Characterization and Release Kinetics Study of Active Packaging Films Based on Modified Starch and Red Cabbage Anthocyanin Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Red Cabbage Anthocyanin Extract (RCAE)
2.3. Preparation of Active Films
2.4. Film Characterization
2.4.1. Microstructural Characteristics
2.4.2. Thermogravimetric Analysis
2.4.3. Color Properties and Opacity
2.4.4. Oxygen Permeability (OP)
2.4.5. Moisture Absorption
2.4.6. Antioxidant Activity
2.4.7. Release Behavior
2.4.8. Cytotoxicity Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification and Relative Quantification of RCAE
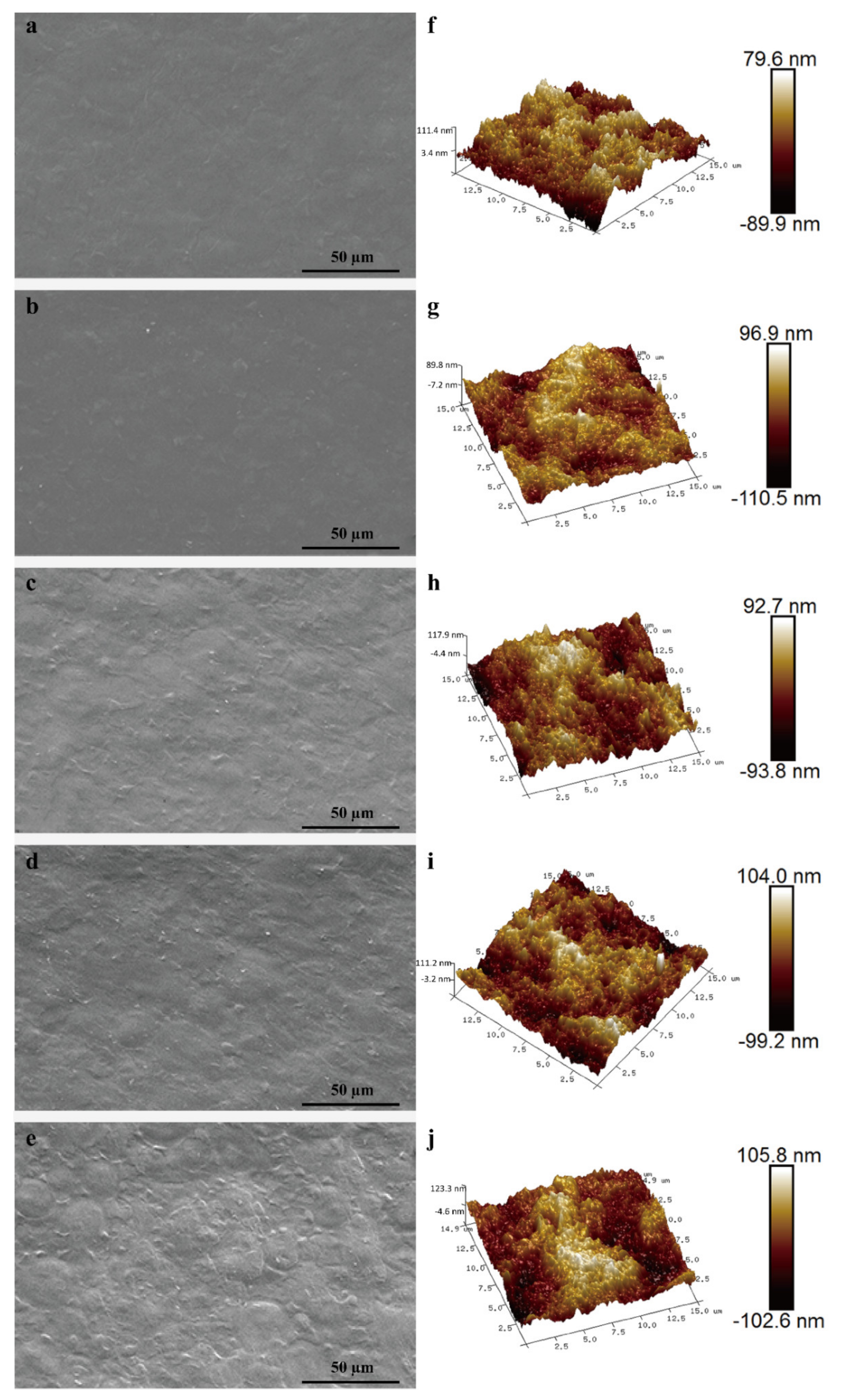
3.2. Morphology and Structure Analysis
3.3. Thermal Analysis
3.4. Color Properties and Opacity
3.5. Oxygen Permeability
3.6. Moisture Absorption
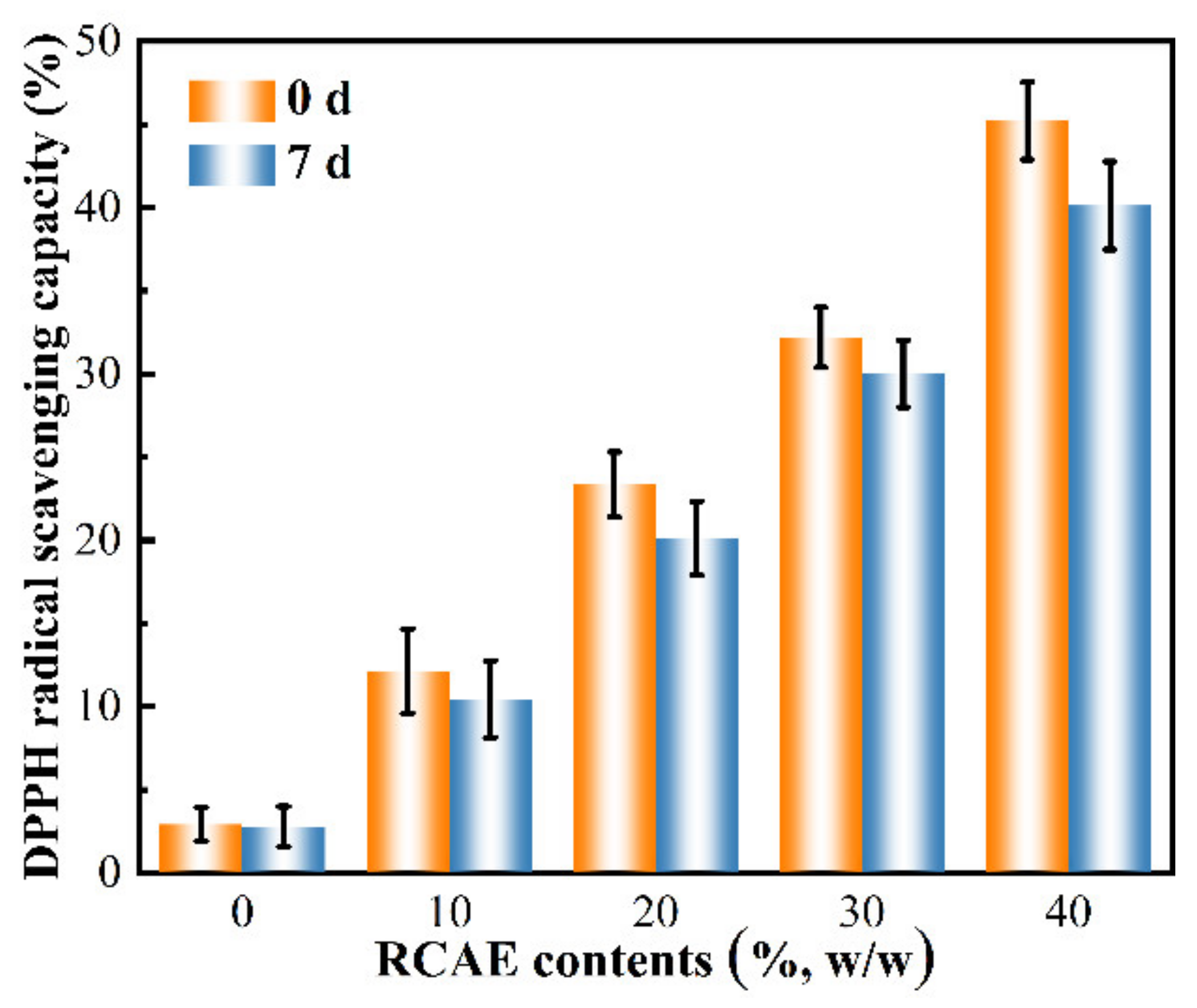
3.7. Antioxidant Activity
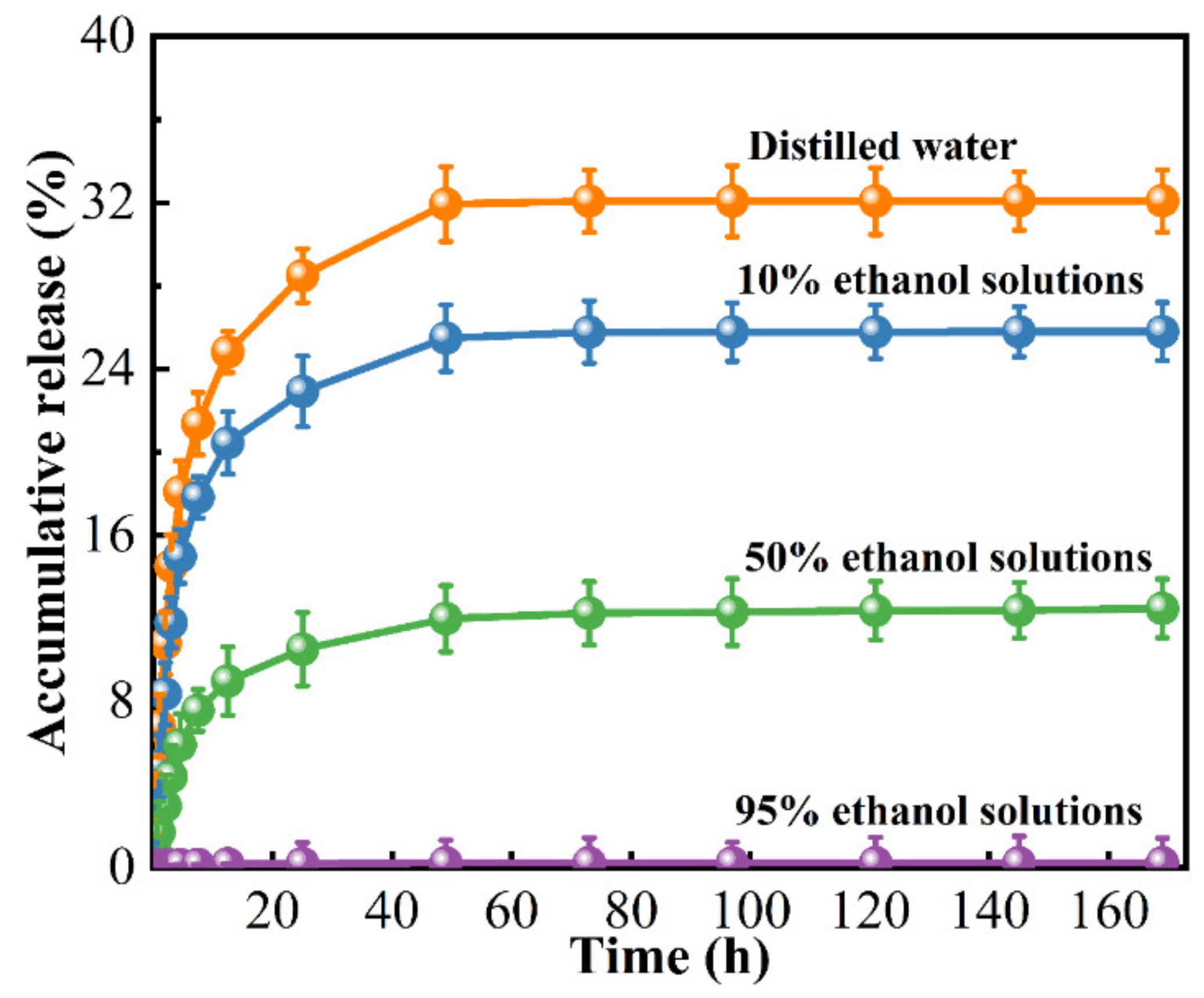
3.8. Release Kinetics
3.9. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, B.U.; Lingayat, S.; Banerjee, A.N.; Kale, R.D. Development of multifunctional food packaging films based on waste Garlic peel extract and Chitosan. Int. J. Biol. Macromol. 2021, 192, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Ciannamea, E.M.; Castillo, L.A.; Barbosa, S.E.; De Angelis, M.G. Barrier properties and mechanical strength of bio-renewable, heat-sealable films based on gelatin, glycerol and soybean oil for sustainable food packaging. React. Funct. Polym. 2018, 125, 29–36. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Alvarez Igarzabal, C.I. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, Y.; Lu, P. Novel active starch films incorporating tea polyphenols-loaded porous starch as food packaging materials. Int. J. Biol. Macromol. 2021, 192, 1123–1133. [Google Scholar] [CrossRef]
- Sooch, B.S.; Mann, M.K. Nanoreinforced biodegradable gelatin based active food packaging film for the enhancement of shelf life of tomatoes (Solanum lycopersicum L.). Food Control 2021, 130, 108322. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, H.; Ma, Q.; Cheng, D.; Zhang, Y.; Wang, W.; Wang, J.; Sun, J. Development of chitosan/potato peel polyphenols nanoparticles driven extended-release antioxidant films based on potato starch. Food Packag. Shelf Life 2022, 31, 100793. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhang, H.; Dong, M.; Li, L.; Zhangsun, H.; Wang, L. Dual-functional intelligent gelatin based packaging film for maintaining and monitoring the shrimp freshness. Food Hydrocoll. 2022, 124, 107258. [Google Scholar] [CrossRef]
