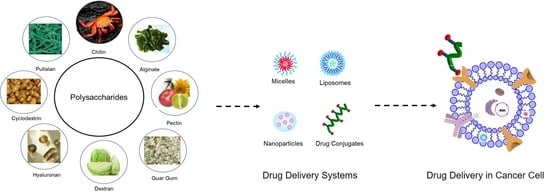
Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy
Abstract
:1. Introduction
2. Properties of Polysaccharides Carriers
3. Biopolymer Based Materials
4. Polysaccharides Based Drug Carriers
4.1. Chitosan
4.1.1. Chitosan with Doxorubicin (DOX)
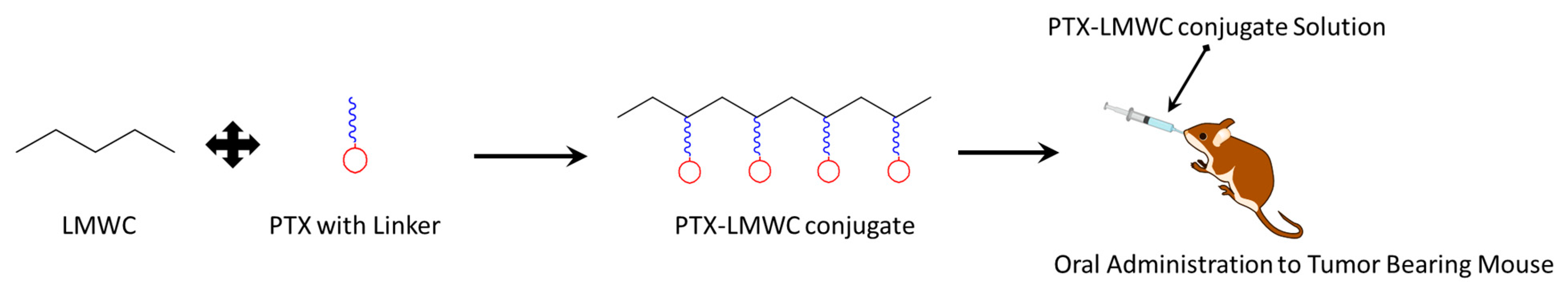
4.1.2. Chitosan with Paclitaxel (PTX)
4.1.3. Chitosan with DTX
4.1.4. Chitosan with MTX
4.1.5. Chitosan with Curcumin
4.1.6. Chitosan with Oxaliplatin
4.2. Alginate
4.2.1. Alginate with DOX
4.2.2. Alginate with Curcumin
4.2.3. Alginate with Exemestane (EXE)
4.2.4. Alginate with Tamoxifen (TMX)
4.3. Pectin
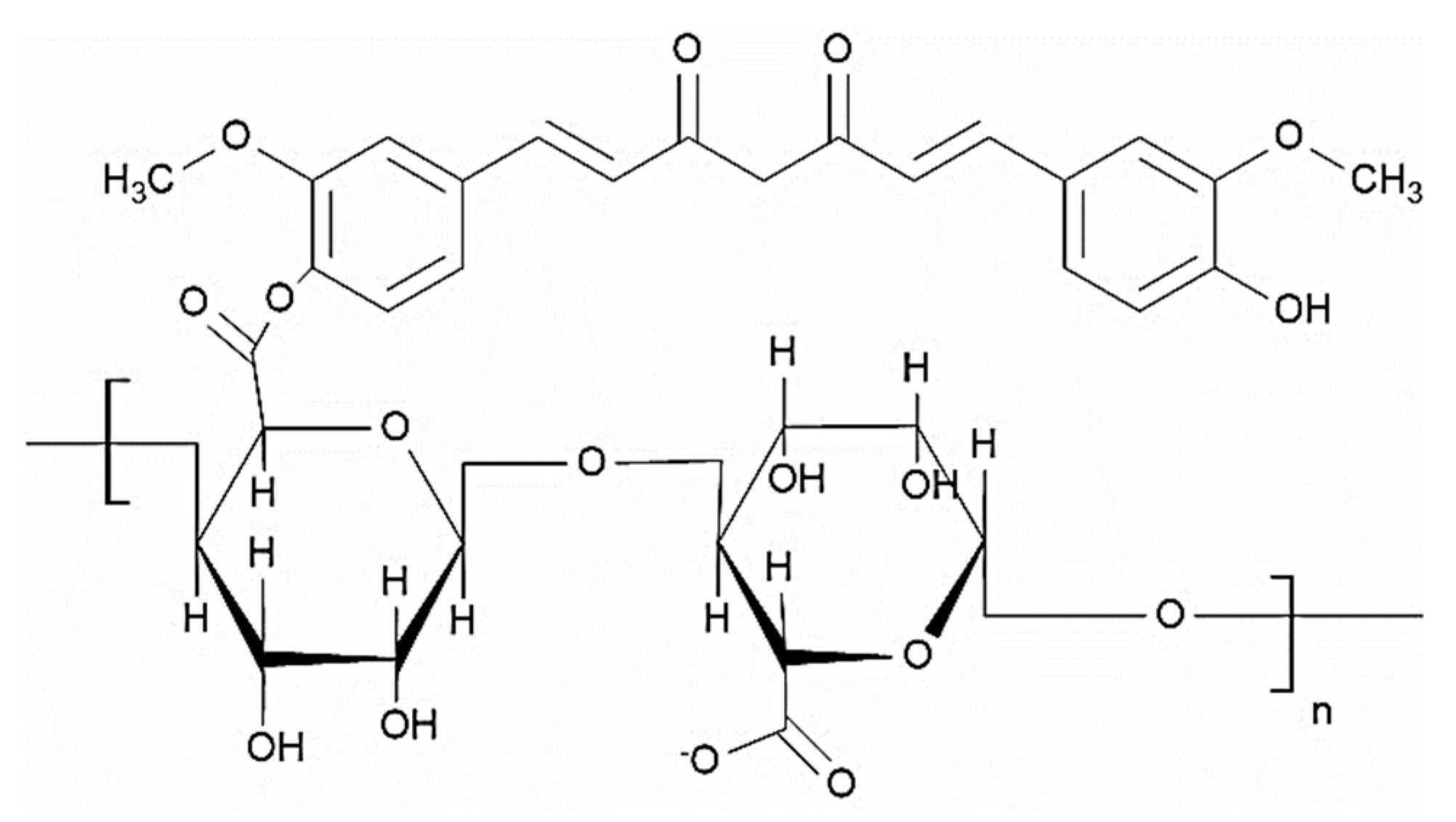
4.3.1. Pectin with Curcumin
4.3.2. Pectin with DOX
4.3.3. Pectin with Cisplatin
4.4. Guar Gum
4.4.1. Guar Gum with 5-Fluorouracil (5FU)
4.4.2. Guar Gum with Tamoxifen Citrate (TMX)
4.4.3. Guar Gum with MTX
4.4.4. Guar Gum with Curcumin
4.5. Dextran
4.5.1. Dextran with DOX
4.5.2. Dextran with PTX
4.5.3. Dextran with Phenoxodiol (PXD)
4.5.4. Dextran with MTX
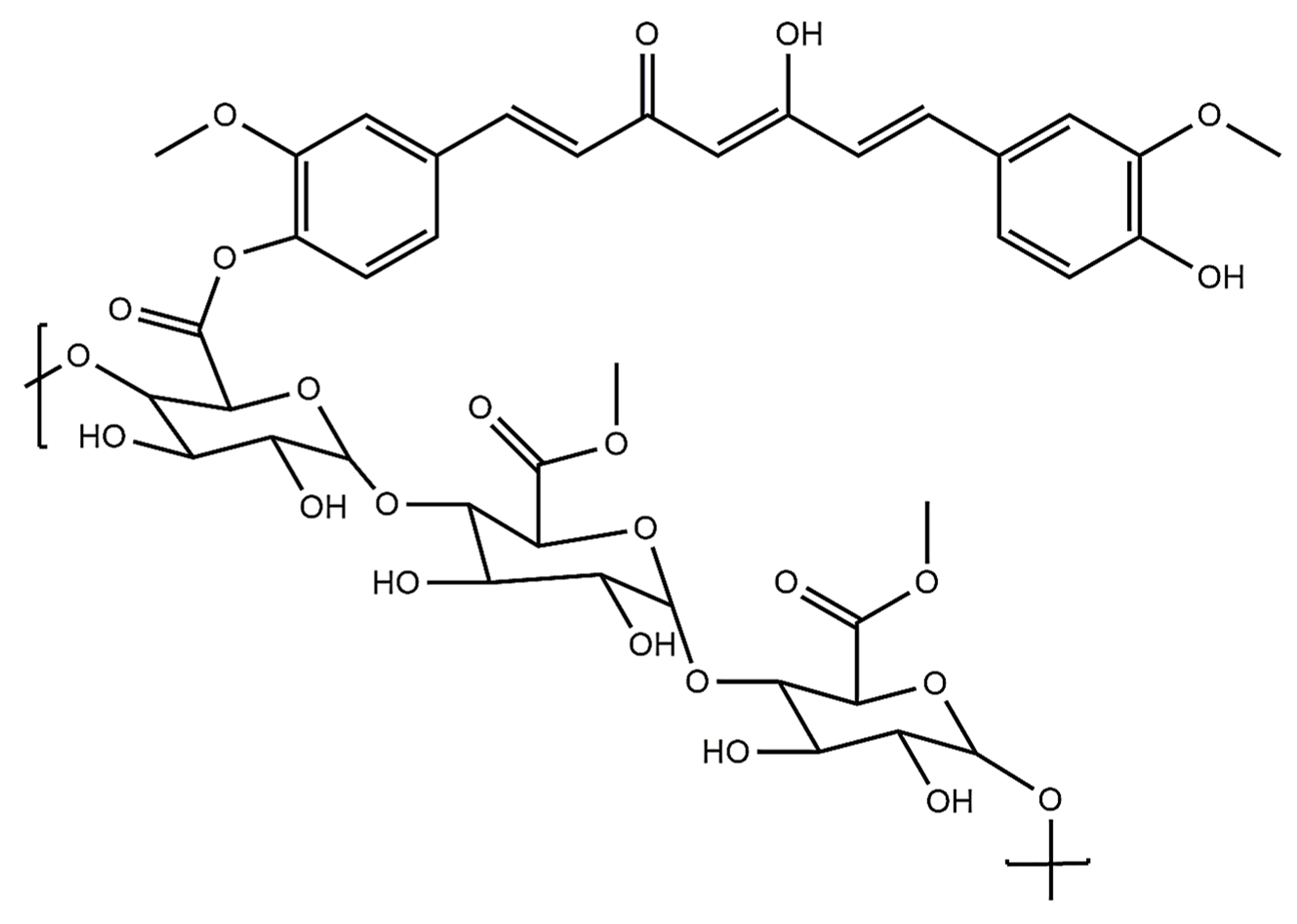
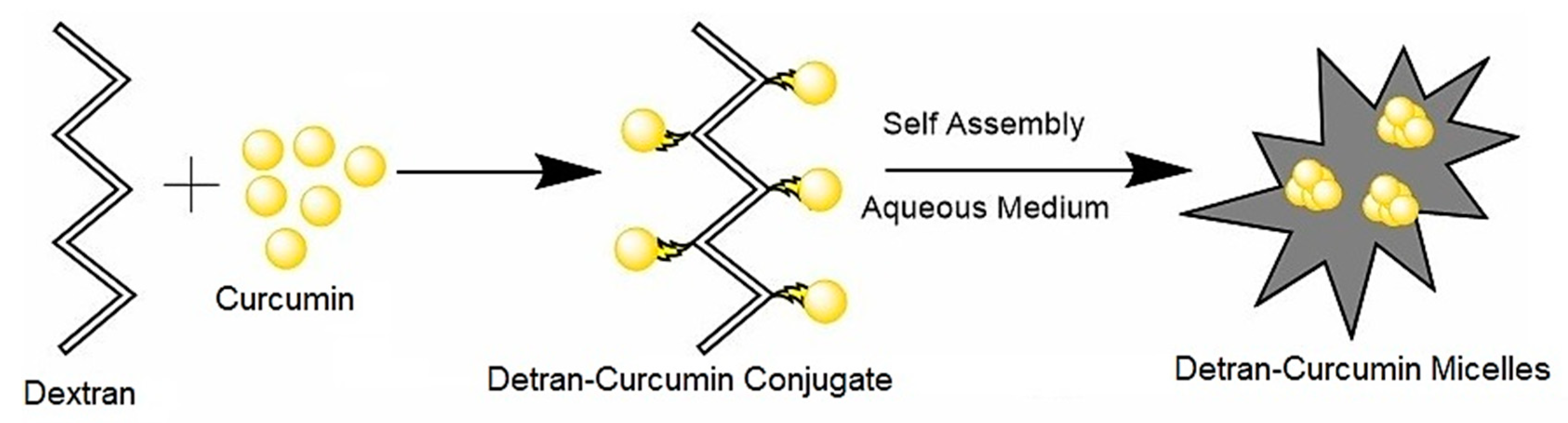
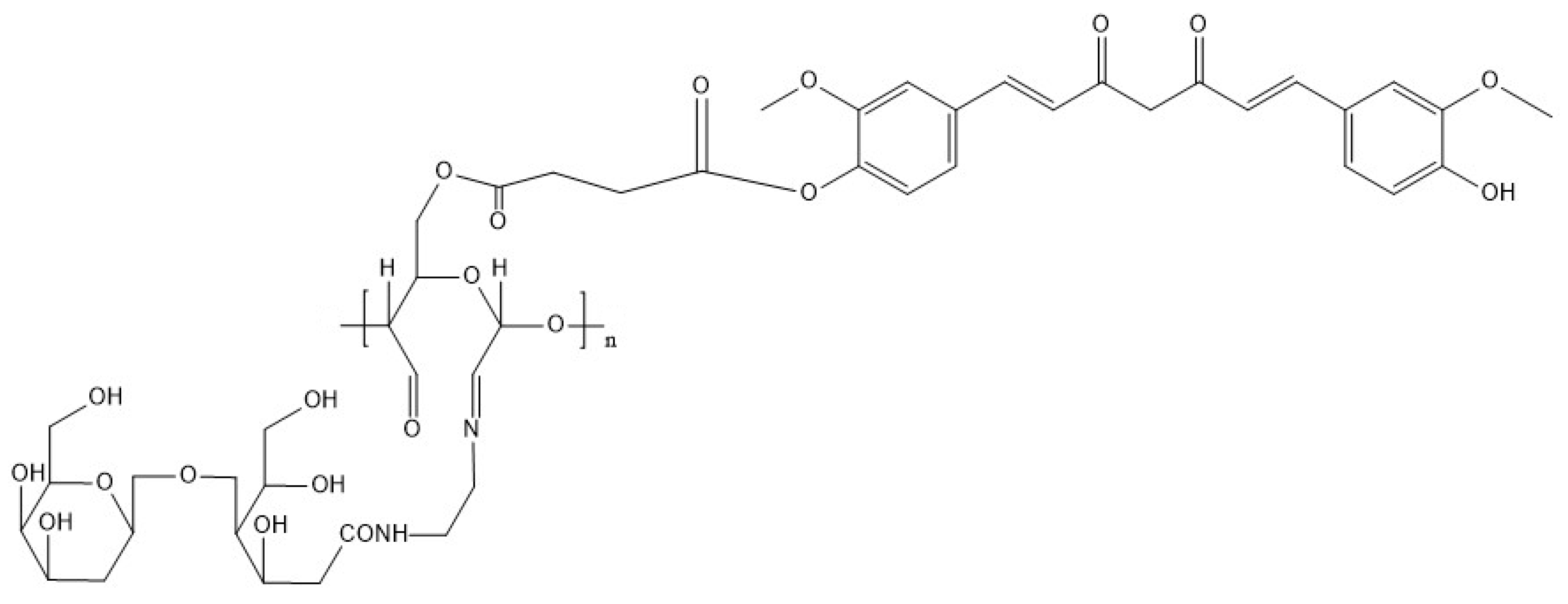
4.5.5. Dextran with Curcumin
4.6. Hyaluronic Acid (HA)
4.6.1. HA with PTX
4.6.2. HA with DOX
4.6.3. HA with Cisplatin
4.6.4. HA with Camptothecin (CPT)
4.7. Cyclodextrin
4.7.1. Cyclodextrin with DOX
4.7.2. Cyclodextrin with CPT
4.7.3. Cyclodextrin with Curcumin
4.7.4. Cyclodextrin with PTX
4.8. Pullulan
4.8.1. Pullulan with DOX
4.8.2. Pullulan with Mitoxantrone
4.8.3. Pullulan with Curcumin
5. Potentials and Prospective of Polysaccharides in Cancer Drug Delivery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dagenais, G.R.; Leong, D.P.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Gupta, R.; Diaz, R.; Avezum, A.; Oliveira, G.B.F.; Wielgosz, A.; et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2020, 395, 785–794. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yahya, E.B.; Alqadhi, A.M. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 2021, 269, 119087. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. ECancerMedicalScience 2012, 6, ed16. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 2019, 31, e1904197. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides-based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Ai, J.-W.; Liao, W.; Ren, Z.-L. Enhanced anticancer effect of copper-loaded chitosan nanoparticles against osteosarcoma. RSC Adv. 2017, 7, 15971–15977. [Google Scholar] [CrossRef] [Green Version]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, I.I.; Lazim, N.A.M.; Selvakumaran, S. Natural polysaccharide-based composites for drug delivery and biomedical applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 419–440. ISBN 9780128170557. [Google Scholar]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yao, F.; Ming, K.; Wang, D.; Hu, Y.; Liu, J. Polysaccharides from Traditional Chinese Medicines: Extraction, Purification, Modification, and Biological Activity. Molecules 2016, 21, 1705. [Google Scholar] [CrossRef]
- Ngwuluka, N.C. Responsive polysaccharides and polysaccharides-based nanoparticles for drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Salam, A., Makhlouf, H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2018; Volume 1, pp. 531–554. ISBN 9780081019979. [Google Scholar]
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef]
- Ravi, P.R.; Vats, R.; Balija, J.; Adapa, S.P.N.; Aditya, N. Modified pullulan nanoparticles for oral delivery of lopinavir: Formulation and pharmacokinetic evaluation. Carbohydr. Polym. 2014, 110, 320–328. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, D.; Hu, Y.; Kim, Y.; Hong, I.K.; Kim, M.S.; Jung, S. pH-Responsive Succinoglycan-Carboxymethyl Cellulose Hydrogels with Highly Improved Mechanical Strength for Controlled Drug Delivery Systems. Polymers 2021, 13, 3197. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Gholamali, I.; Hosseini, S.N.; Alipour, E. Doxorubicin-loaded oxidized starch/poly (vinyl alcohol)/CuO bio-nanocomposite hydrogels as an anticancer drug carrier agent. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 967–980. [Google Scholar] [CrossRef]
- Gholamali, I.; Yadollahi, M. Bio-nanocomposite Polymer Hydrogels Containing Nanoparticles for Drug Delivery: A Review. Regen. Eng. Transl. Med. 2021, 7, 129–146. [Google Scholar] [CrossRef]
