Study of the Dilution-Induced Deposition of Concentrated Mixtures of Polyelectrolytes and Surfactants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Model Technical Formulation
2.3. Characterization of the Adsorption onto Solid Surfaces
3. Results and Discussion
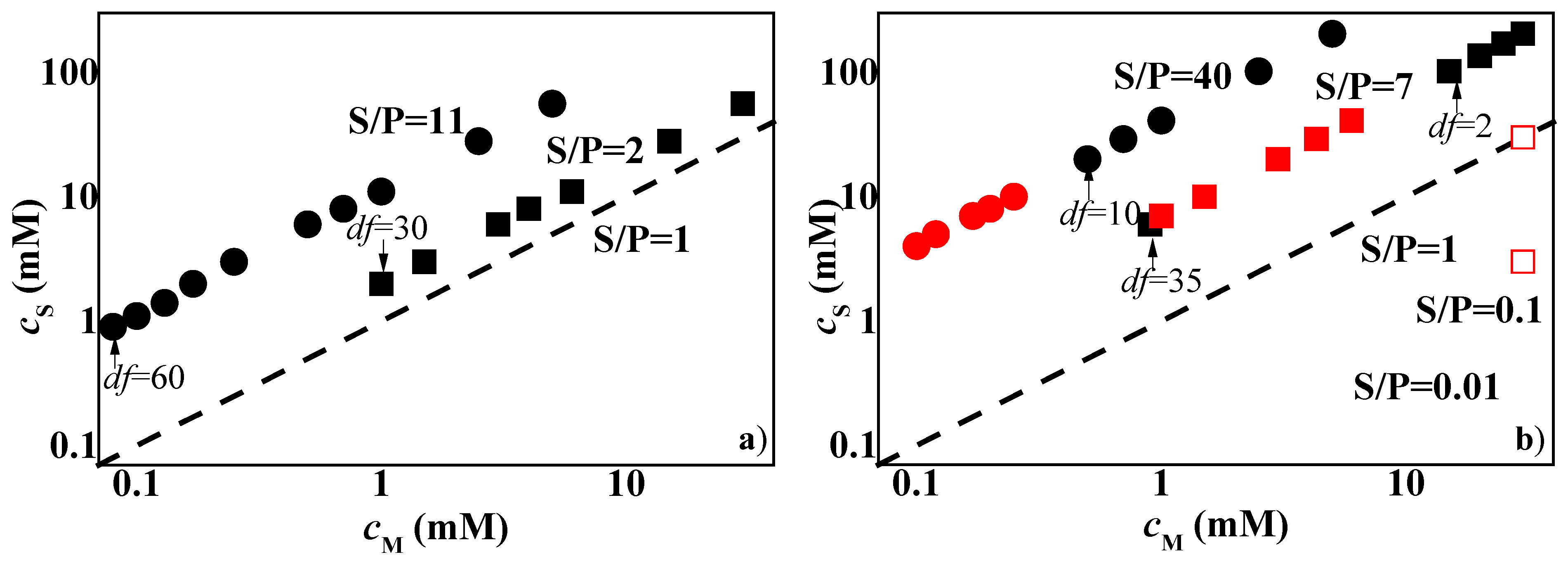
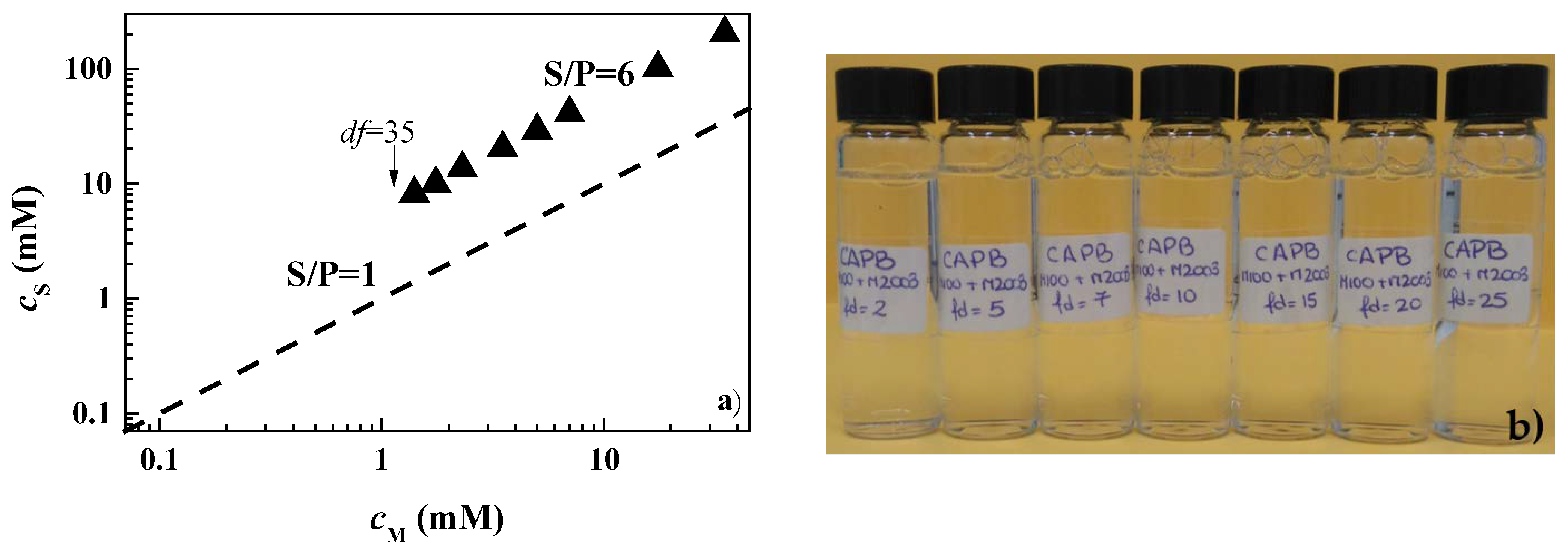
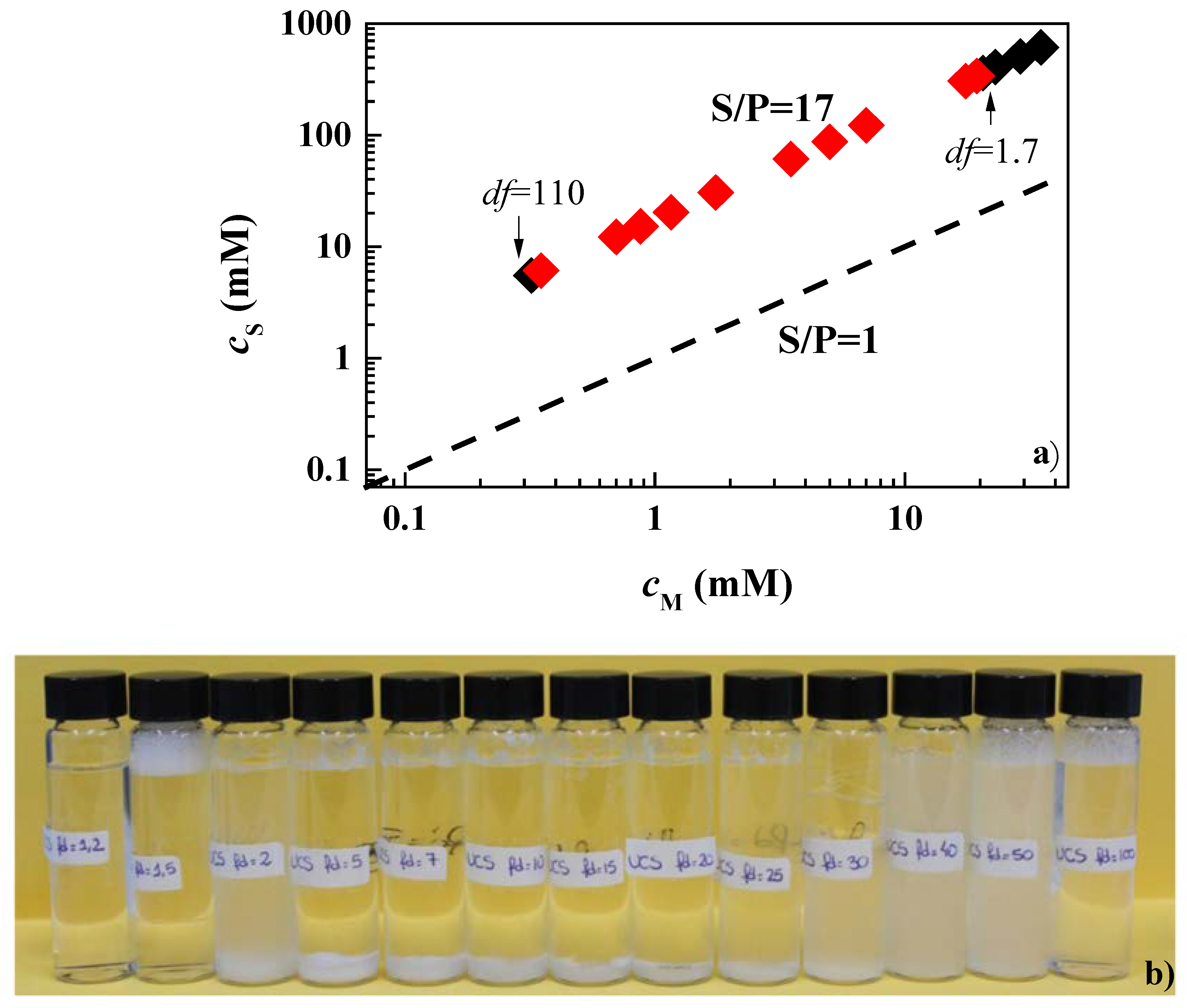
3.1. Determination of the Phase Separation upon Dilution for Concentrated Pseudo-Binary Polymer–Surfactant Mixtures
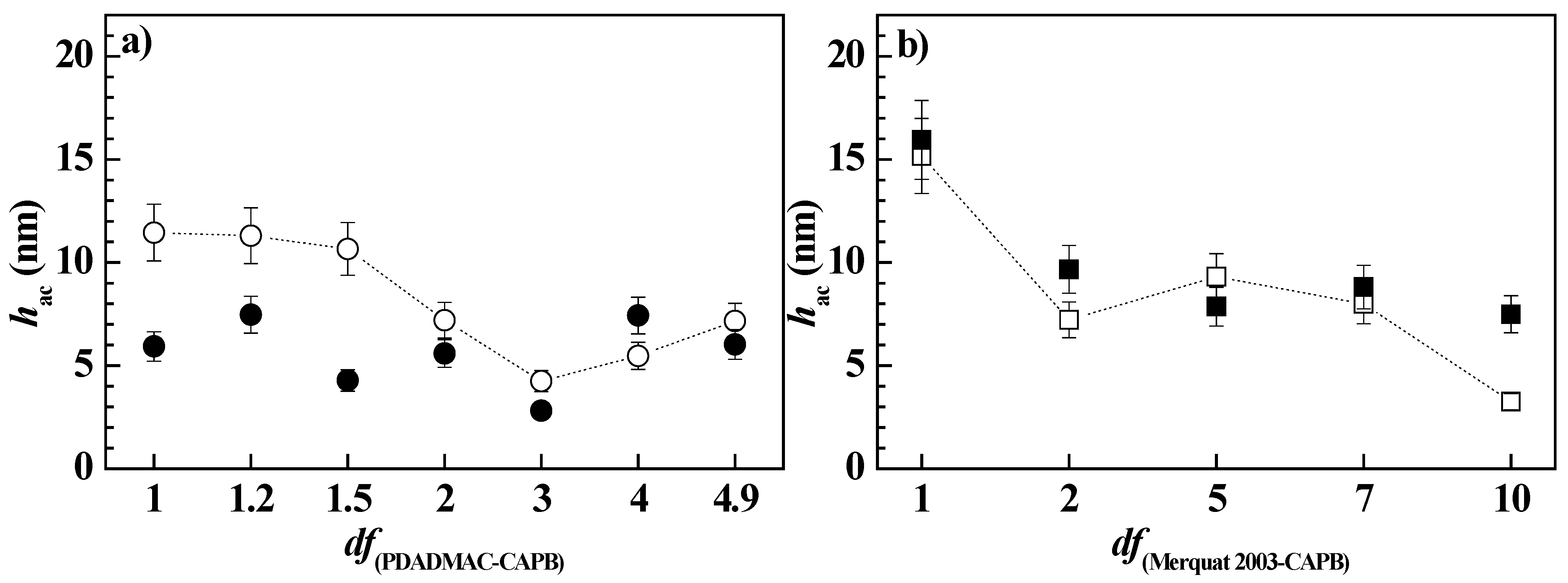
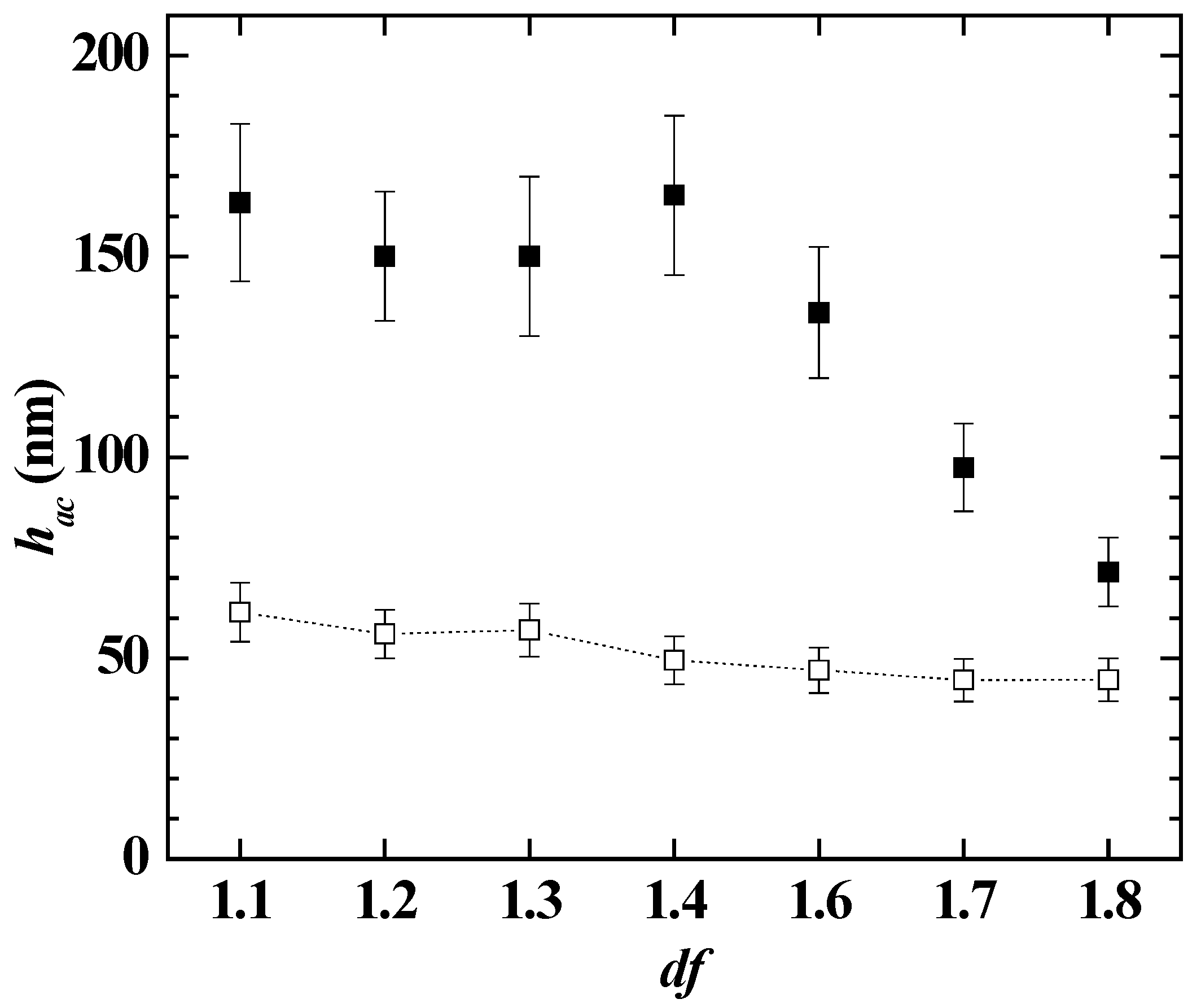
3.2. Study of the Deposition of MonoPhasic Pseudo-Binary Polymer-Surfactant Mixtures
3.3. Study of the Deposition Enhanced by Dilution for Pseudo-Binary Polymer–CAPB Mixtures
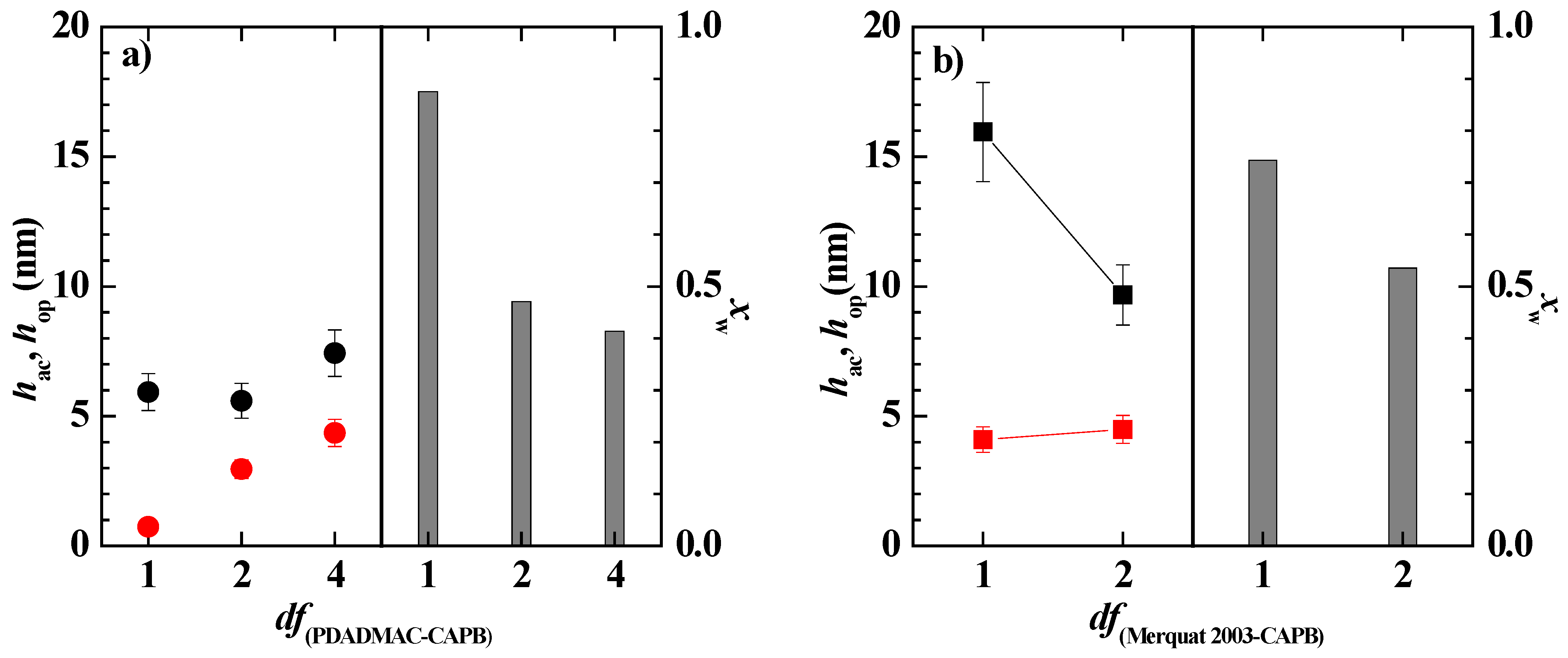
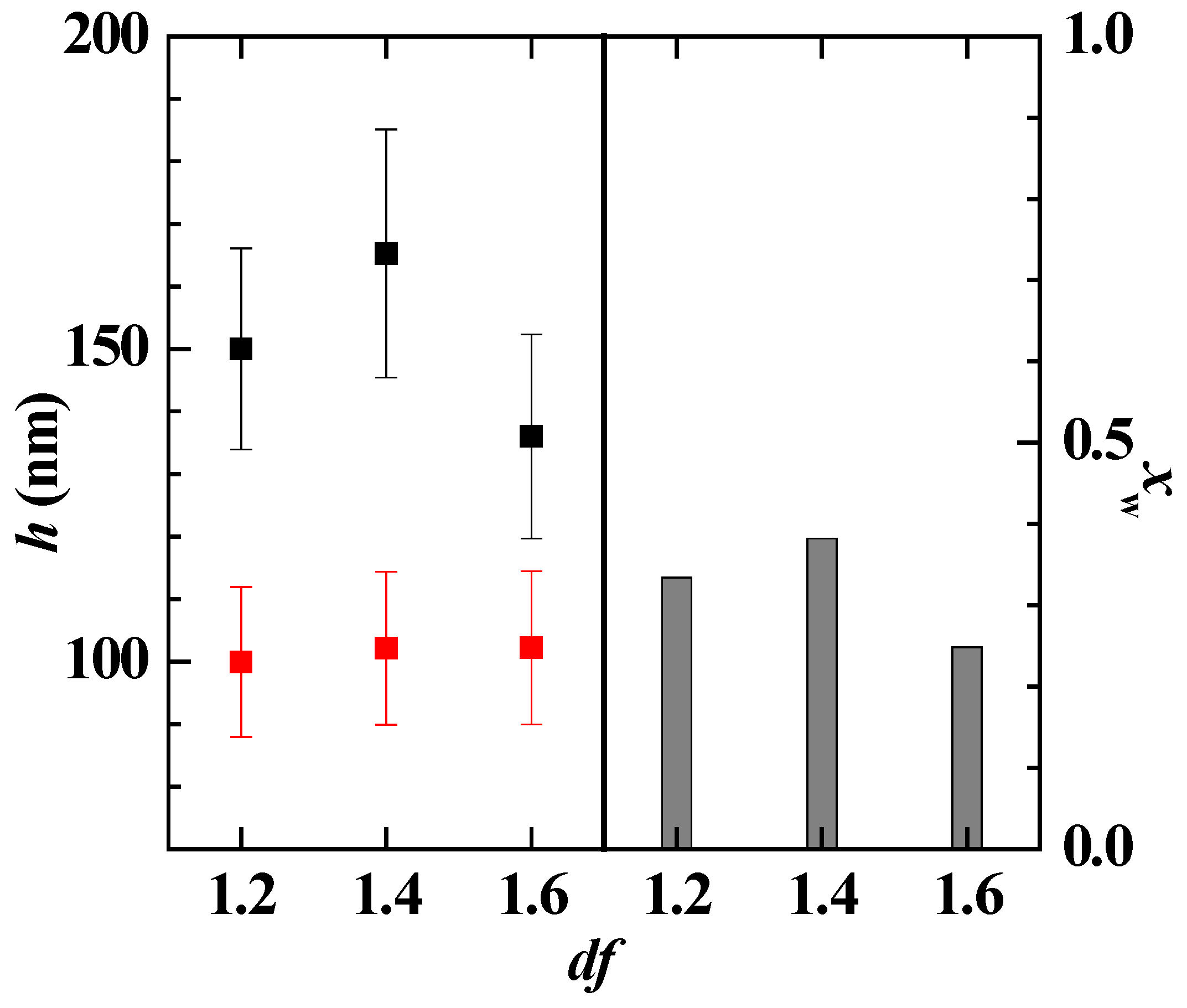
3.4. Bulk and Interfacial Behavior of Pseudo-Ternary Mixtures Containing Two Polymers and CAPB
3.5. Behavior of the Model Mixture upon Dilution: Towards the Real Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lochhead, R.Y. Shampoo and conditioner science. In Practical Modern Hair Science; Evans, T., Wickett, R.R., Eds.; Allured Pub. Corp.: Carol Stream, IL, USA, 2012; pp. 75–115. [Google Scholar]
- Goddard, E.D.; Gruber, J.V. Principles of Polymer Science and Technology in Cosmetics and Personal Care; CRC Press: Boca Ratón, FL, USA, 1999. [Google Scholar]
- Lindman, B.; Antunes, F.; Aidarova, S.; Miguel, M.; Nylander, T. Polyelectrolyte-surfactant association—From fundamentals to applications. Colloid J. 2014, 76, 585–594. [Google Scholar] [CrossRef]