- Lu, W.; Chen, M.; Cheng, M.; Yan, X.; Zhang, R.; Kong, R.; Wang, J.; Wang, X. Development of antioxidant and antimicrobial bioactive films based on Oregano essential oil/mesoporous nano-silica/sodium alginate. Food Packag. Shelf Life 2021, 29, 100691. [Google Scholar] [CrossRef]
- Tanwar, R.; Gupta, V.; Kumar, P.; Kumar, A.; Singh, S.; Gaikwad, K.K. Development and characterization of PVA-starch incorporated with coconut shell extract and sepiolite clay as an antioxidant film for active food packaging applications. Int. J. Biol. Macromol. 2021, 185, 451–461. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Hu, Z.; Li, G.; Hu, L.; Chen, X.; Hu, Y. Chitosan-based films with antioxidant of bamboo leaves and ZnO nanoparticles for application in active food packaging. Int. J. Biol. Macromol. 2021, 189, 363–369. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Tavassoli, M.; Salim, S.A.; Azizi-lalabadi, M.; McClements, D.J. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle- and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocoll. 2022, 124, 107324. [Google Scholar] [CrossRef]
- Kadam, A.A.; Singh, S.; Gaikwad, K.K. Chitosan based antioxidant films incorporated with pine needles (Cedrus deodara) extract for active food packaging applications. Food Control 2021, 124, 107877. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempoura, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Y.; Wang, L.; Yin, W.; Liang, J. Antioxidant activity and subcritical water extraction of anthocyanin from raspberry process optimization by response surface methodology. Food Biosci. 2021, 44, 101394. [Google Scholar] [CrossRef]
- Machado, M.H.; Almeida, A.D.R.; Maciel, M.V.D.O.B.; Vitorino, V.B.; Bazzo, G.C.; da Rosa, C.G.; Sganzerla, W.G.; Mendes, C.; Barreto, P.L.M. Microencapsulation by spray drying of red cabbage anthocyanin-rich extract for the production of a natural food colorant. Biocatal. Agric. Biotechnol. 2022, 39, 102287. [Google Scholar] [CrossRef]
- Yao, L.; Xu, J.; Zhang, L.; Zheng, T.; Liu, L.; Zhang, L. Physicochemical stability-increasing effects of anthocyanin via a co-assembly approach with an amphiphilic peptide. Food Chem. 2021, 362, 130101. [Google Scholar] [CrossRef]
