Toward Mechanically Robust Crosslinked Elastomers through Phase Transfer Agent Tuning the Solubility of Zn2+ in the Organic Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
3. Results
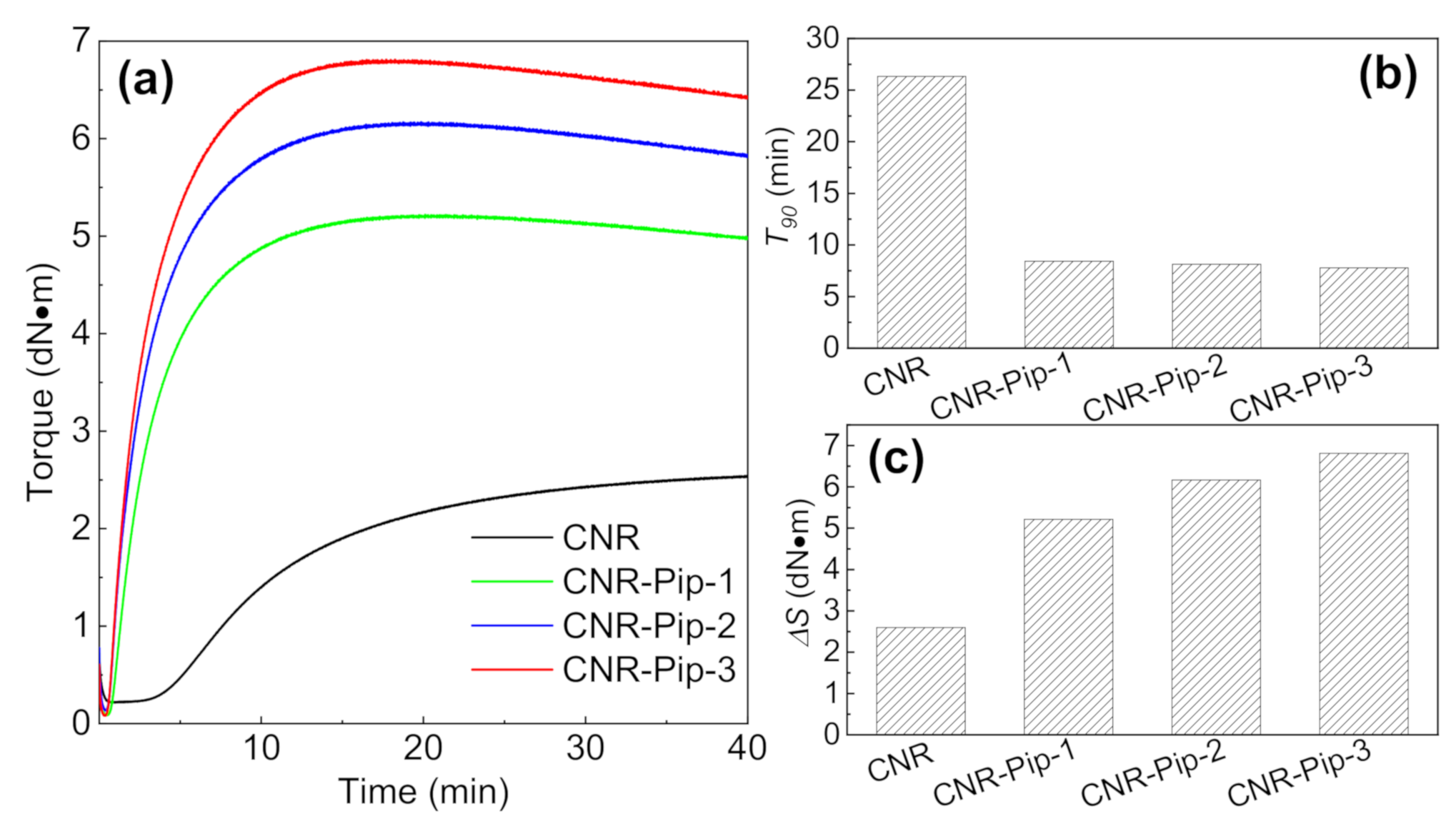
3.1. Changes in Crosslinking Process Induced by Phase Transfer Agent
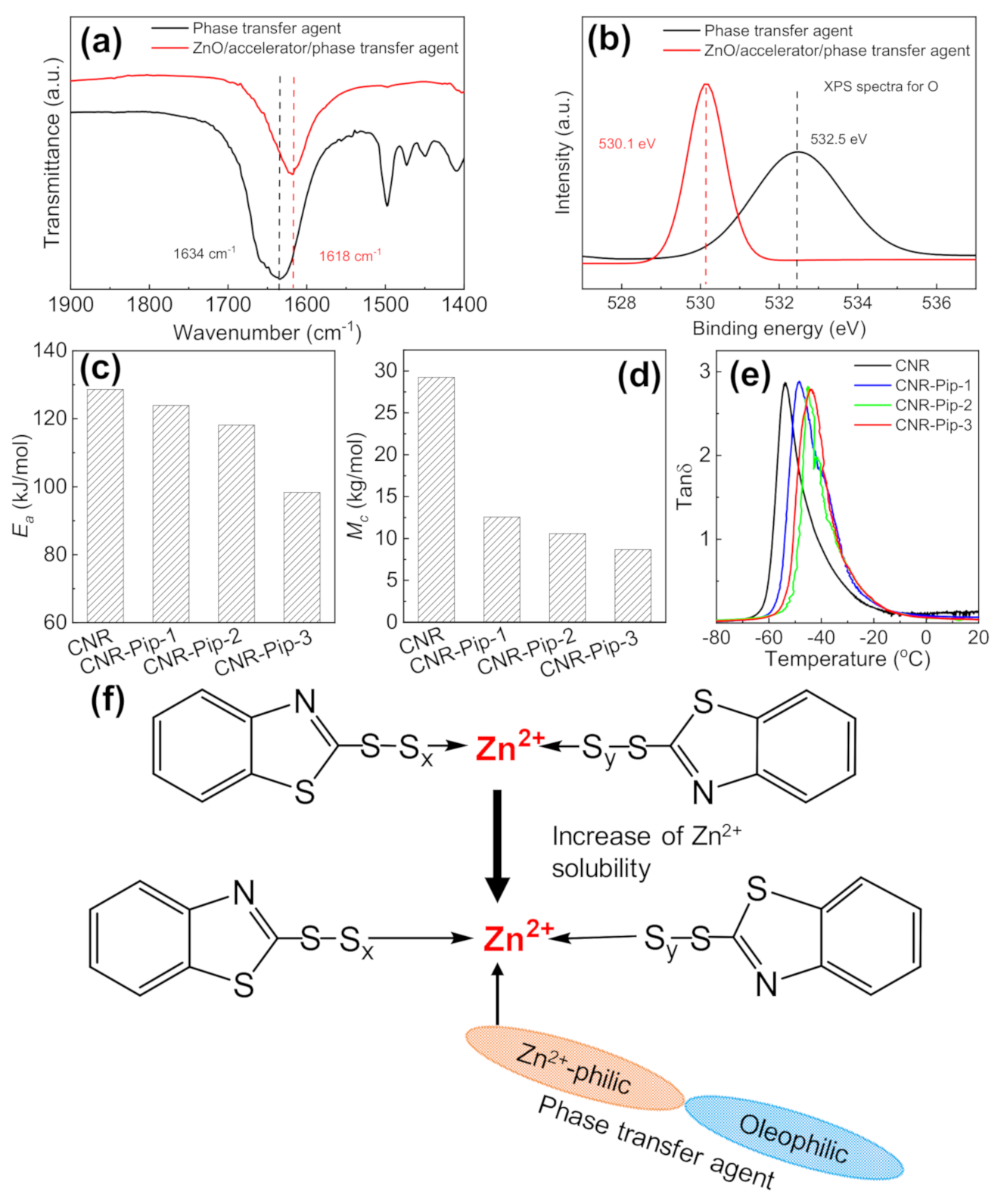
3.2. Effect of Phase Transfer Agent on Crosslinking Structure
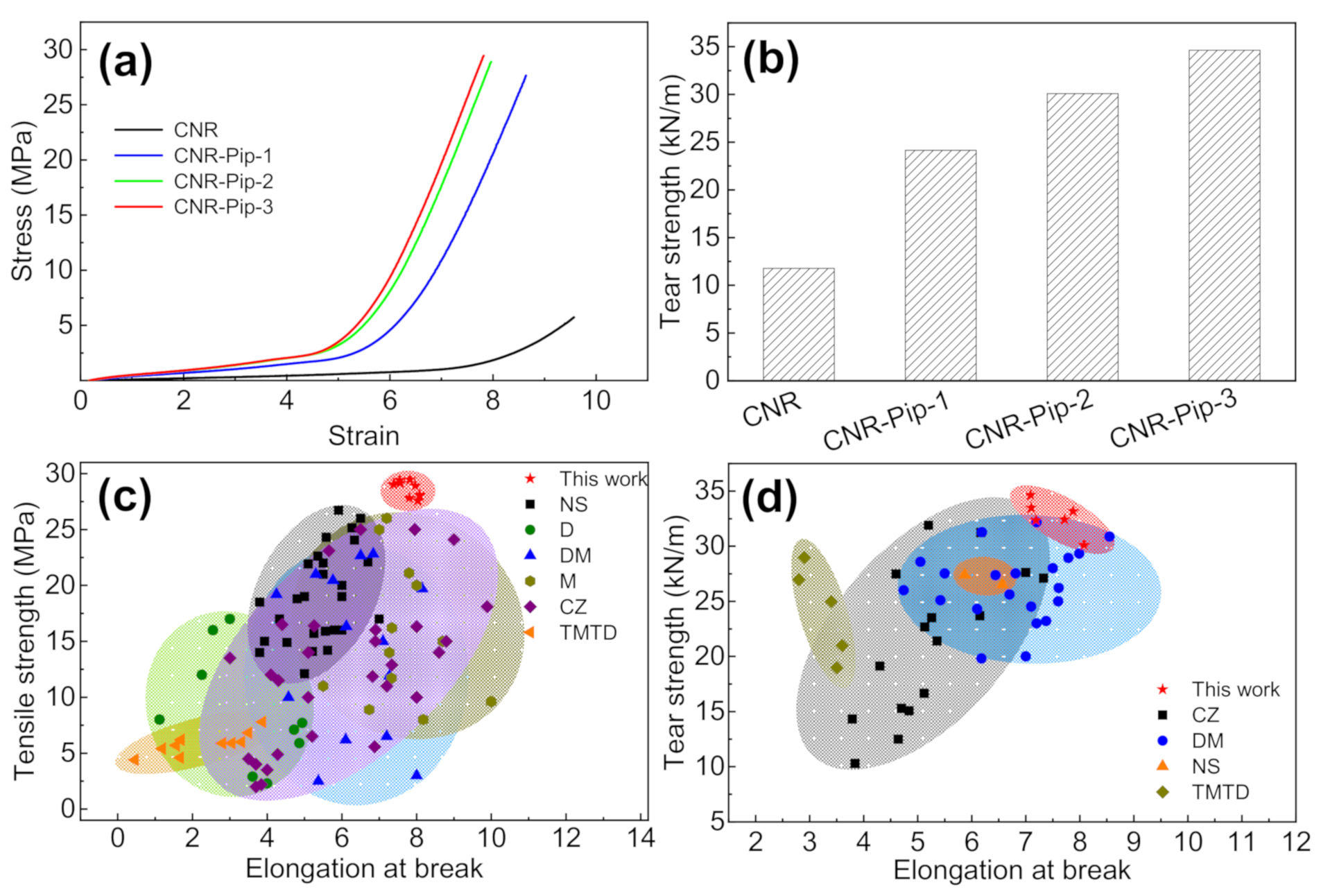
3.3. Effect of Phase Transfer Agent on Mechanical Properties
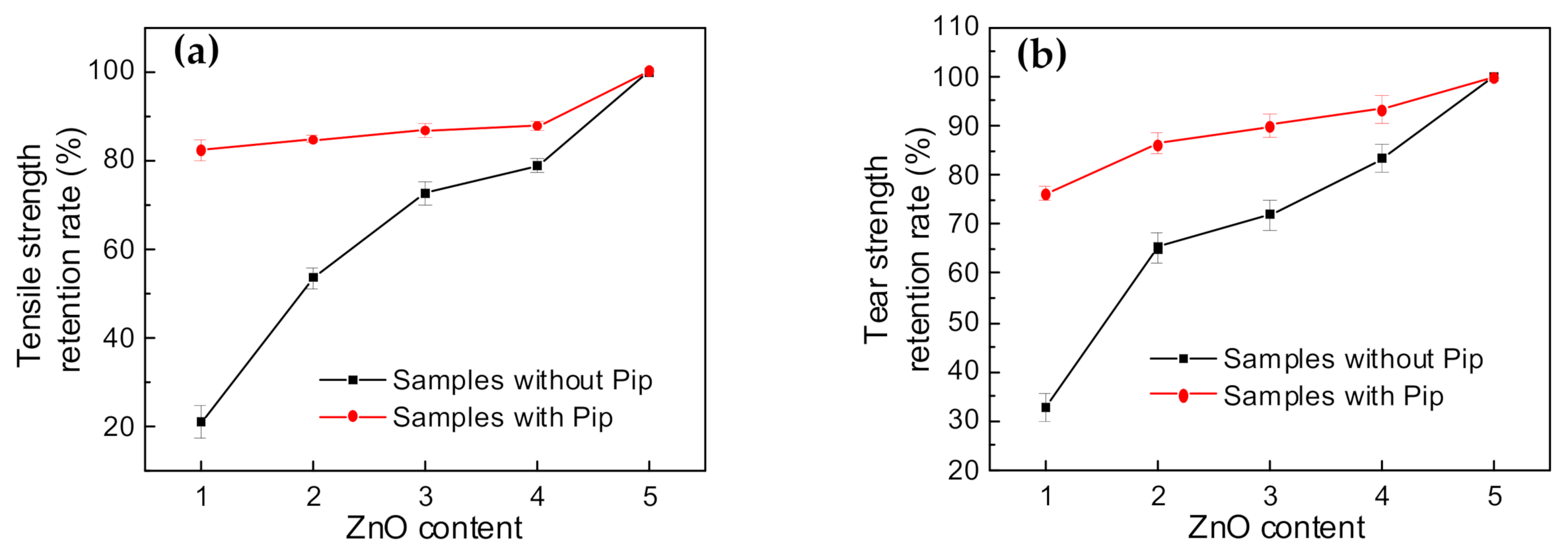
3.4. Changes in Properties upon Lowering ZnO Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Shen, Q.; Wei, L.; Fu, X.; Huang, C.; Zhu, Y.; Zhao, L.; Huang, G.; Wu, J. Ultra-Tough, Strong, and Defect-Tolerant Elastomers with Self-Healing and Intelligent-Responsive Abilities. ACS Appl. Mater. Interfaces 2019, 11, 29373–29381. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-C.; Liu, G.-X.; Zhang, L.; Xu, W.-Z.; Liao, S.; Luo, M.-C. Mimicking the Mechanical Robustness of Natural Rubber Based on a Sacrificial Network Constructed by Phospholipids. ACS Appl. Mater. Interfaces 2020, 12, 14468–14475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, Z.; Liu, Y.; Wu, S.; Guo, B. Mechanically Robust, Self-Healable, and Reprocessable Elastomers Enabled by Dynamic Dual Cross-Links. Macromolecules 2019, 52, 3805–3812. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, K.; Song, W.; Yan, S.; Zhao, X.; Lu, Y.; Zhang, L. The Effect of Epoxidation on Strain-Induced Crystallization of Epoxidized Natural Rubber. Macromol. Rapid Commun. 2019, 40, 1900042–1900046. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Y.; Niu, Z.; Wu, R.; Fan, W.; Dai, Q.; He, J.; Bai, C. Catalyst-Free Vitrimer Cross-Linked by Biomass-Derived Compounds with Mechanical Robustness, Reprocessability, and Multishape Memory Effects. Macromol. Rapid Commun. 2021, 42, 2100432–2100441. [Google Scholar] [CrossRef] [PubMed]
- Akiba, M.; Hashim, A.S. Vulcanization and Crosslinking in Elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Aprem, A.S.; Joseph, K.; Thomas, S. Recent Developments in Crosslinking of Elastomers. Rubber Chem. Technol. 2005, 78, 458–488. [Google Scholar] [CrossRef]
- Ghosh, P.; Katare, S.; Patkar, P.; Caruthers, J.M.; Venkatasubramanian, V.; Walker, K.A. Sulfur Vulcanization of Natural Rubber for Benzothiazole Accelerated Formulations: From Reaction Mechanisms to a Rational Kinetic Model. Rubber Chem. Technol. 2003, 76, 592–693. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Synthesis and Applications of ZnO/Polymer Nanohybrids. ACS Mater. Lett. 2021, 3, 599–621. [Google Scholar] [CrossRef]