- Yahya, E.B.; Jummaat, F.; Amirul, A.A.; Adnan, A.S.; Olaiya, N.G.; Abdullah, C.K.; Rizal, S.; Mohamad Haafiz, M.K.; Abdul Khalil, H.P.S. A Review on Revolutionary Natural Biopolymer-Based Aerogels for Antibacterial Delivery. Antibiotics 2020, 9, 648. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An Opinion Paper on Aerogels for Biomedical and Environmental Applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Atta, S.; Khaliq, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable biopolymer based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2015, 80, 240–245. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Yang, J.; Li, F.; Li, M.; Zhang, S.; Liu, J.; Liang, C.; Sun, Q.; Xiong, L. Fabrication and characterization of hollow starch nanoparticles by gelation process for drug delivery application. Carbohydr. Polym. 2017, 173, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, Y.; Zhou, Q.; Ao, Y.; Yu, C.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. Redox-Sensitive Hydroxyethyl Starch–Doxorubicin Conjugate for Tumor Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 30833–30844. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.; Cheung, P.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Nauts, H.C.; Swift, W.E.; Coley, B.L. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res. 1946, 6, 205–216. [Google Scholar] [PubMed]
- Fritz, H.; Kennedy, D.A.; Ishii, M.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, D. Polysaccharide K and Coriolus versicolor Extracts for Lung Cancer: A Systematic Review. Integr. Cancer Ther. 2015, 14, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, X.; Wong, K.-H. Novel nanoparticle materials for drug/food delivery-polysaccharides. Phys. Sci. Rev. 2016, 1, 20160053. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Babu, A.; Ramesh, R. Multifaceted Applications of Chitosan in Cancer Drug Delivery and Therapy. Mar. Drugs 2017, 15, 96. [Google Scholar] [CrossRef] [Green Version]
- Yogeshkumar, N.G.; GuravAtul, S.; Adhikrao, V.Y. Chitosan and Its Applications: A Review of Literature. Int. J. Res. Pharm. Biomed. Sci. 2013, 4, 312–331. [Google Scholar]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Nahar, K.; Hossain, K.; Khan, T.A. Alginate and Its Versatile Application in Drug Delivery. J. Pharm. Sci. Res. 2017, 9, 606–617. [Google Scholar]
- Lee, M.; Nah, J.; Kwon, Y.; Koh, J.J.; Ko, K.S.; Kim, S.W. Water-Soluble and Low Molecular Weight Chitosan-Based Plasmid DNA Delivery. Pharm. Res. 2001, 18, 427–431. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, J.; Dutta, P. Preparation and characterization of N-heterocyclic chitosan derivative based gels for biomedical applications. Int. J. Biol. Macromol. 2009, 45, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gim, S.; Zhu, Y.; Seeberger, P.H.; Delbianco, M. Carbohydrate-based nanomaterials for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.-A. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.K.; Chung, M.J.; Na Choi, H.; Park, Y.I. Effects of the Molecular Weight and the Degree of Deacetylation of Chitosan Oligosaccharides on Antitumor Activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Andreadis, C.; Vahtsevanos, K.; Sidiras, T.; Thomaidis, I.; Antoniadis, K.; Mouratidou, D. 5-Fluorouracil and cisplatin in the treatment of advanced oral cancer. Oral Oncol. 2003, 39, 380–385. [Google Scholar] [CrossRef]
- Remesh, A. Toxicities of anticancer drugs and its management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef] [Green Version]
- Arima, H.; Hagiwara, Y.; Hirayama, F.; Uekama, K. Enhancement of antitumor effect of doxorubicin by its complexation with γ-cyclodextrin in pegylated liposomes. J. Drug Target. 2006, 14, 225–232. [Google Scholar] [CrossRef]
- Park, J.M.; Lee, S.Y.; Lee, G.H.; Chung, E.Y.; Chang, K.M.; Kwak, B.K.; Kuh, H.-J.; Lee, J. Design and characterisation of doxorubicin-releasing chitosan microspheres for anti-cancer chemoembolisation. J. Microencapsul. 2012, 29, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 2014, 62, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, X.; Wu, J.; Liu, Y.; Liu, P.; Xu, X.; Yu, H.; Lu, L.; Che, X. Folic acid conjugated glycol chitosan micelles for targeted delivery of doxorubicin: Preparation and preliminary evaluation in vitro. J. Biomater. Sci. Polym. Ed. 2013, 24, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Trickler, W.J.; Nagvekar, A.A.; Dash, A.K. A Novel Nanoparticle Formulation for Sustained Paclitaxel Delivery. AAPS PharmSciTech 2008, 9, 486–493. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.; Lee, I.-H.; Yu, M.; Kim, H.; Chae, S.Y.; Jon, S. Conjugated Chitosan as a Novel Platform for Oral Delivery of Paclitaxel. J. Med. Chem. 2008, 51, 6442–6449. [Google Scholar] [CrossRef]
- He, R.; Yin, C. Trimethyl chitosan based conjugates for oral and intravenous delivery of paclitaxel. Acta Biomater. 2017, 53, 355–366. [Google Scholar] [CrossRef]
