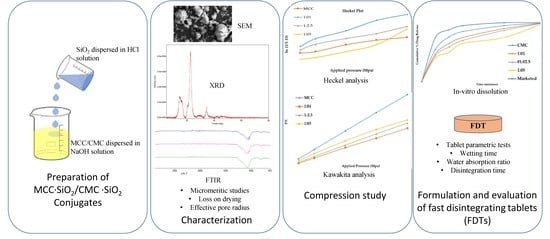
Development and Evaluation of MCC-SiO2/CMC-SiO2 Conjugates as Tablet Super-Disintegrants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MCC-SiO2 and CMC-SiO2 Conjugates
2.3. Characterization of MCC-SiO2 and CMC-SiO2 Conjugates
2.3.1. Micromeritic Studies
2.3.2. Loss on Drying (LOD)
2.3.3. Effective Pore Radius (Reff.p)
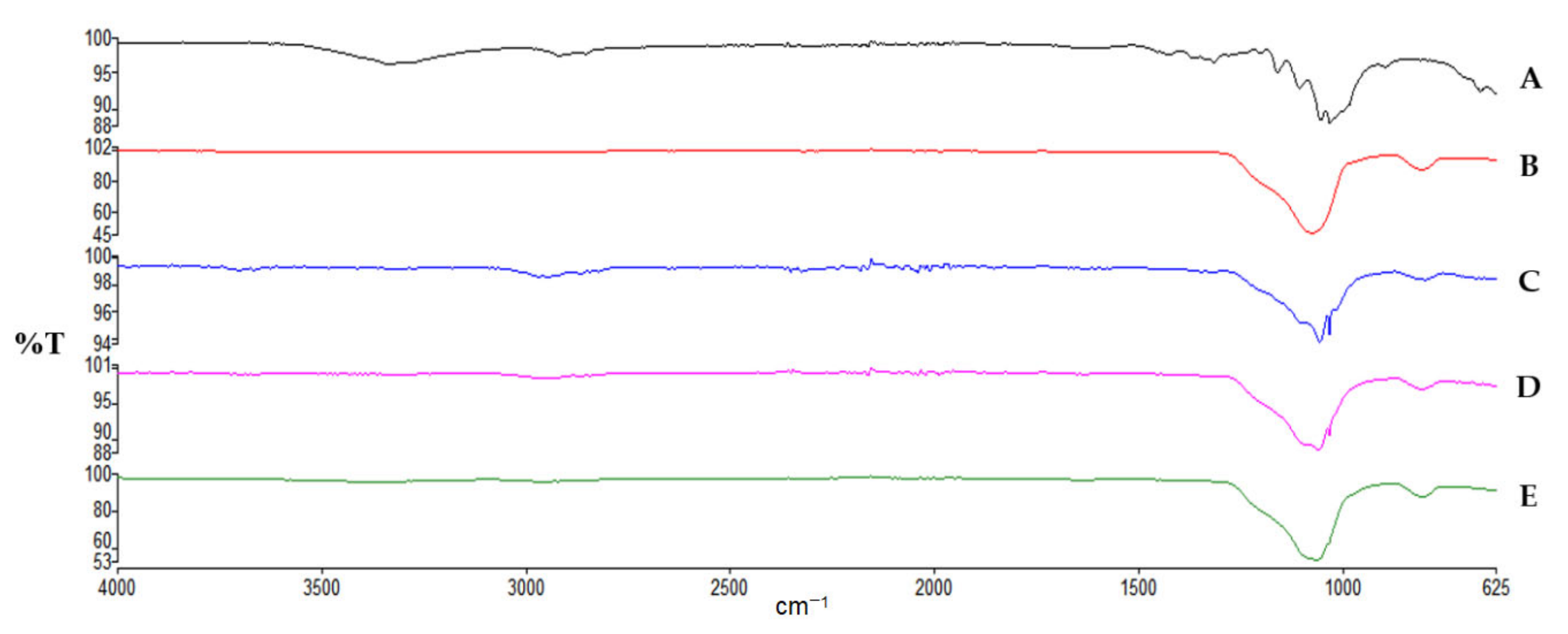
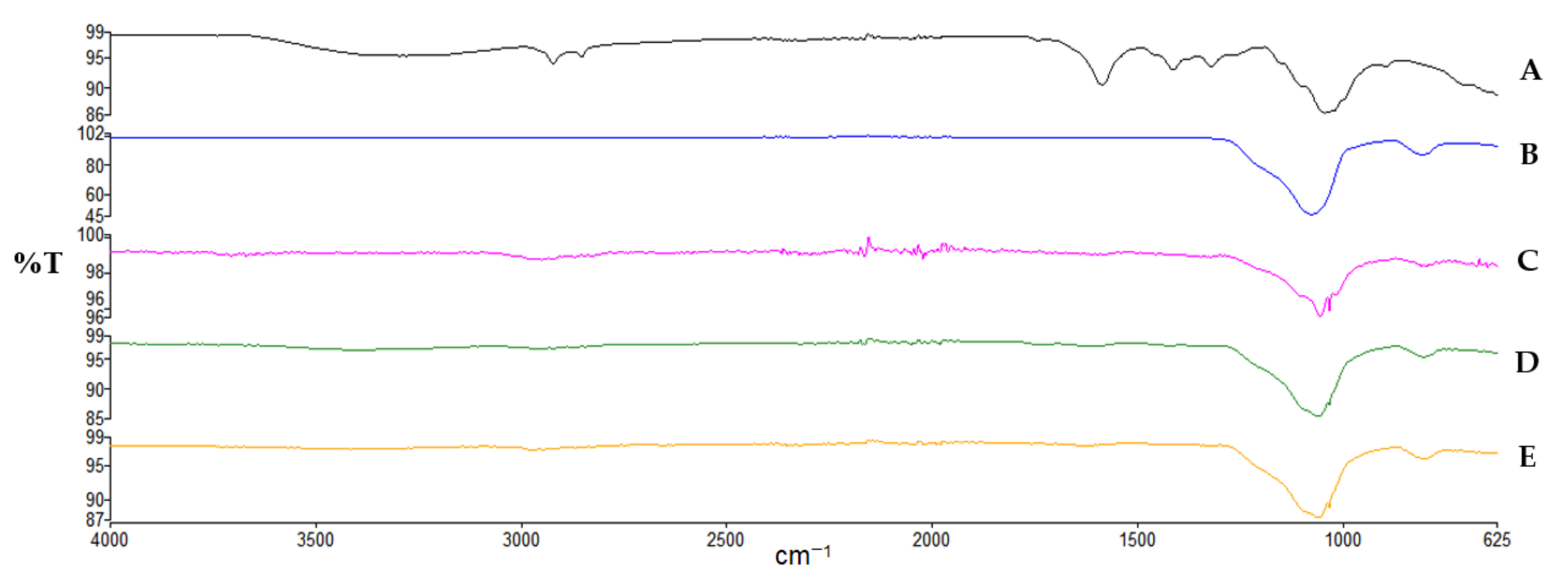
2.3.4. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.3.5. X-ray Diffraction (XRD)
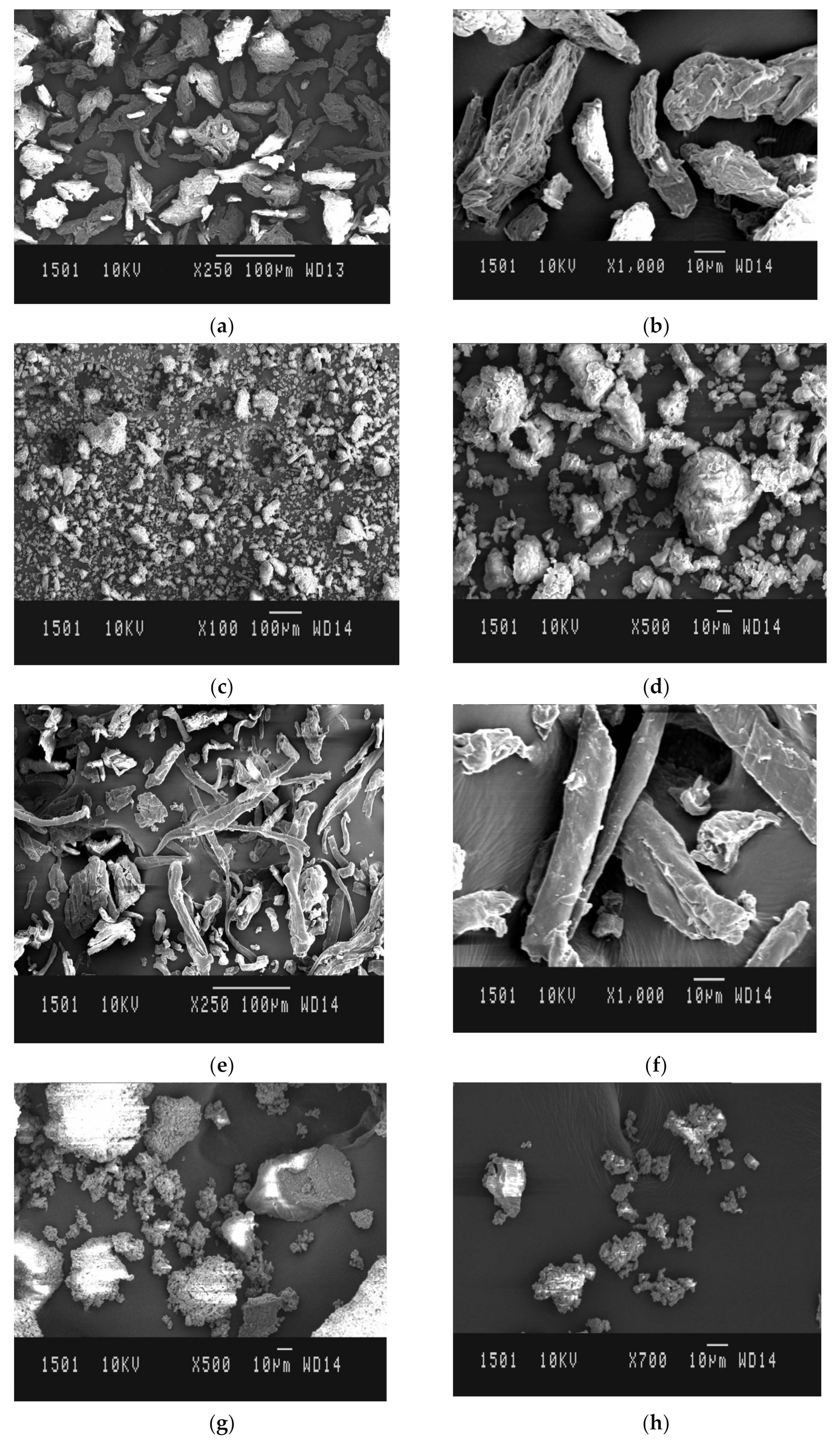
2.3.6. Scanning Electron Microscopy (SEM)
2.4. Preparation of Tablets
2.4.1. Compression Studies
2.4.2. Heckel Function
2.4.3. Kawakita Function
2.5. Preparation of Fast Disintegrating Tablets
2.5.1. Wetting Time
2.5.2. Water Absorption Ratio
2.5.3. In Vitro Disintegration Time
2.5.4. In Vitro Dissolution Study
2.5.5. Friability Test
2.5.6. Hardness
2.5.7. Tensile Strength
3. Results
3.1. Pre-Compression Evaluation
3.2. Instrumental Evaluation
3.2.1. ATR-FTIR Analysis
3.2.2. XRD Analysis
3.2.3. SEM Analysis
3.3. Compression Study
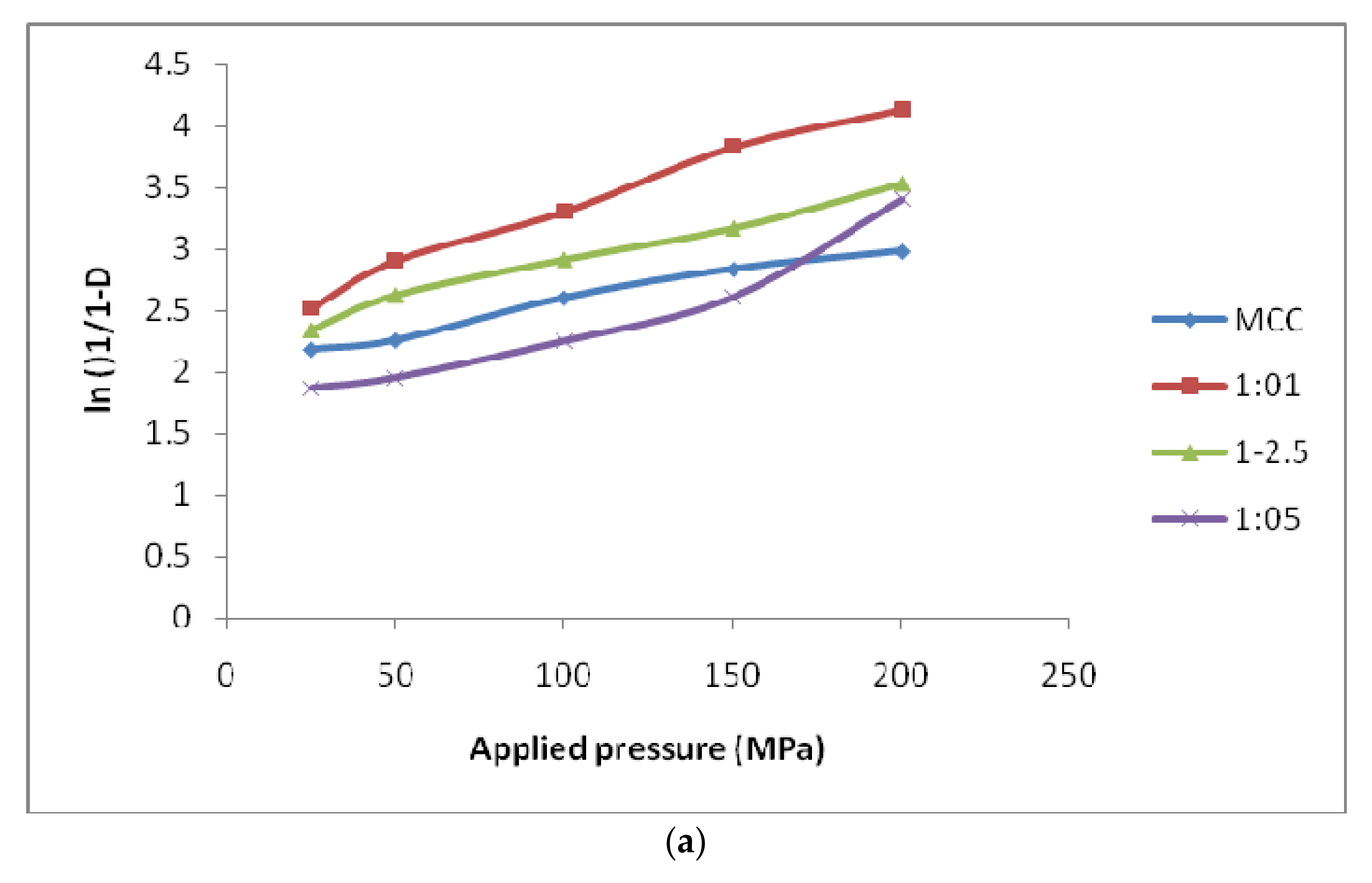
3.3.1. Heckel Function Analysis
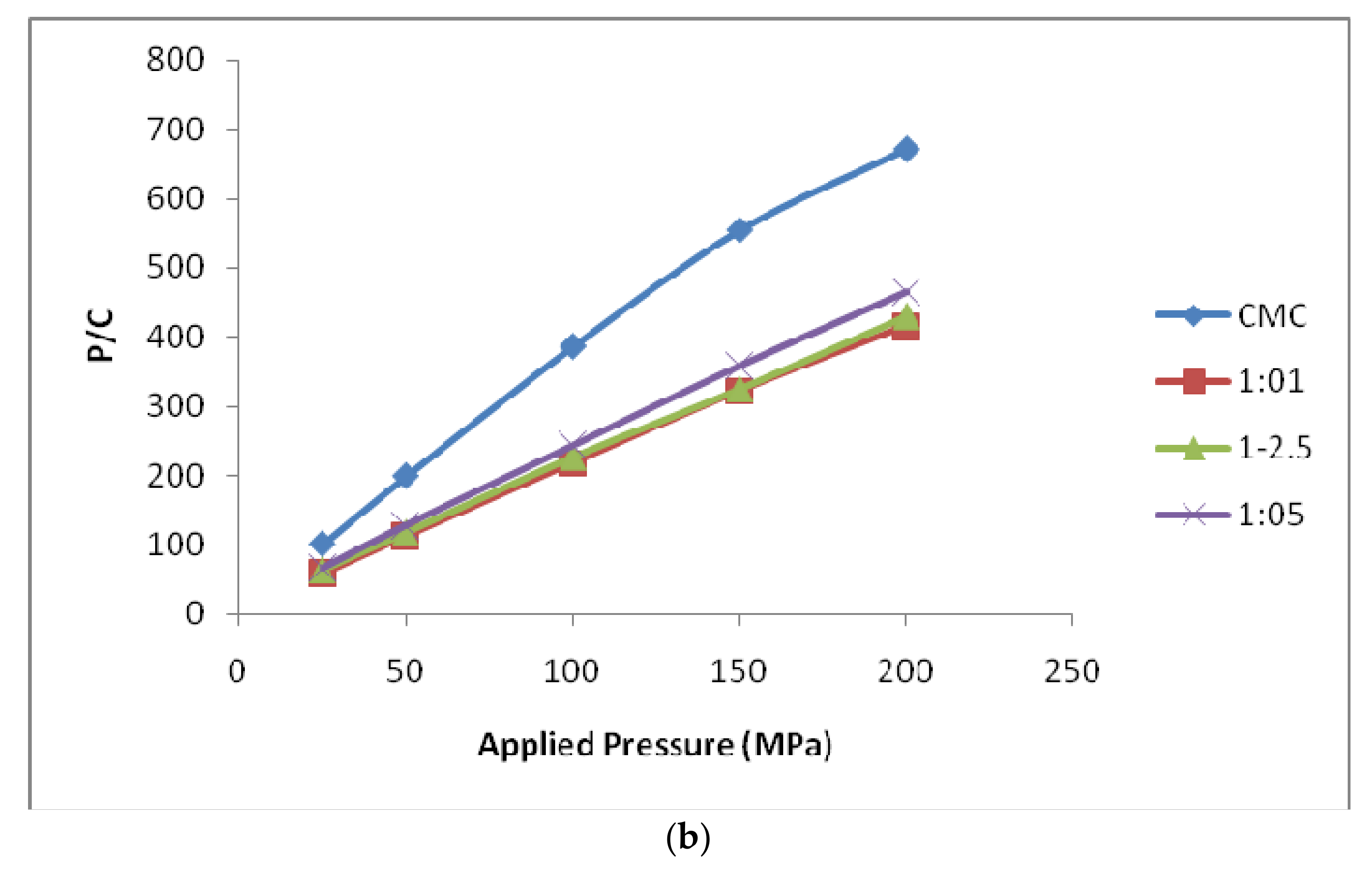
3.3.2. Kawakita Function Analysis
3.4. Post-Compression Evaluation
3.4.1. Size, Friability, Hardness and Tensile Strength of the Formulated FDTs
3.4.2. Wetting Time, Water Absorption Ratio, Disintegration Time and Drug Content of the Prepared FDTs
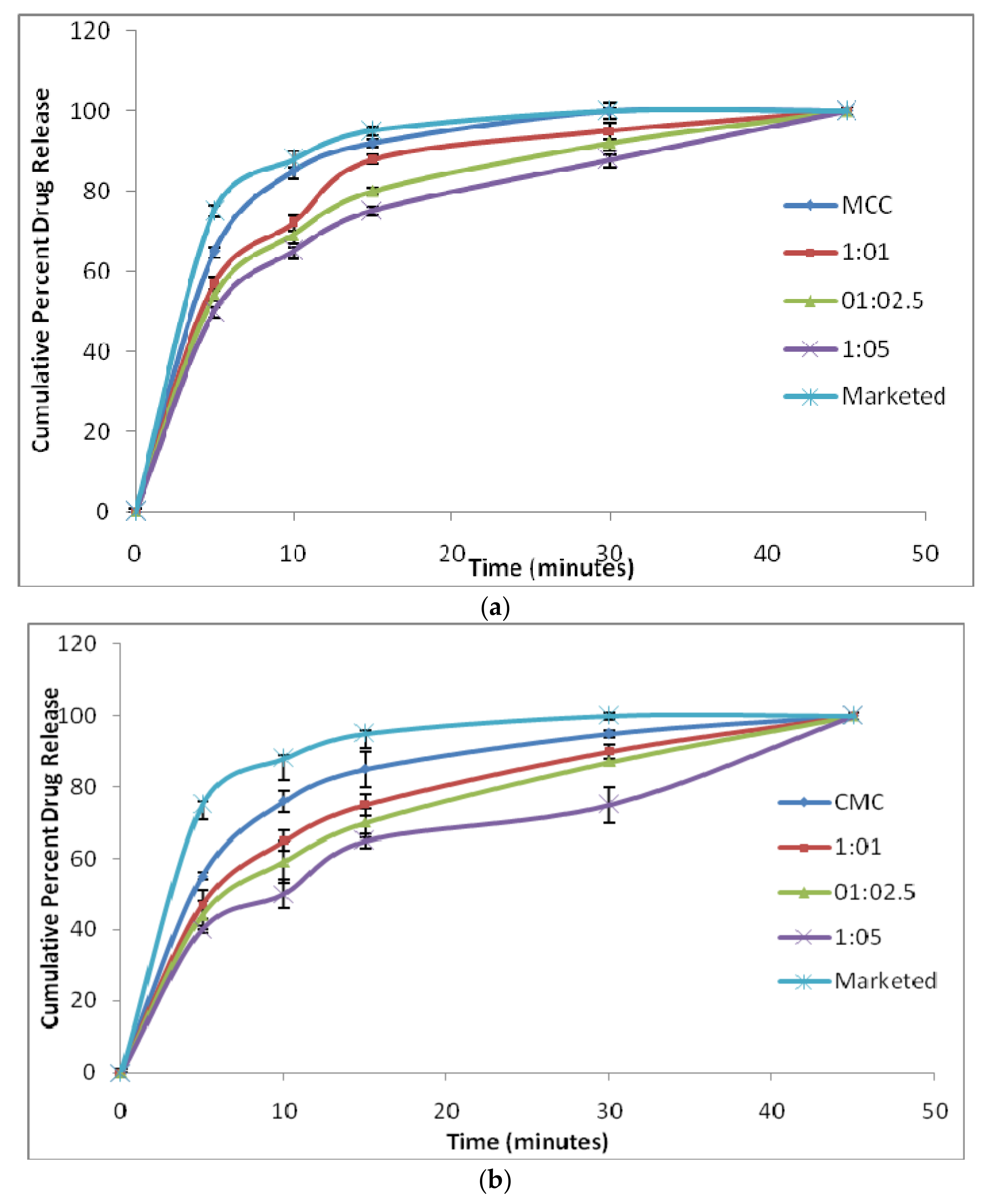
3.4.3. In Vitro Dissolution Studies and Similarity Factor (f2)
