EGDMA- and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Instruments
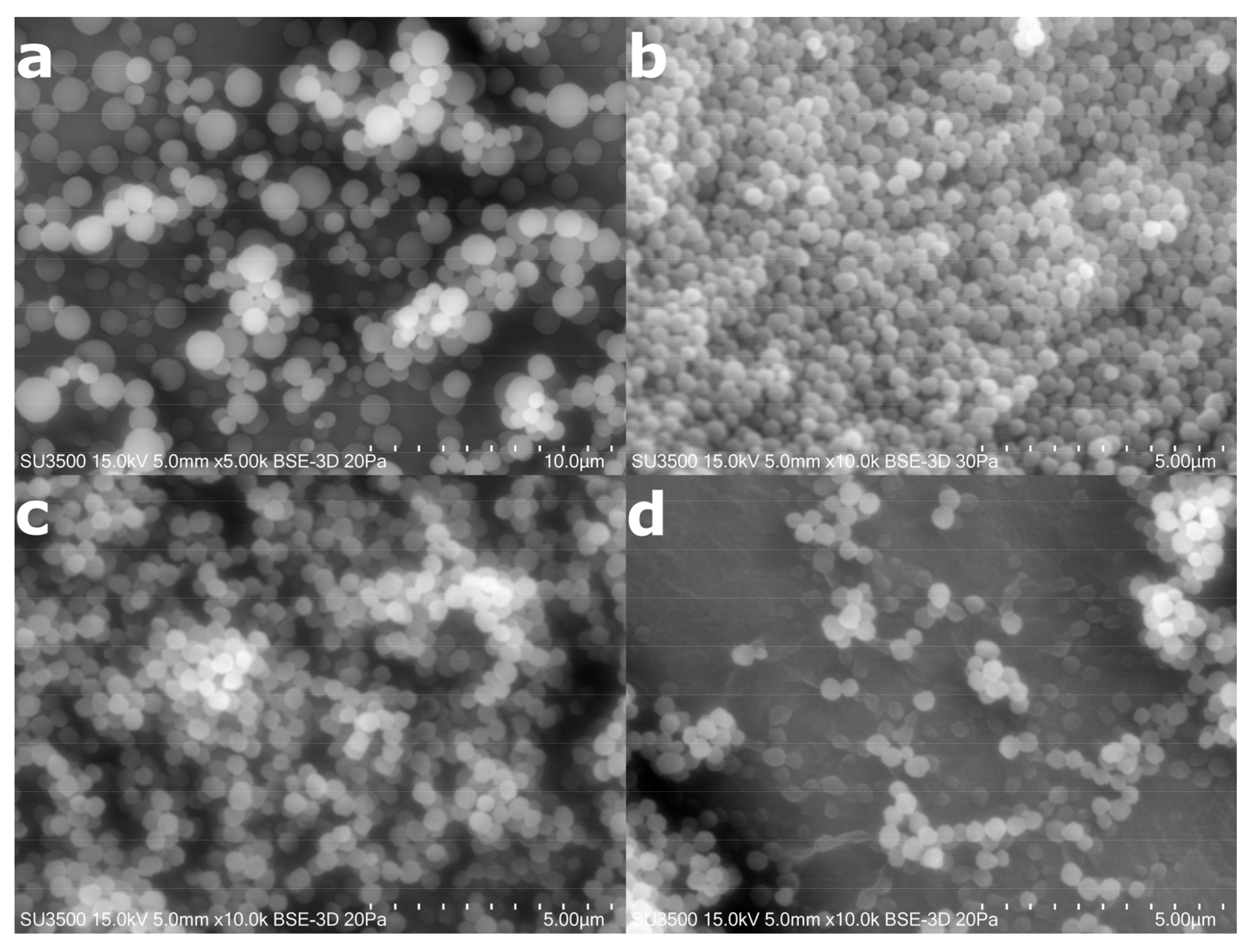
2.3. Synthesis of Microparticles
2.4. Adsorption Studies
2.5. Release Experiments
2.6. Cytotoxicity Assays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Adsorption Isotherms
3.2. Adsorption Kinetics
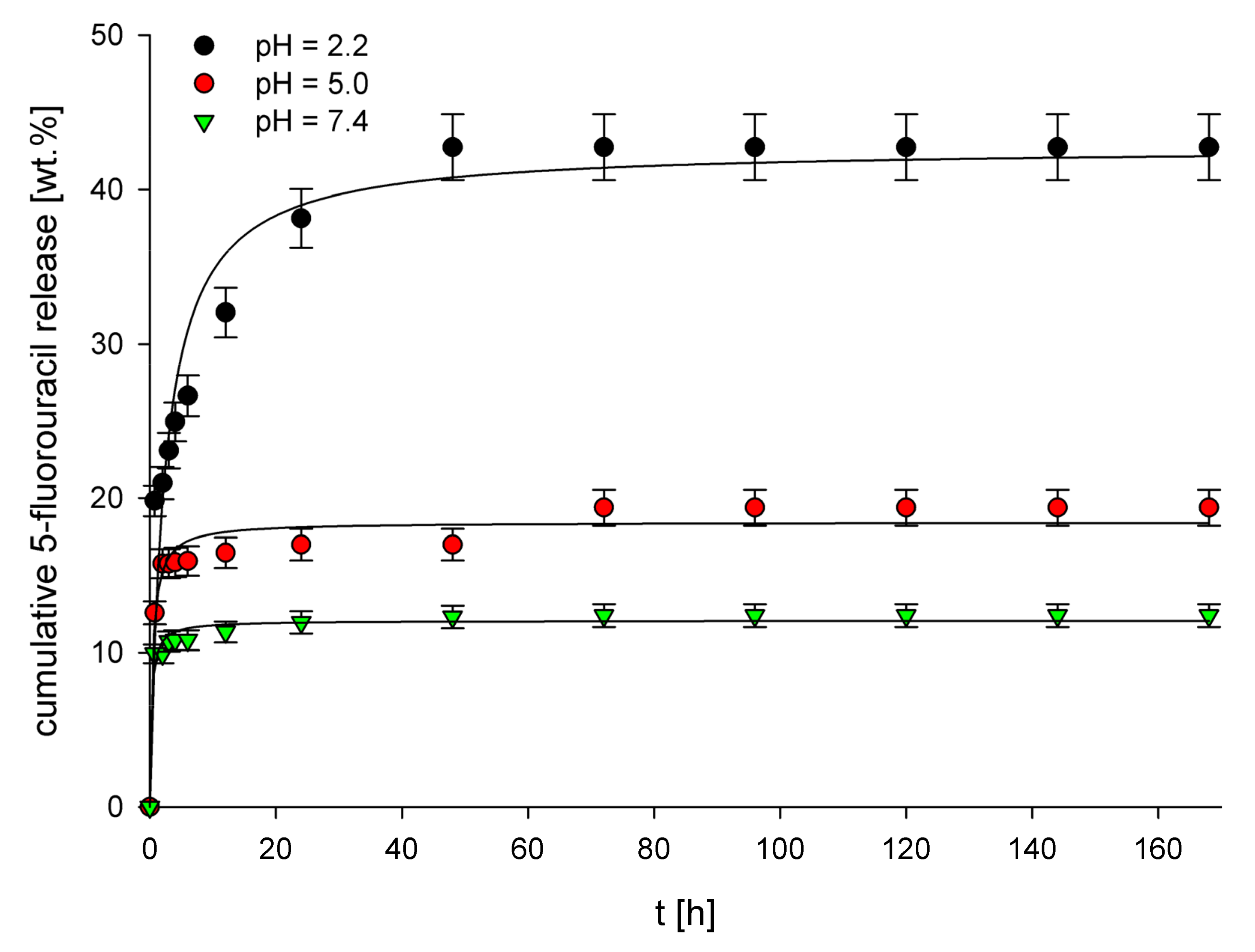
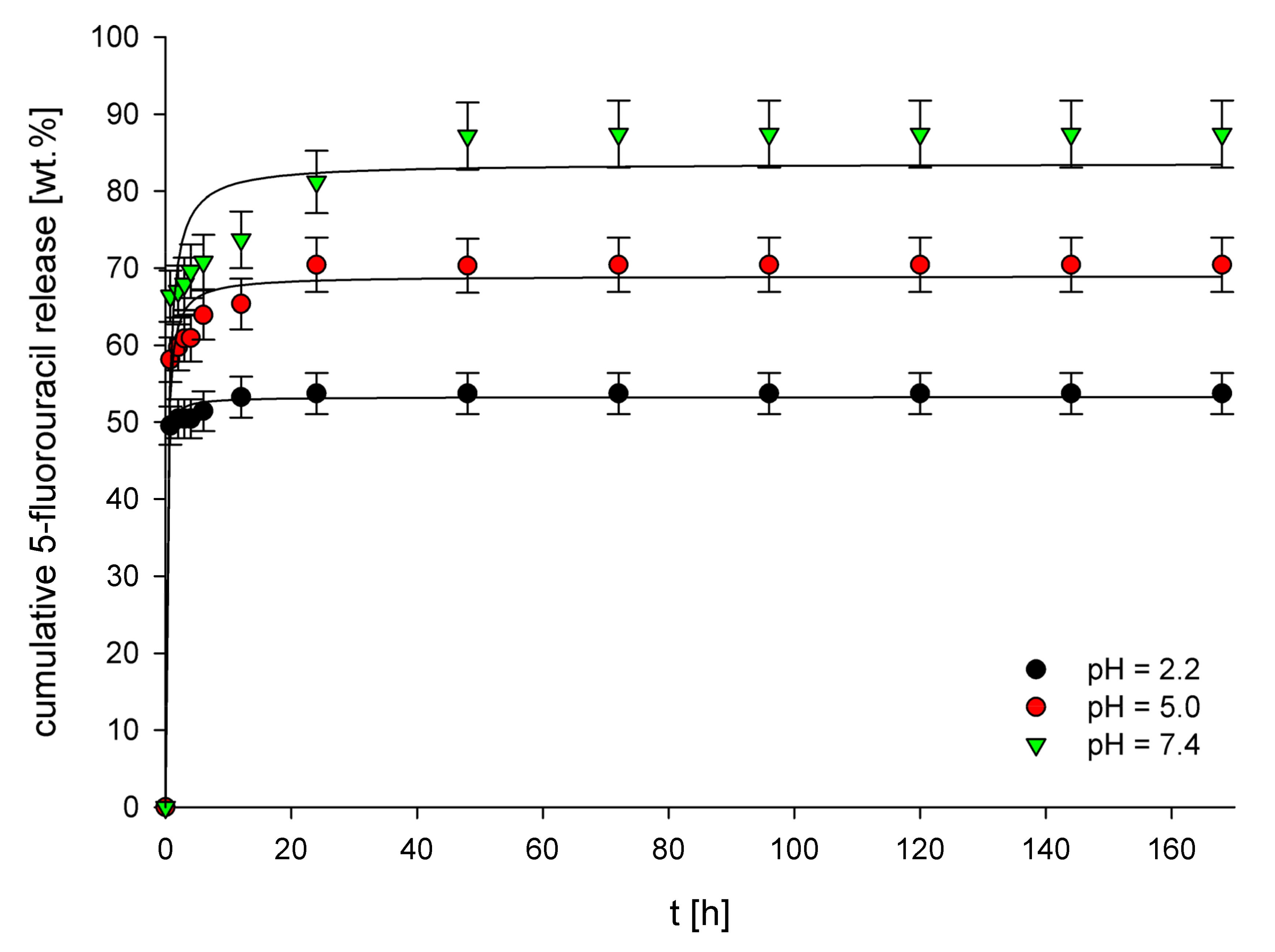
3.3. In Vitro Release Studies
3.4. In Vitro Cell Viability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Abdou, E.M.; Fayed, M.A.A.; Helal, D.; Ahmed, K.A. Assessment of the hepatoprotective effect of developed lipid-polymer hybrid nanoparticles (LPHNPs) encapsulating naturally extracted β-Sitosterol against CCl4 induced hepatotoxicity in rats. Sci. Rep. 2019, 9, 19779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iachetta, G.; Falanga, A.; Molino, Y.; Masse, M.; Jabès, F.; Mechioukhi, Y.; Laforgia, V.; Khrestchatisky, M.; Galdiero, S.; Valiante, S. gH625-liposomes as tool for pituitary adenylate cyclase-activating polypeptide brain delivery. Sci. Rep. 2019, 9, 9183. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Huang, Y.-H.; Cheng, K.-C.; Song, Y.-L. Investigations of the Influences of Processing Conditions on the Properties of Spray Dried Chitosan-Tripolyphosphate Particles loaded with Theophylline. Sci. Rep. 2020, 10, 1155. [Google Scholar] [CrossRef]
- Babu, A.; Amreddy, N.; Muralidharan, R.; Pathuri, G.; Gali, H.; Chen, A.; Zhao, Y.D.; Munshi, A.; Ramesh, R. Chemodrug delivery using integrin-targeted PLGA-Chitosan nanoparticle for lung cancer therapy. Sci. Rep. 2017, 7, 14674. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Yoshikawa, C.; Welch, N.G.; Pasic, P.; Thissen, H.; Voelcker, N.H. Spatially Controlled Surface Modification of Porous Silicon for Sustained Drug Delivery Applications. Sci. Rep. 2019, 9, 1367. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Liu, L.; Han, C.; Ma, X.; Qian, J.; Zhou, J.; Zhu, W. Cancer cell membrane-coated mesoporous silica loaded with superparamagnetic ferroferric oxide and Paclitaxel for the combination of Chemo/Magnetocaloric therapy on MDA-MB-231 cells. Sci. Rep. 2019, 9, 14475. [Google Scholar] [CrossRef]