- Hwang, H.-Y.; Kim, I.-S.; Kwon, I.C.; Kim, Y.-H. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2008, 128, 23–31. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, K.; Kush, P.; Jain, U.K. Docetaxel loaded chitosan nanoparticles: Formulation, characterization and cytotoxicity studies. Int. J. Biol. Macromol. 2014, 69, 546–553. [Google Scholar] [CrossRef]
- Mirzaie, Z.H.; Irani, S.; Mirfakhraie, R.; Atyabi, S.M.; Dinarvand, M.; Dinarvand, R.; Varshochian, R.; Atyabi, F. Docetaxel-Chitosan nanoparticles for breast cancer treatment: Cell viability and gene expression study. Chem. Biol. Drug Des. 2016, 88, 850–858. [Google Scholar] [CrossRef]
- Wu, P.; He, X.; Wang, K.; Tan, W.; He, C.; Zheng, M. A Novel Methotrexate Delivery System Based on Chitosan-Methotrexate Covalently Conjugated Nanoparticles. J. Biomed. Nanotechnol. 2009, 5, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Methotrexate-conjugated chitosan-grafted pH- and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int. J. Biol. Macromol. 2020, 154, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Saranya, T.; Rajan, V.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Synthesis, characterisation and biomedical applications of curcumin conjugated chitosan microspheres. Int. J. Biol. Macromol. 2018, 110, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Tian, F.; Dahmani, F.Z.; Yang, H.; Yue, D.; He, S.; Zhou, J.; Yao, J. Curcumin-carboxymethyl chitosan (CNC) conjugate and CNC/LHR mixed polymeric micelles as new approaches to improve the oral absorption of P-gp substrate drugs. Drug Deliv. 2016, 23, 3424–3435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakurup, V.; Thulasidasan, A.T.; Shankar, G.M.; Retnakumari, A.P.; Nandan, C.D.; Somaraj, J.; Antony, J.; Alex, V.V.; Vinod, B.S.; Liju, V.B.; et al. Chitosan Encapsulation Enhances the Bioavailability and Tissue Retention of Curcumin and Improves its Efficacy in Preventing B[a]P-induced Lung Carcinogenesis. Cancer Prev. Res. 2019, 12, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Esfandiarpour-Boroujeni, S.; Bagheri-Khoulenjani, S.; Mirzadeh, H.; Amanpour, S. Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohydr. Polym. 2017, 168, 14–21. [Google Scholar] [CrossRef]
- Chuah, L.H.; Roberts, C.; Billa, N.; Abdullah, S.; Rosli, R. Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 116, 228–236. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Y.; Han, S.; Chen, Y.; Li, B.; Liao, M.; Chen, W.; Deng, X.; Zhao, J.; Huang, B. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2010, 400, 211–220. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Nipunbabu, V.; Ponraj, T.; Kannan, S. Oxaliplatin-chitosan nanoparticles induced intrinsic apoptotic signaling pathway: A “smart” drug delivery system to breast cancer cell therapy. Int. J. Biol. Macromol. 2014, 65, 289–297. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Rajaonarivony, M.; Vauthier, C.; Couarraze, G.; Puisieux, F.; Couvreur, P. Development of a New Drug Carrier Made from Alginate. J. Pharm. Sci. 1993, 82, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhanjana, G.; Verma, R.K.; Dhingra, D.; Dilbaghi, N.; Kim, K.-H. Metformin-loaded alginate nanoparticles as an effective antidiabetic agent for controlled drug release. J. Pharm. Pharmacol. 2017, 69, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.R.; Narayan, P.; Liu, G.; Panyam, J. Polymer-surfactant nanoparticles for improving oral bioavailability of doxorubicin. J. Pharm. Investig. 2017, 47, 65–73. [Google Scholar] [CrossRef]
- Abdelghany, S.; Alkhawaldeh, M.; AlKhatib, H.S. Carrageenan-stabilized chitosan alginate nanoparticles loaded with ethionamide for the treatment of tuberculosis. J. Drug Deliv. Sci. Technol. 2017, 39, 442–449. [Google Scholar] [CrossRef]
- Zahoor, A.; Sharma, S.; Khuller, G. Inhalable alginate nanoparticles as antitubercular drug carriers against experimental tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, S.; Kheiri, M.T.; Abnous, K.; Eskandari, M.; Tafaghodi, M. Preparation, characterization and immunological evaluation of alginate nanoparticles loaded with whole inactivated influenza virus: Dry powder formulation for nasal immunization in rabbits. Microb. Pathog. 2018, 115, 74–85. [Google Scholar] [CrossRef]
- Hefnawy, A.; Khalil, I.A.; El-Sherbiny, I.M. Facile development of nanocomplex-in-nanoparticles for enhanced loading and selective delivery of doxorubicin to brain. Nanomedicine 2017, 12, 2737–2761. [Google Scholar] [CrossRef]
- Najafabadi, A.H.; Azodi-Deilami, S.; Abdouss, M.; Payravand, H.; Farzaneh, S. Synthesis and evaluation of hydroponically alginate nanoparticles as novel carrier for intravenous delivery of propofol. J. Mater. Sci. Mater. Electron. 2015, 26, 145. [Google Scholar] [CrossRef]
