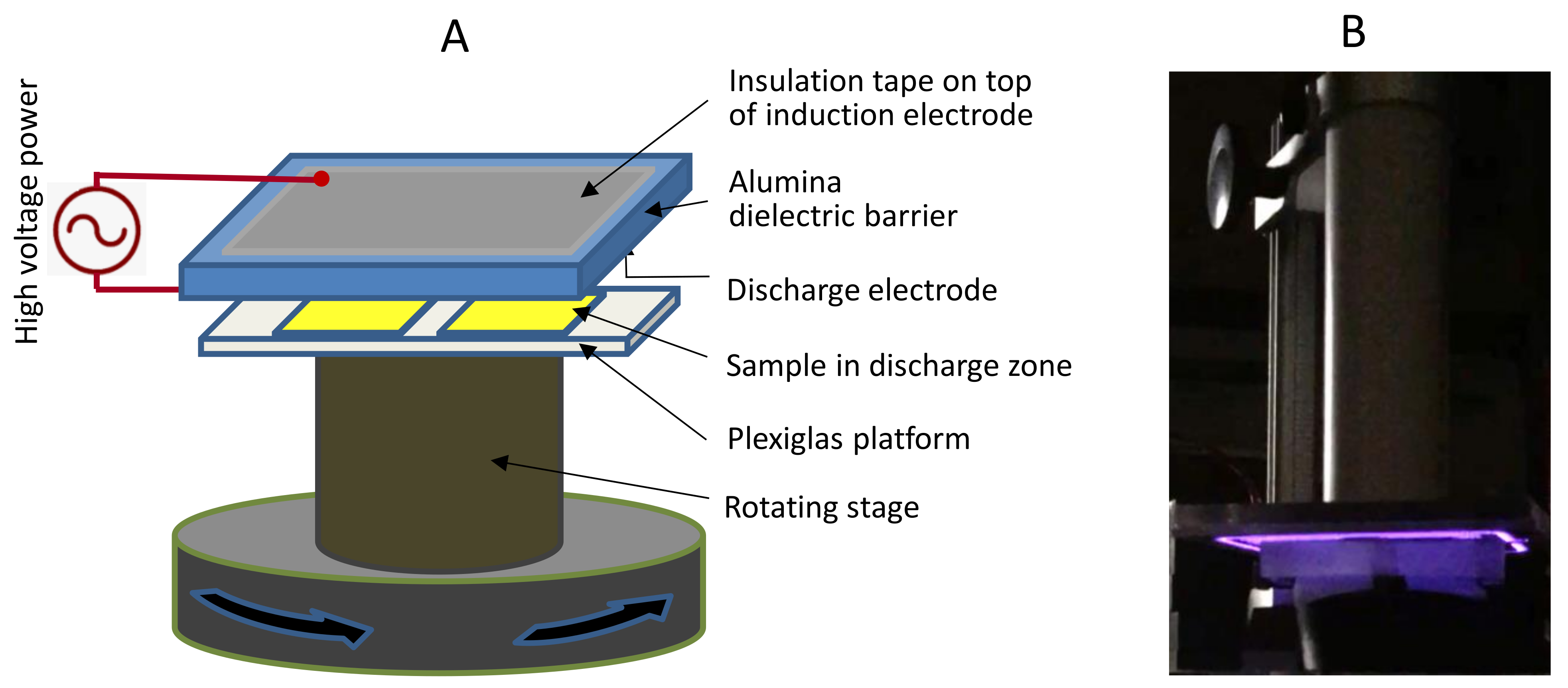
3.1. Analysis of Dyeing Experiments
We designed Experiment A to investigate the impact of AESO concentration, the use of benzyl alcohol (Benzyl-OH) as a swelling agent, and NaCl as an electrolyte on dyeing. Furthermore, we tested the NTP treatment prior to or post AESO pretreatment in order to understand the function of NTP in improving the dyeing color strength. The design of this 4-factor, 2-level, full factorial experiment and results are presented in
Table 1.