3.4.4. Stability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.; Yang, S.; Jeong, S.H.; Kimura, S.; Park, K. Orally fast disintegrating tablets: Developments, technologies, taste-masking and clinical studies. Crit. Rev. Drug Carr. Syst. 2004, 21, 433–475. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Tran, T.T. Recent Strategic Developments in the Use of Super-disintegrants for Drug Delivery. Curr. Pharma. Desi. 2020, 26, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Parkash, V.; Maan, S.; Deepika, S.K.; Hemlata, V.J. Fast disintegrating tablets: Opportunity in drug delivery system. J. Adv. Pharm. Technol. Res. 2011, 2, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Al-Khattawi, A.; Mohammed, A.R. Challenges and emerging solutions in the development of compressed orally disintegrating tablets. Expert. Opin. Drug Discov. 2014, 9, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Bhalekar, M.R.; Desale, S.S.; Madgulkar, A.R. Synthesis of MCC–PEG Conjugate and Its Evaluation as a Superdisintegrant. AAPS Pharm. Sci. Tech. 2010, 11, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Vora, N.; Rana, V. Preparation and Optimization of Mouth/Orally Dissolving Tablets Using a Combination of Glycine, Carboxymethyl Cellulose and Sodium Alginate: A Comparison with Super-disintegrants. Pharm. Develop. Technol. 2008, 13, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Rashid, I.; Al-Remawi, M.; Eftaiha, A.A.; Badwan, A. Chitin–silicon dioxide coprecipitate as a novel superdisintegrant. J. Pharm. Sci. 2008, 97, 4955–4969. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Goyal, A.; Kumar, S.; Singh, I. Starch-silicon dioxide coprecipitate as super-disintegrant: Formulation and evaluation of fast disintegrating tablets. Int. J. Drug Deliv. 2012, 4, 164–174. [Google Scholar]