- Cornwell, P.A. A review of shampoo surfactant technology: Consumer benefits, raw materials and recent developments. Int. J. Cosmet. Sci. 2017, 40, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Llamas, S.; Fernández-Peña, L.; Akanno, A.; Guzmán, E.; Ortega, V.; Ortega, F.; Csaky, A.G.; Campbell, R.A.; Rubio, R.G. Towards understanding the behavior of polyelectrolyte–surfactant mixtures at the water/vapor interface closer to technologically-relevant conditions. Phys. Chem. Chem. Phys. 2018, 20, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Llamas, S.; Guzmán, E.; Baghdadli, N.; Ortega, F.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of poly(diallyldimethylammonium chloride)—Sodium methyl-cocoyl-taurate complexes onto solid surfaces. Colloids Surf. A 2016, 505, 150–157. [Google Scholar] [CrossRef]
- Varga, I.; Campbell, R.A. General Physical Description of the Behavior of Oppositely Charged Polyelectrolyte/Surfactant Mixtures at the Air/Water Interface. Langmuir ACS J. Surf. Colloids 2017, 33, 5915–5924. [Google Scholar] [CrossRef]
- Hoèssel, P.; Dieing, R.; Noèrenberg, R.; Pfau, A.; Sander, R. Conditioning polymers in today’s shampoo formulations—Efficacy, mechanism and test methods. Int. J. Cosmet. Sci. 2000, 22, 1–10. [Google Scholar] [CrossRef]
- Kogej, K. Association and structure formation in oppositely charged polyelectrolyte–surfactant mixtures. Adv. Colloid Interface Sci. 2010, 158, 68–83. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; Ortega, F.; Rubio, R.G. Equilibrium and kinetically trapped aggregates in polyelectrolyte–oppositely charged surfactant mixtures. Curr. Opin. Colloid Interface Sci. 2020, 48, 91–108. [Google Scholar] [CrossRef]