- Hernández, M.; Ezquerra, T.A.; Verdejo, R.; López-Manchado, M.A. Role of Vulcanizing Additives on the Segmental Dynamics of Natural Rubber. Macromolecules 2012, 45, 1070–1075. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhou, X.; Liang, K.; Guo, B.; Li, X.; Wang, Z.; Zhang, L. Mechanically Robust and Recyclable EPDM Rubber Composites by a Green Cross-Linking Strategy. ACS Sustain. Chem. Eng. 2019, 7, 11712–11720. [Google Scholar] [CrossRef]
- Qin, X.; Xu, H.; Zhang, G.; Wang, J.; Wang, Z.; Zhao, Y.; Wang, Z.; Tan, T.; Bockstaller, M.R.; Zhang, L.; et al. Enhancing the Performance of Rubber with Nano ZnO as Activators. ACS Appl. Mater. Interfaces 2020, 12, 48007–48015. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Yasuda, Y.; Ohashi, T.; Yokohama, H.; Minoda, S.; Kobayashi, H.; Honma, T. Dinuclear Bridging Bidentate Zinc/Stearate Complex in Sulfur Cross-Linking of Rubber. Macromolecules 2015, 48, 462–475. [Google Scholar] [CrossRef]
- Dodd, L.J.; Omar, Ö.; Wu, X.; Hasell, T. Investigating the Role and Scope of Catalysts in Inverse Vulcanization. ACS Catal. 2021, 11, 4441–4455. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Liu, G.-X.; Zhang, L.; Zhao, F.; Liao, S.; Luo, M.-C. Exploring the Unique Characteristics of Natural Rubber Induced by Coordination Interaction between Proteins and Zn2+. Polymer 2020, 193, 122357–122363. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Liu, G.-X.; Zhang, H.-F.; Zhao, F.; Luo, M.-C.; Liao, S. Non-Rubber Components Tuning Mechanical Properties of Natural Rubber from Vulcanization Kinetics. Polymer 2019, 183, 121911–121917. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sakaki, Y.; Yasuda, Y.; Junkong, P.; Ohashi, T.; Miyaji, K.; Kobayashi, H. Roles of Dinuclear Bridging Bidentate Zinc/Stearate Complexes in Sulfur Cross-Linking of Isoprene Rubber. Organometallics 2019, 38, 2363–2380. [Google Scholar] [CrossRef]
- Miyaji, K.; Sugiyama, T.; Ohashi, T.; Saalwächter, K.; Ikeda, Y. Study on Homogeneity in Sulfur Cross-Linked Network Structures of Isoprene Rubber by TD-NMR and AFM–Zinc Stearate System. Macromolecules 2020, 53, 8438–8449. [Google Scholar] [CrossRef]