- Rashid, I.; Daraghmeh, N.; Al-Remawi, M.; Leharne, S.A.; Chowdhry, B.Z.; Badwan, A. Characterization of chitin–metal silicates as binding. J. Pharm. Sci. 2009, 98, 4887–4901. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Gunn, C. Powder flow and compaction. In Tutorial Pharmacy; CBS Publishers: New Delhi, India, 1986; pp. 211–223. [Google Scholar]
- Goel, H.; Kaur, G.; Tiwary, A.K.; Rana, V. Formulation development of stronger and quick disintegrating tablets: A crucial effect of chitin. Yakugaku Zasshi 2010, 130, 729–735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sonnergaard, J.M. Impact of particle density and initial volume on mathematical compression models. Eur. J. Pharm. Sci. 2000, 11, 307–315. [Google Scholar] [CrossRef]
- Kiekens, F.; Debunne, A.; Vervaet, C.; Baert, L.; Vanhoutte, F.; van Assche, I.; Menard, F.; Remon, J.P. Influence of the punch diameter and curvature on the yield pressure of MCC compacts during Heckel analysis. Eur. J. Pharm. Sci. 2004, 22, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Puttewar, T.Y.; Kshirsagar, M.D.; Chandewar, A.V.; Chikhale, R.V. Formulation and evaluation of orodispersibletabletof taste masked doxylamine succinate using ion exchange resin. J. King Saud Univ. Sci. 2010, 22, 229–240. [Google Scholar] [CrossRef]
- Fini, A.; Bergamante, V.; Ceschel, G.C.; Ronchi, C.; de Moraes, C.A.F. Fast dispersible/slow releasing ibuprofen tablets. Eur. J. Pharm. Biopharm. 2008, 69, 335–341. [Google Scholar] [CrossRef]
- Draksiene, G.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Psyllium (Plantago Ovata Forsk) husk powder as a natural super-disintegrant for orodispersible formulations: A study on meloxicam tablets. Molecules 2019, 24, 3255. [Google Scholar] [CrossRef] [PubMed]