- Martinsen, B.K.; Aaby, K.; Skrede, G. Effect of temperature on stability of anthocyanins, ascorbic acid and color in strawberry and raspberry jams. Food Chem. 2020, 316, 126297. [Google Scholar] [CrossRef]
- Quan, W.; He, W.; Qie, X.; Chen, Y.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effects of β-cyclodextrin, whey protein, and soy protein on the thermal and storage stability of anthocyanins obtained from purple-fleshed sweet potatoes. Food Chem. 2020, 320, 126655. [Google Scholar] [CrossRef]
- Wang, S.; Xia, P.; Wang, S.; Liang, J.; Sun, Y.; Yue, P.; Gao, X. Packaging films formulated with gelatin and anthocyanins nanocomplexes: Physical properties, antioxidant activity and its application for olive oil protection. Food Hydrocoll. 2019, 96, 617–624. [Google Scholar] [CrossRef]
- Qin, Y.; Yun, D.; Xu, F.; Chen, D.; Kan, J.; Liu, J. Smart packaging films based on starch/polyvinyl alcohol and Lycium ruthenicum anthocyanins-loaded nano-complexes: Functionality, stability and application. Food Hydrocoll. 2021, 119, 106850. [Google Scholar] [CrossRef]
- Cheng, M.; Cui, Y.; Yan, X.; Zhang, R.; Wang, J.; Wang, X. Effect of dual-modified cassava starches on intelligent packaging films containing red cabbage extracts. Food Hydrocoll. 2022, 124, 107225. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Kan, J.; Liu, J. Preparation and characterization of active and intelligent films based on fish gelatin and haskap berries (Lonicera caerulea L.) extract. Food Packag. Shelf Life 2019, 22, 100417. [Google Scholar] [CrossRef]
- Chen, C.; Zong, L.; Wang, J.; Xie, J. Microfibrillated cellulose reinforced starch/polyvinyl alcohol antimicrobial active films with controlled release behavior of cinnamaldehyde. Carbohydr. Polym. 2021, 272, 118448. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.A.V.; Silva, R.R.A.; de Oliveira, T.V.; Soares, R.R.A.; Junior, N.S.; Moraes, A.R.F.; Pires, A.C.D.S.; Soares, N.F.F. Development and characterization of intelligent cellulose acetate-based films using red cabbage extract for visual detection of volatile bases. LWT Food Sci. Technol. 2020, 132, 109780. [Google Scholar] [CrossRef]