- Junkong, P.; Morimoto, R.; Miyaji, K.; Tohsan, A.; Sakaki, Y.; Ikeda, Y. Effect of Fatty Acids on the Accelerated Sulfur Vulcanization of Rubber by Active Zinc/Carboxylate Complexes. RSC Adv. 2020, 10, 4772–4785. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Bolados, H.; Brasero, J.; Lopez-Manchado, M.A.; Yazdani-Pedram, M. High performance natural rubber/thermally reduced graphite oxide nanocomposites by latex technology. Compos. Part B Eng. 2014, 67, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Sukmak, G.; Sukmak, P.; Horpibulsuk, S.; Yaowarat, T.; Kunchariyakun, K.; Patarapaiboolchai, O.; Arulrajah, A. Physical and mechanical properties of natural rubber modified cement paste. Constr. Build. Mater. 2020, 244, 118319–118329. [Google Scholar] [CrossRef]
- Walteros-León, M.; Álvarez-Láinez, M.L. Colloidal and rheological properties of natural rubber latex concentrated with hydroxyethyl cellulose and sodium dodecyl sulphate. J. Appl. Polym. Sci. 2021, 139, 52034–52046. [Google Scholar] [CrossRef]
- Hui Mei, E.-L.; Misran, M. Effect of Sodium Dodecyl Sulphate and Polyoxyethylene Dodecyl Ether on the Rheological Behaviour and Stability of Natural Rubber Latex. ASM Sci. J. 2018, 11, 44–55. [Google Scholar]
- Sigel, H.; Martin, R.B. Coordinating Properties of the Amide Bond. Stability and Structure of Metal Ion Complexes of Peptides and Related Ligands. Chem. Rev. 1982, 82, 385–426. [Google Scholar] [CrossRef]
- Fockaert, L.I.; Taheri, P.; Abrahami, S.T.; Boelen, B.; Terryn, H.; Mol, J.M.C. Zirconium-Based Conversion Film Formation on Zinc, Aluminium and Magnesium Oxides and Their Interactions with Functionalized Molecules. Appl. Surf. Sci. 2017, 423, 817–828. [Google Scholar] [CrossRef]
- Wu, J.; Xing, W.; Huang, G.; Li, H.; Tang, M.; Wu, S.; Liu, Y. Vulcanization Kinetics of Graphene/Natural Rubber Nanocomposites. Polymer 2013, 54, 3314–3323. [Google Scholar] [CrossRef]
- Tang, M.-Z.; Xing, W.; Wu, J.-R.; Huang, G.-S.; Li, H.; Wu, S.-D. Vulcanization Kinetics of Graphene/Styrene Butadiene Rubber Nanocomposites. Chin. J. Polym. Sci. 2014, 32, 658–666. [Google Scholar] [CrossRef]
- Xie, Z.-T.; Fu, X.; Wei, L.-Y.; Luo, M.-C.; Liu, Y.-H.; Ling, F.-W.; Huang, C.; Huang, G.; Wu, J. New Evidence Disclosed for the Engineered Strong Interfacial Interaction of Graphene/Rubber Nanocomposites. Polymer 2017, 118, 30–39. [Google Scholar] [CrossRef]
- Alam, M.N.; Debnath, S.C.; Choi, J. Nitrosamine-Safe Thiuram Disulfide and Benzothiazole Sulfenamide as a Synergistic Pair of Accelerators for the Vulcanization of Rubber. J. Polym. Res. 2021, 28, 317–326. [Google Scholar] [CrossRef]