- Itiola, O.A.; Pilpel, N. Tableting characteristics of metronidazole formulations. Int. J. Pharm. 1986, 31, 99–105. [Google Scholar] [CrossRef]
- Odeku, O.A.; Itiola, O.A. Evaluation of khaya gum as a binder in a paracetamol tablet formulation. Pharm. Pharmacol. Communi. 1998, 4, 183–188. [Google Scholar]
- Odeku, O.A.; Fell, J.T. Effects of the method of preparation on the compression, mechanical, and release properties of khaya gum matrices. Pharm. Dev. Technol. 2006, 11, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, C.-Y.; Pan, X.; Wu, C. On Identification of Critical Material Attributes for Compression Behavior of Pharmaceutical Diluent Powders. Materials 2017, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Draksiene, G.; Venclovaite, B.; Pudziuvelyte, L.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. Natural polymer chitosan as super disintegrant in fast orally disintegrating meloxicam tablets: Formulation and evaluation. Pharmaceutics 2021, 13, 879. [Google Scholar] [CrossRef] [PubMed]



| Formula of Fast Disintegrating Tablets of MCC and MCC-SiO2 Conjugates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | Ingredients (mg) | ||||||||
| Domperidone | Native CMC | CMC-SiO2 Conjugate | Avicel 102 | Magnesium Stearate | Talc | TW * | |||
| 1:1 | 1:2.5 | 1:5 | |||||||
| F1 | 10 | 10 | - | - | - | 78 | 1 | 1 | 100 |
| F2 | 10 | - | 10 | - | - | 78 | 1 | 1 | 100 |
| F3 | 10 | - | - | 10 | - | 78 | 1 | 1 | 100 |
| F4 | 10 | - | - | - | 10 | 78 | 1 | 1 | 100 |
| Formula of Fast Disintegrating Tablets of CMC and CMC-SiO2 Conjugates | |||||||||
| Code | Ingredients (mg) | ||||||||
| Domperidone | Native MCC | MCC-SiO2 Conjugate | Avicel 102 | Magnesium Stearate | Talc | TW * | |||
