Low-Temperature Mechano-Chemical Rubber Reclamation Using Terpinene as a Swelling Agent to Enhance Bond-Breaking Selectivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.2.1. Swelling
2.2.2. Rubber Reclamation
2.2.3. Mixing and Vulcanization
2.3. Measurements and Characterizations
- Sol Fraction
- Crosslinking Density and Devulcanization Ratio
Gel Permeation Chromatography (GPC)
- Mooney Viscosity
- Curing Characteristics
- Physical Properties
- Dynamic Mechanical Properties
3. Results and Discussion
3.1. Swelling Effect of Terpinene on Vulcanized Rubbers
3.2. Effect of Terpinene on Bond Scissions during the Rubber Reclamation Process
3.2.1. Characteristics of the Reclaimed Rubbers
3.2.2. Horikx
3.3. Properties of Reclaimed Rubbers
3.3.1. Curing Characteristics
3.3.2. Physical Properties
3.3.3. Dynamic Mechanical Properties
3.4. Production Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowles, A.J.; Fowler, G.D.; O’Sullivan, C.; Parker, K. Sustainable rubber recycling from waste tyres by waterjet: A novel mechanistic and practical analysis. SM&T 2020, 25, 15. [Google Scholar]
- Liu, H.L.; Wang, X.P.; Jia, D.M. Recycling of waste rubber powder by mechano-chemical modification. J. Clean Prod. 2020, 245, 10. [Google Scholar] [CrossRef]
- El-Nemr, K.F.; Raslan, H.A.; Ali, M.A.M.; Hasan, M.M. Innovative gamma rays irradiated styrene butadiene rubber/reclaimed waste tire rubber blends: A comparative study using mechano-chemical and microwave devulcanizing methods. J. Polym. Eng. 2020, 40, 267–277. [Google Scholar] [CrossRef]
- Hassan, M.M.; Badway, N.A.; Elnaggar, M.Y.; Hegazy, E.S.A. Thermo-mechanical properties of devulcanized rubber/high crystalline polypropylene blends modified by ionizing radiation. J. Ind. Eng. Chem. 2013, 19, 1241–1250. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Karthikeyan, S. Recycling of waste tires and its energy storage application of by-products—A review. SM&T 2019, 22, 6. [Google Scholar]
- Wang, Z.F.; Zeng, D.P. Preparation of devulcanized ground tire rubber with supercritical carbon dioxide jet pulverization. MatL 2021, 282, 5. [Google Scholar] [CrossRef]
- Hassan, M.M.; Aly, R.O.; Aal, S.E.A.; El-Masry, A.M.; Fathy, E.S. Styrene butadiene-based blends containing waste rubber powder: Physico-mechanical effects of mechanochemical devulcanization and gamma irradiation. J. Ind. Eng. Chem. 2013, 19, 1735–1742. [Google Scholar] [CrossRef]