- Ghorai, S.; Jalan, A.K.; Roy, M.; Das, A.; De, D. Tuning of Accelerator and Curing System in Devulcanized Green Natural Rubber Compounds. Polym. Test. 2018, 69, 133–145. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, J.; Guo, B.; Zhang, L.; Liu, F. Bioinspired Engineering of Sacrificial Metal-Ligand Bonds into Elastomers with Supramechanical Performance and Adaptive Recovery. Macromolecules 2016, 49, 1781–1789. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Tang, Z.; Huang, J.; Guo, B.; Huang, G. Bioinspired Engineering of Two Different Types of Sacrificial Bonds into Chemically Cross-Linked cis-1,4-Polyisoprene Toward a High-Performance Elastomer. Macromolecules 2016, 49, 8593–8604. [Google Scholar] [CrossRef]
- Wu, S.; Qiu, M.; Tang, Z.; Liu, J.; Guo, B. Carbon Nanodots as High-Functionality Cross-Linkers for Bioinspired Engineering of Multiple Sacrificial Units toward Strong yet Tough Elastomers. Macromolecules 2017, 50, 3244–3253. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, Z.; Guo, B.; Zhang, L. Concurrently Improved Dispersion and Interfacial Interaction in Rubber/Nanosilica Composites via Efficient Hydrosilane Functionalization. Compos. Sci. Technol. 2019, 169, 217–223. [Google Scholar] [CrossRef]
- Zheng, L.; Jerrams, S.; Su, T.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. Enhanced Covalent Interface, Crosslinked Network and Gas Barrier Property of Functionalized Graphene Oxide/Styrene-Butadiene Rubber Composites Triggered by Thiol-Ene Click Reaction. Compos. Part B Eng. 2020, 197, 108186–108194. [Google Scholar] [CrossRef]
- Guo, H.; Jerrams, S.; Xu, Z.; Zhou, Y.; Jiang, L.; Zhang, L.; Liu, L.; Wen, S. Enhanced Fatigue and Durability of Carbon Black/Natural Rubber Composites Reinforced with Graphene Oxide and Carbon Nanotubes. Eng. Fract. Mech. 2020, 223, 106764–106775. [Google Scholar] [CrossRef]
- Zheng, L.; Jerrams, S.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. Enhanced Gas Barrier Properties of Graphene Oxide/Rubber Composites with Strong Interfaces Constructed by Graphene Oxide and Sulfur. Chem. Eng. J. 2020, 383, 123100–123109. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, Z.; An, X.; Fang, S.; Wu, S.; Guo, B. Generic Method to Create Segregated Structures toward Robust, Flexible, Highly Conductive Elastomer Composites. ACS Appl. Mater. Interfaces 2021, 13, 24154–24163. [Google Scholar] [CrossRef]