- Wong, T.W.; Dhanawat, M.; Rathbone, M.J. Vaginal drug delivery: Strategies and concerns in polymeric nanoparticle development. Expert Opin. Drug Deliv. 2014, 11, 1419–1434. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.; Khar, R.K. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Gujarathi, P.; Pansare, P.; Tripathi, S. A comprehensive review on alginate-based delivery systems for the delivery of chemotherapeutic agent: Doxorubicin. Carbohydr. Polym. 2021, 259, 117696. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, S.; Wang, J.; Qian, H.; Wu, W.; Jiang, X. In vitro and in vivo Antitumor Activity of Doxorubicin-Loaded Alginic-Acid-Based Nanoparticles. Macromol. Biosci. 2012, 12, 1326–1335. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saralkar, P.; Dash, A.K. Alginate Nanoparticles Containing Curcumin and Resveratrol: Preparation, Characterization, and In Vitro Evaluation Against DU145 Prostate Cancer Cell Line. AAPS PharmSciTech 2017, 18, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sreenivasan, K. Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin. Carbohydr. Polym. 2014, 99, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.M. Exemestane: A potent irreversible aromatase inactivator and a promising advance in breast cancer treatment. Expert Rev. Anticancer Ther. 2002, 2, 267–275. [Google Scholar] [CrossRef]
- Jayapal, J.J.; Dhanaraj, S. Exemestane loaded alginate nanoparticles for cancer treatment: Formulation and in vitro evaluation. Int. J. Biol. Macromol. 2017, 105, 416–421. [Google Scholar] [CrossRef]
- Legha, S.S. Tamoxifen. Use in treatment of metastatic breast cancer refractory to combination chemotherapy. JAMA 1979, 242, 49–52. [Google Scholar] [CrossRef]
- Martínez, A.; Benito-Miguel, M.; Iglesias, I.; Teijón, J.M.; Blanco, M.D. Tamoxifen-loaded thiolated alginate-albumin nanoparticles as antitumoral drug delivery systems. J. Biomed. Mater. Res. Part A 2012, 100A, 1467–1476. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; El-Deeb, N.M.; Abbas, H.; Elmasry, S.M.; El-Aassar, M. Alginate based tamoxifen/metal dual core-folate decorated shell: Nanocomposite targeted therapy for breast cancer via ROS-driven NF-κB pathway modulation. Int. J. Biol. Macromol. 2020, 146, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P. Application of pectin in oral drug delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Features, K. The chemistry and technology of pectin. In Food Science and Technology; Academic Press: Cambridge, MA, USA, 1991; ISBN 9780080926445. [Google Scholar]
- Morris, G.A.; Samil Kök, M.; Harding, S.E.; Adams, G.G. Polysaccharide Drug Delivery Systems Based on Pectin and Chitosan. Biotechnol. Genet. Eng. Rev. 2010, 27, 257–284. [Google Scholar]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Huang, S.; Beevers, C.S. Pharmacological and clinical properties of curcumin. Bot. Targets Ther. 2011, 1, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [Green Version]
- Shih, F.-Y.; Su, I.-J.; Chu, L.-L.; Lin, X.; Kuo, S.-C.; Hou, Y.-C.; Chiang, Y.-T. Development of Pectin-Type B Gelatin Polyelectrolyte Complex for Curcumin Delivery in Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 3625. [Google Scholar] [CrossRef] [Green Version]
- Mundlia, J.; Ahuja, M.; Kumar, P.; Pillay, V. Pectin–curcumin composite: Synthesis, molecular modeling and cytotoxicity. Polym. Bull. 2019, 76, 3153–3173. [Google Scholar] [CrossRef]
- Cheewatanakornkool, K.; Niratisai, S.; Manchun, S.; Dass, C.R.; Sriamornsak, P. Thiolated pectin–doxorubicin conjugates: Synthesis, characterization and anticancer activity studies. Carbohydr. Polym. 2017, 174, 493–506. [Google Scholar] [CrossRef]
- Tao, Y.; Zheng, D.; Zhao, J.; Liu, K.; Liu, J.; Lei, J.; Wang, L. Self-Assembling PH-Responsive Nanoparticle Platform Based on Pectin-Doxorubicin Conjugates for Codelivery of Anticancer Drugs. ACS Omega 2021, 6, 9998–10004. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.K.; Chanchal, A.; Chutani, K. Augmentation of anti-tumour activity of cisplatin by pectin nano-conjugates in B-16 mouse model: Pharmacokinetics and in-vivo biodistribution of radio-labelled, hydrophilic nano-conjugates. Int. J. Nanotechnol. 2012, 9, 872–886. [Google Scholar] [CrossRef]
- Verma, A.; Sachin, A. Novel Hydrophilic Drug Polymer Nano-Conjugates of Cisplatin Showing Long Blood Retention Profile: Its Release Kinetics, Cellular Uptake and Bio-Distribution. Curr. Drug Deliv. 2008, 5, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.J.; Karve, M.; Patel, N.K. View of Guar Gum: A Versatile Material for Pharmaceutical Industries. Int. J. Pharm. Pharm. Sci. 2014, 6, 13–19. [Google Scholar]