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: A review. J. Clean Prod. 2019, 236, 16. [Google Scholar] [CrossRef]
- Formela, K.; Wasowicz, D.; Formela, M.; Hejna, A.; Haponiuk, J. Curing characteristics, mechanical and thermal properties of reclaimed ground tire rubber cured with various vulcanizing systems. Iran. Polym. J. 2015, 24, 289–297. [Google Scholar] [CrossRef]
- Gagol, M.; Boczkaj, G.; Haponiuk, J.; Formela, K. Investigation of volatile low molecular weight compounds formed during continuous reclaiming of ground tire rubber. Polym. Degrad. Stab. 2015, 119, 113–120. [Google Scholar] [CrossRef]
- Sripornsawat, B.; Saiwari, S.; Pichaiyut, S.; Nakason, C. Influence of ground tire rubber devulcanization conditions on properties of its thermoplastic vulcanizate blends with copolyester. Eur. Polym. J. 2016, 85, 279–297. [Google Scholar] [CrossRef]
- Hassan, M.M.; Aly, R.O.; Aal, S.E.A.; El-Masry, A.M.; Fathy, E.S. Mechanochemical devulcanization and gamma irradiation of devulcanized waste rubber/high density polyethylene thermoplastic elastomer. J. Ind. Eng. Chem. 2013, 19, 1722–1729. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, L.; Colom, X.; Canavate, J. Microwave treatment in waste rubber recycling-recent advances and limitations. Express Polym. Lett. 2019, 13, 565–588. [Google Scholar] [CrossRef]
- Simon, D.A.; Pirityi, D.; Tamas-Benyei, P.; Barany, T. Microwave devulcanization of ground tire rubber and applicability in SBR compounds. J. Appl. Polym. Sci. 2020, 137, 8. [Google Scholar] [CrossRef]
- Elnaggar, M.Y.; Fathy, E.; Amdeha, E.; Hassan, M.M. Impact of gamma irradiation on virgin styrene butadiene rubber blended with ultrasonically devulcanized waste rubber. Polym. Eng. Sci. 2019, 59, 807–813. [Google Scholar] [CrossRef]
- Sun, X.M.; Isayev, A.I. Continuous ultrasonic devulcanization comparison of carbon black filled synthetic isoprene and natural rubbers. Rubber Chem. Technol. 2008, 81, 19–46. [Google Scholar] [CrossRef]
- Seghar, S.; Asaro, L.; Rolland-Monnet, M.; Hocine, N.A. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recycl. 2019, 144, 180–186. [Google Scholar] [CrossRef]
