scholar] [CrossRef]
- Marwood, C.; McAtee, B.; Kreider, M.; Ogle, R.S.; Finley, B.; Sweet, L.; Panko, J. Acute aquatic toxicity of tire and road wear particles to alga, daphnid, and fish. Ecotoxicology 2011, 20, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Shokouhian, M.; Ni, S.B. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere 2001, 44, 997–1009. [Google Scholar] [CrossRef]
- Park, J.K.; Edil, T.B.; Kim, J.Y.; Huh, M.; Lee, S.H.; Lee, J.J. Suitability of shredded tyres as a substitute for a landfill leachate collection medium. Waste Manag. Res. 2003, 21, 278–289. [Google Scholar] [CrossRef]
- Bocca, B.; Forte, G.; Petrucci, F.; Costantini, S.; Izzo, P. Metals contained and leached from rubber granulates used in synthetic turf areas. Sci. Total Environ. 2009, 407, 2183–2190. [Google Scholar] [CrossRef]
- Watterson, A. Artificial Turf: Contested Terrains for Precautionary Public Health with Particular Reference to Europe? Int. J. Environ. Res. Public Health 2017, 14, 16. [Google Scholar] [CrossRef] [Green Version]
- Testolin, R.C.; Tischer, V.; Lima, A.O.S.; Cotelle, S.; Ferard, J.F.; Radetski, C.M. Aquatic ecotoxicity assessment of a new natural formicide. Environ. Sci. Pollut. Res. 2012, 19, 2186–2194. [Google Scholar] [CrossRef]
- Gagol, M.; Boczkaj, G.; Haponiuk, J.; Formela, K. Investigation of volatile low molecular weight compounds formed during continuous reclaiming of ground tire rubber. Polym. Degrad. Stab. 2015, 119, 113–120. [Google Scholar] [CrossRef]
- Evariste, L.; Barret, M.; Mottier, A.; Mouchet, F.; Gauthier, L.; Pinelli, E. Gut microbiota of aquatic organisms: A key endpoint for ecotoxicological studies. Environ. Pollut. 2019, 248, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.B.; Laabir, M.; Yahia, M.N.D. A novel index based on planktonic copepod reproductive traits as a tool for marine ecotoxicology studies. Sci. Total Environ. 2020, 727, 138621. [Google Scholar] [CrossRef] [PubMed]
