Impact of Drying Regimes and Different Coating Layers on Carboxymethyl Cellulose Cross-Linked with Citric Acid on Cotton Thread Fibers for Wound Dressing Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of CMC and Citric Acid Coating Solution
2.3. Design of Coating Processes
2.4. Determination of Physical Characteristic of Uncoated and Coated Cotton Threads
2.5. Evaluation of Mechanical Properties
2.6. Determination of Water Absorption and Moisture Content
2.6.1. Water Absorption
2.6.2. Moisture Content
2.7. Surface Analysis
2.7.1. Surface Morphology
2.7.2. Attenuated Total Reflection–Fourier-Transform Infrared Spectroscopy (ATR–FTIR)
2.8. Antibacterial Analysis
2.9. Statistical Analysis
3. Results and Discussion
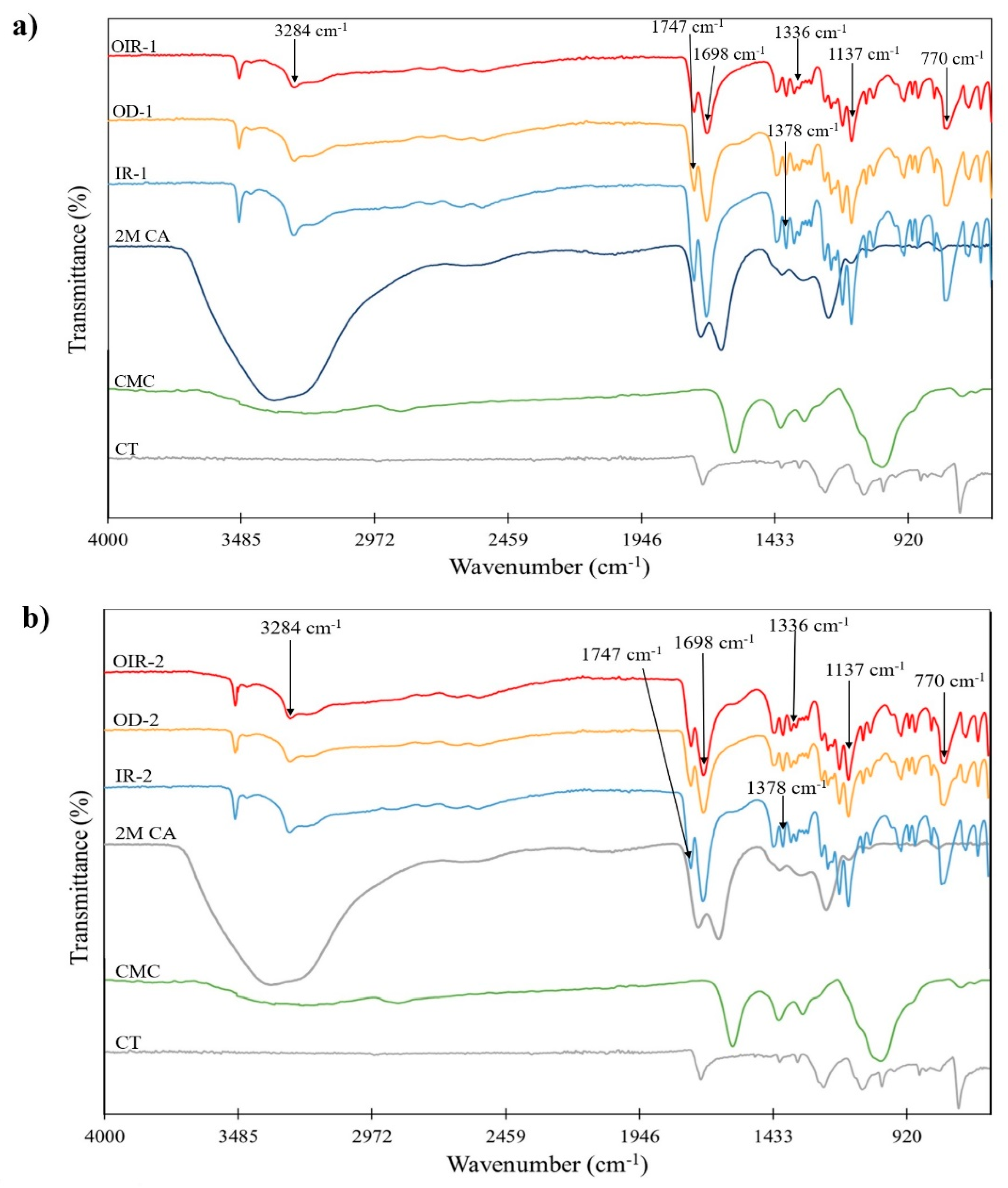
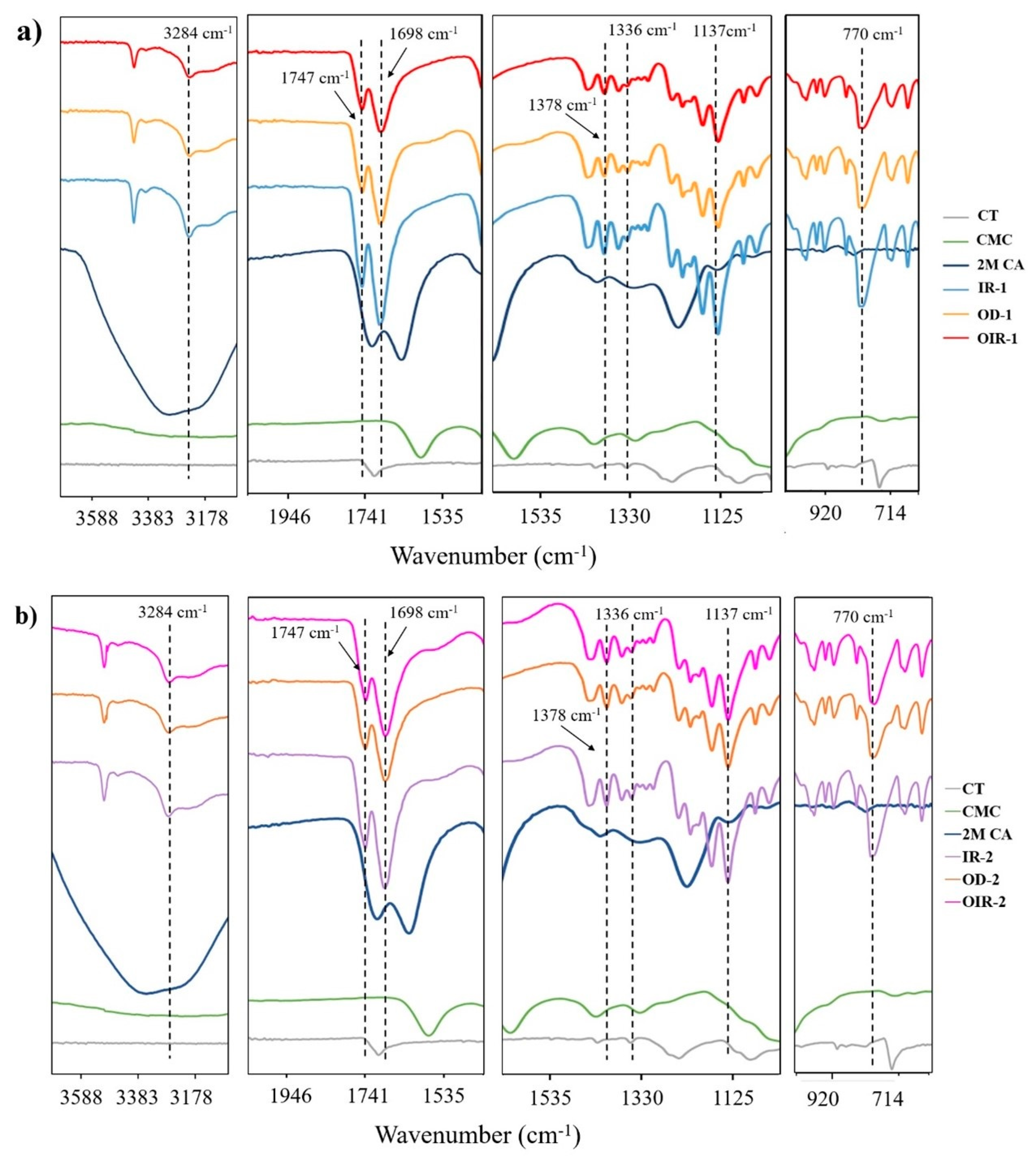
3.1. ATR–FTIR Characterization of Uncoated CT and CT Coated with CMC Cross-Linked with CA (CT/CMC + CA)
3.2. Physical Properties of Uncoated CT and Coated CT/CMC + CA
3.2.1. Basis Weight and Thickness
3.2.2. Moisture Content
3.3. Surface Morphology of Uncoated CT and Coated CT/CMC + CA
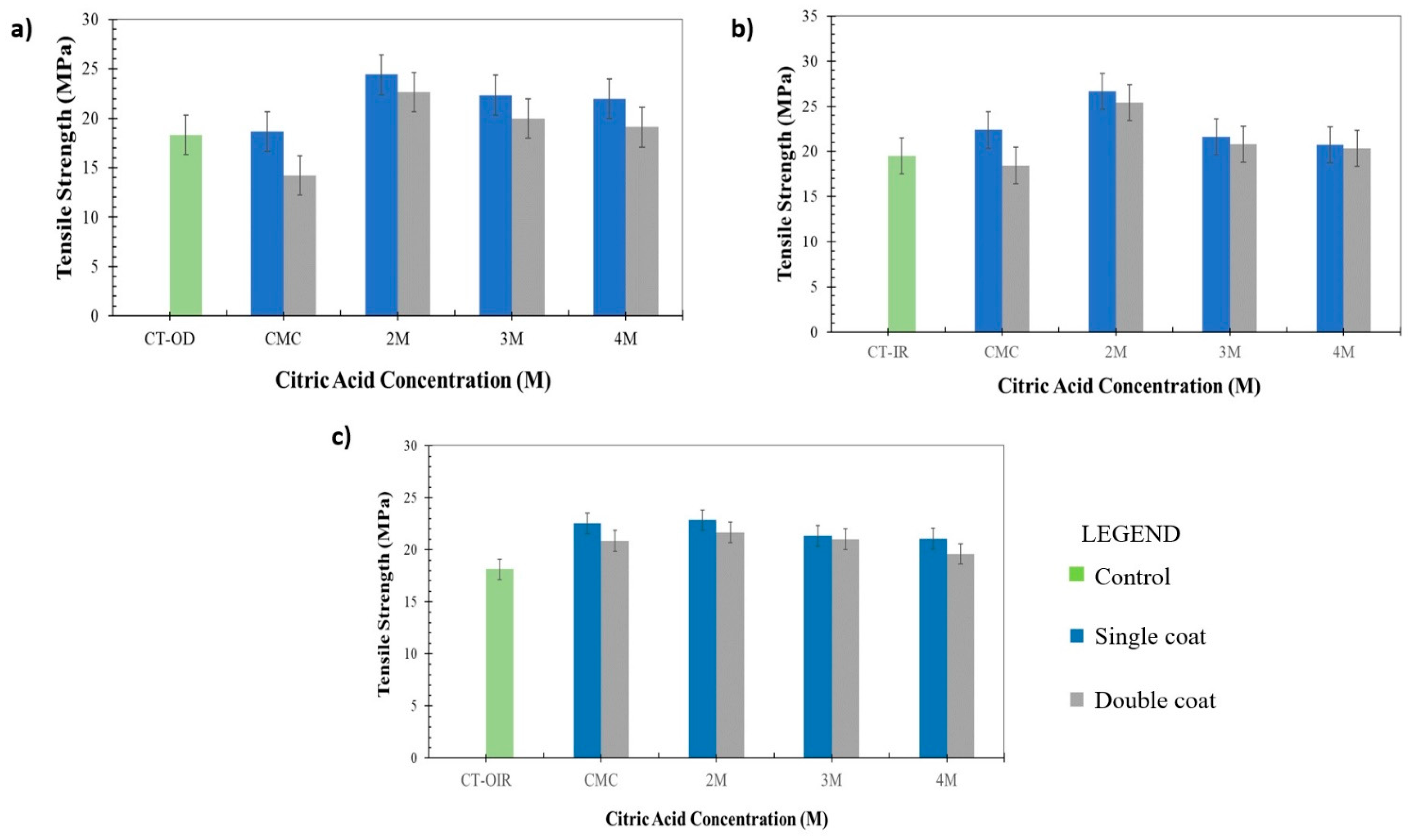
3.4. Mechanical Properties of Uncoated CT and Coated CT/CMC + CA
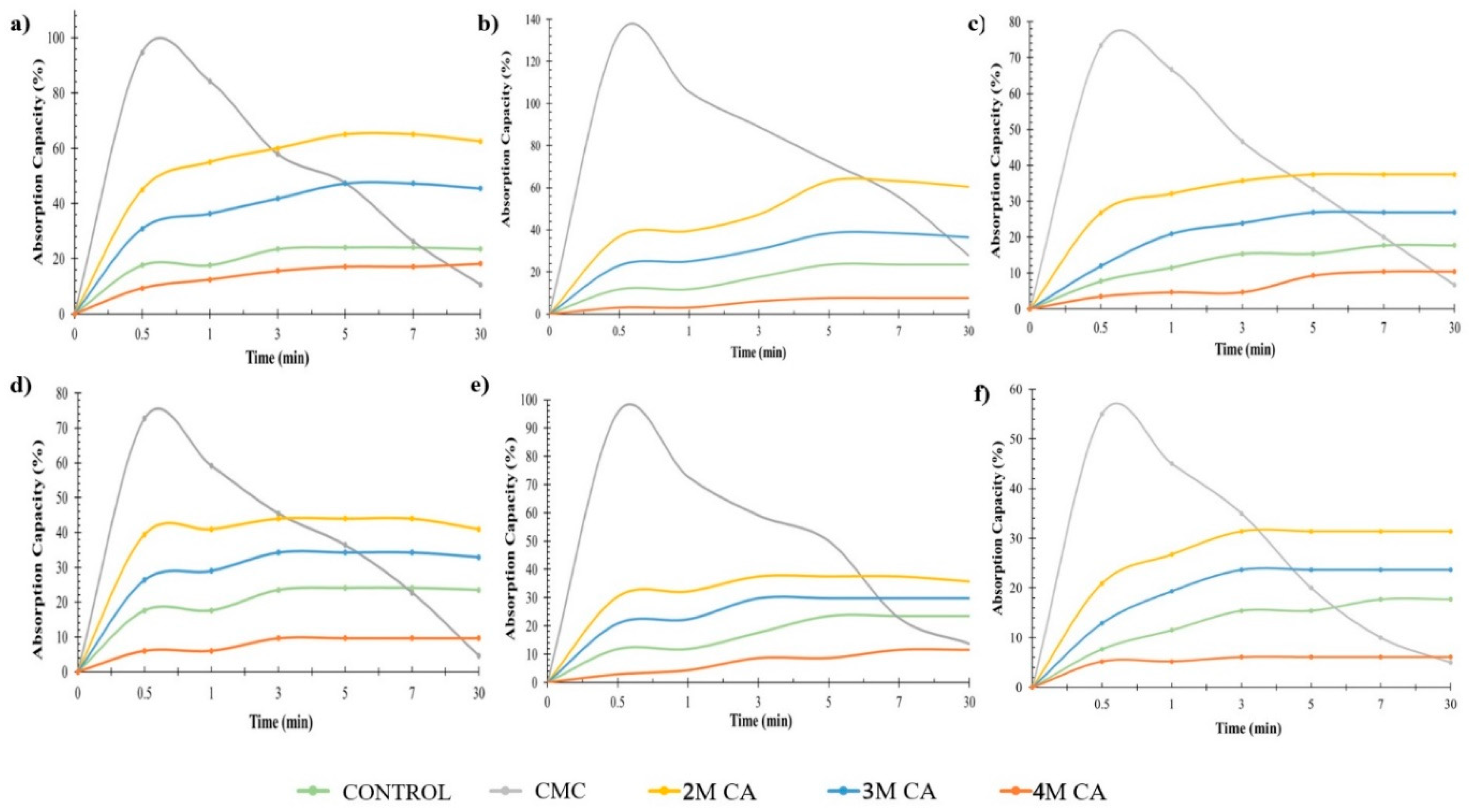
3.5. Effects of Drying and Coating Layers on Water Absorption
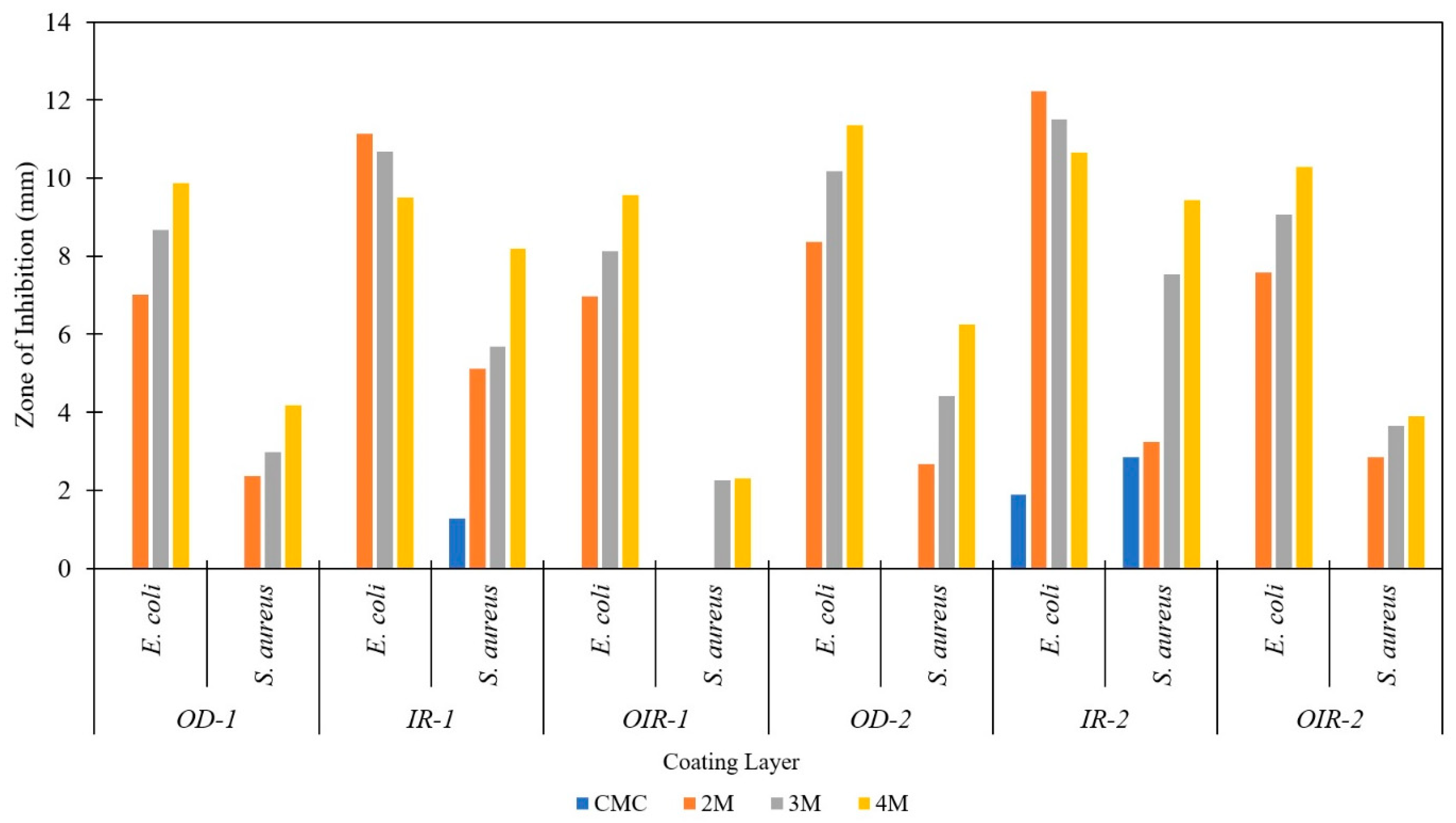
3.6. Antibacterial Activity of Coated CT
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gadkari, R.R.; Ali, S.W.; Joshi, M.; Rajendran, S.; Das, A.; Alagirusamy, R. Leveraging Antibacterial Efficacy of Silver Loaded Chitosan Nanoparticles on Layer-by-Layer Self-Assembled Coated Cotton Fabric. Int. J. Biol. Macromol. 2020, 162, 548–560. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Khattab, T.A.; Abdelrahman, M.S.; Aldalbahi, A.; Hatshan, M.R. Development of antimicrobial, UV blocked and photocatalytic self-cleanable cotton fibers decorated with silver nanoparticles using silver carbamate and plasma activation. Cellulose 2021, 28, 1105–1121. [Google Scholar] [CrossRef]
- Li, Y.D.; Li, W.Y.; Chai, H.H.; Fang, C.; Kang, Y.J.; Li, C.M.; Yu, L. Chitosan functionalization to prolong stable hydrophilicity of cotton thread for thread-based analytical device application. Cellulose 2018, 25, 4831–4840. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Wang, C.; Hu, W.; Chen, Z. Cotton thread modified with ionic liquid copolymerized polymer for online in-tube solid-phase microextraction and HPLC analysis of nonsteroidal anti-inflammatory drugs. J. Sep. Sci. 2020, 43, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Montaser, A.S.; Rehan, M. Designing strategy for coating cotton gauze fabrics and its application in wound healing. Carbohydr. Polym. 2020, 244, 116479. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Vinklárková, L.; Masteiková, R.; Foltýnová, G. Film wound dressing with local anesthetic based on insoluble carboxymethycellulose matrix. J. Appl. Biomed. 2017, 15, 313–320. [Google Scholar] [CrossRef]