- Prabaharan, M. Prospective of guar gum and its derivatives as controlled drug delivery systems. Int. J. Biol. Macromol. 2011, 49, 117–124. [Google Scholar] [CrossRef]
- Pardini, B.; Kumar, R.; Naccarati, A.; Novotny, J.; Prasad, R.B.; Forsti, A.; Hemminki, K.; Vodicka, P.; Bermejo, J.L. 5-Fluorouracil-based chemotherapy for colorectal cancer and MTHFR/MTRR genotypes. Br. J. Clin. Pharmacol. 2011, 72, 162–163. [Google Scholar] [CrossRef] [Green Version]
- Kamal, T.; Sarfraz, M.; Arafat, M.; Mikov, M.; Rahman, N. Cross-linked guar gum and sodium borate based microspheres as colon-targeted anticancer drug delivery systems for 5-fluorouracil. Pak. J. Pharm. Sci. 2017, 30, 2329–2336. [Google Scholar]
- Lashley, M.R.; Niedzinski, E.J.; Rogers, J.M.; Denison, M.S.; Nantz, M.H. Synthesis and estrogen receptor affinity of a 4-hydroxytamoxifen-Labeled ligand for diagnostic imaging. Bioorgan. Med. Chem. 2002, 10, 4075–4082. [Google Scholar] [CrossRef]
- Krishnaiah, Y.; Karthikeyan, R.; Satyanarayana, V. A three-layer guar gum matrix tablet for oral controlled delivery of highly soluble metoprolol tartrate. Int. J. Pharm. 2002, 241, 353–366. [Google Scholar] [CrossRef]
- Soppirnath, K.S.; Aminabhavi, T.M. Water transport and drug release study from cross-linked polyacrylamide grafted guar gum hydrogel microspheres for the controlled release application. Eur. J. Pharm. Biopharm. 2002, 53, 87–98. [Google Scholar] [CrossRef]
- Chaurasia, M.; Chourasia, M.K.; Jain, N.K.; Jain, A.; Soni, V.; Gupta, Y.; Jain, S.K. Cross-linked guar gum microspheres: A viable approach for improved delivery of anticancer drugs for the treatment of colorectal cancer. AAPS PharmSciTech 2006, 7, E143–E151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, E.J.; Anil, S.; Ahmad, S.; Daud, A. Colon Targeted Curcumin Delivery Using Guar Gum. Nat. Prod. Commun. 2010, 5, 915–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Huang, G. Preparation and drug delivery of dextran-drug complex. Drug Deliv. 2019, 26, 252–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Montes, E. Dextran: Sources, Structures, and Properties. Polysaccharides 2021, 2, 554–565. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional Polymers Based on Dextran. Adv. Polym. Sci. 2006, 205, 199–291. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of dextran as nanoscale drug carriers. Nanomedicine 2018, 13, 3149–3158. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Mukerabigwi, J.F.; Liu, M.; Luo, S.; Lei, S.; Cao, Y.; Huang, X.; He, H. Self-organized nanoparticle drug delivery systems from a folate-targeted dextran–doxorubicin conjugate loaded with doxorubicin against multidrug resistance. RSC Adv. 2015, 5, 71164–71173. [Google Scholar] [CrossRef]
- Cao, D.; He, J.; Xu, J.; Zhang, M.; Zhao, L.; Duan, G.; Cao, Y.; Zhou, R.; Ni, P. Polymeric prodrugs conjugated with reduction-sensitive dextran–camptothecin and pH-responsive dextran–doxorubicin: An effective combinatorial drug delivery platform for cancer therapy. Polym. Chem. 2016, 7, 4198–4212. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Ma, X.; Wang, Y.; Lu, Y.; Jia, D.; Huang, X.; Chen, J.; Xu, Z.; Wen, F. The design and synthesis of dextran-doxorubicin prodrug-based pH-sensitive drug delivery system for improving chemotherapy efficacy. Asian J. Pharm. Sci. 2019, 15, 605–616. [Google Scholar] [CrossRef]
- Nakamura, J.; Nakajima, N.; Matsumura, K.; Hyon, S.-H. Water-soluble taxol conjugates with dextran and targets tumor cells by folic acid immobilization. Anticancer Res. 2010, 30, 903–909. [Google Scholar]
- Kanwal, S.; Naveed, M.; Arshad, A.; Arshad, A.; Firdous, F.; Faisal, A.; Yameen, B. Reduction-Sensitive Dextran-Paclitaxel Polymer-Drug Conjugate: Synthesis, Self-Assembly into Nanoparticles, and in Vitro Anticancer Efficacy. Bioconjugate Chem. 2021, 32, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Georgaki, S.; Skopeliti, M.; Tsiatas, M.; Nicolaou, K.A.; Ioannou, K.; Husband, A.; Bamias, A.; Dimopoulos, M.A.; Constantinou, A.I.; Tsitsilonis, O.E. Phenoxodiol, an anticancer isoflavene, induces immunomodulatory effects in vitro and in vivo. J. Cell. Mol. Med. 2009, 13, 3929–3938. [Google Scholar] [CrossRef] [Green Version]
- Gamble, J.R.; Xia, P.; Hahn, C.; Drew, J.J.; Drogemuller, C.J.; Brown, D.; Vadas, M.A. Phenoxodiol, an experimental anticancer drug, shows potent antiangiogenic properties in addition to its antitumour effects. Int. J. Cancer 2006, 118, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Yee, E.M.H.; Cirillo, G.; Brandl, M.B.; Black, D.S.; Vittorio, O.; Kumar, N. Synthesis of Dextran–Phenoxodiol and Evaluation of Its Physical Stability and Biological Activity. Front. Bioeng. Biotechnol. 2019, 7, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevozhay, D.; Budzynska, R.; Jagiello, M.; Kanska, U.; Omar, M.S.; Opolski, A.; Wietrzyk, J.; Boratynski, J. The effect of the substitution level of some dextran-methotrexate conjugates on their antitumor activity in experimental cancer models. Anticancer Res. 2006, 26, 2179–2186. [Google Scholar]