- Manzetti, S. Ecotoxicity of polycyclic aromatic hydrocarbons, aromatic amines, and nitroarenes through molecular properties. Environ. Chem. Lett. 2012, 10, 349–361. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediation 2018, 20, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimbabaie, P.; Meeinkuirt, W.; Pichtel, J. Phytoremediation of engineered nanoparticles using aquatic plants: Mechanisms and practical feasibility. J. Environ. Sci. 2020, 93, 151–163. [Google Scholar] [CrossRef]
- Emenike, C.U.; Jayanthi, B.; Agamuthu, P.; Fauziah, S.H. Biotransformation and removal of heavy metals: A review of phytoremediation and microbial remediation assessment on contaminated soil. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef] [Green Version]
- Bidleman, T.F.; Andersson, A.; Haglund, P.; Tysklind, M. Will Climate Change Influence Production and Environmental Pathways of Halogenated Natural Products? Environ. Sci. Technol. 2020, 54, 6468–6485. [Google Scholar] [CrossRef]
- Xu, E.G.; Lin, N.; Cheong, R.S.; Ridsdale, C.; Tahara, R.; Du, T.Y.; Das, D.; Zhu, J.P.; Silva, L.P.; Azimzada, A.; et al. Artificial turf infill associated with systematic toxicity in an amniote vertebrate. Proc. Natl. Acad. Sci. USA 2019, 116, 25156–25161. [Google Scholar] [CrossRef]
- Teixido, E.; Leuthold, D.; de Croze, N.; Leonard, M.; Scholz, S. Comparative Assessment of the Sensitivity of Fish Early-Life Stage, Daphnia, and Algae Tests to the Chronic Ecotoxicity of Xenobiotics: Perspectives for Alternatives to Animal Testing. Environ. Toxicol. Chem. 2020, 39, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Su, Y.; Ji, Y.; Ji, R. Release of Zinc and Polycyclic Aromatic Hydrocarbons from Tire Crumb Rubber and Toxicity of Leachate to Daphnia magna: Effects of Tire Source and Photoaging. Bull. Environ. Contam. Toxicol. 