- Xu, Z.; Jerrams, S.; Guo, H.; Zhou, Y.; Jiang, L.; Gao, Y.; Zhang, L.; Liu, L.; Wen, S. Influence of Graphene Oxide and Carbon Nanotubes on the Fatigue Properties of Silica/Styrene-Butadiene Rubber Composites under Uniaxial and Multiaxial Cyclic Loading. Int. J. Fatigue 2020, 131, 105388–105397. [Google Scholar] [CrossRef]
- Wu, S.; Qiu, M.; Tang, Z.; Guo, B. Interphase Percolation Mechanism Underlying Elastomer Reinforcement. J. Phys. Chem. C 2017, 121, 28594–28603. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, C.; Zhu, L.; Guo, B. Low Permeability Styrene Butadiene Rubber/Boehmite Nanocomposites Modified with Tannic Acid. Mater. Des. 2016, 103, 25–31. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Q.; Wang, R.; Guo, B.; Lvov, Y.; Hu, G.-H.; Zhang, L. Preparation and Performance of Bio-Based Carboxylic Elastomer/Halloysite Nanotubes Nanocomposites with Strong Interfacial Interaction. Compos. Part A Appl. Sci. Manuf. 2017, 102, 253–262. [Google Scholar] [CrossRef]
- Meera, A.P.; Thomas, S.; Zachariah, A.K.; Yang, W. Effect of Organoclay on the Solvent Diffusion Behavior and Mechanical Properties of Natural Rubber Nanocomposites. Polym. Compos. 2018, 39, 3110–3118. [Google Scholar] [CrossRef]
- Yin, B.; Li, G.; Wang, D.; Wang, L.; Wang, J.; Jia, H.; Ding, L.; Sun, D. Enhanced Mechanical Properties of Styrene-Butadiene Rubber with Low Content of Bacterial Cellulose Nanowhiskers. Adv. Polym. Technol. 2018, 37, 1323–1334. [Google Scholar] [CrossRef]
- Xue, X.; Yin, Q.; Jia, H.; Zhang, X.; Wen, Y.; Ji, Q.; Xu, Z. Enhancing Mechanical and Thermal Properties of Styrene-Butadiene Rubber/Carboxylated Acrylonitrile Butadiene Rubber Blend by the Usage of Graphene Oxide with Diverse Oxidation Degrees. Appl. Surf. Sci. 2017, 423, 584–591. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Yin, Q.; Wu, J.; Song, W.; Mohamed, A.; Jia, H.; Yang, F.; Rui, X. Highly Improved Compatibility and Mechanical Properties of Carboxylated Nitrile Rubber/Styrene Butadiene Rubber by Incorporating Modified Kevlar Nanofibers. Mater. Chem. Phys. 2019, 238, 121926–121931. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, J.; Yuan, R.; Wan, H.; Chen, L.; Li, J.; Zhou, H.; Chen, J. Investigate on Mechanical and Tribological Properties of Solution Styrene Butadiene and Butadiene Rubber Composites. Polym. Adv. Technol. 2018, 29, 2674–2682. [Google Scholar] [CrossRef]