- Llamas, S.; Guzmán, E.; Ortega, F.; Baghdadli, N.; Cazeneuve, C.; Rubio, R.G.; Luengo, G.S. Adsorption of polyelectrolytes and polyelectrolytes-surfactant mixtures at surfaces: A physico-chemical approach to a cosmetic challenge. Adv. Colloid Interface Sci. 2015, 222, 461–487. [Google Scholar] [CrossRef]
- Benhur, A.M.; Diaz, J.; Amin, S. Impact of polyelectrolyte-surfactant interactions on the rheology and wet lubrication performance of conditioning shampoo. Int. J. Cosmet. Sci. 2021, 43, 246–253. [Google Scholar] [CrossRef]
- Yorke, K.; Amin, S. High Performance Conditioning Shampoo with Hyaluronic Acid and Sustainable Surfactants. Cosmetics 2021, 8, 71. [Google Scholar] [CrossRef]
- Miyake, M. Recent progress of the characterization of oppositely charged polymer/surfactant complex in dilution deposition system. Adv. Colloid Interface Sci. 2017, 239, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Guzmán, E. Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics 2020, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Goddard, E.D.; Phillips, T.S.; Hannan, R.B. Water Soluble Polymer-Surfactant Interaction—Part I. J. Soc. Cosmet. Chem. 1975, 26, 461–475. [Google Scholar]
