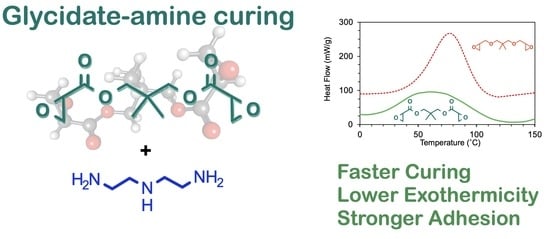
Glycidate as a High-Strength Epoxy Adhesive Curable with Amine under Ambient Conditions
Abstract
:1. Introduction
2. Materials and Methods
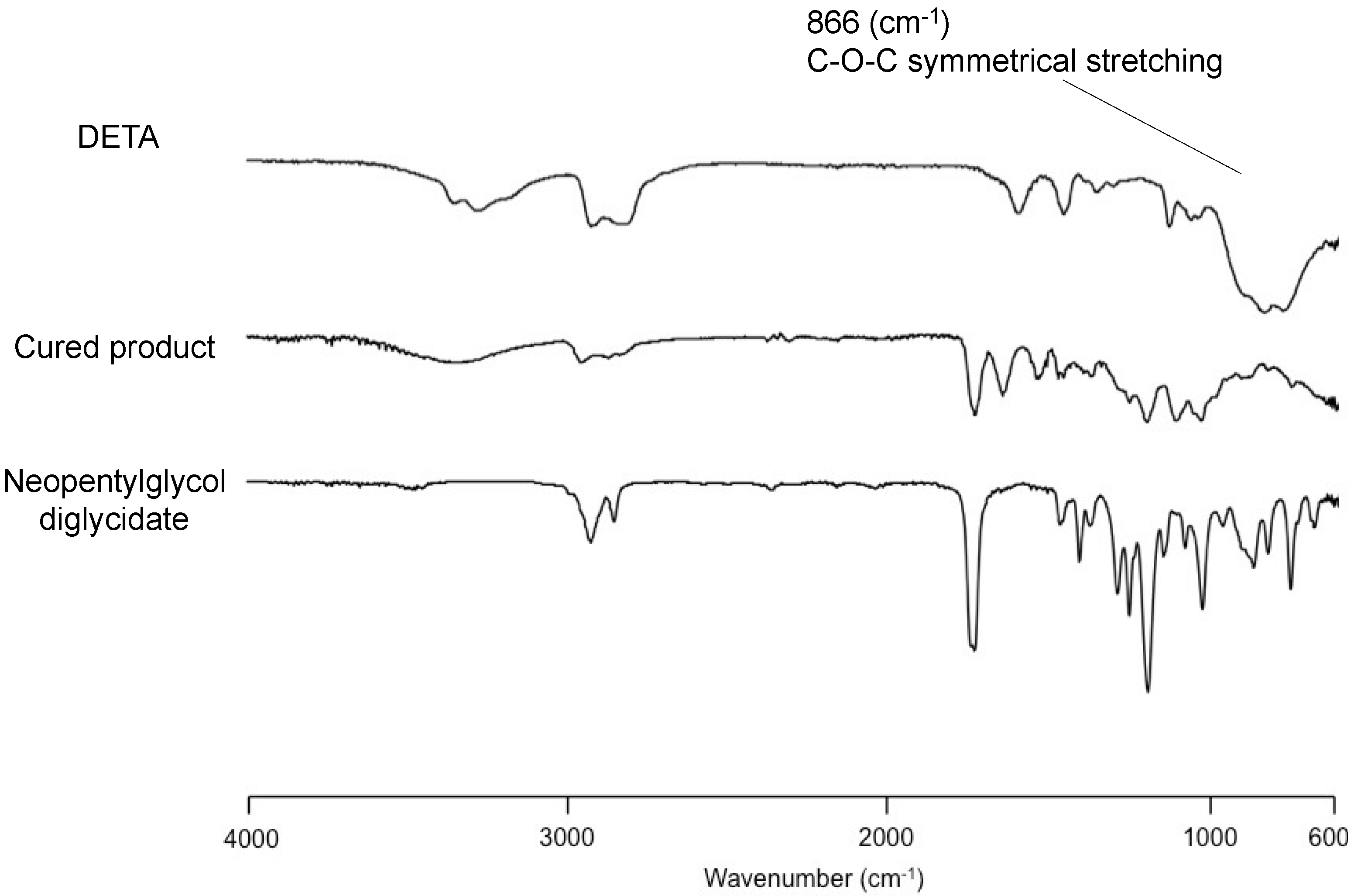
2.1. Measurements
2.2. Materials
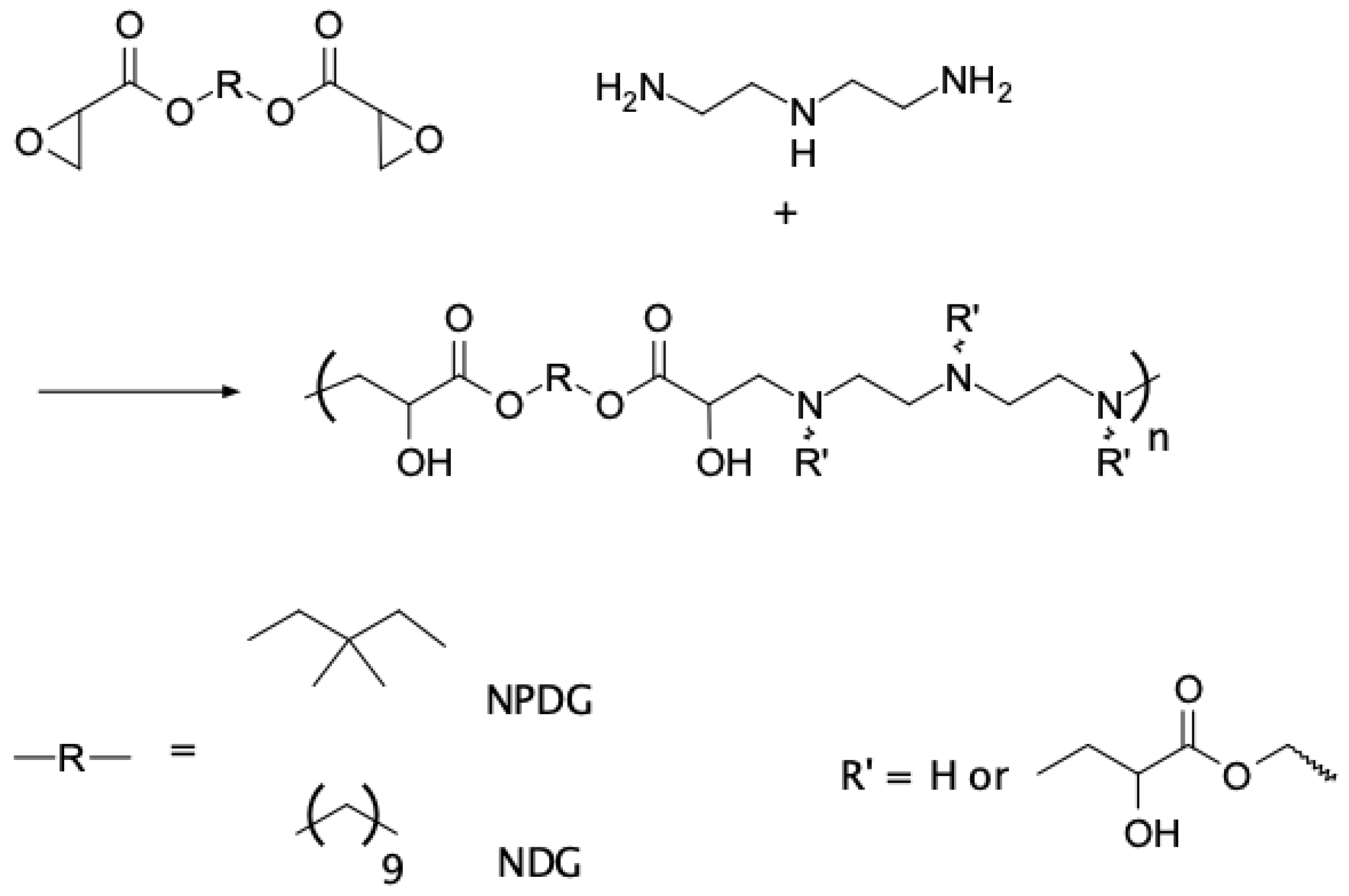
2.3. Synthesis of Neopentyl Glycol Diglycidate (NPDG)
2.4. Synthesis of 1,9-Nonanediol Diglycidate (NDG)
2.5. Curing of Glycidate and DETA (Typical Procedure)
2.6. Tensile Test Procedure
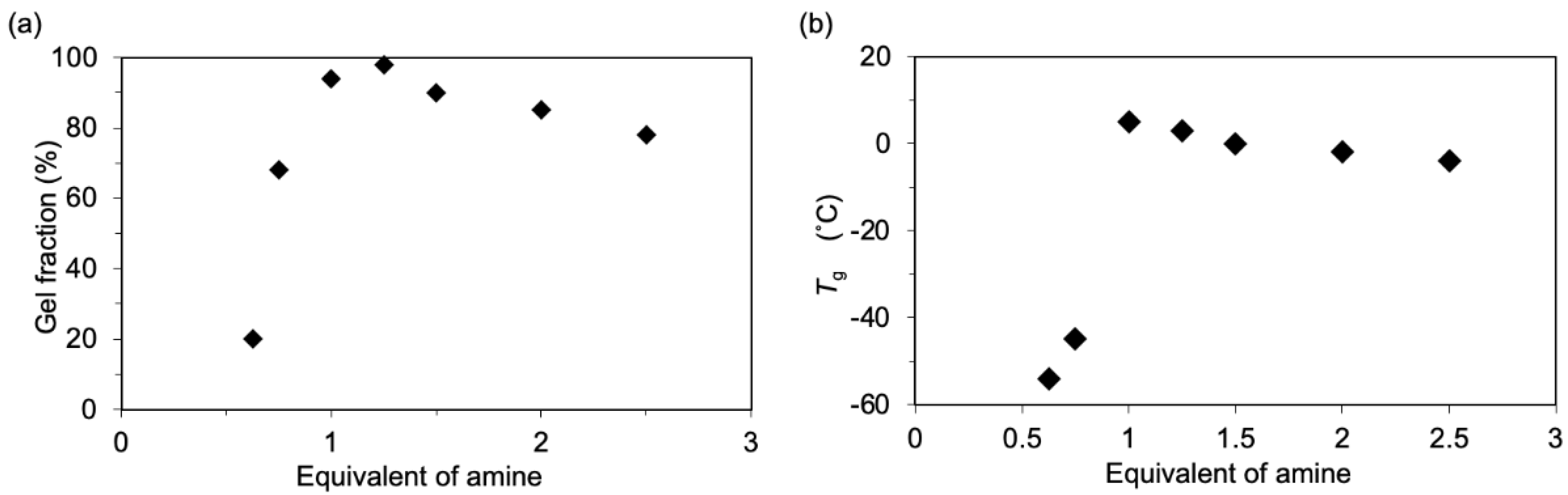
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rozenberg, B.A. Kinetics, thermodynamics and mechanism of reactions of epoxy oligomers with amines. Adv. Polym. Sci. 1986, 75, 113–165. [Google Scholar]
- Garg, A.C.; Mai, Y.-W. Failure mechanisms in toughened epoxy resins—A review. Compos. Sci. Technol. 1988, 31, 179–223. [Google Scholar] [CrossRef]
- Unnikrishnan, K.P.; Thachil, E.T. Toughening of epoxy resins. Des. Monomers Polym. 2006, 9, 129–152. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Matharu, A.S. Recent developments on biobased curing agents: A review of their preparation and use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Jin, F.L.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef] [Green Version]
- Tosti, A.; Guerra, L.; Vincenzi, C.; Peluso, A.M. Occupational skin hazards from synthetic plastics. Toxicol. Ind. Health 1993, 9, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Rigano, L.; Foti, C.; Grandolfo, M.; Vena, G.A.; Bonamonte, D.; Soleo, L.; Scorpinitt, A.A. Occupational sensitization to epoxy resin and reactive diluents in marble workers. Contact Dermat. 1996, 33, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bangsgaard, N.; Thyssen, J.P.; Menné, T.; Andersen, K.E.; Mortz, C.G.; Paulsen, E.; Sommerlund, M.; Veien, N.K.; Laurberg, G.; Kaaber, K.; et al. Contact allergy to epoxy resin: Risk occupations and consequences. Contact Dermat. 2012, 67, 73–77. [Google Scholar] [CrossRef]
- Dios-Guillán, V.; Matellanes-Palacios, M.; Bou-Boluda, L.; Fernández-Romero, C.; Miquel-Miquel, J. Non-occupational, recreational epoxy resin contact allergy: Report of two cases. Contact Dermat. 2021, 85, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Castell, P.; Serra, A.; Cádiz, V.; Galià, M. Crosslinking of trimellitimide glycidyl ester derivatives. J. Appl. Polym. Sci. 1999, 72, 537–542. [Google Scholar] [CrossRef]
- Ahmetli, G.; Deveci, H.; Soydal, U.; Gurler, S.P.; Altun, A. Epoxy resin/polymer blends: Improvement of thermal and mechanical properties. J. Appl. Polym. Sci. 2012, 125, 38–45. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Z.; Zhang, J.; Li, S.; Li, M.; Xia, J.; Zhou, Y. Epoxy monomers derived from tung oil fatty acids and its regulable thermosets cured in two synergistic ways. Biomacromolecules 2014, 15, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.A.; Striemer, G.; Webster, D.C. Synthesis, characterization and self-crosslinking of glycidyl carbamate functional resins. Prog. Org. Coat. 2006, 57, 129–139. [Google Scholar] [CrossRef]
- Harkal, U.D.; Muehlberg, A.J.; Webster, D.C. UV curable glycidyl carbamate based resins. Prog. Org. Coat. 2012, 73, 19–25. [Google Scholar] [CrossRef]