- Li, J.; Isayev, A.I.; Ren, X.; Soucek, M.D. Norbornylized Soybean Oil as a Sustainable New Plasticizer for Rubbers with Hybrid Fillers. Polym. Int. 2017, 66, 820–829. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Wu, W.; Zhang, Z.; Xian, Y.; Lin, Y.; Lu, Y.; Zhang, L. Advanced Flexible rGO-BN Natural Rubber Films with High Thermal Conductivity for Improved Thermal Management Capability. Carbon 2020, 162, 46–55. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wang, W.; Geng, X.; Zhang, L.; Guo, B.; Nishi, T.; Hu, G.-H. Significantly Improving Strength and Damping Performance of Nitrile Rubber via Incorporating Sliding Graft Copolymer. Ind. Eng. Chem. Res. 2018, 57, 16692–16700. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Kuang, W.; Tang, Z.; Guo, B. Coating Polyrhodanine onto Boron Nitride Nanosheets for Thermally Conductive Elastomer Composites. Compos. Part A Appl. Sci. Manuf. 2017, 94, 77–85. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Z.; Zhang, L.; Guo, B. Dispersion of Graphene in Chlorosulfonated Polyethylene by Slurry Compounding. Compos. Sci. Technol. 2018, 162, 156–162. [Google Scholar] [CrossRef]
- Hernández, M.; Bernal, M.d.M.; Verdejo, R.; Ezquerra, T.A.; López-Manchado, M.A. Overall Performance of Natural Rubber/Graphene Nanocomposites. Compos. Sci. Technol. 2012, 73, 40–46. [Google Scholar] [CrossRef]
- Weng, P.; Tang, Z.; Huang, J.; Wu, S.; Guo, B. Promoted Dispersion of Silica and Interfacial Strength in Rubber/Silica Composites by Grafting with Oniums. J. Appl. Polym. Sci. 2019, 136, 48243–48252. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, J.; Liao, R.; Yang, G.; Wu, X.; Tang, Z.; Guo, B.; Zhang, L.; Ma, Y.; Nie, Q.; et al. Rational Design of Covalent Interfaces for Graphene/Elastomer Nanocomposites. Compos. Sci. Technol. 2016, 132, 68–75. [Google Scholar] [CrossRef]
- Delgado, E.; Espitia, A.; Aperador, W. Comparative Evaluation of Clusia Multiflora Wood Flour, against Mineral Fillers, as Reinforcement in SBR Rubber Composites. Iran. Polym. J. 2019, 29, 13–23. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Jia, H.; Yin, B.; Ding, L.; Xu, Z.; Ji, Q. Polyvinyl Pyrrolidone Modified Graphene Oxide for Improving the Mechanical, Thermal Conductivity and Solvent Resistance Properties of Natural Rubber. RSC Adv. 2016, 6, 54668–54678. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.F.; Li, K.J.; Liao, S.; Luo, M.C. Enabling Superior Thermo-Oxidative Resistance Elastomers Based on a Structure Recovery Strategy. Macromol. Rapid Commun. 2021, 42, 2000762–2000768. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, D.; Xu, Z.; Zhang, L.; Liu, L.; Wen, S. High Barrier Properties against Sulfur Mustard of Graphene Oxide/Butyl Rubber Composites. Compos. Sci. Technol. 2019, 170, 141–147. [Google Scholar] [CrossRef]