2021, 107, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Andrioletti, M.; Vismara, C.; Milani, M.; Camatini, M. Toxicity of tire debris leachates. Environ. Int. 2005, 31, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Wik, A.; Dave, G. Acute toxicity of leachates of tire wear material to Daphnia magna—Variability and toxic components. Chemosphere 2006, 64, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Pochron, S.T.; Fiorenza, A.; Sperl, C.; Ledda, B.; Patterson, C.L.; Tucker, C.C.; Tucker, W.; Ho, Y.L.; Panico, N. The response of earthworms (Eisenia fetida) and soil microbes to the crumb rubber material used in artificial turf fields. Chemosphere 2017, 173, 557–562. [Google Scholar] [CrossRef]
- Jayasri, M.A.; Suthindhiran, K. Effect of zinc and lead on the physiological and biochemical properties of aquatic plant Lemna minor: Its potential role in phytoremediation. Appl. Water Sci. 2017, 7, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)-A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 2020, 733, 19. [Google Scholar] [CrossRef]
- Wik, A.; Dave, G. Occurrence and effects of tire wear particles in the environment—A critical review and an initial risk assessment. Environ. Pollut. 2009, 157, 1–11. [Google Scholar] [CrossRef]
- Capolupo, M.; Sorensen, L.; Jayasena, K.D.; Booth, A.M.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef]
- Phiri, M.M.; Phiri, M.J.; Formela, K.; Hlangothi, S.P. Chemical surface etching methods for ground tire rubber as sustainable approach for environmentally-friendly composites development—A review. Compos. Part B Eng. 2020, 204, 108429. [Google Scholar] [CrossRef]
- Huber, M.; Welker, A.; Helmreich, B. Critical review of heavy metal pollution of traffic area runoff: Occurrence, influencing factors, and partitioning. Sci. Total Environ. 2016, 541, 895–919. [Google Scholar] [CrossRef]