- Webster, D.C. Glycidyl carbamate functional resins and their applications: A review. Polym. Int. 2021, 70, 710–719. [Google Scholar] [CrossRef]
- Wang, R.-M.; Zheng, S.-R.; Zheng, Y.-P. Matrix materials. In Polymer Matrix Composites and Technology; Wang, R.-M., Zheng, S.-R., Zheng, Y.-P., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 101–167. [Google Scholar] [CrossRef]
- Ochi, M.; Ichikawa, N.; Shiota, R.; Hattori, Y.; Harada, M. Surface properties of alicyclic epoxy coatings modified with reactive copolymer containing fluorinated side chains. J. Coat. Technol. Res. 2014, 11, 913–921. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Cornwall, R.G.; Shi, Y. Organocatalytic asymmetric epoxidation and aziridination of olefins and their synthetic applications. Chem. Rev. 2014, 114, 8199–8256. [Google Scholar] [CrossRef] [PubMed]
- Emmons, W.D.; Pagano, A.S. Peroxytrifluoroacetic acid. IV. The epoxidation of olefins. J. Am. Chem. Soc. 1955, 77, 89–92. [Google Scholar] [CrossRef]
- Moriwaki, Y.; Akaishi, R. Mehod for synthesis of α,β-epoxy carboxylic acid derivatives. JP-Patent 2892866, 26 February 1999. [Google Scholar]
- Yao, H.; Richardson, D.E. Epoxidation of alkenes with bicarbonate-activated hydrogen peroxide J. Am. Chem. Soc. 2000, 122, 3220–3221. [Google Scholar] [CrossRef]
- Ochiai, B.; Hirano, T. Facile synthesis of glycidates via oxidation of acrylates with aqueous solution of NaOCl in the presence of ammonium salts. Heterocycles 2014, 89, 487–493. [Google Scholar] [CrossRef]
- Ji, N.; Tian, Q.; Yang, Q.; Li, M.; He, W. Chiral urea-catalyzed enantioselective epoxidation of α, β-unsaturated esters. Tetrahedron Lett. 2021, 68, 152909. [Google Scholar] [CrossRef]
- Basak, R.; Dharuman, S.; Reddy, Y.S.; Doddi, V.R.; Vankar, Y.D. HClO4·SiO2-mediated improved isomerization of glycidic esters to α-hydroxy-β,γ-unsaturated esters: Application in the formal synthesis of (R)-baclofen and β-phenyl GABA analogues. Chem. Lett. 2012, 41, 325–327. [Google Scholar] [CrossRef]