- Amolegbe, S.A.; Hirano, Y.; Adebayo, J.O.; Ademowo, O.G.; Balogun, E.A.; Obaleye, J.A.; Krettli, A.U.; Yu, C.; Hayami, S. Mesoporous silica nanocarriers encapsulated antimalarials with high therapeutic performance. Sci. Rep. 2018, 8, 3078. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Gao, Y.; Song, W.; Zhao, Q.; Zhang, H.; Zhang, H. Halloysite nanotube-based H2O2-responsive drug delivery system with a turn on effect on fluorescence for real-time monitoring. Chem. Eng. J. 2020, 380, 122474. [Google Scholar] [CrossRef]
- Sharif, S.; Abbas, G.; Hanif, M.; Bernkop-Schnürch, A.; Jalil, A.; Yaqoob, M. Mucoadhesive micro-composites: Chitosan coated halloysite nanotubes for sustained drug delivery. Colloids Surf. B 2019, 184, 110527. [Google Scholar] [CrossRef]
- Nagiah, N.; Murdock, C.J.; Bhattacharjee, M.; Nair, L.; Laurencin, C.T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Sci. Rep. 2020, 10, 609. [Google Scholar] [CrossRef] [Green Version]
- Fortuni, B.; Inose, T.; Ricci, M.; Fujita, Y.; Van Zundert, I.; Masuhara, A.; Fron, E.; Mizuno, H.; Latterini, L.; Rocha, S.; et al. Polymeric Engineering of Nanoparticles for Highly Efficient Multifunctional Drug Delivery Systems. Sci. Rep. 2019, 9, 2666. [Google Scholar] [CrossRef]
- Kharaghani, D.; Gitigard, P.; Ohtani, H.; Kim, K.O.; Ullah, S.; Saito, Y.; Khan, M.Q.; Kim, I.S. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci. Rep. 2019, 9, 12640. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Dutta, K.; Zhuang, J.; Thayumanavan, S. Cellular- and Subcellular-Targeted Delivery Using a Simple All-in-One Polymeric Nanoassembly. Angew. Chem. Int. Ed. 2020, 59, 23466–23470. [Google Scholar] [CrossRef]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef]