- Rooj, S.; Basak, G.C.; Maji, P.K.; Bhowmick, A.K. New Route for Devulcanization of Natural Rubber and the Properties of Devulcanized Rubber. J. Polym. Environ. 2011, 19, 382–390. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, K.; Ren, D.; Zou, H.; Wang, Y.; Lv, X.; Zhang, L. Structure and performance of reclaimed rubber obtained by different methods. J. Appl. Polym. Sci. 2013, 129, 999–1007. [Google Scholar] [CrossRef]
- Guo, L.; Lv, D.; Ren, D.; Qu, L.; Wang, W.; Hao, K.; Guo, X.; Chen, T.; Sun, J.; Wang, C.; et al. Effectiveness of original additives in waste rubbers for revulcanization after reclamation with a low-temperature mechanochemical devulcanization method. J. Clean Prod. 2021, 297, 126620. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Lv, D.; Ren, D.; Zhai, T.; Sun, C.; Liu, H. Rubber reclamation with high bond-breaking selectivity using a low-temperature mechano-chemical devulcanization method. J. Clean Prod. 2021, 279, 123266. [Google Scholar] [CrossRef]
- De Sousa, F.D.B.; Scuracchio, C.H.; Hu, G.H.; Hoppe, S. Devulcanization of waste tire rubber by microwaves. Polym. Degrad. Stab. 2017, 138, 169–181. [Google Scholar] [CrossRef]
- Asaro, L.; Gratton, M.; Seghar, S.; Hocine, N.A. Recycling of rubber wastes by devulcanization. Resour. Conserv. Recycl. 2018, 133, 250–262. [Google Scholar] [CrossRef]
- Meysami, M.; Tzoganakis, C.; Mutyala, P.; Zhu, S.H.; Bulsari, M. Devulcanization of Scrap Tire Rubber with Supercritical CO2: A Study of the Effects of Process Parameters on the Properties of Devulcanized Rubber. Int. Polym. Proc. 2017, 32, 183–193. [Google Scholar] [CrossRef]
- Asaro, L.; Gratton, M.; Poirot, N.; Seghar, S.; Hocine, N.A. Devulcanization of natural rubber industry waste in supercritical carbon dioxide combined with diphenyl disulfide. Waste Manag. 2020, 118, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Shi, C.P.; Zhang, L.; Zhang, Y.C. Effects of shear stress and subcritical water on devulcanization of styrene-butadiene rubber based ground tire rubber in a twin-screw extruder. J. Appl. Polym. Sci. 2013, 130, 1845–1854. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, J.X.; Wang, X.J.; Zhang, L.; Zhang, Y.C. Influences of process variables and subcritical fluids on epdm-assisted devulcanization of sbr-based ground tire rubber. Rubber Chem. Technol. 2018, 91, 401–416. [Google Scholar] [CrossRef]
