Water-Based Highly Stretchable PEDOT:PSS/Nonionic WPU Transparent Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stretchable Electrodes
Fabrication of Stretchable Electrode Films
2.3. Preparation of Alternative Current Electroluminescence (ACEL) Device
2.4. Sample Preparation and Characterization
3. Results
3.1. PEDOT:PSS/Nonionic WPU Composite
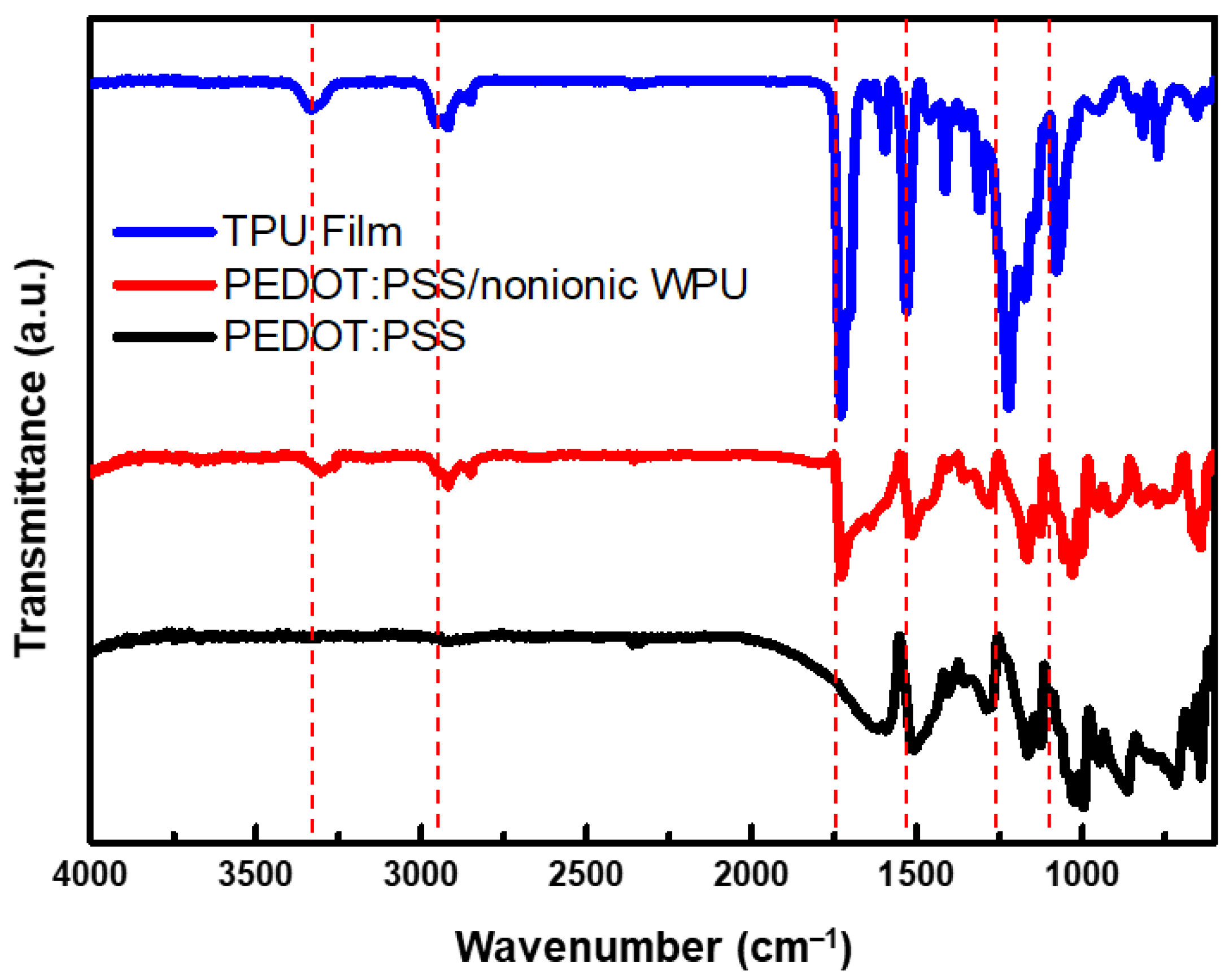
3.1.1. Compatibility of PEDOT:PSS and Nonionic WPU
3.1.2. Preparation and Surface Morphology of the PEDOT:PSS/Nonionic WPU
3.1.3. Improvement in Mechanical Properties of PEDOT:PSS/Nonionic WPU
3.1.4. Mechanical Properties of Other Substrate Materials
3.2. Alternating Current Electroluminescent (ACEL) Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W.; et al. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Sekitani, T.; Iba, S.; Kato, Y.; Kawaguchi, H.; Sakurai, T. A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc. Natl. Acad. Sci. USA 2004, 101, 9966–9970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Niu, X.; Liu, Z.; Pei, Q. Intrinsically stretchable polymer light-emitting devices using carbon nanotube-polymer composite electrodes. Adv. Mater. 2011, 23, 3989–3994. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.; Kang, I.; Jung, M.S.; Kim, S.J.; Kim, J.K.; Cho, S.M.; Kim, J.H.; Park, J.H. Hybrid silver mech electrode for ITO-free flexible polymer solar cells with good mechanical stability. ChemSusChem 2016, 9, 1042–1049. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.Y.; Kim, J.; Kim, J.H. Highly reliable AgNW/PEDOT:PSS hybrid films: Efficient methods for enhancing transparency and lowering resistance and haziness. J. Mater. Chem. C 2014, 2, 5636. [Google Scholar] [CrossRef]
- Kim, Y.U.; Ma, B.S.; Kim, Y.; Park, S.H.; Kang, H.; Yoon, H.J.; Cho, M.J.; Kim, T.-S.; Kim, J.H.; Choi, D.H. Comparison of the mechanical properties of polymer blend and main-chain conjugated copolymer films with donor–acceptor heterojunctions. Chem. Eng. J. 2021, 415, 128952. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Kim, J.H. Highly conductive PEDOT:PSS thin films with two-dimensional lamellar stacked multi-layers. Nanomaterials 2020, 10, 2211. [Google Scholar] [CrossRef]
- Palumbiny, C.M.; Liu, F.; Russell, T.P.; Hexemer, A.; Wang, C.; Muller-Buschbaum, P. The Crystallization of PEDOT:PSS Polymeric Electrodes Probed In Situ during Printing. Adv. Mater. 2015, 27, 3391–3397. [Google Scholar] [CrossRef]
- Duc, C.; Vlandas, A.; Malliaras, G.G.; Senez, V. Wettability of PEDOT:PSS films. Soft Matter 2016, 12, 5146–5153. [Google Scholar] [CrossRef]