- Gao, J.; Wu, P.; Fernandez, A.; Zhuang, J.; Thayumanavan, S. Cellular AND Gates: Synergistic Recognition to Boost Selective Uptake of Polymeric Nanoassemblies. Angew. Chem. Int. Ed. 2020, 59, 10456–10460. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef] [Green Version]
- Kokil, G.R.; Veedu, R.N.; Le, B.T.; Ramm, G.A.; Parekh, H.S. Self-assembling asymmetric peptide-dendrimer micelles—a platform for effective and versatile in vitro nucleic acid delivery. Sci. Rep. 2018, 8, 4832. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Xie, Y.; An, Y.; Huang, Y.; Zhu, Z.; Yang, C.J. A Controllable Aptamer-Based Self-Assembled DNA Dendrimer for High Affinity Targeting, Bioimaging and Drug Delivery. Sci. Rep. 2015, 5, 10099. [Google Scholar] [CrossRef]
- Smoluch, M.; Ceglowski, M.; Kurczewska, J.; Babij, M.; Gotszalk, T.; Silberring, J.; Schroeder, G. Molecular Scavengers as Carriers of Analytes for Mass Spectrometry Identification. Anal. Chem. 2014, 86, 11226–11229. [Google Scholar] [CrossRef]
- Narkiewicz, U.; Pełech, I.; Podsiadły, M.; Cegłowski, M.; Schroeder, G.; Kurczewska, J. Preparation and characterization of magnetic carbon nanomaterials bearing APTS–silica on their surface. J. Mater. Sci. 2010, 45, 1100–1106. [Google Scholar] [CrossRef]
- Sellergren, B.; Allender, C.J. Molecularly imprinted polymers: A bridge to advanced drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1733–1741. [Google Scholar] [CrossRef]
- Mokhtari, P.; Ghaedi, M. Water compatible molecularly imprinted polymer for controlled release of riboflavin as drug delivery system. Eur. Polym. J. 2019, 118, 614–618. [Google Scholar] [CrossRef]