- Barbosa, R.; Nunes, A.T.; Ambrosio, J.D. Devulcanization of Natural Rubber in Composites with Distinct Crosslink Densities by Twin-Screw Extruder. Mater. Res-Ibero-Am. J. 2017, 20, 77–83. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Shen, A.Q.; Lyu, Z.H.; Wang, S.F.; Formela, K.; Zhang, G.T. Ground tire rubber thermo-mechanically devulcanized in the presence of waste engine oil as asphalt modifier. Constr. Build. Mater. 2019, 222, 588–600. [Google Scholar] [CrossRef]
- Sabzekar, M.; Zohuri, G.; Chenar, M.P.; Mortazavi, S.M.; Kariminejad, M.; Asadi, S. A new approach for reclaiming of waste automotive EPDM rubber using waste oil. Polym. Degrad. Stab. 2016, 129, 56–62. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, Y.S.; Ma, L.; Zhou, L.; He, H. Tailoring the properties of ground tire rubber/high-density polyethylene blends by combining surface devulcanization and in-situ grafting technology. MCP 2018, 220, 161–170. [Google Scholar] [CrossRef]
- Horikx, M.M. Chain scissions in a polymer network. J. Polym. Sci. 1956, 19, 445–454. [Google Scholar] [CrossRef]
- Tao, G.L.; He, Q.H.; Xia, Y.P.; Jia, G.C.; Yang, H.C.; Ma, W.Z. The effect of devulcanization level on mechanical properties of reclaimed rubber by thermal-mechanical shearing devulcanization. J. Appl. Polym. Sci. 2013, 129, 2598–2605. [Google Scholar] [CrossRef]
- Ghorai, S.; Bhunia, S.; Roy, M.; De, D. Mechanochemical devulcanization of natural rubber vulcanizate by dual function disulfide chemicals. Polym. Degrad. Stab. 2016, 129, 34–46. [Google Scholar] [CrossRef]
- Zhang, X.; Saha, P.; Cao, L.; Li, H.; Kim, J. Devulcanization of waste rubber powder using thiobisphenols as novel reclaiming agent. Waste Manag. 2018, 78, 980–991. [Google Scholar] [CrossRef]
- Zedler, L.; Klein, M.; Saeb, M.R.; Colom, X.; Canavate, J.; Formela, K. Synergistic Effects of Bitumen Plasticization and Microwave Treatment on Short-Term Devulcanization of Ground Tire Rubber. Polymers 2018, 10, 1265. [Google Scholar] [CrossRef] [PubMed]

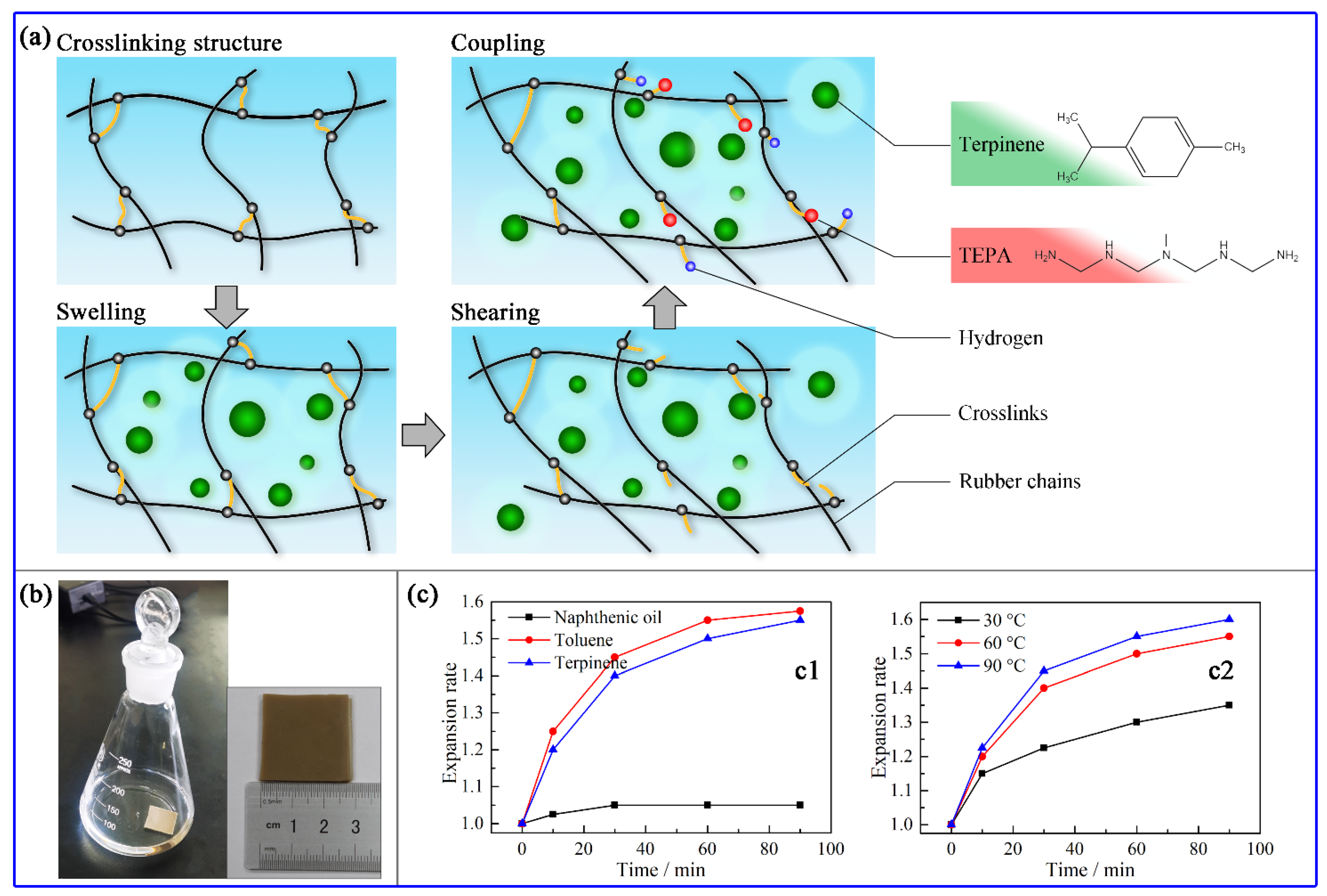

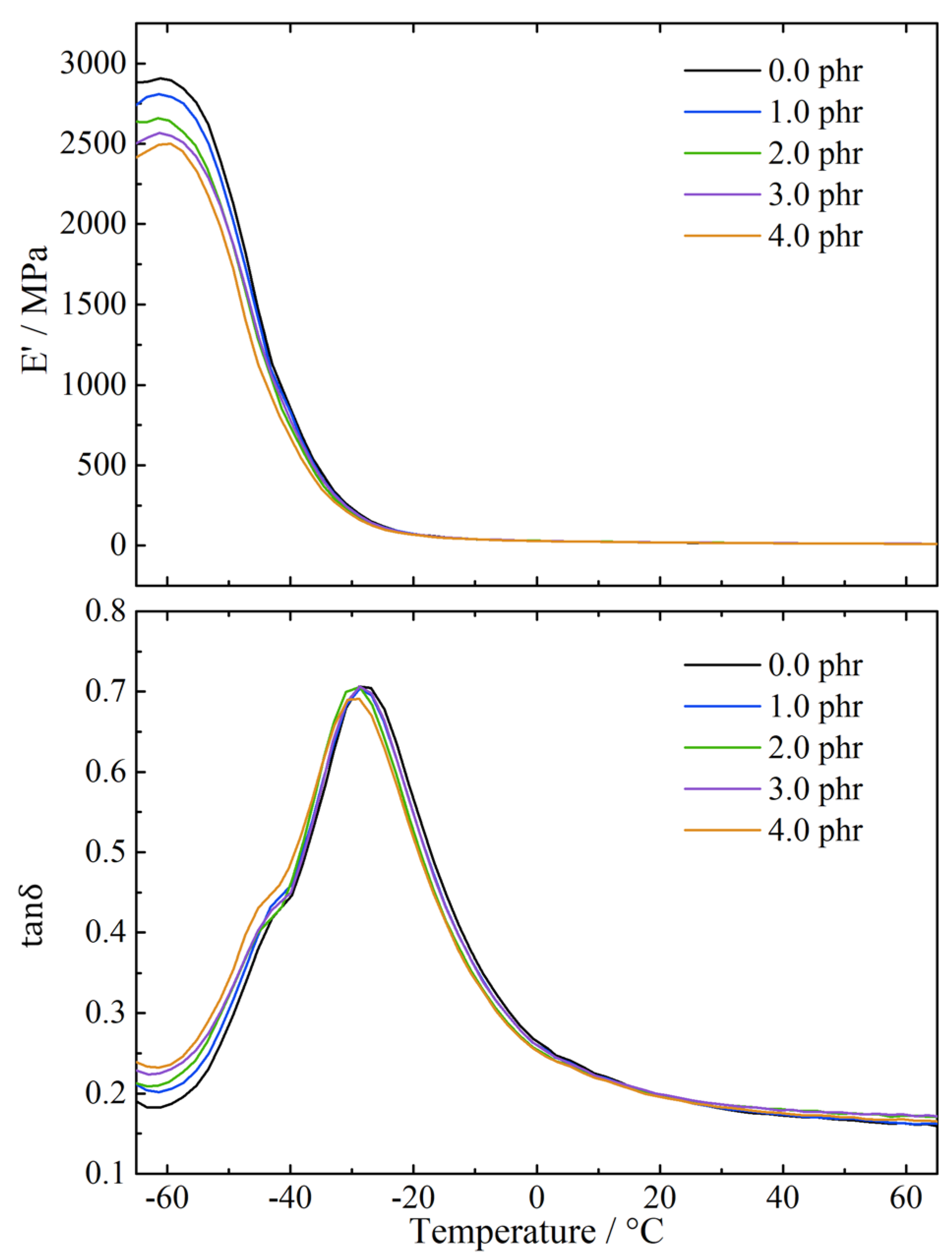

| Terpinene Amount/phr | Mn | Sol Fraction | Mooney Viscosity | Devulcanization Ratio |
|---|---|---|---|---|
| 0.0 | 18,987 | 16.7% | 105.7 | 75.2% |
| 0.5 | 20,540 | 17.1% | 104.7 | 77.9% |
| 1.0 | 20,750 | 18.0% | 103.5 | 80.3% |