- Basu, P.; Narendrakumar, U.; Arunachalam, R.; Devi, S.; Manjubala, I. Characterization and Evaluation of Carboxymethyl Cellulose-Based Films for Healing of Full-Thickness Wounds in Normal and Diabetic Rats. ACS Omega 2018, 3, 12622–12632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.W.; Ramli, N.A. Carboxymethylcellulose film for bacterial wound infection control and healing. Carbohydr. Polym. 2014, 112, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Kaco, H. Superabsorbent hydrogel from oil palm empty fruit bunch cellulose and sodium carboxymethylcellulose. Int. J. Biol. Macromol. 2019, 131, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Salleh, K.M.; Armir, N.A.Z.; Mazlan, N.S.N.; Wang, C.; Zakaria, S. Cellulose and Its Derivatives in Textiles: Primitive Application to Current Trend; Semantic Scholar: Seattle, WA, USA, 2021; ISBN 9780128214831. [Google Scholar]
- Nangare, S.; Vispute, Y.; Tade, R.; Dugam, S.; Patil, P. Pharmaceutical Applications of Citric Acid. Future J. Pharm. 2021, 7, 1–23. [Google Scholar] [CrossRef]
- Nirmaan, A.M.C.; Rohitha Prasantha, B.D.; Peiris, B.L. Comparison of Microwave Drying and Oven-Drying Techniques for Moisture Determination of Three Paddy (Oryza sativa L.) Varieties. Chem. Biol. Technol. Agric. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Park, Y.W. Chapter 3 Moisture and Water Activity. In Handbook of Processed Meats and Poultry Analysis; Nollet, L., Toldra, F., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 35–67. [Google Scholar]
- Rihana, H.; Lubna; Shravya, K.; Jhansi, A.; Tasleem, P.; Vellanki, B. Comparative Study of Tray Dryer, Infrared Dryer and Hot Air Oven for Drying of Chickpeas. Int. J. Food Sci. Nutr. 2019, 4, 90–92. [Google Scholar]
- Mazandarani, Z.; Mirsaeidghazi, N.; Kaviani, M.; Shariati, M.A. Drying of Agriculture Products Using Hot Air Oven and Microwave Method. Indian J. Res. Pharm. Biotechnol. 2014, 5674, 1522–1523. [Google Scholar]
- Lyu, J.; Chen, Q.; Bi, J.; Zeng, M.; Wu, X. Drying Characteristics and Quality of Kiwifruit Slices with/without Osmotic Dehydration under Short-and Medium-Wave Infrared Radiation Drying. Int. J. Food Eng. 2017, 13, 1–15. [Google Scholar] [CrossRef]