- Tuwahatu, C.A.; Yeung, C.C.; Lam, Y.W.; Roy, V.A.L. The molecularly imprinted polymer essentials: Curation of anticancer, ophthalmic, and projected gene therapy drug delivery systems. J. Control. Release 2018, 287, 24–34. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, X.; Zhang, Z.; Yin, J.; Wang, D.; Xu, Y.; Zheng, W.; Li, X.; Zhang, Q.; Liu, L. Synthesis of dummy-template molecularly imprinted polymer adsorbents for solid phase extraction of aminoglycosides antibiotics from environmental water samples. Talanta 2020, 208, 120385. [Google Scholar] [CrossRef]
- Tamayo, F.G.; Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers for solid-phase extraction and solid-phase microextraction: Recent developments and future trends. J. Chromatogr. A 2007, 1152, 32–40. [Google Scholar] [CrossRef]
- Andersson, L.I. Molecular imprinting for drug bioanalysis: A review on the application of imprinted polymers to solid-phase extraction and binding assay. J. Chromatogr. B 2000, 739, 163–173. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef] [Green Version]
- Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Saarma, M.; Syritski, V. Molecularly imprinted polymer-based SAW sensor for label-free detection of cerebral dopamine neurotrophic factor protein. Sens. Actuat. B-Chem. 2020, 308, 127708. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Wang, Y.; Wang, S.; Wang, J. Hollow molecularly imprinted polymer based quartz crystal microbalance sensor for rapid detection of methimazole in food samples. Food Chem. 2020, 309, 125787. [Google Scholar] [CrossRef] [PubMed]
- Hashemi-Moghaddam, H.; Kazemi-Bagsangani, S.; Jamili, M.; Zavareh, S. Evaluation of magnetic nanoparticles coated by 5-fluorouracil imprinted polymer for controlled drug delivery in mouse breast cancer model. Int. J. Pharm. 2016, 497, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, L.; Zhang, H.; Yang, Y.; Liu, X.; Chen, Y. Temperature and magnetism bi-responsive molecularly imprinted polymers: Preparation, adsorption mechanism and properties as drug delivery system for sustained release of 5-fluorouracil. Mater. Sci. Eng. C 2016, 61, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Parisi, O.I.; Morelli, C.; Puoci, F.; Saturnino, C.; Caruso, A.; Sisci, D.; Trombino, G.E.; Picci, N.; Sinicropi, M.S. Magnetic molecularly imprinted polymers (MMIPs) for carbazole derivative release in targeted cancer therapy. J. Mater. Chem. B 2014, 2, 6619–6625. [Google Scholar] [CrossRef]
- Norell, M.C.; Andersson, H.S.; Nicholls, I.A. Theophylline molecularly imprinted polymer dissociation kinetics: A novel sustained release drug dosage mechanism. J. Mol. Recognit. 1998, 11, 98–102. [Google Scholar] [CrossRef]
- Cegłowski, M.; Kurczewska, J.; Ruszkowski, P.; Liberska, J.; Schroeder, G. The influence of cross-linking agent onto adsorption properties, release behavior and cytotoxicity of doxorubicin-imprinted microparticles. Colloids Surf. B 2019, 182, 110379. [Google Scholar] [CrossRef]
- Cegłowski, M.; Kurczewska, J.; Ruszkowski, P.; Schroeder, G. Application of paclitaxel-imprinted microparticles obtained using two different cross-linkers for prolonged drug delivery. Eur. Polym. J. 2019, 118, 328–336. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, M.; Zhang, Y.; Ma, Z.-B.; Xiao, N.-N.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Preparation of Dual-Template Epitope Imprinted Polymers for Targeted Fluorescence Imaging and Targeted Drug Delivery to Pancreatic Cancer BxPC-3 Cells. ACS Appl. Mater. Interfaces 2019, 11, 32431–32440. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Zavareh, S.; Karimpour, S.; Madanchi, H. Evaluation of molecularly imprinted polymer based on HER2 epitope for targeted drug delivery in ovarian cancer mouse model. React. Funct. Polym. 2017, 121, 82–90. [Google Scholar] [CrossRef]
- Marcelo, G.; Ferreira, I.C.; Viveiros, R.; Casimiro, T. Development of itaconic acid-based molecular imprinted polymers using supercritical fluid technology for pH-triggered drug delivery. Int. J. Pharm. 2018, 542, 125–131. [Google Scholar] [CrossRef]