- Yu, W.W.; Xu, W.Z.; Wei, Y.C.; Liao, S.; Luo, M.C. Mechanically Robust Elastomers Enabled by a Facile Interfacial Interactions-Driven Sacrificial Network. Macromol. Rapid Commun. 2021, 42, 2100509–2100517. [Google Scholar] [CrossRef]
- Peter, R.; Sreelekshmi, R.V.; Menon, A.R.R. Cetyltrimethyl Ammonium Bromide Modified Kaolin as a Reinforcing Filler for Natural Rubber. J. Polym. Environ. 2016, 26, 39–47. [Google Scholar] [CrossRef]
- Sreelekshmi, R.V.; Sudha, J.D.; Menon, A.R.R. Novel Organomodified Kaolin/Silica Hybrid Fillers in Natural Rubber and Its Blend with Polybutadiene Rubber. Polym. Bull. 2016, 74, 783–801. [Google Scholar] [CrossRef]
- Raji Vijay, V.; Anitha, A.M.; Ravindranatha Menon, A.R. Studies on Blends of Natural Rubber and Butadiene Rubber Containing Silica-Organomodified Kaolin Hybrid Filler Systems. Polymer 2016, 89, 135–142. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Guo, X.; Yi, M.; Wan, L.; Liao, S.; Wang, Z.; Fang, L. Xanthate-Modified NanoTiO2 as a Novel Vulcanization Accelerator Enhancing Mechanical and Antibacterial Properties of Natural Rubber. Nanotechnol. Rev. 2021, 10, 478–487. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, Y.; Wang, H.; Guo, X.; Liao, S.; Wang, Z.; Fang, L. Xanthate-Modified Silica as a Novel Multifunctional Additive for Properties Improvement of Natural Rubber. Compos. Sci. Technol. 2021, 203, 108567. [Google Scholar] [CrossRef]
- Liu, G.-X.; Yang, Y.-D.; Zhu, D.; Wei, Y.-C.; Liao, S.; Luo, M. MXene Enabling the Long-Term Superior Thermo-Oxidative Resistance for Elastomers. Polymers 2021, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, Y.; Han, D.; Zheng, J.; Wu, X.; Wang, Z.; Li, X.; Ye, X.; Zhang, L. New Designed Coupling Agents for Silica Used in Green Tires with Low VOCs and Low Rolling Resistance. Appl. Surf. Sci. 2021, 558, 149819–149828. [Google Scholar] [CrossRef]
- Han, T.; Nagarajan, S.; Zhao, H.; Sun, C.; Wen, S.; Zhao, S.; Zhao, S.; Zhang, L. Novel Reinforcement Behavior in Nanofilled Natural Rubber (NR)/Butadiene-Acrylonitrile Rubber (NBR) Blends: Filling-Polymer Network and Supernanosphere. Polymer 2020, 186, 122005–122013. [Google Scholar] [CrossRef]