- Moon, B.; Han, S.; Kim, D. Efficient synthesis of highly functionalized cyclic aminimides. Org. Lett. 2005, 7, 3359–3361. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Clifton, I.J.; Stok, J.E.; Adlington, R.M.; Baldwin, J.E.; Rutledge, P.J. Isopenicillin N synthase mediates thiolate oxidation to sulfenate in a depsipeptide substrate analogue: Implications for oxygen binding and a link to nitrile hydratase? J. Am. Chem. Soc. 2008, 130, 10096–10102. [Google Scholar] [CrossRef] [PubMed]
- Barykina, O.V.; Snider, B.B. Synthesis of (±)-Eusynstyelamide A. Org. Lett. 2010, 12, 2664–2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Lin, X.; Chin, S.; Grinstaff, M.W. Synthesis and characterization of poly(glyceric acid carbonate): A degradable analogue of poly(acrylic acid). J. Am. Chem. Soc. 2015, 137, 12660–12666. [Google Scholar] [CrossRef] [PubMed]
- Beharaj, A.; Ekladious, I.; Grinstaff, M.W. Poly(alkyl glycidate carbonate)s as degradable pressure-sensitive adhesives. Angew. Chem. Int. Ed. 2019, 58, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Lakes, A.L.; Jordan, C.T.; Gupta, P.; Puleo, D.A.; Hilt, J.Z.; Dziubla, T.D. Reducible disulfide poly(beta-amino ester) hydrogels for antioxidant delivery. Acta Biomater. 2018, 68, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, H.J.; Daniels, F. The application of differential thermal analysis to the study of reaction kinetics. J. Am. Chem. Soc. 1957, 79, 41–46. [Google Scholar] [CrossRef]
- Javdanitehran, M.; Berg, D.C.; Duemichen, E.; Ziegmann, G. An iterative approach for isothermal curing kinetics modelling of an epoxy resin system. Thermochim. Acta 2016, 623, 72–79. [Google Scholar] [CrossRef]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Kinetic Analysis of the Curing of a Partially Biobased Epoxy Resin Using Dynamic Differential Scanning Calorimetry. Polymers 2019, 11, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
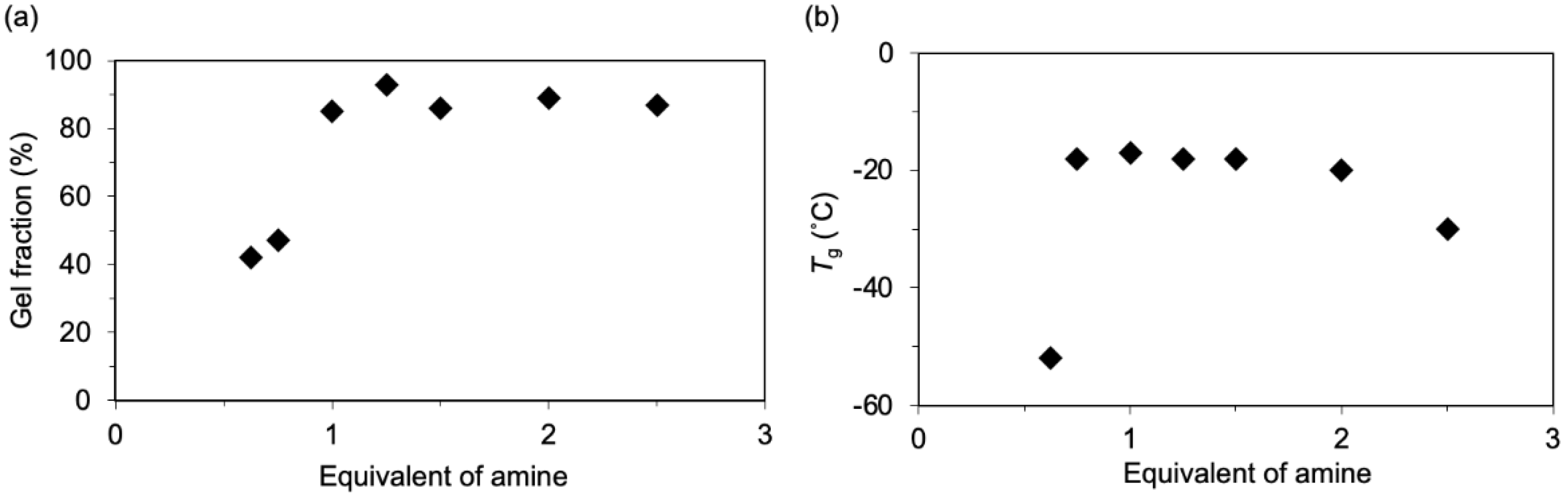

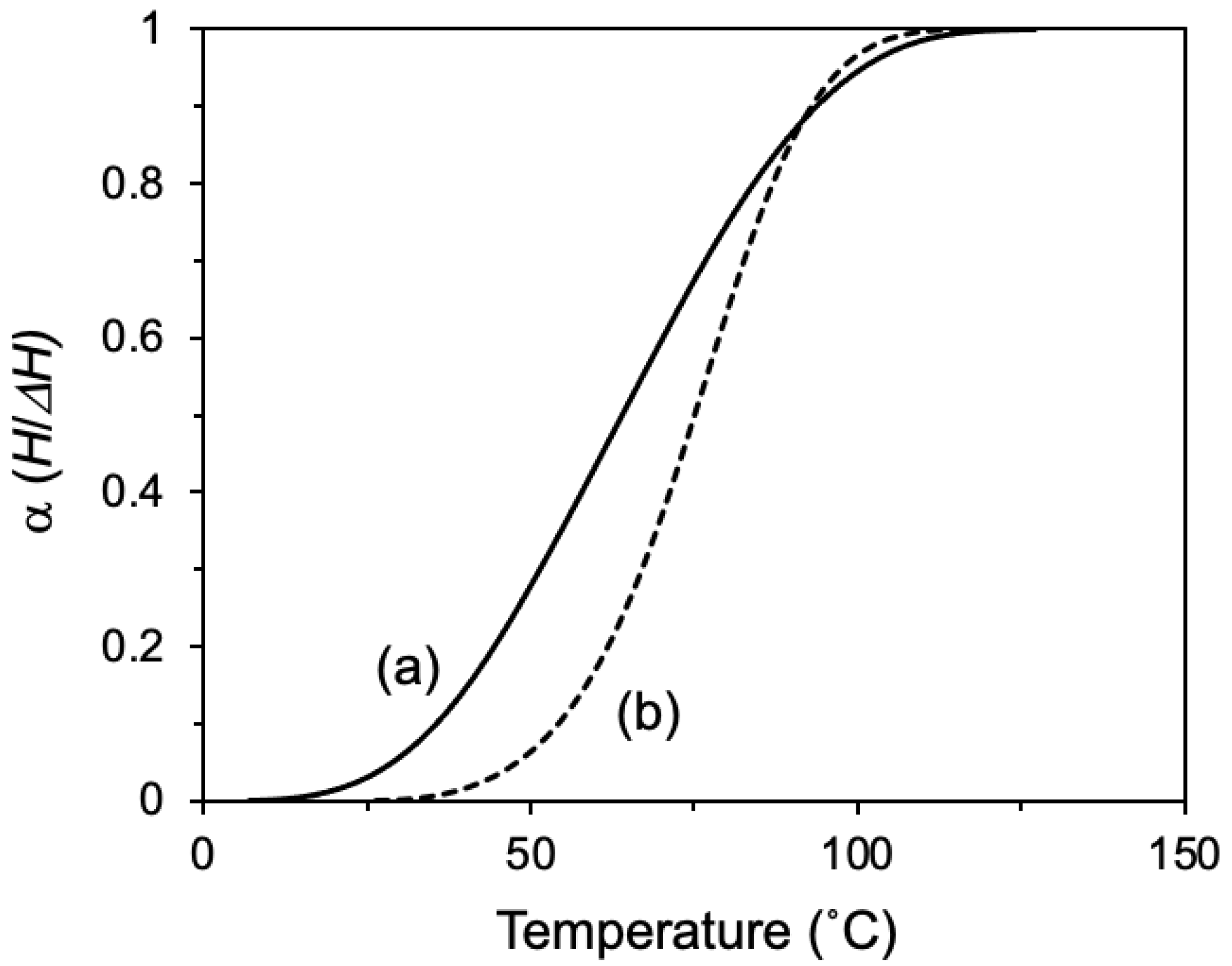
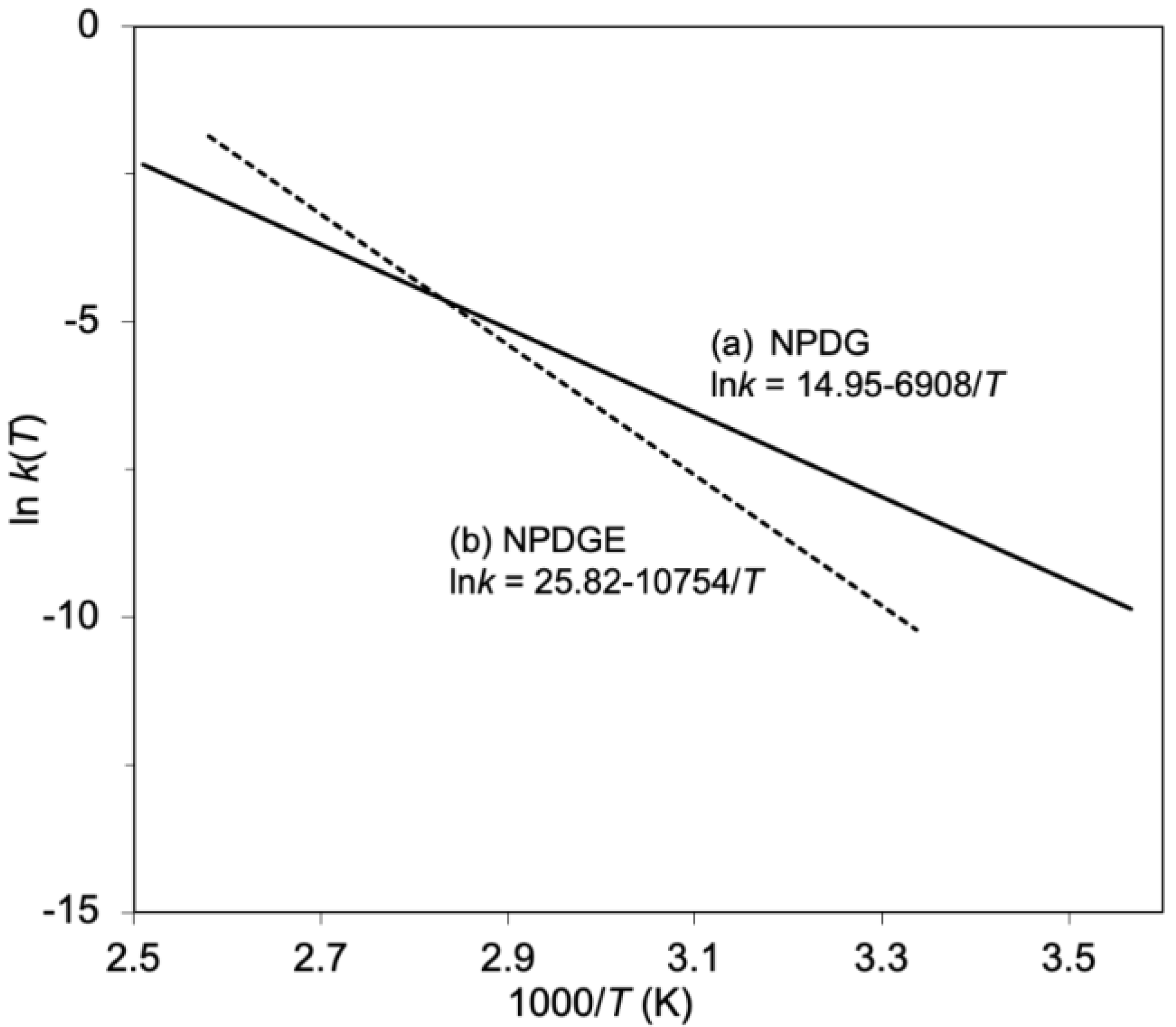
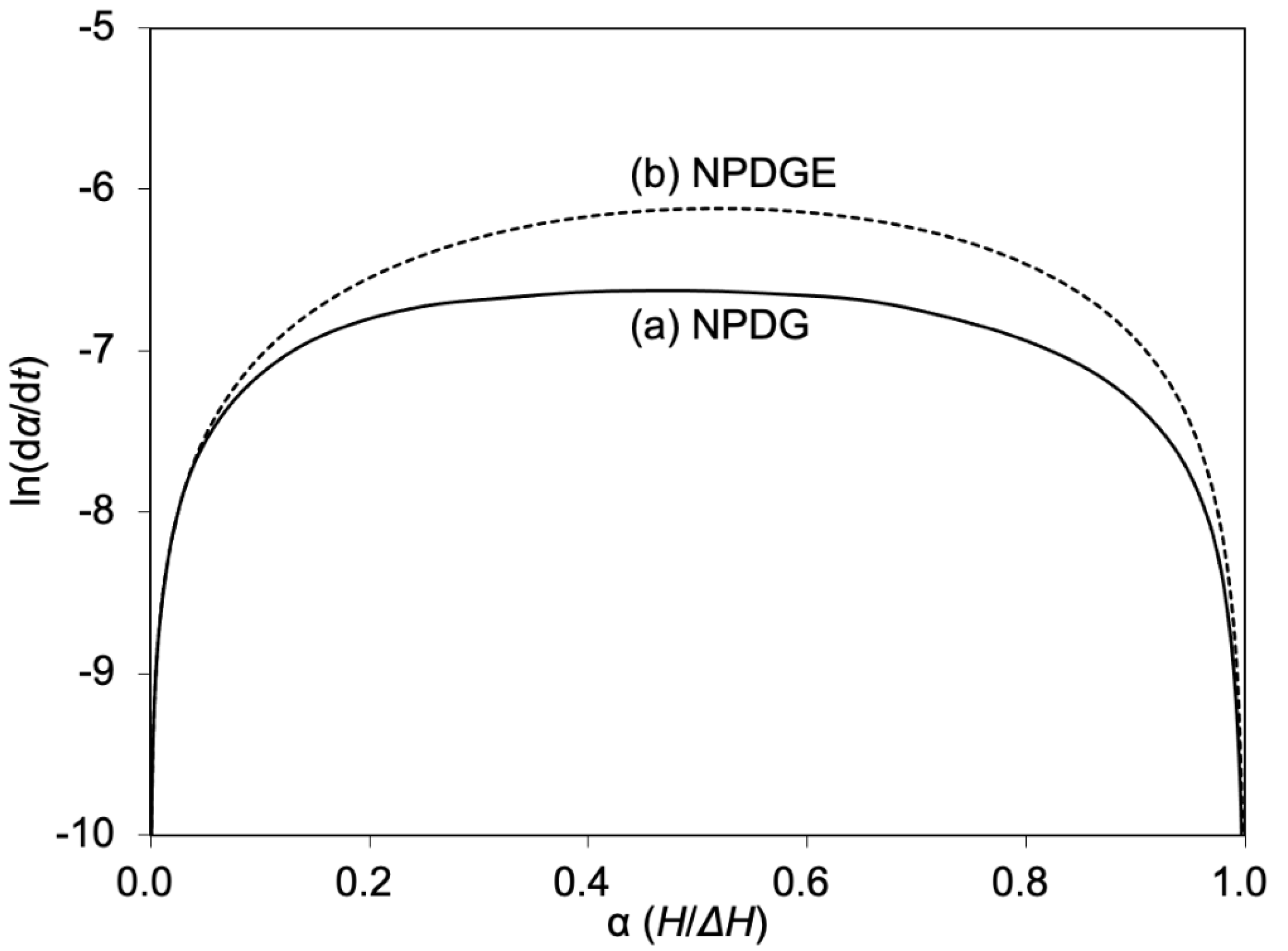