| 1:1 | 1:2.5 | 1:5 | |||||||
| F1 | 10 | 10 | - | - | - | 78 | 1 | 1 | 100 |
| F2 | 10 | - | 10 | - | - | 78 | 1 | 1 | 100 |
| F3 | 10 | - | - | 10 | - | 78 | 1 | 1 | 100 |
| F4 | 10 | - | - | - | 10 | 78 | 1 | 1 | 100 |
| Parameter | Observation | |||
|---|---|---|---|---|
| MCC | MCC-SiO2 Conjugate | |||
| 1:1 | 1:2.5 | 1:5 | ||
| Bulk density (g/cm3) | 0.368 | 0.581 | 0.532 | 0.476 |
| Tapped density (g/cm3) | 0.461 | 0.721 | 0.628 | 0.538 |
| Carr’s index (%) | 20.17 | 19.42 | 15.29 | 11.52 |
| Hausner ratio | 1.25 | 1.24 | 1.18 | 1.13 |
| Angle of repose (°) | 35.66 | 34.52 | 32.64 | 29.19 |
| LOD (%) | 11.10 ± 0.15 | 9.53 ± 0.20 | 9.29 ± 0.37 | 9.11 ± 0.46 |
| Effective pore radius (µm) | 12.21 ± 1.23 | 13.35 ± 0.31 | 15.66 ± 1.17 | 18.38 ± 0.44 |
| Parameter | Observation | |||
|---|---|---|---|---|
| CMC | CMC-SiO2 Conjugate | |||
| 1:1 | 1:2.5 | 1:5 | ||
| Bulk density (g/cm3) | 0.568 | 0.556 | 0.506 | 0.504 |
| Tapped density (g/cm3) | 0.705 | 0.668 | 0.602 | 0.574 |
| Carr’s index (%) | 19.43 | 16.77 | 15.95 | 12.19 |
| Hausner ratio | 1.24 | 1.20 | 1.19 | 1.14 |
| Angle of repose (°) | 36.56 | 33.24 | 31.66 | 28.89 |
| LOD (%) | 9.88 ± 0.09 | 9.60 ± 0.12 | 9.45 ± 0.26 | 9.28 ± 0.22 |
| Effective pore radius (µm) | 13.65 ± 0.21 | 16.81 ± 0.24 | 20.12 ± 0.39 | 26.37 ± 0.24 |
| Heckel Analysis | Kawakita Analysis | |||||
|---|---|---|---|---|---|---|
| D0 | DA | DB | Py | DI | Pk | |
| MCC | 0.791 | 0.916 | 0.126 | 68.54 | 0.733 | 1.789 |
| 1:1 | 0.786 | 0.917 | 0.137 | 62.10 | 0.510 | 2.758 |
| 1:2.5 | 0.750 | 0.894 | 0.144 | 56.33 | 0.562 | 3.995 |
| 1:5 | 0.569 | 0.784 | 0.224 | 51.85 | 0.582 | 5.141 |
| Heckel Analysis | Kawakita Analysis | |||||
|---|---|---|---|---|---|---|