- Dileep, P.; Narayanankutty, S.K. Styrenated Phenol Modified Nanosilica for Improved Thermo-Oxidative and Mechanical Properties of Natural Rubber. Polym. Test. 2020, 82, 106302–106310. [Google Scholar]
- Ojogbo, E.; Tzoganakis, C.; Mekonnen, T.H. Effect of Extrusion, Batch-Mixing, and Co-Coagulation on the Dispersion of CNCs in Natural Rubber-CNC Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106580–106589. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, M.; Liu, C.; Xue, B.; Yu, L.; Zhu, Y.; Wang, X. Surface Modification of Rice Husk Ash by Ethanol-Assisted Milling to Reinforce the Properties of Natural Rubber/Butadiene Rubber Composites. Chem. Res. Chin. Univ. 2021, 37, 757–762. [Google Scholar] [CrossRef]
- Ghorai, S.; Bhunia, S.; Roy, M.; De, D. Mechanochemical Devulcanization of Natural Rubber Vulcanizate by Dual Function Disulfide Chemicals. Polym. Degrad. Stabil. 2016, 129, 34–46. [Google Scholar] [CrossRef]
- Zedler, Ł.; Przybysz, M.; Klein, M.; Saeb, M.R.; Formela, K. Processing, Physico-Mechanical and Thermal Properties of Reclaimed GTR and NBR/Reclaimed GTR Blends as Function of Various Additives. Polym. Degrad. Stabil. 2017, 143, 186–195. [Google Scholar] [CrossRef]
- Najipoor, M.; Haroonabadi, L.; Dashti, A. Assessment of Failures of Nitrile Rubber Vulcanizates in Rapid Gas Decompression (RGD) Testing: Effect of Physico-Mechanical Properties. Polym. Test. 2018, 72, 377–385. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Quan, X.-Y.; Wang, H.-R.; Liao, S.; Luo, M.-C. Toward Mechanically Robust Crosslinked Elastomers through Phase Transfer Agent Tuning the Solubility of Zn2+ in the Organic Phase. Polymers 2022, 14, 1234. https://doi.org/10.3390/polym14061234
Liu S, Quan X-Y, Wang H-R, Liao S, Luo M-C. Toward Mechanically Robust Crosslinked Elastomers through Phase Transfer Agent Tuning the Solubility of Zn2+ in the Organic Phase. Polymers. 2022; 14(6):1234. https://doi.org/10.3390/polym14061234
Chicago/Turabian StyleLiu, Shuang, Xin-Yao Quan, Hao-Ran Wang, Shuangquan Liao, and Ming-Chao Luo. 2022. "Toward Mechanically Robust Crosslinked Elastomers through Phase Transfer Agent Tuning the Solubility of Zn2+ in the Organic Phase" Polymers 14, no. 6: 1234. https://doi.org/10.3390/polym14061234
APA StyleLiu, S., Quan, X.-Y., Wang, H.-R., Liao, S., & Luo, M.-C. (2022). Toward Mechanically Robust Crosslinked Elastomers through Phase Transfer Agent Tuning the Solubility of Zn2+ in the Organic Phase. Polymers, 14(6), 1234. https://doi.org/10.3390/polym14061234