- Formela, K.; Marc, M.; Wang, S.; Saeb, M.R. Interrelationship between total volatile organic compounds emissions, structure and properties of natural rubber/polycaprolactone bio-blends cross-linked with peroxides. Polym. Test. 2017, 60, 405–412. [Google Scholar] [CrossRef]
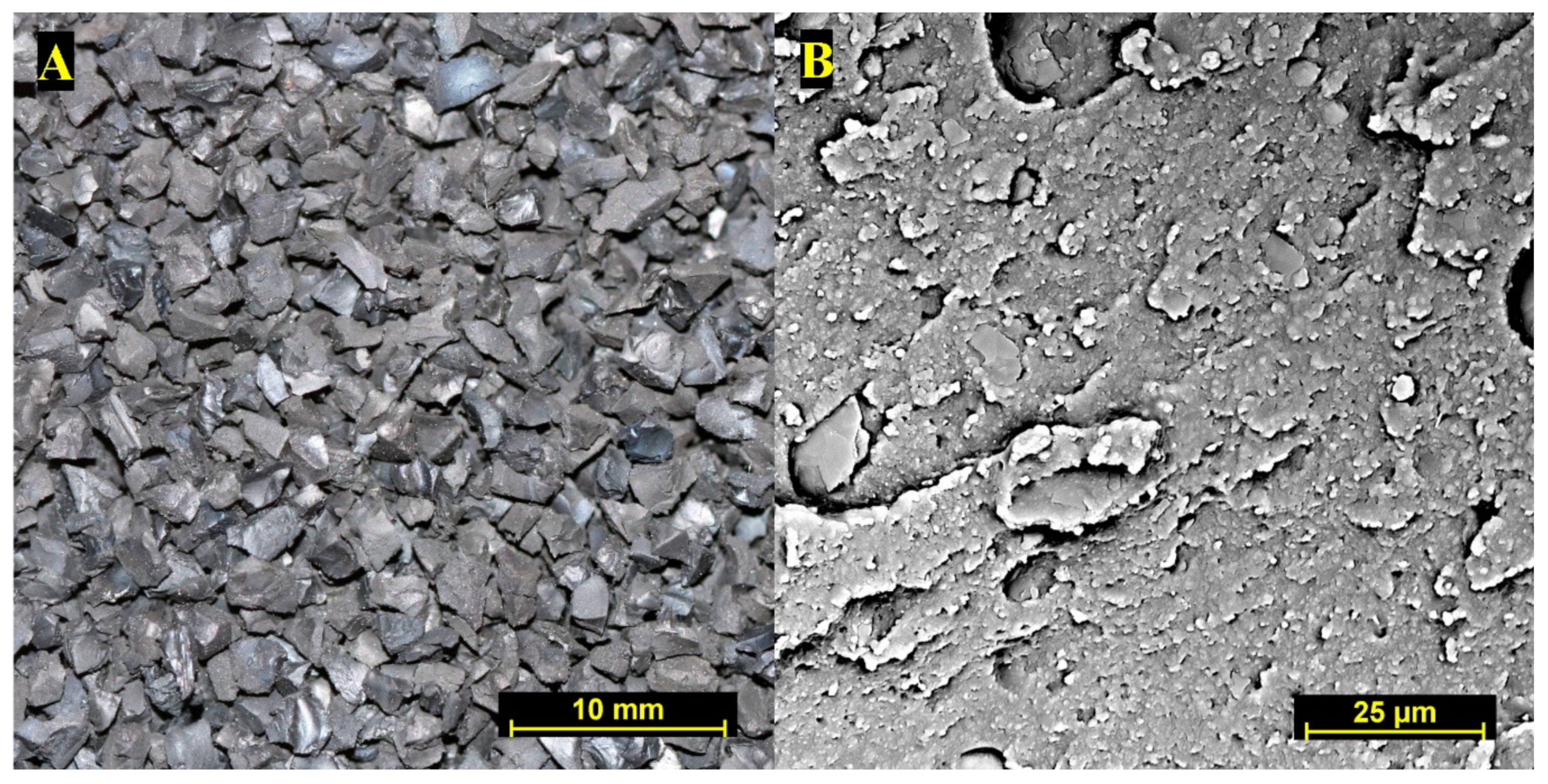
| Component | Weight Fraction (%) |
|---|---|
| Rubber | 47.3 |
| Carbon black | 31.6 |
| Additives | 12.7 |
| Acetone extract | 6.4 |
| Ash | 2.1 |
| Concentration (mg·L−1) | |||
|---|---|---|---|
| Element | 24 h | 21 Days | Limit (According to WHO, Environmental Health Criteria 221) |
| B (249.77 nm) | 0 | 0.231 | 0.5 |
| Ba (455.40 nm) | 0.021 | 0.056 | 0.7 |
| Fe (259.94 nm) | 0 | 0.059 | - |
| Mn (257.61 nm) | 0 | 0.113 | 0.4 |
| Si (251.61 nm) | 0 | 2.271 | - |
| Zn (206.20 nm) | 0.273 | 7.041 | 3 |
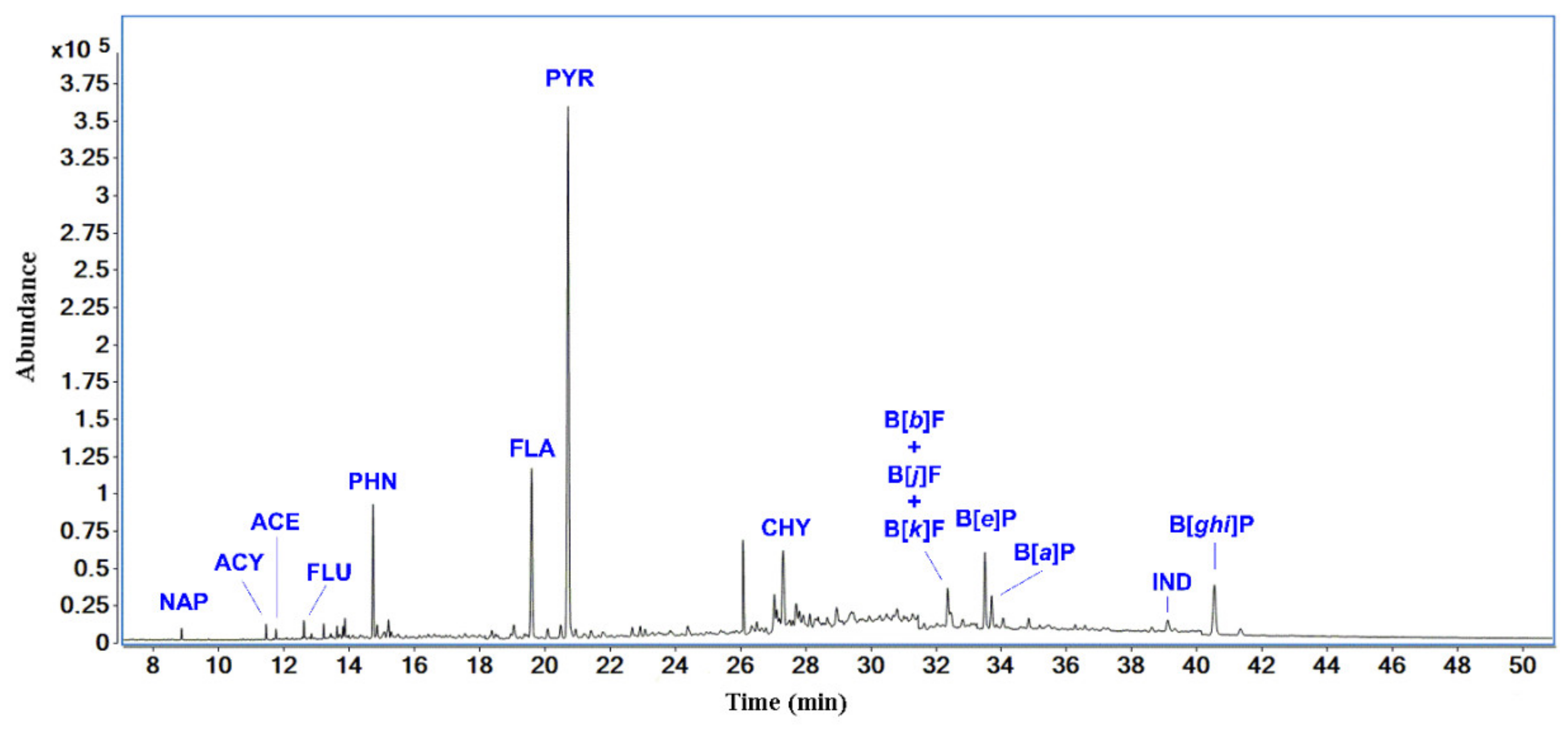
| Compound | Abbrev. | CAS | Retention Time (min.) | Quantifier | Qualifier 1 | Qualifier 2 | Qualifier 3 |
|---|---|---|---|---|---|---|---|
| naphthalene | NAP | 91-20-3 | 8.878 | 128 | 127 | 129 | 102 |
| acenaphthylene | ACY | 208-96-8 | 11.484 | 152 | 151 | 153 | 76 |
| acenaphthene | ACE | 83-32-9 | 11.784 | 153 | 154 | 151 | 155 |
| fluorene | FLU | 86-73-7 | 12.642 | 166 | 165 | 163 | 167 |
| phenanthrene | PHN | 85-01-8 | 14.771 | 178 | 179 | 177 | 152 |
| fluoranthene | FLA | 206-44-0 | 19.656 | 202 | 203 | 201 | 101 |
| pyrene | PYR | 129-00-0 | 20.782 | 202 | 203 | 201 | 101 |
| chrysene | CHY | 218-01-9 | 27.382 | 228 | 226 | 229 | 114 |
| benzo[b]fluoranthene + benzo[j]fluoranthene + benzo[k]fluoranthene | B[b]F B[j]F B[k]F | 205-99-2 205-82-3 207-08-9 | 32.442 | 252 | 126 | – | – |
| benzo[e]pyrene | B[e]P | 192-97-2 | 33.585 | 252 | 253 | 126 | 250 |
| benzo[a]pyrene | B[a]P | 50-32-8 | 33.790 | 252 | 253 | 250 | 126 |
| indeno[1,2,3-cd]pyrene | IND | 193-39-5 | 39.208 | 276 | 138 | 277 | 137 |
| benzo[ghi]perylene | B[ghi]P | 191-24-2 | 40.042 | 276 | 138 | 277 | 137 |
| Analyte | ISTD | Cm (mg·kg−1) | Standard Deviation | Variation Coeff. (%) | Average Linearity (R2) |