- Lepilleur, C.; Mullay, J.; Kyer, C.; McCalister, P.; Clifford, T. Use of statistical modeling to predict the effect of formulation composition on coacervation, silicone deposition, and conditioning sensory performance of cationic cassia polymers. J. Cosmet. Sci. 2011, 62, 161–177. [Google Scholar]
- Svensson, A.V.; Huang, L.; Johnson, E.S.; Nylander, T.; Piculell, L. Surface Deposition and Phase Behavior of Oppositely Charged Polyion/Surfactant Ion Complexes. 1. Cationic Guar versus Cationic Hydroxyethylcellulose in Mixtures with Anionic Surfactants. ACS Appl. Mat. Interface 2009, 1, 2431–2442. [Google Scholar] [CrossRef]
- Svensson, A.V.; Johnson, E.S.; Nylander, T.; Piculell, L. Surface Deposition and Phase Behavior of Oppositely Charged Polyion−Surfactant Ion Complexes. 2. A Means to Deliver Silicone Oil to Hydrophilic Surfaces. ACS Appl. Mat. Interfaces 2010, 2, 143–156. [Google Scholar] [CrossRef]
- Dhopatkar, N.; Park, J.H.; Chari, K.; Dhinojwala, A. Adsorption and Viscoelastic Analysis of Polyelectrolyte–Surfactant Complexes on Charged Hydrophilic Surfaces. Langmuir ACS J. Surf. Colloids 2015, 31, 1026–1037. [Google Scholar] [CrossRef]
- Bain, C.D.; Claesson, P.M.; Langevin, D.; Meszaros, R.; Nylander, T.; Stubenrauch, C.; Titmuss, S.; von Klitzing, R. Complexes of surfactants with oppositely charged polymers at surfaces and in bulk. Adv. Colloid Interface Sci. 2010, 155, 32–49. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Miyake, M. Creation of New Functions by Combination of Surfactant and Polymer—Complex Coacervation with Oppositely Charged Polymer and Surfactant for Shampoo and Body Wash. J. Oleo Sci. 2019, 68, 525–539. [Google Scholar] [CrossRef] [Green Version]