| 2.0 | 20,964 | 19.6% | 97.1 | 84.8% |
| 3.0 | 21,484 | 21.2% | 90.3 | 86.6% |
| 4.0 | 19,570 | 22.1% | 87.4 | 86.5% |
| Terpinene Amount/phr | T10/Min | T90/Min | ML/N m | MH/N m | MD/N m |
|---|---|---|---|---|---|
| 0.0 | 0.63 | 8.91 | 2.80 | 13.78 | 10.98 |
| 0.5 | 0.63 | 8.67 | 2.71 | 13.59 | 10.88 |
| 1.0 | 0.56 | 6.97 | 2.67 | 13.30 | 10.63 |
| 2.0 | 0.53 | 6.44 | 2.66 | 12.66 | 10.01 |
| 3.0 | 0.51 | 5.89 | 2.64 | 12.13 | 9.49 |
| 4.0 | 0.50 | 5.50 | 2.60 | 12.01 | 9.41 |
| Terpinene Amount/phr | Tensile Strength/MPa | Breaking Elongation/% | 100% Modulus/MPa | 300% Modulus/MPa | Hardness (Shore A) |
|---|---|---|---|---|---|
| 0.0 | 15.52 ± 0.54 | 325.5 ± 6.6 | 4.10 ± 0.03 | 14.23 ± 0.24 | 75.5 ± 0.5 |
| 0.5 | 16.36 ± 0.53 | 351.9 ± 12.0 | 3.83 ± 0.10 | 13.95 ± 0.25 | 75.0 ± 0.5 |
| 1.0 | 16.73 ± 0.77 | 357.7 ± 17.9 | 3.81 ± 0.07 | 13.79 ± 0.25 | 74.5 ± 0.5 |
| 2.0 | 16.81 ± 0.35 | 365.7 ± 12.4 | 3.47 ± 0.08 | 12.51 ± 0.17 | 73.5 ± 1.0 |
| 3.0 | 17.03 ± 0.64 | 400.7 ± 23.4 | 3.39 ± 0.12 | 11.95 ± 0.70 | 73.0 ± 1.0 |
| 4.0 | 15.64 ± 0.47 | 387.5 ± 33.9 | 3.17 ± 0.13 | 11.54 ± 0.56 | 72.5 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Ren, D.; Wang, W.; Hao, K.; Guo, X.; Liu, F.; Xu, Y.; Liu, M.; Liu, H. Low-Temperature Mechano-Chemical Rubber Reclamation Using Terpinene as a Swelling Agent to Enhance Bond-Breaking Selectivity. Polymers 2021, 13, 4272. https://doi.org/10.3390/polym13244272
Guo L, Ren D, Wang W, Hao K, Guo X, Liu F, Xu Y, Liu M, Liu H. Low-Temperature Mechano-Chemical Rubber Reclamation Using Terpinene as a Swelling Agent to Enhance Bond-Breaking Selectivity. Polymers. 2021; 13(24):4272. https://doi.org/10.3390/polym13244272
Chicago/Turabian StyleGuo, Lei, Donghui Ren, Wenchao Wang, Kuanfa Hao, Xiurui Guo, Fumin Liu, Yuan Xu, Miaomiao Liu, and Haichao Liu. 2021. "Low-Temperature Mechano-Chemical Rubber Reclamation Using Terpinene as a Swelling Agent to Enhance Bond-Breaking Selectivity" Polymers 13, no. 24: 4272. https://doi.org/10.3390/polym13244272
APA StyleGuo, L., Ren, D., Wang, W., Hao, K., Guo, X., Liu, F., Xu, Y., Liu, M., & Liu, H. (2021). Low-Temperature Mechano-Chemical Rubber Reclamation Using Terpinene as a Swelling Agent to Enhance Bond-Breaking Selectivity. Polymers, 13(24), 4272. https://doi.org/10.3390/polym13244272