For the analysis of variance (ANOVA) of the factorial design, model term selection (including factorial interactions) was first conducted based on the half-normal probability plot, showing the factorial impact in the ascending order of (AESO conc.) < (Benzyl-OH) < (NaCl) < (NTP order). This resulted in an overall significant model (F-test,
p < 0.0001). T-tests for each model term coefficients showed that all model terms were significant (
p < 0.002), except for the main effect of (AESO conc.) (
p = 0.89). This was not surprising because we did not know a suitable AESO concentration in this first experiment and randomly selected the two AESO concentrations. Since all factors were involved in significant interactions, the statistical inferences are based on the interaction plots as shown in
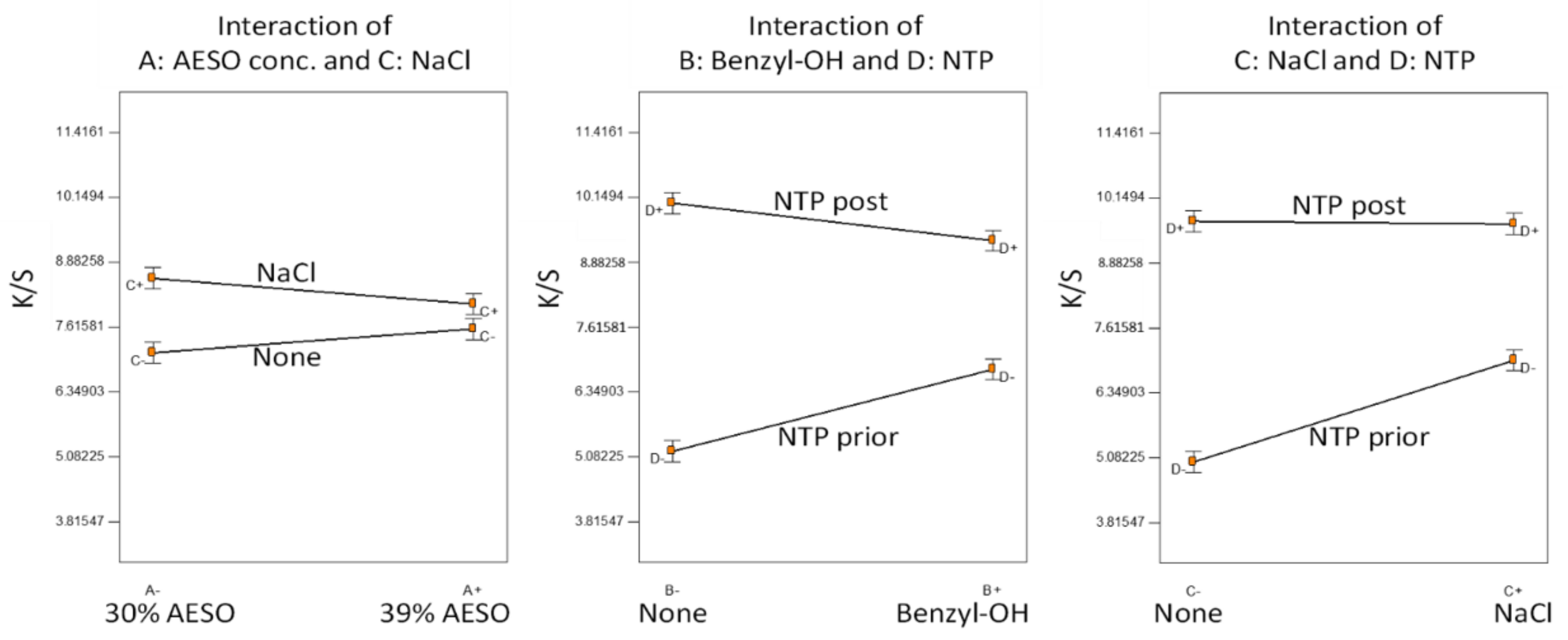
Figure 2.
The difference between the two selected AESO concentrations was insignificant. Addition of NaCl in the dye bath slightly improved color strength, apparently when the NTP was applied prior to AESO pretreatment. The order of NTP application played an important role; NTP application post AESO pretreatment greatly increased the dyed color strength, indicating that the chemical reactions induced by NTP on AESO were the key. It appeared that the effect of NTP was confounded by the addition of benzyl alcohol and NaCl in the dye bath, so the two did not show an obvious effect if the fabrics were NTP-treated post AESO pretreatment. A reasonable explanation would be that NTP application prior to AESO pretreatment facilitated the attraction of polar NaCl and benzyl alcohol to the fabrics, while in the case of NTP application post AESO pretreatment of the fabrics was covered by AESO, and NTP was mainly used to induce reactions on the AESO.
Because the interaction between NTP and AESO was important, in Experiment B, we used a central composite design to find optimal AESO concentration and NTP treatment time post AESO pretreatment, without the addition of benzyl alcohol and NaCl in the dye bath. The detailed design and results are presented in
Table 2.
Stepwise regression resulted in the selection of a reduced quadratic model as the best fit (F-test, p < 0.001), including only three terms of the main effect of NTP time, main effect of AESO concentration, and the interaction of the two. The quadratic terms of both NTP time and AESO concentration were not significant.
The response surface plot for Experiment B is shown in
Figure 3. Increasing both NTP time and AESO concentration increased color strength. At low AESO concentration, increasing NTP time only slightly increased color strength. However, at higher AESO concentration, increasing NTP time prominently improved color strength. This is reasonable because higher concentration of AESO and longer NTP time induced more polymerized AESO on the fabric surface that attracted more dye molecules, again corroborating the importance of AESO pretreatment followed by NTP treatment.