- Duc, C.; Vlandas, A.; Malliaras, G.G.; Senez, V. Electrowetting on immersed conducting hydrogel. J. Phys. Chem. 2017, 121, 9947–9956. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.; Müller, E.; Naujoks, N.; Dual, J. Microscopical Investigations of PEDOT:PSS Thin Films. Adv. Funct. Mater. 2009, 19, 1215–1220. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savagatrup, S.; Chan, E.; Renteria-Garcia, S.M.; Printz, A.D.; Zaretski, A.V.; O’Connor, T.F.; Rodriquez, D.; Darren, E.V.; Lipomi, D.J. Plasticization of PEDOT:PSS by common additives for mechanically robust organic solar cells and wearable sensors. Adv. Funct. Mater. 2015, 25, 427–436. [Google Scholar] [CrossRef]
- Tan, Z.; Li, H.; Huang, Y.; Gong, X.; Qi, J.; Li, J.; Chen, X.; Ji, D.; Lv, W.; Li, L.; et al. Breathing-effect assisted transferring large-area PEDOT:PSS to PDMS substrate with robust adhesion for stable flexible pressure sensor. Compos. Appl. Sci. 2021, 143, 106299. [Google Scholar] [CrossRef]
- Kayser, L.V.; Russell, M.D.; Rodriquez, D.; Abuhamdieh, S.N.; Dhong, C.; Khan, S.; Stein, A.N.; Ramirez, J.; Lipomi, D.J. RAFT Polymerization of an Intrinsically Stretchable Water-Soluble Block Copolymer Scaffold for PEDOT. Chem. Mater. 2018, 30, 4459–4468. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Lee, H.; Park, C.; Im, S.; Kim, J.H. Synthesis of stretchable, environmentally stable, conducting polymer PEDOT using a modified acid template random copolymer. Macromol. Chem. Phys. 2020, 221, 1900465. [Google Scholar] [CrossRef]
- Kim, Y.; Park, C.; Im, S.; Kim, J.H. Design of intrinsically stretchable and highly conductive polymers for fully stretchable electrochromic devices. Sci. Rep. 2020, 10, 16488. [Google Scholar] [CrossRef]
- Luo, R.; Li, H.; Du, B.; Zhou, S.; Zhu, Y. A simple strategy for high stretchable, flexible and conductive polymer films based on PEDOT:PSS-PDMS blends. Org. Electron. 2020, 76, 105451. [Google Scholar] [CrossRef]
- Taroni, P.J.; Santagiuliana, G.; Wan, K.; Calado, P.; Qiu, M.; Zhang, H.; Pugno, N.M.; Palma, M.; Stingelin-Stutzman, N.; Heeney, M.; et al. Toward stretchable self-powered sensors based on the thermoelectric response of PEDOT:PSS/Polyurethane blends. Adv. Funct. Mater. 2018, 28, 1704285. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv. 2017, 3, e1602076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyedin, M.Z.; Razal, J.M.; Innis, P.C.; Wallace, G.G. Strain-responsive polyyrethane/PEDOT:PSS elastomeric composite fibers with high electrical conductivity. Adv. Funct. Mater. 2014, 24, 2957–2966. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Chen, G.; Liang, J. The preparation method of nonionic waterborne polyurethane. Model. Meas. Control. 2018, 79, 222–228. [Google Scholar] [CrossRef]
- He, H.; Ouyang, J. Enhancements in the mechanical stretchability and thermoelectric properties of PEDOT:PSS for flexible electronics applications. Acc. Mater. Res. 2020, 1, 146–157. [Google Scholar] [CrossRef]