| D0 | DA | DB | Py | DI | Pk | |
| CMC | 0.772 | 0.871 | 0.101 | 75.11 | 0.697 | 2.541 |
| 1:1 | 0.748 | 0.867 | 0.127 | 67.84 | 0.513 | 4.519 |
| 1:2.5 | 0.760 | 0.838 | 0.138 | 60.96 | 0.521 | 6.122 |
| 1:5 | 0.679 | 0.833 | 0.203 | 57.63 | 0.562 | 6.904 |
| Formulation Code | Diameter (mm) | Thickness (mm) | Friability (%) | Hardness (Kg/cm2) | Tensile Strength (MN/m2) |
|---|---|---|---|---|---|
| MCC | 6.73 ± 0.02 | 3.03 ± 0.01 | 0.80 ± 0.02 | 2.65 ± 0.01 | 0.827 ± 0.015 |
| 1:1 | 6.73 ± 0.03 | 3.03 ± 0.03 | 0.64 ± 0.01 | 2.90 ± 0.05 | 0.905 ± 0.005 |
| 1:2.5 | 6.72 ± 0.01 | 3.04 ± 0.04 | 0.59 ± 0.03 | 3.09 ± 0.09 | 0.963 ± 0.018 |
| 1:5 | 6.72 ± 0.04 | 3.04 ± 0.04 | 0.56 ± 0.02 | 3.11 ± 0.15 | 0.969 ± 0.011 |
| CMC | 6.73 ± 0.01 | 3.04 ± 0.01 | 0.86 ± 0.02 | 2.95 ± 0.08 | 0.887 ± 0.011 |
| 1:1 | 6.74 ± 0.02 | 3.04 ± 0.03 | 0.80 ± 0.03 | 3.01 ± 0.03 | 0.935 ± 0.001 |
| 1:2.5 | 6.73 ± 0.02 | 3.03 ± 0.02 | 0.73 ± 0.04 | 3.20 ± 0.14 | 0.999 ± 0.014 |
| 1:5 | 6.74 ± 0.02 | 3.03 ± 0.03 | 0.67 ± 0.02 | 3.30 ± 0.05 | 1.029 ± 0.020 |
| Formulation Code | Wetting Time (s) | Water Absorption Ratio (%) | Disintegration Time (s) | Drug Content (%) |
|---|---|---|---|---|
| MCC | 35 ± 1.15 | 38 ± 0.40 | 40 ± 1 | 97.15 ± 0.3 |
| 1:1 | 30 ± 1.33 | 42 ± 0.28 | 23 ± 2 | 97.82 ± 0.2 |
| 1:2.5 | 24 ± 1.56 | 46 ± 0.56 | 18 ± 3 | 98.75 ± 1.0 |
| 1:5 | 19 ± 1.21 | 49 ± 0.47 | 15 ± 2 | 99.23 ± 0.7 |
| CMC | 45 ± 1.21 | 39 ± 0.77 | 43 ± 2 | 97.48 ± 0.8 |
| 1:1 | 40 ± 1.17 | 42 ± 0.94 | 20 ± 3 | 98.66 ± 0.3 |
| 1:2.5 | 30 ± 1.33 | 45 ± 0.08 | 18 ± 1 | 98.89 ± 1.0 |
| 1:5 | 21 ± 1.13 | 49 ± 0.57 | 12 ± 2 | 99.35 ± 0.2 |
| Formulation Code | f2 Value | Formulation Code | f2 Value |
|---|---|---|---|
| MCC | 70 | CMC | 52 |