| Epoxide | ΔH (J/g) | Ti 1 (°C) | Tp 2 (°C) | Tf 3 (°C) |
|---|---|---|---|---|
| NPDG | −58 | 7 | 62 | 127 |
| NPDGE | −82 | 27 | 77 | 116 |
| Epoxide | Ea 1 (kJ/mol) | A2 (s−1) | n3 |
|---|---|---|---|
| NPDG | 57 | 3.1 × 106 | 1.3 |
| NPDGE | 89 | 1.6 × 1011 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, B.; Soegawa, K. Glycidate as a High-Strength Epoxy Adhesive Curable with Amine under Ambient Conditions. Polymers 2022, 14, 957. https://doi.org/10.3390/polym14050957
Ochiai B, Soegawa K. Glycidate as a High-Strength Epoxy Adhesive Curable with Amine under Ambient Conditions. Polymers. 2022; 14(5):957. https://doi.org/10.3390/polym14050957
Chicago/Turabian StyleOchiai, Bungo, and Katsutaka Soegawa. 2022. "Glycidate as a High-Strength Epoxy Adhesive Curable with Amine under Ambient Conditions" Polymers 14, no. 5: 957. https://doi.org/10.3390/polym14050957
APA StyleOchiai, B., & Soegawa, K. (2022). Glycidate as a High-Strength Epoxy Adhesive Curable with Amine under Ambient Conditions. Polymers, 14(5), 957. https://doi.org/10.3390/polym14050957