- Wu, B.; Pan, Z.; Xu, B.; Bai, J.; El-Mashad, H.M.; Wang, B.; Zhou, C.; Ma, H. Drying Performance and Product Quality of Sliced Carrots by Infrared Blanching Followed by Different Drying Methods. Int. J. Food Eng. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Aboud, S.A.; Altemimi, A.B.; Al-HiIphy, A.R.S.; Yi-Chen, L.; Cacciola, F. A Comprehensive Review on Infrared Heating Applications in Food Processing. Molecules 2019, 24, 4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz Celma, A.; Rojas, S.; Lopez-Rodríguez, F. Mathematical Modelling of Thin-Layer Infrared Drying of Wet Olive Husk. Chem. Eng. Process. Process Intensif. 2008, 47, 1810–1818. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.J.; Gao, J.; Wei, Z.; Zhou, J.; Chen, Y.M. Facile Fabrication of Self-Healing Carboxymethyl Cellulose Hydrogels. Eur. Polym. J. 2015, 72, 514–522. [Google Scholar] [CrossRef]
- Uranga, J.; Nguyen, B.T.; Si, T.T.; Guerrero, P.; De la Caba, K. The Effect of Cross-Linking with Citric Acid on the Properties of Agar/Fish Gelatin Films. Polymers 2020, 12, 291. [Google Scholar] [CrossRef] [Green Version]
- Capanema, N.S.V.; Mansur, A.A.P.; Mansur, H.S.; de Jesus, A.C.; Carvalho, S.M.; Chagas, P.; de Oliveira, L.C. Eco-Friendly and Biocompatible Cross-Linked Carboxymethylcellulose Hydrogels as Adsorbents for the Removal of Organic Dye Pollutants for Environmental Applications. Environ. Technol. 2017, 39, 2856–2872. [Google Scholar] [CrossRef] [PubMed]
- Syafiuddin, A.; Fulazzaky, M.A.; Salmiati, S.; Roestamy, M.; Fulazzaky, M.; Sumeru, K.; Yusop, Z. Sticky Silver Nanoparticles and Surface Coatings of Different Textile Fabrics Stabilised by Muntingia Calabura Leaf Extract. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, H.; Naseri, M.; Shanmugam, K.; Dehghani, M.; Browne, C.; Miri, S.; Garnier, G.; Batchelor, W. An Energy Efficient Production of High Moisture Barrier Nanocellulose/Carboxymethyl Cellulose Films via Spray-Deposition Technique. Carbohydr. Polym. 2020, 250, 116911. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, P. Surface Coating Modified Polyglycolide (PGA) Braided Threads as Potential Thread-Embedding Materials. Fibers Polym. 2020, 21, 2401–2406. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of Citric Acid Induced Crosslinking on the Structure and Properties of Potato Starch/Chitosan Composite Films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J.; Mali, K.K.; Pargaonkar, S.S.; Shinde, P.V.; Dhane, N.S. Citric Acid Crosslinked Carboxymethylcellulose-Poly(Ethylene Glycol) Hydrogel Films for Delivery of Poorly Soluble Drugs. Int. J. Biol. Macromol. 2018, 118, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Seligra, P.G.; Medina Jaramillo, C.; Famá, L.; Goyanes, S. Biodegradable and Non-Retrogradable Eco-Films Based on Starch-Glycerol with Citric Acid as Crosslinking Agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Kanafi, N.M.; Rahman, N.A.; Rosdi, N.H. Citric Acid Cross-Linking of Highly Porous Carboxymethyl Cellulose/Poly(Ethylene Oxide) Composite Hydrogel Films for Controlled Release Applications. Mater. Today Proc. 2019, 7, 721–731. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Anaya, N.M.; Schifman, L.A. Fourier Transform Infrared Spectroscopy to Assess Molecular-Level Changes in Microorganisms Exposed to Nanoparticles. Nanotechnol. Environ. Eng. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem 2006, 10815–10837. [Google Scholar] [CrossRef]