- Dang, W.; Colvin, O.M.; Brem, H.; Saltzman, W.M. Covalent coupling of methotrexate to dextran enhances the penetration of cytotoxicity into a tissue-like matrix. Cancer Res. 1994, 54, 1729–1735. [Google Scholar] [PubMed]
- Huang, Q.; Zhang, L.; Sun, X.; Zeng, K.; Li, J.; Liu, Y.-N. Coating of carboxymethyl dextran on liposomal curcumin to improve the anticancer activity. RSC Adv. 2014, 4, 59211–59217. [Google Scholar] [CrossRef]
- Raveendran, R.; Bhuvaneshwar, G.; Sharma, C.P. Hemocompatible curcumin–dextran micelles as pH sensitive pro-drugs for enhanced therapeutic efficacy in cancer cells. Carbohydr. Polym. 2016, 137, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Sarkati, M.N.; Tashakkorian, H.; Partovi, R.; Rahaiee, S. Dextran-immobilized curcumin: An efficient agent against food pathogens and cancer cells. J. Bioact. Compat. Polym. 2019, 34, 309–320. [Google Scholar] [CrossRef]
- Curcio, M.; Cirillo, G.; Tucci, P.; Farfalla, A.; Bevacqua, E.; Vittorio, O.; Iemma, F.; Nicoletta, F.P. Dextran-Curcumin Nanoparticles as a Methotrexate Delivery Vehicle: A Step Forward in Breast Cancer Combination Therapy. Pharmaceuticals 2020, 13, 2. [Google Scholar] [CrossRef] [Green Version]
- Alaniz, L.; Cabrera, P.V.; Blanco, G.G.; Ernst, G.; Rimoldi, G.; Alvarez, E.; Hajos, S.E. Interaction of CD44 with Different Forms of Hyaluronic Acid. Its Role in Adhesion and Migration of Tumor Cells. Cell Commun. Adhes. 2002, 9, 117–130. [Google Scholar] [CrossRef]
- Jong, A.; Wu, C.-H.; Gonzales-Gomez, I.; Kwon-Chung, K.J.; Chang, Y.C.; Tseng, H.-K.; Cho, W.-L.; Huang, S.-H. Hyaluronic Acid Receptor CD44 Deficiency Is Associated with Decreased Cryptococcus neoformans Brain Infection. J. Biol. Chem. 2012, 287, 15298–15306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widjaja, L.K.; Bora, M.; Chan, P.N.P.H.; Lipik, V.; Wong, T.T.L.; Venkatraman, S.S. Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications. J. Biomed. Mater. Res. Part A 2014, 102, 3056–3065. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Huai, J.; Chen, X.; Yang, Y.; Zhang, X.; Gan, Y.; Wang, G.; Gu, X.; Li, J. Intracellular delivery and antitumor effects of a redox-responsive polymeric paclitaxel conjugate based on hyaluronic acid. Acta Biomater. 2015, 26, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic Acid−Paclitaxel Conjugate Micelles: Synthesis, Characterization, and Antitumor Activity. Bioconj. Chem. 2008, 19, 1319–1325. [Google Scholar] [CrossRef]
- Wickens, J.M.; Alsaab, H.O.; Kesharwani, P.; Bhise, K.; Amin, M.C.I.M.; Tekade, R.K.; Gupta, U.; Iyer, A.K. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today 2017, 22, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Huang, P.; Chang, L.; Long, X.; Dong, A.; Liu, J.; Chu, L.; Hu, F.; Liu, J.; Deng, L. Tumor targeting and pH-responsive polyelectrolyte complex nanoparticles based on hyaluronic acid-paclitaxel conjugates and Chitosan for oral delivery of paclitaxel. Macromol. Res. 2013, 21, 1331–1337. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Cai, S.; Thati, S.; Bagby, T.R.; Diab, H.-M.; Davies, N.M.; Cohen, M.S.; Forrest, M.L. Localized doxorubicin chemotherapy with a biopolymeric nanocarrier improves survival and reduces toxicity in xenografts of human breast cancer. J. Control. Release 2010, 146, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Simanek, E.E. Cancer Therapies Utilizing the Camptothecins: A Review of the in Vivo Literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzolato, J.F.; Saltz, L.B. The camptothecins. Lancet 2003, 361, 2235–2242. [Google Scholar] [CrossRef]
- Serafino, A.; Zonfrillo, M.; Andreola, F.; Psaila, R.; Mercuri, L.; Moroni, N.; Renier, D.; Campisi, M.; Secchieri, C.; Pierimarchi, P. CD44-targeting for antitumor drug delivery: A new SN-38-hyaluronan bioconjugate for locoregional treatment of peritoneal carcinomatosis. Curr. Cancer Drug Targets 2011, 11, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Montagner, I.M.; Merlo, A.; Zuccolotto, G.; Renier, D.; Campisi, M.; Pasut, G.; Zanovello, P.; Rosato, A. Peritoneal Tumor Carcinomatosis: Pharmacological Targeting with Hyaluronan-Based Bioconjugates Overcomes Therapeutic Indications of Current Drugs. PLoS ONE 2014, 9, e112240. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H. Formulation. In Drug-Like Properties Concepts, Structure Design and Methods from ADME to Toxicity Optimization; Di, L., Kerns, E.H., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 497–510. [Google Scholar]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2016, 23, 739–757. [Google Scholar] [CrossRef]