- Miyake, M.; Kakizawa, Y. Morphological study of cationic polymer-anionic surfactant complex precipitated in solution during the dilution process. J. Cosmet. Sci. 2010, 61, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Terada, E.; Samoshina, Y.; Nylander, T.; Lindman, B. Adsorption of cationic cellulose derivative/anionic surfactant complexes onto solid surfaces. II. Hydrophobized silica surfaces. Langmuir ACS J. Surf. Colloids 2004, 20, 6692–6701. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Abelenda-Nuñez, I.; Hernández-Rivas, M.; Ortega, F.; Rubio, R.G.; Guzmán, E. Impact of the bulk aggregation on the adsorption of oppositely charged polyelectrolyte-surfactant mixtures onto solid surfaces. Adv. Colloid Interface Sci. 2020, 282, 102203. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, R.Y.; Huisinga, L.R.; Waller, T. Deposition from conditioning shampoo: Optimizing coacervate formation. Cosmet. Toil. 2006, 121, 1. [Google Scholar]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Pour Khiabani, N.; Darabad, J.B.; Azin, R.; Arya, S. Wettability Alteration in Carbonates using Zirconium Oxide Nanofluids: EOR Implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Philippe, M.; Haridon, J.; Portal, J.; Chodorowski, S.; Luengo, G. Eco-friendly polymers for cosmetic formulations. L’Actualité Chim. 2020, 456–458, 101–107. [Google Scholar]
- Champú Suave Piel Sana. Available online: https://institutoespanol.com/producto/champu-suave-piel-sana/#1591723784510-d3f1e6b3-8a5f (accessed on 9 February 2022).