| 1:1 | 52 | 1:1 | 41 |
| 1:2.5 | 47 | 1:2.5 | 37 |
| 1:5 | 42 | 1:5 | 32 |
| Formulation Code | Time Interval (Months) | Test Parameters (MCC-SiO2) | |||
|---|---|---|---|---|---|
| Hardness (kg/cm2) | Friability (%) | Drug Content (%) | Disintegration Time (s) | ||
| MCC | 0 | 3.10 ± 0.02 | 0.80 ± 0.02 | 97.15 ± 0.3 | 40 ± 1 |
| 1 | 3.09 ± 0.15 | 0.81 ± 0.06 | 97.28 ± 0.7 | 41 ± 3 | |
| 2 | 3.09 ± 0.33 | 0.82 ± 0.05 | 96.41 ± 0.4 | 42 ± 1 | |
| 3 | 3.08 ± 0.26 | 0.83 ± 0.03 | 96.20 ± 0.32 | 43 ± 1 | |
| 1:1 | 0 | 3.13 ± 0.05 | 0.64 ± 0.01 | 98.20 ± 0.11 | 23 ± 1 |
| 1 | 3.11 ± 0.12 | 0.65 ± 0.15 | 97.95 ± 0.17 | 24 ± 2 | |
| 2 | 3.10 ± 0.22 | 0.67 ± 0.04 | 97.90 ± 0.20 | 25 ± 1 | |
| 3 | 3.08 ± 0.34 | 0.68 ± 0.06 | 97.86 ± 0.36 | 26 ± 3 | |
| 1:2.5 | 0 | 3.14 ± 0.09 | 0.59 ± 0.03 | 98.75 ± 1.0 | 18 ± 3 |
| 1 | 3.13 ± 0.92 | 0.60 ± 0.08 | 98.40 ± 0.71 | 19 ± 2 | |
| 2 | 3.11 ± 0.21 | 0.62 ± 0.15 | 97.33 ± 0.23 | 21 ± 4 | |
| 3 | 3.09 ± 0.52 | 0.62 ± 0.06 | 97.04 ± 0.87 | 21 ± 3 | |
| 1:5 | 0 | 3.11 ± 0.06 | 0.56 ± 0.02 | 99.23 ± 0.07 | 15 ± 2 |
| 1 | 3.11 ± 0.11 | 0.57 ± 0.08 | 98.76 ± 0.10 | 15 ± 7 | |
| 2 | 3.10 ± 0.56 | 0.57 ± 0.19 | 98.11 ± 0.18 | 16 ± 2 | |
| 3 | 3.09 ± 0.41 | 0.58 ± 0.03 | 97.99 ± 0.84 | 17 ± 4 | |
| Formulation Code | Time Interval (Months) | Test Parameters (CMC-SiO2) | |||
|---|---|---|---|---|---|
| Hardness (kg/cm2) | Friability (%) | Drug Content (%) | Disintegration Time (s) | ||
| CMC | 0 | 3.09 ± 0.08 | 0.86 ± 0.02 | 97.8 ± 0.80 | 43 ± 2 |
| 1 | 3.07 ± 0.23 | 0.88 ± 0.12 | 97.0 ± 0.54 | 46 ± 2 | |
| 2 | 3.04 ± 0.78 | 0.90 ± 0.19 | 96.27 ± 0.67 | 48 ± 3 | |
| 3 | 3.00 ± 0.12 | 0.93 ± 0.23 | 95.28 ± 0.45 | 48 ± 2 | |
| 1:1 | 0 | 3.11 ± 0.03 | 0.80 ± 0.03 | 98.66 ± 0.30 | 20 ± 3 |