- Çırpanlı, Y.; Allard, E.; Passirani, C.; Bilensoy, E.; Lemaire, L.; Çalış, S.; Benoit, J.-P. Antitumoral activity of camptothecin-loaded nanoparticles in 9L rat glioma model. Int. J. Pharm. 2011, 403, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Çirpanli, Y.; Bilensoy, E.; Doğan, A.L.; Çaliş, S. Comparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camptothecin delivery. Eur. J. Pharm. Biopharm. 2009, 73, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-Applied Curcumin for Different Diseases Therapy. BioMed Res. Int. 2014, 2014, 394264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef]
- Jing, J.; Szarpak-Jankowska, A.; Guillot, R.; Pignot-Paintrand, I.; Picart, C.; Auzély-Velty, R. Cyclodextrin/Paclitaxel Complex in Biodegradable Capsules for Breast Cancer Treatment. Chem. Mater. 2013, 25, 3867–3873. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Wang, X.; Zhen, X.; Zhang, Z.; Wu, W.; Jiang, X. Synthesis of Paclitaxel-Conjugated β-Cyclodextrin Polyrotaxane and Its Antitumor Activity. Angew. Chem. Int. Ed. 2013, 52, 7272–7277. [Google Scholar] [CrossRef] [PubMed]
- Yusheng, S.; Chenjun, M.; Yingying, H.; Tiantian, W.; Liefeng, Z. Multifunctional nanoparticles of paclitaxel and cyclodextrin–polypeptide conjugates with in vitro anticancer activity. Pharm. Dev. Technol. 2020, 25, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Suneetha, V.; Ramalingam, C. An overview of Mechanistic Characterization and optimization of Pullulan producing microorganism. South Asian J. Exp. Biol. 2011, 1, 147–151. [Google Scholar] [CrossRef]
- Mishra, B.; Vuppu, S.; Rath, K. The Role of Microbial Pullulan, a Biopolymer in Pharmaceutical Approaches: A Review. J. Appl. Pharm. Sci. 2011, 1, 45–50. [Google Scholar]
- Jeans, A. Dextrans and pullulans: Industrially significant α-d-glucans. In Encyclopedia of Polymer Science and Technology; Jeans, A., Ed.; John Wiley and Sons Inc: New York, NY, USA, 1966; Volume 4, pp. 819–821. [Google Scholar]
- Yuen, S. Pullulan and Its Applications. Process Biochem. 1974, 22, 7–9. [Google Scholar]
- Gao, F.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Tan, L.; Li, H.; Huang, X.; Peng, B.; Yan, C.; et al. Doxorubicin loaded silica nanorattles actively seek tumors with improved anti-tumor effects. Nanoscale 2012, 4, 3365–3372. [Google Scholar] [CrossRef]
- Posner, L.E.; Dukart, G.; Goldberg, J.D.; Bernstein, T.; Cartwright, K. Mitoxantrone: An overview of safety and toxicity. Investig. New Drugs 1985, 3, 123–132. [Google Scholar] [CrossRef]
- Tao, X.; Tao, T.; Wen, Y.; Yi, J.; He, L.; Huang, Z.; Nie, Y.; Yao, X.; Wang, Y.; He, C.; et al. Novel Delivery of Mitoxantrone with Hydrophobically Modified Pullulan Nanoparticles to Inhibit Bladder Cancer Cell and the Effect of Nano-drug Size on Inhibition Efficiency. Nanoscale Res. Lett. 2018, 13, 345. [Google Scholar] [CrossRef] [Green Version]
- Falvo, E.; Malagrinò, F.; Arcovito, A.; Fazi, F.; Colotti, G.; Tremante, E.; Di Micco, P.; Braca, A.; Opri, R.; Giuffrè, A.; et al. The presence of glutamate residues on the PAS sequence of the stimuli-sensitive nano-ferritin improves in vivo biodistribution and mitoxantrone encapsulation homogeneity. J. Control. Release 2018, 275, 177–185. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan and pullulan derivatives as promising biomolecules for drug and gene targeting. Carbohydr. Polym. 2015, 123, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Ganeshkumar, M.; Ponrasu, T.; Subamekala, M.K.; Janani, M.; Suguna, L. Curcumin loaded on pullulan acetate nanoparticles protects the liver from damage induced by DEN. RSC Adv. 2016, 6, 5599–5610. [Google Scholar] [CrossRef]
- Sarika, P.; James, N.R.; Nishna, N.; Kumar, P.A.; Raj, D.K. Galactosylated pullulan–curcumin conjugate micelles for site specific anticancer activity to hepatocarcinoma cells. Colloids Surf. B Biointerfaces 2015, 133, 347–355. [Google Scholar] [CrossRef] [PubMed]



| Polysaccharides | Sources | Physicochemical Properties | Applications and Benefits |
|---|---|---|---|
| Chitosan | Shells of crab, shrimp, and krill | Soluble in weak acids, mucoadhesive, reacts with negatively charged surfaces | Tissue regenerative medicine, pulmonary delivery, ionotropic gelation |
| Alginates | Marine brown algae | Water soluble, anionic coacervation with ions and polycations | pH-dependent swelling nontoxic, diffusion, erosion, in situ forming hydrogels |