- Ramos, G.A.R.; Akanji, L.T.; Afzal, W. A Novel Surfactant–Polymer/Alkaline–Surfactant–Polymer Formulation for Enhanced Oil Recovery (EOR) Processes. Energy Fuels 2020, 34, 1230–1239. [Google Scholar] [CrossRef]
- Fernández-Peña, L.; Guzmán, E.; Ortega, F.; Bureau, L.; Leonforte, F.; Velasco, D.; Rubio, R.G.; Luengo, G.S. Physico-chemical study of polymer mixtures formed by a polycation and a zwitterionic copolymer in aqueous solution and upon adsorption onto negatively charged surfaces. Polymer 2021, 217, 123442. [Google Scholar] [CrossRef]
- Guzmán, E.; Llamas, S.; Fernández-Peña, L.; Léonforte, F.; Baghdadli, N.; Cazeneuve, C.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Effect of a natural amphoteric surfactant in the bulk and adsorption behavior of polyelectrolyte-surfactant mixtures. Colloids Surf. A 2020, 585, 124178. [Google Scholar] [CrossRef]
- Akanno, A.; Guzmán, E.; Fernández-Peña, L.; Ortega, F.; G Rubio, R. Surfactant-Like Behavior for the Adsorption of Mixtures of a Polycation and Two Different Zwitterionic Surfactants at the Water/Vapor Interface. Molecules 2019, 24, 3442. [Google Scholar] [CrossRef] [Green Version]
- Akanno, A.; Guzmán, E.; Fernández-Peña, L.; Llamas, S.; Ortega, F.; Rubio, R.G. Equilibration of a Polycation–Anionic Surfactant Mixture at the Water/Vapor Interface. Langmuir ACS J. Surf. Colloids 2018, 34, 7455–7464. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Yanez Arteta, M.; Angus-Smyth, A.; Nylander, T.; Noskov, B.A.; Varga, I. Direct Impact of Nonequilibrium Aggregates on the Structure and Morphology of Pdadmac/SDS Layers at the Air/Water Interface. Langmuir ACS J. Surf. Colloids 2014, 30, 8664–8674. [Google Scholar] [CrossRef] [PubMed]
- Angus-Smyth, A.; Bain, C.D.; Varga, I.; Campbell, R.A. Effects of bulk aggregation on PEI–SDS monolayers at the dynamic air–liquid interface: Depletion due to precipitation versus enrichment by a convection/spreading mechanism. Soft Matter 2013, 9, 6103–6117. [Google Scholar] [CrossRef]
- Mezei, A.; Mészáros, R.; Varga, I.; Gilányi, T. Effect of Mixing on the Formation of Complexes of Hyperbranched Cationic Polyelectrolytes and Anionic Surfactants. Langmuir ACS J. Surf. Colloids 2007, 23, 4237–4247. [Google Scholar] [CrossRef]
- Mészáros, R.; Thompson, L.; Bos, M.; Varga, I.; Gilányi, T. Interaction of Sodium Dodecyl Sulfate with Polyethyleneimine: Surfactant-Induced Polymer Solution Colloid Dispersion Transition. Langmuir ACS J. Surf. Colloids 2003, 19, 609–615. [Google Scholar] [CrossRef]
- Gavazzoni Dias, M.F. Hair cosmetics: An overview. Int. J. Trichol. 2015, 7, 2–15. [Google Scholar] [CrossRef]