- Su, C.; Zhang, X.; Ge, X.; Shen, H.; Zhang, Q.; Lu, Y.; Sun, X.; Sun, Z.; Li, W. Structural, physical and degradation characteristics of polyvinyl alcohol/esterified mung bean starch/gliadin ternary composite plastic. Ind. Crops Prod. 2022, 176, 114365. [Google Scholar] [CrossRef]
- Gürler, N.; Paşa, S.; Temel, H. Silane doped biodegradable starch-PLA bilayer films for food packaging applications: Mechanical, thermal, barrier and biodegradability properties. J. Taiwan Inst. Chem. Eng. 2021, 123, 261–271. [Google Scholar] [CrossRef]
- Hasheminya, S.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhang, B.; Ge, X.; Jiang, Y. Preparation of self-reinforced starch films for use as hard capsule material. Int. J. Biol. Macromol. 2021, 189, 715–721. [Google Scholar] [CrossRef]
- Prachayawarakorn, J.; Kansanthia, P. Characterization and properties of singly and dually modified hydrogen peroxide oxidized and glutaraldehyde crosslinked biodegradable starch films. Int. J. Biol. Macromol. 2022, 194, 331–337. [Google Scholar] [CrossRef]
- Cheng, M.; Kong, R.; Zhang, R.; Wang, X.; Wang, J.; Chen, M. Effect of glyoxal concentration on the properties of corn starch/poly(vinyl alcohol)/carvacrol nanoemulsion active films. Ind. Crops Prod. 2021, 171, 113864. [Google Scholar] [CrossRef]
- Wu, L.T.; Tsai, I.L.; Ho, Y.C.; Hang, Y.H.; Lin, C.; Tsai, M.L.; Mi, F.L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr. Polym. 2021, 254, 117410. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Li, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Extract from Lycium ruthenicum Murr. Incorporating κ-carrageenan colorimetric film with a wide pH-sensing range for food freshness monitoring. Food Hydrocoll. 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Bi, F.; Qin, Y.; Chen, D.; Kan, J.; Liu, J. Development of active packaging films based on chitosan and nano-encapsulated luteolin. Int. J. Biol. Macromol. 2021, 182, 545–553. [Google Scholar] [CrossRef]
- Campoccia, D.; Ravaioli, S.; Santi, S.; Mariani, V.; Santarcangelo, C.; De Filippis, A.; Montanaro, L.; Arciola, C.R.; Daglia, M. Exploring the anticancer effects of standardized extracts of poplar-type propolis: In vitro cytotoxicity toward cancer and normal cell lines. Biomed. Pharmacother. 2021, 141, 111895. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocya-nins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.; Dias, A.R.G.; Zavareze, E.D.R. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci. Technol. 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Yuan, B.; Cao, Y.; Tang, Q.; Yuan, Z.; Zhou, Y.; McClemenys, D.J.; Cao, C. Enhanced performance and functionality of active edible films by incorporating tea polyphenols into thin calcium alginate hydrogels. Food Hydrocoll. 2019, 97, 105197. [Google Scholar] [CrossRef]