- Hu, Z.; Xiang, Z.; Song, T.; Lu, F. Effects of Crosslinking Degree on the Coating Properties of Arabinoxylan. BioResources 2019, 14, 70–86. [Google Scholar] [CrossRef]
- Kimna, C.; Tamburaci, S.; Tihminlioglu, F. Novel Zein-Based Multilayer Wound Dressing Membranes with Controlled Release of Gentamicin. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2018, 107, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Kurtz, I. Quantitative Interrelationship between Gibbs-Donnan Equilibrium, Osmolality of Body Fluid Compartments, and Plasma Water Sodium Concentration. J. Appl. Physiol. 2006, 100, 1293–1300. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Gan, S.; Baharin, K.W.; Ibrahim, N.A.; Zamzamin, R. Interconnected Macropores Cryogel with Nano-Thin Crosslinked Network Regenerated Cellulose. Int. J. Biol. Macromol. 2020, 148, 11–19. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhi, X.; Du, B.; Yuan, S. Preparation of Elastic and Antibacterial Chitosan-Citric Membranes with High Oxygen Barrier Ability by in Situ Cross-Linking. ACS Omega 2020, 5, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, K.; Anandalakshmi, R. Fabrication, Characterization and Drug Loading Efficiency of Citric Acid Crosslinked NaCMC-HPMC Hydrogel Films for Wound Healing Drug Delivery Applications. Int. J. Biol. Macromol. 2019, 134, 815–829. [Google Scholar] [CrossRef]
- Kamble, Z.; Behera, B.K. Mechanical Properties and Water Absorption Characteristics of Composites Reinforced with Cotton Fibres Recovered from Textile Waste. J. Eng. Fibers Fabr. 2020, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Antioxidant and Antibacterial Polyelectrolyte Wound Dressing Based on Chitosan/Hyaluronan/Phosphatidylcholine Dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Valachová, K.; Hassan, M.A.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Chitosan/Hyaluronan/Edaravone Membranes for Anti-Inflammatory Wound Dressing: In Vitro and in Vivo Evaluation Studies. Mater. Sci. Eng. C 2018, 90, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Radwan, H.A.; Abdelaal, S.A.; Al-radadi, N.S.; Ahmed, M.K.; Shoueir, K.; Hady, M.A. Polycaprolactone Based Electrospun Matrices Loaded with Ag/Hydroxyapatite as Wound Dressings: Morphology, Cell Adhesion and Antibacterial Activity. Int. J. Pharm. 2021, 596, 1–30. [Google Scholar] [CrossRef]