|---|---|---|---|---|---|
| phenanthrene | phenantrene-d10 | 5.61 | 0.06 | 1.08 | 0.999 |
| fluoranthene | fluoranthene-d10 | 9.12 | 0.13 | 1.40 | 0.999 |
| pyrene | pyrene-d10 | 31.24 | 0.52 | 1.65 | 0.999 |
| chrysene | chrysene-d12 | 3.24 | 0.04 | 1.25 | 0.999 |
| benzo[e]pyrene | benzo[a]pyrene-d12 | 3.76 | 0.03 | 0.70 | 0.999 |
| benzo[ghi]perylene | benzo[a]pyrene-d12 | 5.06 | 0.08 | 1.66 | 0.999 |
| Organism | Parameter | Inhibition (%) | Exposure (h) |
|---|---|---|---|
| Lemna minor | Growth rate | 48 | 168 |
| Biomass | 48 | ||
| Desmodesmus subspicatus | Growth rate | 27 | 72 |
| Biomass | 47 | ||
| Daphnia magna | Mortality | 100 | 24 |
| Sinapis alba | Roots prolongation | 31 | 96 |
| Eisenia fetida | Mortality | 100 | 168 |
| Lactuca sativa | Roots prolongation | 44 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fořt, J.; Kobetičová, K.; Böhm, M.; Podlesný, J.; Jelínková, V.; Vachtlová, M.; Bureš, F.; Černý, R. Environmental Consequences of Rubber Crumb Application: Soil and Water Pollution. Polymers 2022, 14, 1416. https://doi.org/10.3390/polym14071416
Fořt J, Kobetičová K, Böhm M, Podlesný J, Jelínková V, Vachtlová M, Bureš F, Černý R. Environmental Consequences of Rubber Crumb Application: Soil and Water Pollution. Polymers. 2022; 14(7):1416. https://doi.org/10.3390/polym14071416
Chicago/Turabian StyleFořt, Jan, Klára Kobetičová, Martin Böhm, Jan Podlesný, Veronika Jelínková, Martina Vachtlová, Filip Bureš, and Robert Černý. 2022. "Environmental Consequences of Rubber Crumb Application: Soil and Water Pollution" Polymers 14, no. 7: 1416. https://doi.org/10.3390/polym14071416
APA StyleFořt, J., Kobetičová, K., Böhm, M., Podlesný, J., Jelínková, V., Vachtlová, M., Bureš, F., & Černý, R. (2022). Environmental Consequences of Rubber Crumb Application: Soil and Water Pollution. Polymers, 14(7), 1416. https://doi.org/10.3390/polym14071416