- Vedove, T.M.R.D.; Maniglia, B.C.; Tadini, C.C. Production of sustainable smart packaging based on cassava starch and anthocyanin by an extrusion process. J. Food Eng. 2021, 289, 110274. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, G.; Cao, L.; Wang, L. Accurately intelligent film made from sodium carboxymethyl starch/κ-carrageenan reinforced by mulberry anthocyanins as an indicator. Food Hydrocoll. 2020, 108, 106012. [Google Scholar] [CrossRef]
- Eze, F.N.; Jayeoye, T.J.; Singh, S. Fabrication of intelligent pH-sensing films with antioxidant potential for monitoring shrimp freshness via the fortification of chitosan matrix with broken Riceberry phenolic extract. Food Chem. 2022, 366, 130574. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Alvarez, V.A. Bionanocomposite films developed from corn starch and natural and modified nano-clays with or without added blueberry extract. Food Hydrocoll. 2018, 77, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH sensitive film based on starch/carbon nano dots incorporating anthocyanin for monitoring spoilage of pork. Food Control 2021, 126, 108039. [Google Scholar] [CrossRef]
- Rammak, T.; Boonsuk, P.; Kaewtatip, K. Mechanical and barrier properties of starch blend films enhanced with kaolin for application in food packaging. Int. J. Biol. Macromol. 2021, 192, 1013–1020. [Google Scholar] [CrossRef]
- Kong, R.; Wang, J.; Cheng, M.; Lu, W.; Chen, M.; Zhang, R.; Wang, X. Development and characterization of corn starch/PVA active films incorporated with carvacrol nanoemulsions. Int. J. Biol. Macromol. 2020, 164, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2019, 87, 858–868. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Bai, R.; Zhang, X.; Yong, H.; Wang, X.; Liu, Y.; Liu, J. Development and characterization of antioxidant active packaging and intelligent Al3+-sensing films based on carboxymethyl chitosan and quercetin. Int. J. Biol. Macromol. 2019, 126, 1074–1084. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, G.; Li, J.; Wang, L. A green strategy for maintaining intelligent response and improving antioxidant properties of κ-carrageenan-based film via cork bark extractive addition. Food Hydrocoll. 2021, 113, 106470. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Lei, L.; Zhao, G. Combined effects of octenylsuccination and oregano essential oil on sweet potato starch films with an emphasis on water resistance. Int. J. Biol. Macromol. 2018, 115, 547–553. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhi, C.; Jin, Z.; Miao, M. Improving properties of normal maize starch films using dual-modification: Combination treatment of debranching and hydroxypropylation. Int. J. Biol. Macromol. 2019, 130, 197–202. [Google Scholar] [CrossRef]
- Chi, W.; Cao, L.; Sun, G.; Meng, F.; Zhang, C.; Li, J.; Wang, L. Developing a highly pH-sensitive κ-carrageenan-based intelligent film incorporating grape skin powder via a cleaner process. J. Clean. Prod. 2020, 244, 118862. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Gani, A.; Punoo, H.A.; Masoodi, F.A. Use of pomegranate peel extract incorporated zein film with improved properties for prolonged shelf life of fresh Himalayan cheese (Kalari/kradi). Innov. Food Sci. Emerg. Technol. 2018, 48, 25–32. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Shi, Q.; Zhang, Y.; Liu, J.; Wu, X.; Fang, Z. Development and characterization of active and pH-sensitive films based on psyllium seed gum incorporated with free and microencapsulated mulberry pomace extracts. Food Chem. 2021, 352, 129333. [Google Scholar] [CrossRef]
- Zhao, C.L.; Yu, Y.Q.; Chen, Z.J.; Wen, G.S.; Wei, F.G.; Zheng, Q.; Wang, C.D.; Xiao, X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2016, 214, 119–128. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Singh, S.; Nwabor, O.F.; Syukri, D.M.; Voravuthikunchai, S.P. Chitosan-poly(vinyl alcohol) intelligent films fortified with anthocyanins isolated from Clitoria ternatea and Carissa carandas for monitoring beverage freshness. Int. J. Biol. Macromol. 2021, 182, 1015–1025. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Sun, J.; Lu, Y.; Tong, C.; Wang, L.; Yan, Z.; Pang, J. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging. Food Hydrocoll. 2020, 98, 105245. [Google Scholar] [CrossRef]
- ISO 10993−5; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 1 February 2022).