- Jin, L.; Wang, T.; Feng, Z.Q.; Leach, M.K.; Wu, J.; Mo, S.; Jiang, Q. A facile approach for the fabrication of core-shell PEDOT nanofiber mats with superior mechanical properties and biocompatibility. J. Mater. Chem. 2013, 1, 1818–1825. [Google Scholar] [CrossRef]
- Luo, S.-C.; Mohamed Ali, E.; Tansil, N.C.; Yu, H.-H.; Gao, S.; Kantchev, E.A.B.; Ying, J.Y. Poly(3,4-ethylenedioxythiophene) (PEDOT) Nanobiointerfaces: Thin, Ultrasmooth, and Functionalized PEDOT Films with in Vitro and in Vivo Biocompatibility. Langmuir 2008, 24, 8071–8077. [Google Scholar] [CrossRef]
- Poussard, L.; Lazko, J.; Mariage, J.; Raquez, J.M.; Dubois, P. Biobased waterborne polyurethanes for coating applications: How fully biobased polyols may improve the coating properties. Prog. Org. Coat. 2016, 97, 175–183. [Google Scholar] [CrossRef]
- Lövenich, W.; Hill, H. Polymer Coatings Containing Conductive Polymers. U.S. Patent 13/387,775, 12 July 2012. [Google Scholar]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Müller-Meskamp, L.; Leo, K. Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Honarkar, H. Waterborne polyurethanes: A review. J. Dispers. Sci. Technol. 2017, 39, 507–516. [Google Scholar] [CrossRef]
- Zhou, R.; Li, P.; Fan, Z.; Du, D.; Ouyang, J. Stretchable heaters with composites of an intrinsically conductive polymer, reduced graphene oxide and an elastomer for wearable thermotherapy. J. Mater. Chem. 2017, 5, 1544–1551. [Google Scholar] [CrossRef] [Green Version]
- Delpech, M.; Miranda, G. Waterborne polyurethanes: Influence of chain extender in FTIR spectra profiles. Cent. Eur. J. Eng. 2012, 2, 231–238. [Google Scholar] [CrossRef]
- Li, P.; Du, D.; Guo, L.; Guo, Y.; Ouyang, J. Stretchable and conductive polymer films for high-performance electromagnetic interference shielding. J. Mater. Chem. 2016, 4, 6525–6532. [Google Scholar] [CrossRef]
- Ouyang, J. “Secondary doping” methods to significantly enhance the conductivity of PEDOT:PSS for its application as transparent electrode of optoelectronic devices. Display 2013, 34, 423–436. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Yoo, S.; Kim, J.-H. Water-Based Highly Stretchable PEDOT:PSS/Nonionic WPU Transparent Electrode. Polymers 2022, 14, 949. https://doi.org/10.3390/polym14050949
Kim Y, Yoo S, Kim J-H. Water-Based Highly Stretchable PEDOT:PSS/Nonionic WPU Transparent Electrode. Polymers. 2022; 14(5):949. https://doi.org/10.3390/polym14050949
Chicago/Turabian StyleKim, Youngno, Sinseok Yoo, and Jung-Hyun Kim. 2022. "Water-Based Highly Stretchable PEDOT:PSS/Nonionic WPU Transparent Electrode" Polymers 14, no. 5: 949. https://doi.org/10.3390/polym14050949
APA StyleKim, Y., Yoo, S., & Kim, J.-H. (2022). Water-Based Highly Stretchable PEDOT:PSS/Nonionic WPU Transparent Electrode. Polymers, 14(5), 949. https://doi.org/10.3390/polym14050949