- Huang, X.; Fu, Q.; Deng, Y.; Wang, F.; Xia, B.; Chen, Z.; Chen, G. Surface Roughness of Silk Fibroin/Alginate Microspheres for Rapid Hemostasis in Vitro and in Vivo. Carbohydr. Polym. 2021, 253, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, E.; Niemelä, E.; Venu, A.P.; Kummala, R.; Ihalainen, P.; Toivakka, M.; Eriksson, J.; Peltonen, J. Human Dermal Fibroblast Proliferation Controlled by Surface Roughness of Two-Component Nanostructured Latex Polymer Coatings. Colloids Surf. B Biointerfaces 2019, 174, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, N.S.N.; Zakaria, S.; Hu, C.C.; Gan, S. Effect of Coagulant and Drying Methods on Regenerated Cellulose Membrane. AIP Conf. Proc. 2019, 2111, 030007. [Google Scholar] [CrossRef]
- Ozsoy, I.; Demirkol, A.; Mimaroglu, A.; Unal, H.; Demir, Z. The Influence of Micro- And Nano-Filler Content on the Mechanical Properties of Epoxy Composites. Stroj. Vestn./J. Mech. Eng. 2015, 61, 601–609. [Google Scholar] [CrossRef]
- Liang, J.; Wang, R.; Chen, R. The Impact of Cross-Linking Mode on the Physical and Antimicrobial Properties of a Chitosan/Bacterial Cellulose Composite. Polymers 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathangadeera, R.W.; Hequet, E.F.; Kelly, B.; Dever, J.K.; Kelly, C.M. Importance of Cotton Fiber Elongation in Fiber Processing. Ind. Crops Prod. 2020, 147, 112217. [Google Scholar] [CrossRef]
- Dalfi, H.K.; Tausif, M.; Yousaf, Z. Effect of Twist Level on the Mechanical Performance of S-Glass Yarns and Non-Crimp Cross-Ply Composites. J. Ind. Text. 2021, 1528083720987206. [Google Scholar] [CrossRef]
- Tania, I.S.; Ali, M.; Islam, Z. Solaiman Development of Antimicrobial Activity and Mechanical Performances of Cotton Fabric Treated with Silver Nano Particles (AgNPs). AIP Conf. Proc. 2019, 2121, 150003. [Google Scholar] [CrossRef]
- Liang, J.Z.; Wang, F. Tensile Properties of Polyformaldehyde Blends and Nanocomposites. J. Polym. Eng. 2015, 35, 417–422. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Waldron, K.W.; Wilson, D.R.; Cunha, A.P.; Brito, E.S.d.; Rodrigues, T.H.S.; Rosa, M.F.; Azeredo, H.M.C. Wheat Straw Hemicelluloses Added with Cellulose Nanocrystals and Citric Acid. Effect on Film Physical Properties. Carbohydr. Polym. 2017, 164, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Sivaranjana, P.; Nagarajan, E.R.; Rajini, N.; Jawaid, M.; Rajulu, A.V. Cellulose Nanocomposite Films with in Situ Generated Silver Nanoparticles Using Cassia Alata Leaf Extract as a Reducing Agent. Int. J. Biol. Macromol. 2017, 99, 223–232. [Google Scholar] [CrossRef]