- Andonegi, M.; Heras, K.L.; Santos-Vizcaíno, E.; Igartua, M.; Hernandez, R.M.; de la Caba, K.; Guerrero, P. Structure-properties relationship of chitosan/collagen films with potential for biomedical applications. Carbohydr. Polym. 2020, 237, 116159. [Google Scholar] [CrossRef]

| Compound | Molecular Formula | [M+H]+ (m/z) | Retention Time (min) | Relative Quantification (ng/mL) | Relative Content (%) |
|---|---|---|---|---|---|
| Cyanidin 3,5- diglucoside | C27H31O16 | 611.16 | 2.66 | 6938.769 ± 23.841 | 53.913 ± 0.185 |
| Cyanidin 3- galactoside | C21H21O11 | 449.11 | 3.89 | 670.568 ± 10.772 | 5.210 ± 0.084 |
| Cyanidin-3-(sinapoyl)(sinapoyl)-diglicoside-5-glicoside | C43H64N2O36 | 1185 | 4.24 | 593.481 ± 11.219 | 4.611 ± 0.087 |
| Delphinidin | C15H11O7 | 303.05 | 4.71 | 4090.924 ± 16.021 | 31.786 ± 0.124 |
| Procyanidin B4 | C30H26O12 | 579.15 | 5.35 | 7.710 ± 0.318 | 0.060 ± 0.002 |
| Cyanidin | C15H11O6 | 287.06 | 5.39 | 7.444 ± 0.521 | 0.058 ± 0.004 |
| Procyanidin B2 | C30H26O12 | 579.15 | 5.46 | 0.720 ± 0.053 | 0.006 ± 0.004 |
| Petunidin | C16H13O7 | 317.07 | 5.57 | 365.524 ± 5.647 | 2.840 ± 0.044 |
| Pelargonidin | C15H11O5 | 271.06 | 6.02 | 0.866 ± 0.524 | 0.007 ± 0.004 |
| Malvidin | C17H15O7 | 331.08 | 6.21 | 4.278 ± 0.306 | 0.033 ± 0.002 |
| Peonidin | C22H23O11 | 301.07 | 6.18 | 67.232 ± 3.499 | 0.522 ± 0.027 |
| Rutin | C27H30O16 | 611.16 | 6.78 | 3.610 ± 0.041 | 0.028 ± 0.001 |
| Luteolin | C15H10O6 | 287.06 | 8.17 | 37.580 ± 2.314 | 0.292 ± 0.018 |
| Quercetin | C15H10O7 | 303.05 | 8.19 | 50.118 ± 2.597 | 0.389 ± 0.020 |
| Isorhamnetin | C16H12O7 | 317.07 | 8.36 | 14.548 ± 1.573 | 0.113 ± 0.012 |
| Kaempferol | C15H10O6 | 287.06 | 8.35 | 16.808 ± 3.112 | 0.131 ± 0.024 |
| Film Type | Ra (nm) | Rq (nm) | OP (×10−14 cm2 s−1 Pa−1) | Moisture Absorption (%) | Cell Viability (%) |
|---|---|---|---|---|---|
| S0 | 17.7 ± 0.2 a | 22.4 ± 0.4 a | 2.43 ± 0.13 a | 20.36 ± 0.29 a | 97.04 ± 0.31 a |
| S1 | 18.8 ± 0.6 b | 23.5 ± 0.3 b | 2.06 ± 0.18 b | 18.14 ± 0.23 b | 96.38 ± 0.52 ab |
| S2 | 21.0 ± 0.8 c | 26.3 ± 0.5 c | 1.71 ± 0.07 c | 16.98 ± 0.31 c | 95.69 ± 0.78 b |
| S3 | 24.0 ± 0.4 d | 30.2 ± 0.2 d | 1.78 ± 0.09 c | 17.05 ± 0.26 c | 95.72 ± 0.15 b |