We also observed that AESO concentration higher than 25 wt.% resulted in an unnecessarily thick coating on the para-aramid fabrics. Therefore, we considered 25 wt.% as an optimal concentration for AESO pretreatment because the highest K/S in Experiment B was achieved at NTP time = 120 s. and AESO conc. = 25 wt.%.
In Experiment C, based on the results of Experiment A and B, we fixed AESO concentration at 25 wt.% and tested if there is any difference between just applying NTP post AESO pretreatment and applying NTP both before and after AESO pretreatment. We also slightly extended the NTP post AESO pretreatment time to 150 s, based on our observation in
Figure 3. Experiment C focused on the functions of auxiliary additives of a swelling agent (benzyl alcohol), an electrolyte (NaCl), and a surfactant of Polysorbate 80 (denoted as TWEEN hereafter). The factorial design and results are shown in
Table 3.
We determined an ANOVA model as the best fit (F-test,
p < 0.0001) according to the half-normal probability plot, including all the four main factorial effects and only two interaction terms, which were statistically significant based on the
t-test of the model coefficients (
p < 0.05). Statistical inference is presented in
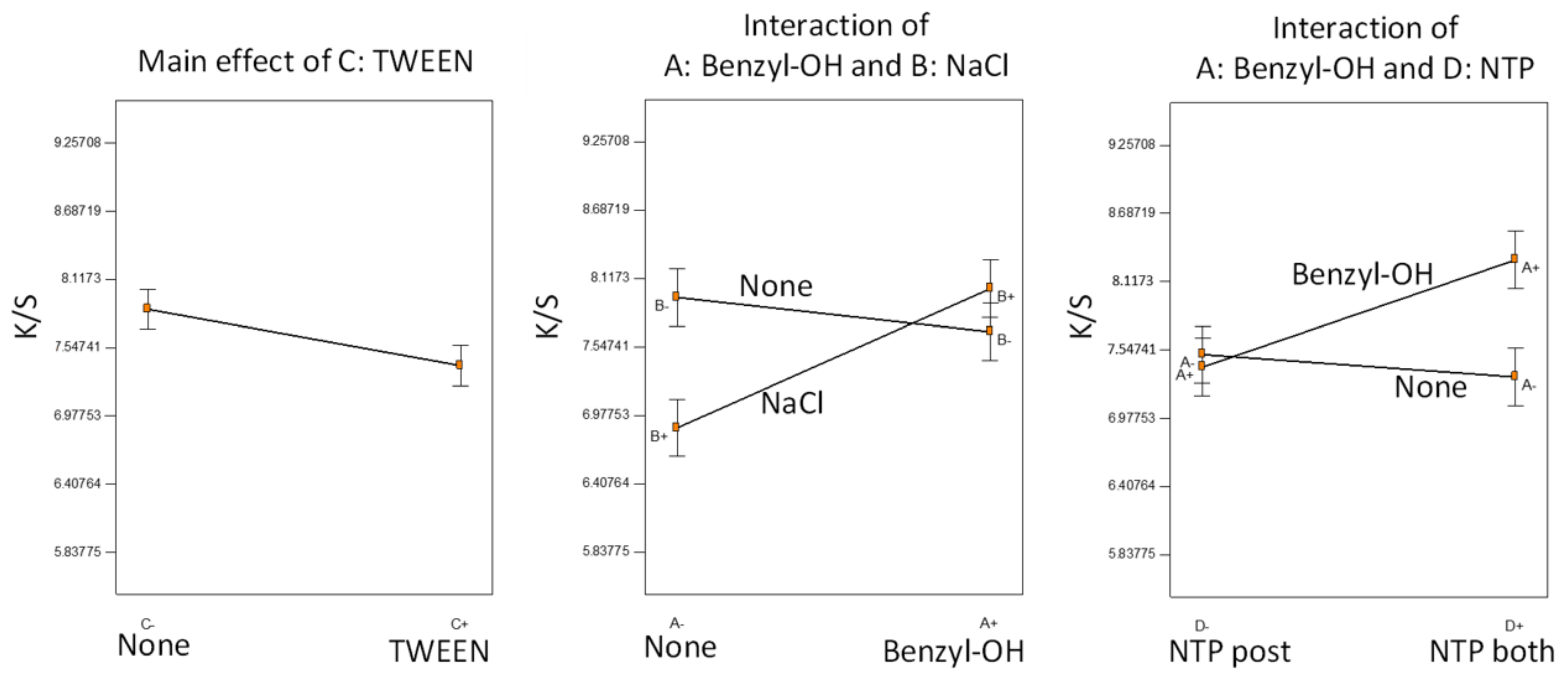
Figure 4.