- Kopacic, S.; Walzl, A.; Hirn, U.; Zankel, A.; Kniely, R.; Leitner, E.; Bauer, W. Application of Industrially Produced Chitosan in the Surface Treatment of Fibre-Based Material: Effect of Drying Method and Number of Coating Layers on Mechanical and Barrier Properties. Polymers 2018, 10, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.S.; Jawaid, M.; Naveen, J. Void Content, Tensile, Vibration and Acoustic Properties of Kenaf/Bamboo Fiber Reinforced Epoxy Hybrid Composites. Materials 2019, 12, 2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Chia, C.H.; Jaafar, S.N.S.; Amran, U.A. Chemically Crosslinked Hydrogel and Its Driving Force towards Superabsorbent Behaviour. Int. J. Biol. Macromol. 2018, 118, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Plotka, M.; Kapusta, M.; Dorawa, S.; Kaczorowska, A.K.; Kaczorowski, T. Ts2631 Endolysin from the Extremophilic Thermus Scotoductus Bacteriophage VB_Tsc2631 as an Antimicrobial Agent against Gram-Negative Multidrug-Resistant Bacteria. Viruses 2019, 11, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliuz, E.A.E. Antimicrobial Activity of Citric Acid against Escherichia Coli, Staphylococcus Aureus and Candida Albicans as a Sanitizer Agent. Eurasian J. For. Sci. 2020, 8, 295–301. [Google Scholar] [CrossRef]
- Al-Rousan, W.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Ajo, R.Y.; Holley, R.A. Use of Acetic and Citric Acids to Inhibit Escherichia Coli O157:H7, Salmonella Typhimurium and Staphylococcus Aureus in Tabbouleh Salad. Food Microbiol. 2018, 73, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shah, Z.H.; Riaz, S.; Ahmad, N.; Islam, S.; Raza, M.A.; Naseem, S. Antimicrobial Activity of Citric Acid Functionalized Iron Oxide Nanoparticles –Superparamagnetic Effect. Ceram. Int. 2020, 46, 10942–10951. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Yoon, S.; Choi, T.; Kim, H.J.; Park, K.J.; Koo, M. The Bactericidal Effect of a Combination of Food-Grade Compounds and Their Application as Alternative Antibacterial Agents for Food Contact Surfaces. Foods 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Drying Techniques | Cost-Effective | Drying Time | Product Quality | Mechanism | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| Oven/ convective | Low | Long. Depends on hot air temperature and air velocity | Depends on the parameter of drying | Moisture exchange between sample and the hot air flow through drying chamber | Long shelf life, simple and easy operation | Exposure to oxidation, crust formation of sample surface due to high temperature | [20,21] |
| Infrared | Low | Short. Increases with air velocity and decreases with IR intensity | High | Sample is exposed to electromagnetic radiation where heat is transferred from heat source to product surface | Environmentally friendly, provides heat homogeneity, short heating time and low energy consumption, has high accuracy, and increases manufacturing efficiency | Exposure to high heat leads to burns; the penetration depth is limited. Prolonged exposure causes tissues rupture. Infrared is not sensitive to reflective properties. | [18,20,22] |