| S4 | 26.3 ± 0.7 e | 32.3 ± 0.6 e | 4.31 ± 0.20 d | 23.07 ± 0.34 d | 95.59 ± 0.27 b |
| Film Type | L* | a* | b* | ΔE | Opacity (mm−1) |
|---|---|---|---|---|---|
| S0 | 96.15 ± 0.06 a | −1.10 ± 0.02 a | 1.88 ± 0.11 a | 0.76 ± 0.09 a | 0.395 ± 0.010 a |
| S1 | 93.67 ± 0.18 b | 1.91 ± 0.23 b | −0.09 ± 0.01 b | 4.08 ± 0.17 b | 0.580 ± 0.010 b |
| S2 | 89.50 ± 0.43 c | 4.92 ± 0.61 c | −1.72 ± 0.52 c | 9.25 ± 0.77 c | 0.752 ± 0.004 c |
| S3 | 86.52 ± 0.39 d | 7.36 ± 0.18 d | −3.42 ± 0.14 d | 14.53 ± 1.22 d | 0.781 ± 0.007 d |
| S4 | 82.74 ± 0.06 e | 11.20 ± 0.20 e | −6.68 ± 0.78 e | 23.07 ± 3.00 e | 1.342 ± 0.009 e |
| Simulated Solutions | K | n | Correlation Coefficients (R2) |
|---|---|---|---|
| Distilled water | 0.356 ± 0.009 | 0.332 ± 0.007 | 0.969 ± 0.001 |
| 10% ethanol aqueous | 0.303 ± 0.003 | 0.333 ± 0.010 | 0.958 ± 0.001 |
| 50% ethanol aqueous | 0.242 ± 0.002 | 0.388 ± 0.006 | 0.963 ± 0.001 |
| 95% ethanol aqueous | 0.196 ± 0.003 | 0.426 ± 0.009 | 0.984 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.; Yan, X.; Cui, Y.; Han, M.; Wang, Y.; Wang, J.; Zhang, R.; Wang, X. Characterization and Release Kinetics Study of Active Packaging Films Based on Modified Starch and Red Cabbage Anthocyanin Extract. Polymers 2022, 14, 1214. https://doi.org/10.3390/polym14061214
Cheng M, Yan X, Cui Y, Han M, Wang Y, Wang J, Zhang R, Wang X. Characterization and Release Kinetics Study of Active Packaging Films Based on Modified Starch and Red Cabbage Anthocyanin Extract. Polymers. 2022; 14(6):1214. https://doi.org/10.3390/polym14061214
Chicago/Turabian StyleCheng, Meng, Xiaoran Yan, Yingjun Cui, Minjie Han, Yirong Wang, Juan Wang, Rongfei Zhang, and Xiangyou Wang. 2022. "Characterization and Release Kinetics Study of Active Packaging Films Based on Modified Starch and Red Cabbage Anthocyanin Extract" Polymers 14, no. 6: 1214. https://doi.org/10.3390/polym14061214
APA StyleCheng, M., Yan, X., Cui, Y., Han, M., Wang, Y., Wang, J., Zhang, R., & Wang, X. (2022). Characterization and Release Kinetics Study of Active Packaging Films Based on Modified Starch and Red Cabbage Anthocyanin Extract. Polymers, 14(6), 1214. https://doi.org/10.3390/polym14061214