- Arias, J.L. Novel strategies to improve the anticancer action of 5-fluorouracil by using drug delivery systems. Molecules 2008, 13, 2340–2369. [Google Scholar] [CrossRef] [Green Version]
- Cegłowski, M.; Jerca, V.V.; Jerca, F.A.; Hoogenboom, R. Reduction-Responsive Molecularly Imprinted Poly(2-isopropenyl-2-oxazoline) for Controlled Release of Anticancer Agents. Pharmaceutics 2020, 12, 506. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Bettencourt, A.; Almeida, A.J. Poly(methyl methacrylate) particulate carriers in drug delivery. J. Microencapsul. 2012, 29, 353–367. [Google Scholar] [CrossRef]
- Farzaneh, S.; Asadi, E.; Abdouss, M.; Barghi-Lish, A.; Azodi-Deilami, S.; Khonakdar, H.A.; Gharghabi, M. Molecularly imprinted polymer nanoparticles for olanzapine recognition: Application for solid phase extraction and sustained release. RSC Adv. 2015, 5, 9154–9166. [Google Scholar] [CrossRef]
- Javanbakht, M.; Attaran, A.M.; Namjumanesh, M.H.; Esfandyari-Manesh, M.; Akbari-adergani, B. Solid-phase extraction of tramadol from plasma and urine samples using a novel water-compatible molecularly imprinted polymer. J. Chromatogr. B 2010, 878, 1700–1706. [Google Scholar] [CrossRef]
- Gupta, A.; Tiwari, G.; Tiwari, R.; Srivastava, R.; Rai, A.K. Enteric coated HPMC capsules plugged with 5-FU loaded microsponges: A potential approach for treatment of colon cancer. Braz. J. Pharm. Sci. 2015, 51, 591–606. [Google Scholar] [CrossRef] [Green Version]
- Haghseresht, F.; Lu, G.Q. Adsorption Characteristics of Phenolic Compounds onto Coal-Reject-Derived Adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Fytianos, K.; Voudrias, E.; Kokkalis, E. Sorption–desorption behaviour of 2,4-dichlorophenol by marine sediments. Chemosphere 2000, 40, 3–6. [Google Scholar] [CrossRef]