| 1 | 3.10 ± 0.88 | 0.82 ± 0.01 | 98.02 ± 0.73 | 22 ± 2 | |
| 2 | 3.08 ± 0.56 | 0.83 ± 0.10 | 97.89 ± 0.44 | 24 ± 3 | |
| 3 | 3.06 ± 0.33 | 0.85 ± 0.31 | 97.66 ± 0.26 | 25 ± 1 | |
| 1:2.5 | 0 | 3.10 ± 0.14 | 0.73 ± 0.04 | 98.89 ± 1.00 | 18 ± 1 |
| 1 | 3.09 ± 0.72 | 0.74 ± 0.11 | 98.55 ± 0.27 | 19 ± 3 | |
| 2 | 3.03 ± 0.19 | 0.76 ± 0.08 | 98.38 ± 0.19 | 21 ± 2 | |
| 3 | 3.00 ± 0.32 | 0.78 ± 0.02 | 97.94 ± 0.38 | 21 ± 2 | |
| 1:5 | 0 | 3.12 ± 0.05 | 0.67 ± 0.02 | 99.35 ± 0.20 | 12 ± 2 |
| 1 | 3.11 ± 0.03 | 0.68 ± 0.03 | 99.13 ± 0.88 | 13 ± 1 | |
| 2 | 3.10 ± 0.52 | 0.70 ± 0.13 | 98.78 ± 0.66 | 14 ± 3 | |
| 3 | 3.09 ± 0.31 | 0.71 ± 0.45 | 98.63 ± 0.17 | 14 ± 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangnim, T.; Zandu, S.K.; Kaur, S.; Odeku, O.A.; Huanbutta, K.; Singh, I. Development and Evaluation of MCC-SiO2/CMC-SiO2 Conjugates as Tablet Super-Disintegrants. Polymers 2022, 14, 1035. https://doi.org/10.3390/polym14051035
Sangnim T, Zandu SK, Kaur S, Odeku OA, Huanbutta K, Singh I. Development and Evaluation of MCC-SiO2/CMC-SiO2 Conjugates as Tablet Super-Disintegrants. Polymers. 2022; 14(5):1035. https://doi.org/10.3390/polym14051035
Chicago/Turabian StyleSangnim, Tanikan, Simran Kaur Zandu, Sukhanpreet Kaur, Oluwatoyin A. Odeku, Kampanart Huanbutta, and Inderbir Singh. 2022. "Development and Evaluation of MCC-SiO2/CMC-SiO2 Conjugates as Tablet Super-Disintegrants" Polymers 14, no. 5: 1035. https://doi.org/10.3390/polym14051035
APA StyleSangnim, T., Zandu, S. K., Kaur, S., Odeku, O. A., Huanbutta, K., & Singh, I. (2022). Development and Evaluation of MCC-SiO2/CMC-SiO2 Conjugates as Tablet Super-Disintegrants. Polymers, 14(5), 1035. https://doi.org/10.3390/polym14051035