| Microwave | Low | Reduces drying time | High | Volumetric heating occurs when electromagnetic waves pass through sample, leading to molecule oscillation that generates thermal energy to remove water | Volumetric heating spread through sample reduces drying time | Leads to damaged product because of improper heat control and mass transfer | [21] |
| Freezing | High | Slow | Low | Two steps: (1) Freezing water molecules in sample (2) Frozen solid samples are heated to induce moisture sublimation | Prevents oxidation, minimal shrinkage and soluble solid shift, volatile compound retention, maintaining porous structure | High facility cost. Could cause major loss of aromatic components | [22,23,24] |
| Samples | Coating Layer | Drying Method |
|---|---|---|
| CT-A | 0 | Oven |
| CT-B | IR | |
| CT-C | Oven + IR | |
| OD-1 | 1 | Oven |
| IR-1 | IR | |
| OIR-1 | Oven + IR | |
| OD-2 | 2 | Oven |
| IR-2 | IR | |
| OIR-2 | Oven + IR |
| Samples | Coating Layers | CA Concentration (M) | Drying Regime | Basis Weight (UCS) (mg) | Basis Weight (CS) (mg) | Average Thickness (µm) |
|---|---|---|---|---|---|---|
| CT-A | 0 | n.a. | Oven | 3.4 ± 0.2 | - | 112.5 ± 1.1 |
| CT-B | n.a. | IR | 3.3 ± 0.2 | - | 112.8 ± 1.0 | |
| CT-C | n.a. | Oven + IR | 3.2 ± 0.3 | - | 106.5 ± 1.3 | |
| OD-1 | 1 | CMC | Oven | 3.2 ± 0.2 | 3.5 ± 0.1 | 127.8 ± 1.1 |
| 2 | 3.3 ± 0.1 | 4.7 ± 0.3 | 183.5 ± 1.2 | |||
| 3 | 3.2 ± 0.2 | 5.1 ± 0.3 | 249.0 ± 1.0 | |||
| 4 | 3.4 ± 0.1 | 6.0 ± 0.2 | 277.0 ± 1.1 | |||
| IR-1 | 1 | CMC | IR | 3.1 ± 0.2 | 3.6 ± 0.3 | 125.3 ± 1.2 |
| 2 | 3.2 ± 0.1 | 4.9 ± 0.6 | 178.5 ± 1.0 | |||
| 3 | 3.0 ± 0.3 | 5.9 ± 0.6 | 182.3 ± 1.0 | |||
| 4 | 3.3 ± 0.1 | 6.7 ± 0.4 | 226.0 ± 1.1 | |||
| OIR-1 | 1 | CMC | Oven + IR | 3.3 ± 0.1 | 3.5 ± 0.3 | 247.8 ± 1.2 |
| 2 | 3.0 ± 0.2 | 3.9 ± 0.3 | 336.0 ± 1.2 | |||
| 3 | 3.2 ± 0.1 | 4.3 ± 0.1 | 362.5 ± 1.3 | |||
| 4 | 3.0 ± 0.3 | 4.8 ± 0.4 | 444.8 ± 1.3 | |||
| OD-2 | 2 | CMC | Oven | 3.4 ± 0.1 | 6.3 ± 0.2 | 136.5 ± 1.1 |
| 2 | 3.4 ± 0.1 | 10.0 ± 0.6 | 256.1 ± 1.3 | |||
| 3 | 3.5 ± 0.1 | 11.7 ± 0.1 | 267.8 ± 1.4 | |||
| 4 | 3.4 ± 0.1 | 14.4 ± 0.1 | 309.0 ± 1.8 | |||
| IR-2 | 2 | CMC | IR | 3.2 ± 0.1 | 6.5 ± 0.2 | 129.5 ± 1.3 |
| 2 | 3.1 ± 0.2 | 10.8 ± 0.1 | 241.7 ± 1.5 | |||
| 3 | 3.3 ± 0.1 | 12.1 ± 0.2 | 283.8 ± 1.1 | |||
| 4 | 3.3 ± 0.1 | 18.1 ± 0.2 | 286.8 ± 1.1 | |||
| OIR-2 | 2 | CMC | Oven + IR | 3.1 ± 0.1 | 6.4 ± 0.2 | 285.8 ± 1.3 |
| 2 | 3.4 ± 0.2 | 9.3 ± 0.1 | 374.0 ± 1.5 | |||
| 3 | 3.0 ± 0.1 | 10.0 ± 0.1 | 381.0 ± 1.1 | |||
| 4 | 3.5 ± 0.2 | 14.4 ± 0.2 | 446.5 ± 1.3 |