- Luengo, G.S.; Galliano, A.; Dubief, C. Aqueous Lubrication in Cosmetic. In Aqueous Lubrication. Natural and Biomimetic Approaches; Spencer, N.D., Ed.; World Scientific Publishing Co., Pte. Ltd.: Singapore, 2014; pp. 103–144. [Google Scholar]
- Guzmán, E.; Ortega, F.; Baghdadli, N.; Luengo, G.S.; Rubio, R.G. Effect of the molecular structure on the adsorption of conditioning polyelectrolytes on solid substrates. Colloids Surf. A 2011, 375, 209–218. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Prolongo, M.G.; Starov, V.M.; Rubio, R.G. Influence of the molecular architecture on the adsorption onto solid surfaces: Comb-like polymers. Phys. Chem. Chem. Phys. 2011, 13, 16416–16423. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, E.; Ortega, F.; Baghdadli, N.; Cazeneuve, C.; Luengo, G.S.; Rubio, R.G. Adsorption of Conditioning Polymers on Solid Substrates with Different Charge Density. ACS Appl. Mater. Interfaces 2011, 3, 3181–3188. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.; Rubio, J.E.F.; Rubio, R.G.; Ortega, F. Salt-induced changes in the growth of polyelectrolyte layers of poly(diallyldimethylammoniumchloride) and poly(4-styrene sulfonate of sodium). Soft Matter 2009, 5, 2130–2142. [Google Scholar] [CrossRef]
- Nestler, P.; Helm, C.A. Determination of refractive index and layer thickness of nm-thin films via ellipsometry. Opt. Express 2017, 25, 27077–27085. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Chuliá-Jordán, R.; Ortega, F.; Rubio, R.G. Influence of the percentage of acetylation on the assembly of LbL multilayers of poly(acrylic acid) and chitosan. Phys. Chem. Chem. Phys. 2011, 13, 18200–18207. [Google Scholar] [CrossRef] [PubMed]
- Palik, E.D. Handbook of Optical Constant of Solid; Academic Press, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Llamas, S.; Ortega, F.; Rubio, R.G. Two Different Scenarios for the Equilibration of Polycation—Anionic Solutions at Water–Vapor Interfaces. Coatings 2019, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Naderi, A.; Claesson, P.M.; Bergström, M.; Dėdinaitė, A. Trapped non-equilibrium states in aqueous solutions of oppositely charged polyelectrolytes and surfactants: Effects of mixing protocol and salt concentration. Colloids Surf. A 2005, 253, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Grządka, E.; Matusiak, J.; Godek, E.; Maciołek, U. Mixtures of cationic guar gum and anionic surfactants as stabilizers of zirconia suspensions. J. Mol. Liq. 2021, 343, 117677. [Google Scholar] [CrossRef]
- Grządka, E.; Matusiak, J.; Godek, E. Alginic acid as a stabilizer of zirconia suspensions in the presence of cationic surfactants. Carbohydr. Polym. 2020, 246, 116634. [Google Scholar] [CrossRef]