- Dand, N.; Patel, P.; Ayre, A.; Kadam, V. Polymeric micelles as a drug carrier for tumor targeting. Chron. Young Sci. 2013, 4, 94–101. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Chen, L.; Yan, H.; Zhang, C.; Liu, L.; Xu, X. Synthesis and characterization of paclitaxel-imprinted microparticles for controlled release of an anticancer drug. Mater. Sci. Eng. C 2018, 92, 338–348. [Google Scholar] [CrossRef]
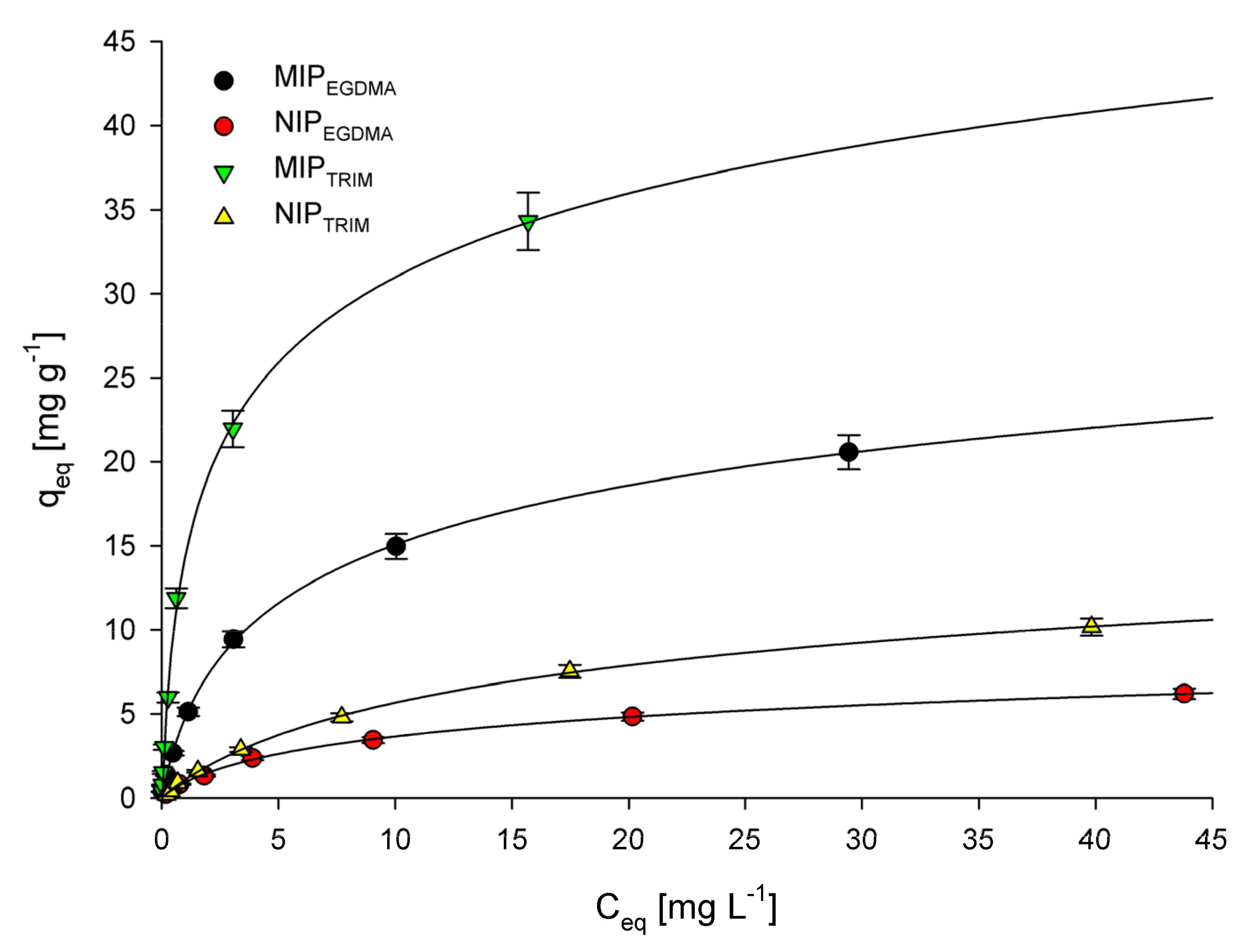
| Polymer | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | K (L mg−1) | R2 | Kf (mg g−1 (L mg−1)1/n) | 1/n | R2 | |
| MIPEGDMA | 22.57 ± 1.96 | 0.285 ± 0.025 | 0.991 | 3.59 ± 0.32 | 0.62 ± 0.06 | 0.973 |
| NIPEGDMA | 7.00 ± 0.64 | 0.142 ± 0.013 | 0.989 | 0.82 ± 0.08 | 0.60 ± 0.06 | 0.977 |
| MIPTRIM | 37.30 ± 3.46 | 0.676 ± 0.064 | 0.997 | 9.72 ± 0.92 | 0.66 ± 0.06 | 0.937 |
| NIPTRIM | 12.89 ± 1.63 | 0.088 ± 0.008 | 0.992 | 0.95 ± 0.09 | 0.73 ± 0.07 | 0.972 |
| Polymer | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||
|---|---|---|---|---|
| k1 (h−1) | R2 | k2 (g mg−1 h−1) | R2 | |
| MIPEGDMA | 3.46 ± 0.31 | 0.987 | 1.03 ± 0.09 | 0.998 |
| NIPEGDMA | 1.50 ± 0.14 | 0.979 | 1.00 ± 0.09 | 0.997 |
| MIPTRIM | 2.47 ± 0.22 | 0.993 | 0.52 ± 0.05 | 0.999 |
| NIPTRIM | 1.74 ± 0.15 | 0.980 | 1.73 ± 0.14 | 0.994 |
| Polymer | pH | Zero-Order | First-Order | Higuchi | Hixson–Crowell | Korsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k0 (h−1) | R2 | k1 (h−1) | R2 | kH (h−1/2) | R2 | kHC (h−1/3) | R2 | kKP (h−n) | n | R2 | ||
| MIPEGDMA | 2.2 | 0.106 ± 0.093 | 0.621 | 1.63 × 10−3 ± 0.14 × 10−3 | 0.634 | 1.73 ± 0.15 | 0.791 | 2.19 × 10−3 ± 0.20 × 10−3 | 0.630 | 20.7 ± 1.8 | 0.16 ± 0.2 | 0.900 |
| 5.0 | 0.110 ± 0.009 | 0.405 | 1.30 × 10−3 ± 0.11 × 10−3 | 0.413 | 0.77 ± 0.07 | 0.555 | 1.91 × 10−3 ± 0.17 × 10−3 | 0.410 | 13.9 ± 1.2 | 0.07 ± 0.01 | 0.726 | |
| 7.4 | 0.047 ± 0.004 | 0.784 | 0.53 × 10−3 ± 0.05 × 10−3 | 0.787 | 0.40 ± 0.04 | 0.910 | 0.79 × 10−3 ± 0.07 × 10−3 | 0.786 | 9.9 ± 0.9 | 0.06 ± 0.01 | 0.944 | |