| Cyclodextrin | Degraded starch derived from potato, corn, rice., etc. | Water soluble, nontoxic | Nanocarrier for controlled drug release, gene and drug delivery |
| Pullulan | Bacterial Homopolysaccharides produced from starch by Aureobasidium pullalans | Neutral polymer underivatized pullulan has high water solubility | Emulsifier sustained-release preparations |
| Hyaluronic acid | Vertebrate organisms | Biodegradable, bioactive, nonimmunogenic | Anti-cancer drug delivery, wound healing and skin regeneration |
| Dextran | Bacterial strains, cell-free supernatant | Neutral polymer, solubility depends on degree of polymerization | Colon-targeted delivery |
| Guar gum | Seeds of Cyamopsis tetragonoloba | Water soluble, non-ionic, galactomannan forms a thixotropic solution, stable at pH 4–10.5 | Controlled release, colon-targeted release, thermoreversible |
| Pectin | Plant cell wall | Negatively charged molecule | In Situ gelling, sustained delivery, drug delivery in colorectal carcinoma |
| Formulation | Model Used | Biological Changes | Reference |
|---|---|---|---|
| PTX-trimethyl chitosan conjugates | H22 tumor-bearing mice | Enhanced mucoadhesion and intestinal transport of PTX, increased tumor retardation and survival rate | [59] |
| Erlotinib-loaded MTX-chitosan magnetic nanoparticles | OVCAR-3 cell lines, | Improved cellular uptake, greater cytotoxicity and target specific delivery in FR-positive cancer cell lines | [64] |
| Curcumin-loaded chitosan nanoparticles | Swiss albino mice | Inhibiting the B[a]P-induced lung carcinogenesis, overexpression of p65 in the nuclei, reduced the overexpression of proliferating cell nuclear antigen | [67] |
| Curcumin-loaded folate-modified-chitosan-nanoparticles | MCF7 cell lines, L929 cell lines | Target specific uptake of curcumin into cancerous cells | [68] |
| Alginate nanoparticles with curcumin and resveratrol | DU145 prostate cancer cells | Increased cell uptake and enhanced cytotoxicity in cancer cells | [87] |
| EXE-loaded alginate nanoparticles | Dalton’s lymphoma ascites cells | Improved cytotoxicity | [90] |
| Ag/Alg-TMX-PEG/FA core shell nanocomposite | MCF7 cell lines | Inducting reactive oxygen species (ROS), downregulation of survival oncogenic genes, G2/M phase arrest | [93] |
| Pectin–curcumin composite | KYSE-30 cell lines | Release of curcumin from the composite at acidic pH, enhanced cytotoxicity | [101] |
| Dihydroartemisinin-loaded DOX–pectin conjugate | MCF-7 cell lines, C57BL/6 mouse | Intranuclear uptake in MCF-7 cell lines, significant reduction in tumor growth | [103] |
| MTX-loaded guar gum microspheres | Albino rats | Target specific delivery to the colon | [114] |
| Dextran–DOX micelles | Balb/C mice bearing 4T1 tumors | Acid-sensitive drug release minimize systemic toxicity in normal tissues Selective accumulation in tumor and enhanced tumor-suppressive efficiency | [122] |
| MTX-loaded dextran–CUR nanoparticles | MCF-7 cell lines | Rapid internalization and enhanced cytotoxicity | [133] |
| PTX–HA conjugate | MCF-7 cell lines BALB/c nude mice | Cellular internalization, and tumor targeting via CD44 caveolae-mediated endocytosis, target specific drug release in the presence of GSH | [137] |
| Paclitaxel-loaded cyclodextrin–polypeptide conjugates | MCF-7 and 4T1 cell lines | Enhanced cellular uptake, inhibit P-gp efflux pumps | [157] |
| Mitoxantrone loaded modified pullulan nanoparticles | MB49 cells | Inhibit the growth migration of MB49 cells | [164] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, N.; Francis, A.P.; Priya, V.V.; Patil, S.; Mustaq, S.; Khan, S.S.; Alzahrani, K.J.; Banjer, H.J.; Mohan, S.K.; Mony, U.; et al. Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy. Polymers 2022, 14, 950. https://doi.org/10.3390/polym14050950
Yadav N, Francis AP, Priya VV, Patil S, Mustaq S, Khan SS, Alzahrani KJ, Banjer HJ, Mohan SK, Mony U, et al. Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy. Polymers. 2022; 14(5):950. https://doi.org/10.3390/polym14050950
Chicago/Turabian StyleYadav, Neena, Arul Prakash Francis, Veeraraghavan Vishnu Priya, Shankargouda Patil, Shazia Mustaq, Sameer Saeed Khan, Khalid J. Alzahrani, Hamsa Jameel Banjer, Surapaneni Krishna Mohan, Ullas Mony, and et al. 2022. "Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy" Polymers 14, no. 5: 950. https://doi.org/10.3390/polym14050950
APA StyleYadav, N., Francis, A. P., Priya, V. V., Patil, S., Mustaq, S., Khan, S. S., Alzahrani, K. J., Banjer, H. J., Mohan, S. K., Mony, U., & Rajagopalan, R. (2022). Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy. Polymers, 14(5), 950. https://doi.org/10.3390/polym14050950