- Aidarova, S.; Sharipova, A.; Krägel, J.; Miller, R. Polyelectrolyte/surfactant mixtures in the bulk and at water/oil interfaces. Adv. Colloid Interface Sci. 2014, 205, 87–93. [Google Scholar] [CrossRef]
- Guzmán, E.; Llamas, S.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubio, R.G. Polymer–surfactant systems in bulk and at fluid interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef]
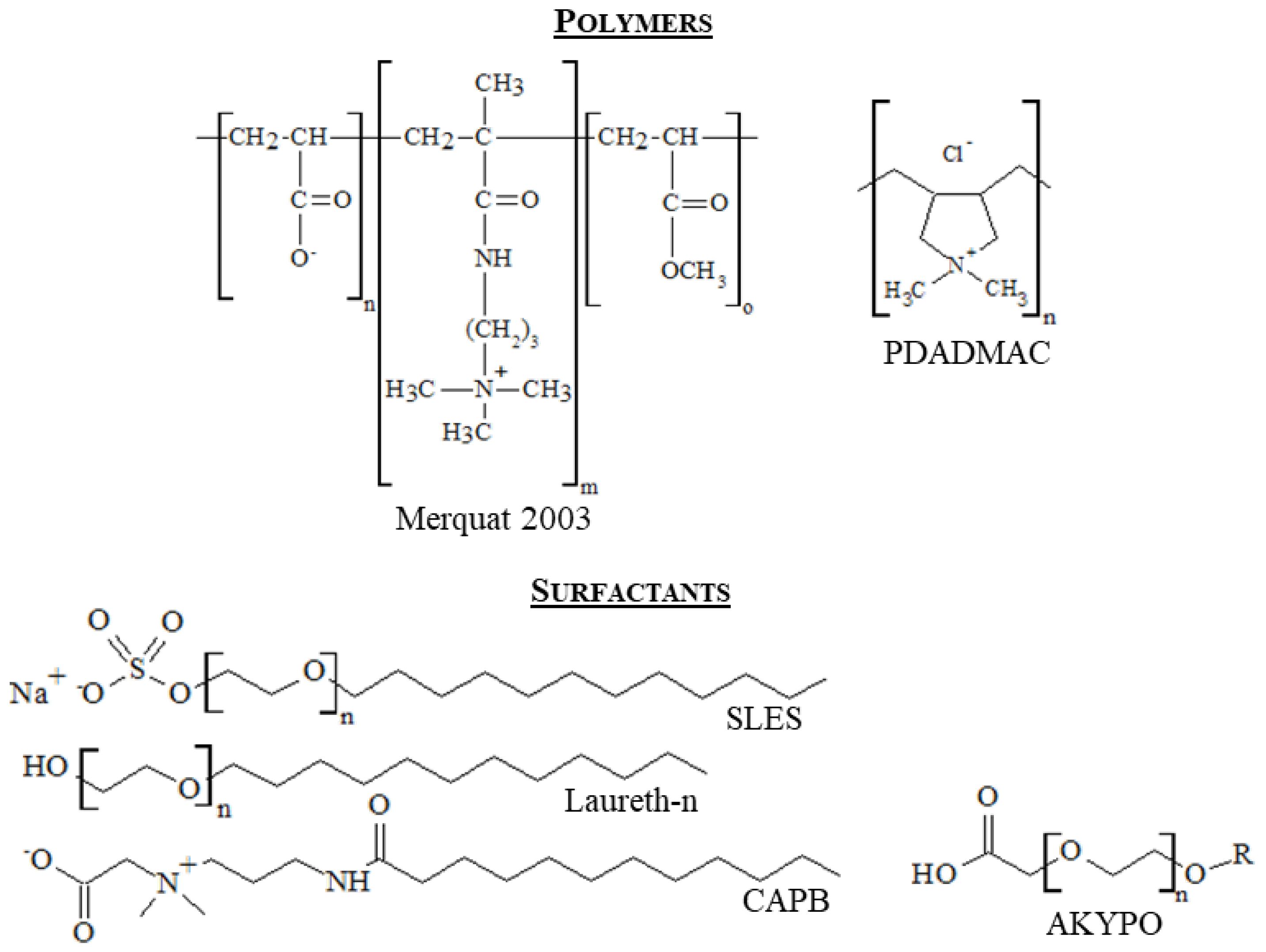



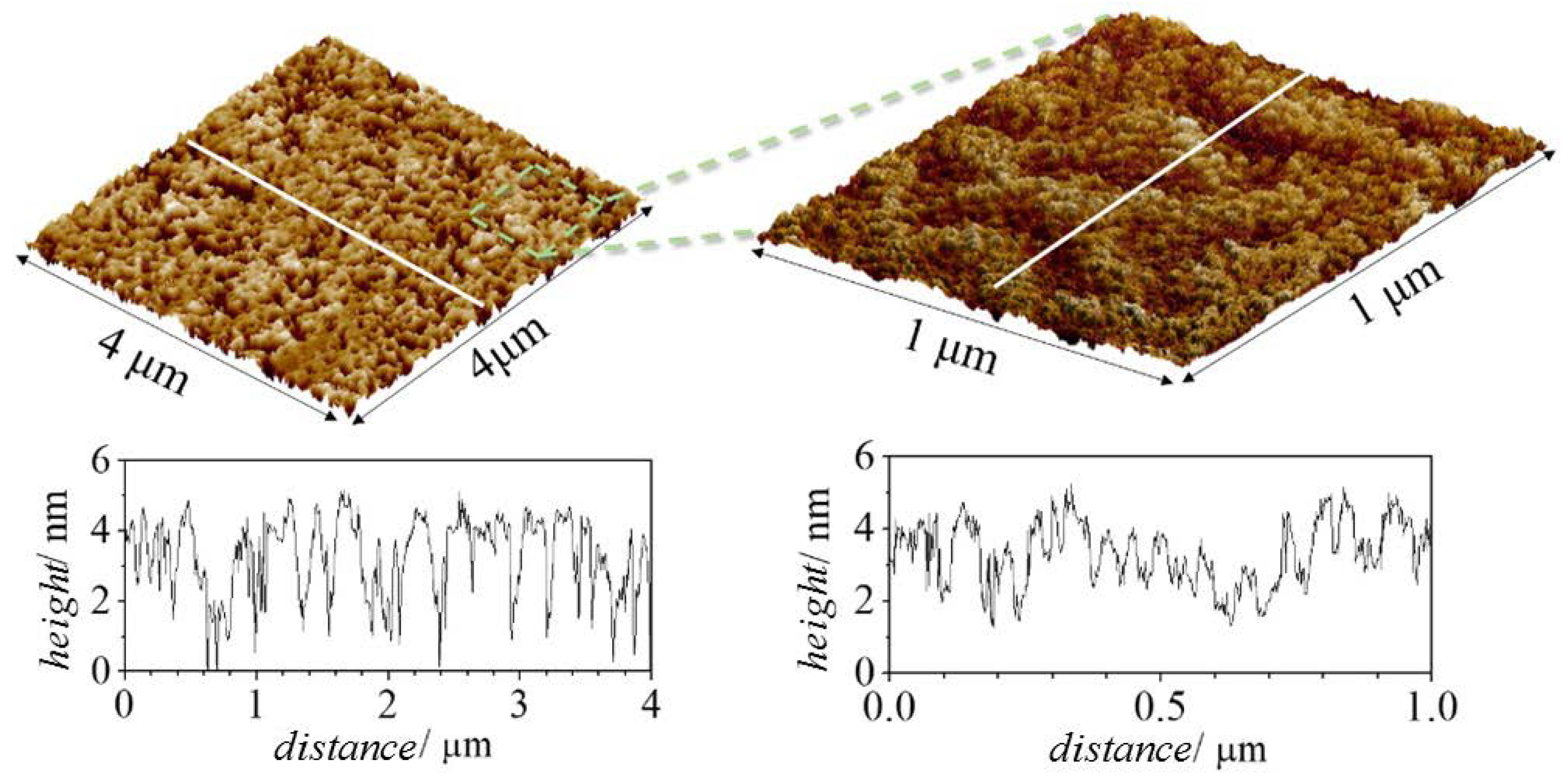
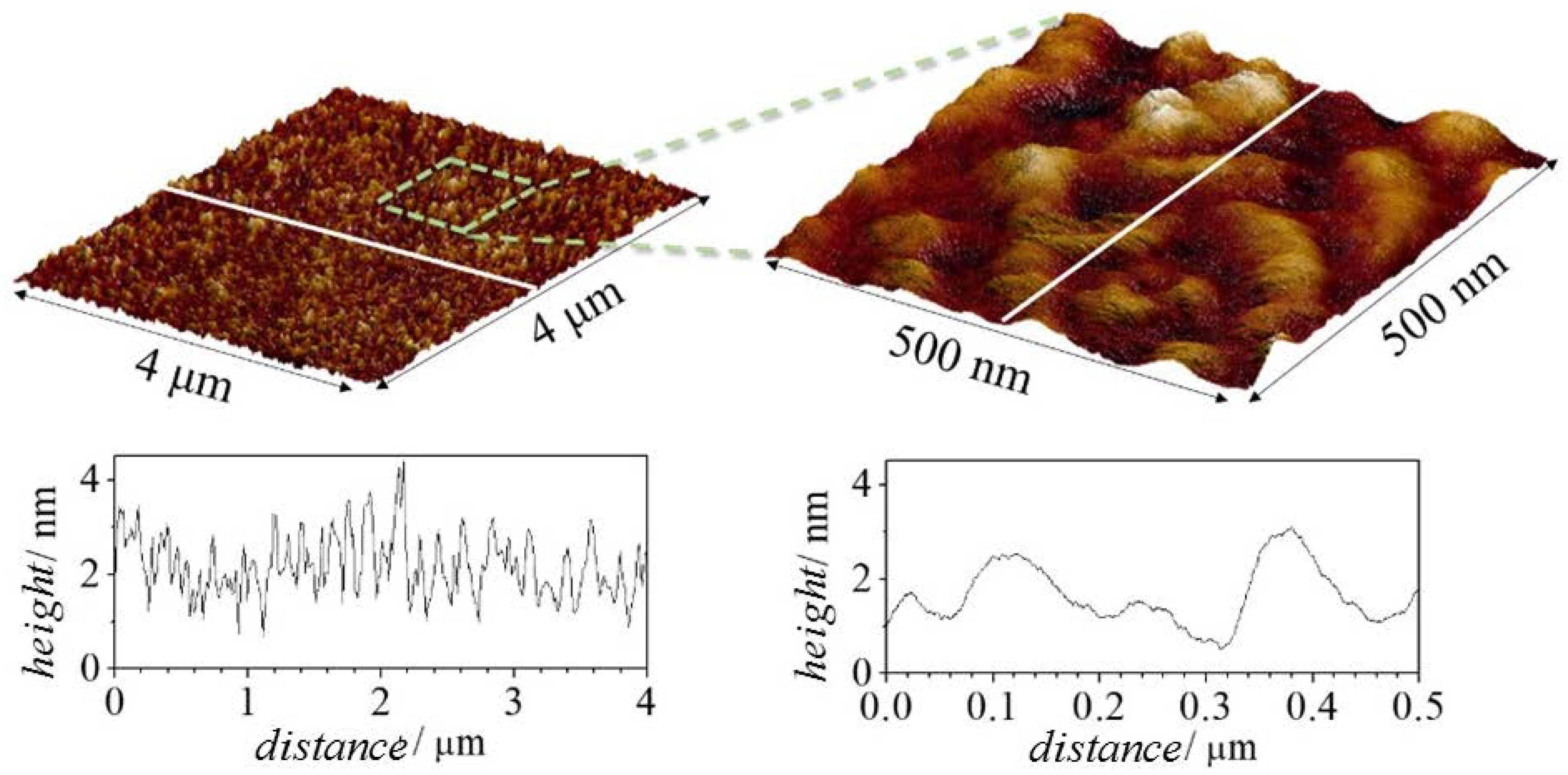


| Component | Type of Molecule |
|---|---|
| copolymer of acrylamide and diallyldimethylammonium chloride | Polymers |
| copolymer of styrene and acrylates | |
| tocopheryl acetate | Surfactants |
| lauryl ether (4 oxyethylene groups) | |
| sodium lauryl ether sulfate | |
| Cocoisopropanolamide | |
| cocoamidopropyl betaine | |
| polyethylenglycolether of glyceryl cocoate (7 oxyethylene groups) |
| Component | Concentration | Type of Molecule |
|---|---|---|
| PDADMAC | 0.5% w/w 1 | Polymers |
| Merquat 2003 | 0.25% w/w 2 | |
| lauryl ether (12 oxyethylene groups) | 56 mM | Surfactants |
| lauryl ether (4 oxyethylene groups) | 28 mM | |
| sodium lauryl ether sulfate | 186 mM | |
| cocamidopropyl Betaine | 204 mM | |
| laureth-5 carboxylic acid | 136 mM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Peña, L.; Guzmán, E.; Fernández-Pérez, C.; Barba-Nieto, I.; Ortega, F.; Leonforte, F.; Rubio, R.G.; Luengo, G.S. Study of the Dilution-Induced Deposition of Concentrated Mixtures of Polyelectrolytes and Surfactants. Polymers 2022, 14, 1335. https://doi.org/10.3390/polym14071335
Fernández-Peña L, Guzmán E, Fernández-Pérez C, Barba-Nieto I, Ortega F, Leonforte F, Rubio RG, Luengo GS. Study of the Dilution-Induced Deposition of Concentrated Mixtures of Polyelectrolytes and Surfactants. Polymers. 2022; 14(7):1335. https://doi.org/10.3390/polym14071335
Chicago/Turabian StyleFernández-Peña, Laura, Eduardo Guzmán, Coral Fernández-Pérez, Irene Barba-Nieto, Francisco Ortega, Fabien Leonforte, Ramón G. Rubio, and Gustavo S. Luengo. 2022. "Study of the Dilution-Induced Deposition of Concentrated Mixtures of Polyelectrolytes and Surfactants" Polymers 14, no. 7: 1335. https://doi.org/10.3390/polym14071335
APA StyleFernández-Peña, L., Guzmán, E., Fernández-Pérez, C., Barba-Nieto, I., Ortega, F., Leonforte, F., Rubio, R. G., & Luengo, G. S. (2022). Study of the Dilution-Induced Deposition of Concentrated Mixtures of Polyelectrolytes and Surfactants. Polymers, 14(7), 1335. https://doi.org/10.3390/polym14071335