| MIPTRIM | 2.2 | 0.085 ± 0.008 | 0.670 | 1.77 × 10−3 ± 0.14 × 10−3 | 0.674 | 0.70 ± 0.07 | 0.803 | 1.93 × 10−3 ± 0.16 × 10−3 | 0.661 | 49.3 ± 4.7 | 0.03 ± 0.01 | 0.896 |
| 5.0 | 0.494 ± 0.42 | 0.944 | 6.82 × 10−3 ± 0.61 × 10−3 | 0.783 | 3.01 ± 0.28 | 0.977 | 1.46 × 10−2 ± 0.11 × 10−2 | 0.966 | 56.5 ± 5.2 | 0.06 ± 0.01 | 0.956 | |
| 7.4 | 0.130 ± 0.011 | 0.705 | 6.40 × 10−3 ± 0.59 × 10−3 | 0.737 | 1.96 ± 0.16 | 0.867 | 5.78 × 10−3 ± 0.53 × 10−3 | 0.728 | 64.8 ± 6.2 | 0.06 ± 0.01 | 0.947 | |
| Material | U87 MG | HeLa | KB | A-549 | MCF-7 | HDF |
|---|---|---|---|---|---|---|
| unloaded MIPEGDMA | 62.13 (0.11) | 67.02 (0.49) | 67.39 (0.07) | 71.08 (0.73) | 63.35 (0.19) | 98.19 (0.51) |
| unloaded MIPTRIM | 81.12 (0.04) | 69.02 (0.31) | 81.01 (1.04) | 77.82 (0.59) | 81.93 (0.94) | 102.02 (0.87) |
| loaded MIPEGDMA | 0.38 (0.07) | 1.02 (0.11) | 0.41 (0.16) | 0.31 (0.91) | 0.19 (0.01) | 0.44 (0.49) |
| loaded MIPTRIM | 0.50 (0.04) | 0.16 (0.01) | 0.21 (0.17) | 0.26 (0.19) | 0.22 (0.01) | 1.94 (0.55) |
| 5-FU | 0.07 (0.01) | 0.16 (0.13) | 0.03 (0.01) | 0.08 (0.03) | 0.08 (0.07) | 0.71 (0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegłowski, M.; Kurczewska, J.; Lusina, A.; Nazim, T.; Ruszkowski, P. EGDMA- and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery. Polymers 2022, 14, 1027. https://doi.org/10.3390/polym14051027
Cegłowski M, Kurczewska J, Lusina A, Nazim T, Ruszkowski P. EGDMA- and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery. Polymers. 2022; 14(5):1027. https://doi.org/10.3390/polym14051027
Chicago/Turabian StyleCegłowski, Michał, Joanna Kurczewska, Aleksandra Lusina, Tomasz Nazim, and Piotr Ruszkowski. 2022. "EGDMA- and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery" Polymers 14, no. 5: 1027. https://doi.org/10.3390/polym14051027
APA StyleCegłowski, M., Kurczewska, J., Lusina, A., Nazim, T., & Ruszkowski, P. (2022). EGDMA- and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery. Polymers, 14(5), 1027. https://doi.org/10.3390/polym14051027