| Samples | Coating Layer | (CA) (M) | Drying Regime | Moisture Content (%) | |
|---|---|---|---|---|---|
| Dry | Wet | ||||
| CT-A | 0 | n.a | Oven | 3.08 ± 0.04 | 92.3 ± 0.02 |
| CT-B | n.a | IR | 3.05 ± 0.03 | 100 ± 0.02 | |
| CT-C | n.a | Oven + IR | 3.11 ± 0.03 | 84.6 ± 0.03 | |
| OD-1 | 1 | CMC | Oven | 3.15 ± 0.04 | 88.9 ± 0.06 |
| 2 | 3.43 ± 0.06 | 95.0 ± 0.03 | |||
| 3 | 3.29 ± 0.06 | 45.8 ± 0.04 | |||
| 4 | 3.17 ± 0.03 | 19.0 ± 0.05 | |||
| IR-1 | CMC | IR | 3.14 ± 0.02 | 63.2 ± 0.03 | |
| 2 | 3.39 ± 0.03 | 120.9 ± 0.04 | |||
| 3 | 3.28 ± 0.02 | 70.9 ± 0.02 | |||
| 4 | 3.15 ± 0.04 | 34.1 ± 0.03 | |||
| OIR-1 | CMC | Oven + IR | 3.33 ± 0.06 | 33.3 ± 0.03 | |
| 2 | 3.45 ± 0.04 | 90.3 ± 0.02 | |||
| 3 | 3.38 ± 0.03 | 44.6 ± 0.03 | |||
| 4 | 3.33 ± 0.04 | 18.0 ± 0.06 | |||
| OD-2 | 2 | CMC | Oven | 3.12 ± 0.02 | 19.5 ± 0.02 |
| 2 | 3.38 ± 0.03 | 36.1 ± 0.05 | |||
| 3 | 3.22 ± 0.04 | 32.1 ± 0.06 | |||
| 4 | 3.11 ± 0.03 | 12.5 ± 0.03 | |||
| IR-2 | CMC | IR | 3.09 ± 0.06 | 70.6 ± 0.03 | |
| 2 | 3.31 ± 0.04 | 71.9 ± 0.04 | |||
| 3 | 3.21 ± 0.03 | 50.0 ± 0.05 | |||
| 4 | 3.11 ± 0.06 | 34.0 ± 0.03 | |||
| OIR-2 | CMC | Oven + IR | 3.31 ± 0.04 | 20.0 ± 0.06 | |
| 2 | 3.43 ± 0.03 | 24.4 ± 0.02 | |||
| 3 | 3.37 ± 0.06 | 17.2 ± 0.06 | |||
| 4 | 3.31 ± 0.03 | 7.1 ± 0.05 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairunnisa-Atiqah, M.K.; Salleh, K.M.; Ainul Hafiza, A.H.; Nyak Mazlan, N.S.; Mostapha, M.; Zakaria, S. Impact of Drying Regimes and Different Coating Layers on Carboxymethyl Cellulose Cross-Linked with Citric Acid on Cotton Thread Fibers for Wound Dressing Modification. Polymers 2022, 14, 1217. https://doi.org/10.3390/polym14061217
Khairunnisa-Atiqah MK, Salleh KM, Ainul Hafiza AH, Nyak Mazlan NS, Mostapha M, Zakaria S. Impact of Drying Regimes and Different Coating Layers on Carboxymethyl Cellulose Cross-Linked with Citric Acid on Cotton Thread Fibers for Wound Dressing Modification. Polymers. 2022; 14(6):1217. https://doi.org/10.3390/polym14061217
Chicago/Turabian StyleKhairunnisa-Atiqah, Mohamad Khalid, Kushairi Mohd Salleh, A. H. Ainul Hafiza, Nyak Syazwani Nyak Mazlan, Marhaini Mostapha, and Sarani Zakaria. 2022. "Impact of Drying Regimes and Different Coating Layers on Carboxymethyl Cellulose Cross-Linked with Citric Acid on Cotton Thread Fibers for Wound Dressing Modification" Polymers 14, no. 6: 1217. https://doi.org/10.3390/polym14061217
APA StyleKhairunnisa-Atiqah, M. K., Salleh, K. M., Ainul Hafiza, A. H., Nyak Mazlan, N. S., Mostapha, M., & Zakaria, S. (2022). Impact of Drying Regimes and Different Coating Layers on Carboxymethyl Cellulose Cross-Linked with Citric Acid on Cotton Thread Fibers for Wound Dressing Modification. Polymers, 14(6), 1217. https://doi.org/10.3390/polym14061217