TWEEN was the only factor not involved in any significant interactions; the addition of the surfactant negatively affected dyeing performance, consistent with our previous observations [
9,
10]. The surfactant, although helping to form a dye dispersion, might also hinder the diffusion of the dye onto the para-aramid fiber fabrics. The positive effect of benzyl alcohol was significant only when the NTP was applied both before and after AESO pretreatment. Furthermore, benzyl alcohol and NaCl were confounding factors. In the absence of benzyl alcohol, the addition of NaCl lowered color strength, but with the presence of benzyl alcohol, the NaCl effect was insignificant. We may speculate that both NaCl and benzyl alcohol participated in the NTP-induced reactions with AESO, leading to different reaction pathways, and therefore, different dyeing effects. Overall, all the samples were dyed to high color strength with small variations because we fixed AESO concentration and NTP treatment time at optimal conditions. The effects of auxiliary additives were limited, compared with the major improvement of color strength caused by AESO pretreatment followed by NTP treatment.
Experiment D was conducted as a starting point to investigate the possibility of replacing AESO with a simple mixture of soybean oil and acrylic acid, because hypothetically a cross-linked network could be formed with the application of NTP to help dyeing. However, longer NTP treatment time is needed based on our preliminary trials. Furthermore, because one soybean oil molecule has an average of 4.6 double bonds and NTP could trigger self-polymerization of soybean oil [
24], we tested two molar ratios of acrylic acid to soybean oil (denoted as AA/Soy hereafter) of 1.6 and 4. We also tested if the application of NTP again after dyeing would help fixing the dye. The design and results of this 3-factor, 2-level factorial experiment are shown in
Table 4.
Based on the half-normal probability plot, we selected an ANOVA model (F-test,
p < 0.0001), which included all three main factors and two interaction terms (
t-test,
p < 0.001). Because all the factors were involved in the interactions, the statistical inference was based on the significant interaction plots as shown in
Figure 5.
Overall, the color strength achieved in Experiment D is comparable with that in Experiment C using AESO, showing the potential of using AA/Soy to replace AESO. Generally, longer NTP treatment time and higher AA/Soy resulted in higher K/S values. It is interesting to observe that at AA/Soy = 1.6, a 30 s NTP treatment after dyeing greatly decreased color strength, whereas at AA/Soy = 4 there was no change. A plausible explanation would be that NTP could quickly degrade the dye molecules [
10]; however, at higher AA/Soy, more chemical functionalities could absorb most NTP energy and reactive species, thus protecting the dye from degradation.
In order to evaluate if the use of auxiliary agents would help improve dyeing with the AA/Soy pretreatment, Experiment E focused on the effects of NaCl and benzyl alcohol. Furthermore, because we observed unevenly dyed samples in Experiment D due to inadequate mixing of the soybean oil and acrylic acid, the AA/Soy well dissolved in ethyl acetate (50 wt.% of AA/Soy in ethyl acetate) was used as the pretreatment solution. The design and results of this 4-factor full factorial experiment are presented in
Table 5.
Based on the half-normal probability plot, we selected the best fit of ANOVA model (F-test,
p < 0.0001). The statistical inference is shown in
Figure 6. Only two interaction terms were significant (
t-test,
p < 0.01). However, the main effect of NTP treatment time was not significant (
t-test,
p = 0.53) due to the confounding effect of NaCl.
The swelling agent of benzyl alcohol was the only significant main factor not involved in any interactions; it negatively affected the dyeing color strength. In comparison with Experiment D, Experiment E showed similar interactions between the NTP treatment time and the AA/Soy ratio. Specifically, fixing the NTP time = 360 s, increasing AA/Soy from 1.6 to 4, did not improve color strength, while fixing NTP time = 240 s, increasing AA/Soy, significantly increased color strength.
A confounding effect of NaCl was observed. Without NaCl, higher AA/Soy yielded higher color strength; with the addition of NaCl in the dye bath, AA/Soy = 4 and AA/Soy = 1.6 resulted in statistically the same K/S. A plausible explanation would be that both NTP and NaCl would induce reactions in AA/Soy (as revealed in our FTIR analysis in
Section 3.2), and the higher dosage of both would result in overreactions that decreased surface functionality for binding with the dye molecules.
Because of the kinetic nature of the reactions catalyzed by NTP and influenced by the presence of NaCl, we speculated that there exists an optimal combination of NTP treatment time, AA/Soy, and NaCl concentration. Therefore, in Experiment F, we utilized a Box–Behnken response surface design in an effort to find this optimal combination. The detailed experimental design and results are presented in
Table 6.
A reduced quadratic model of response surface was selected as the best fit (F-test,
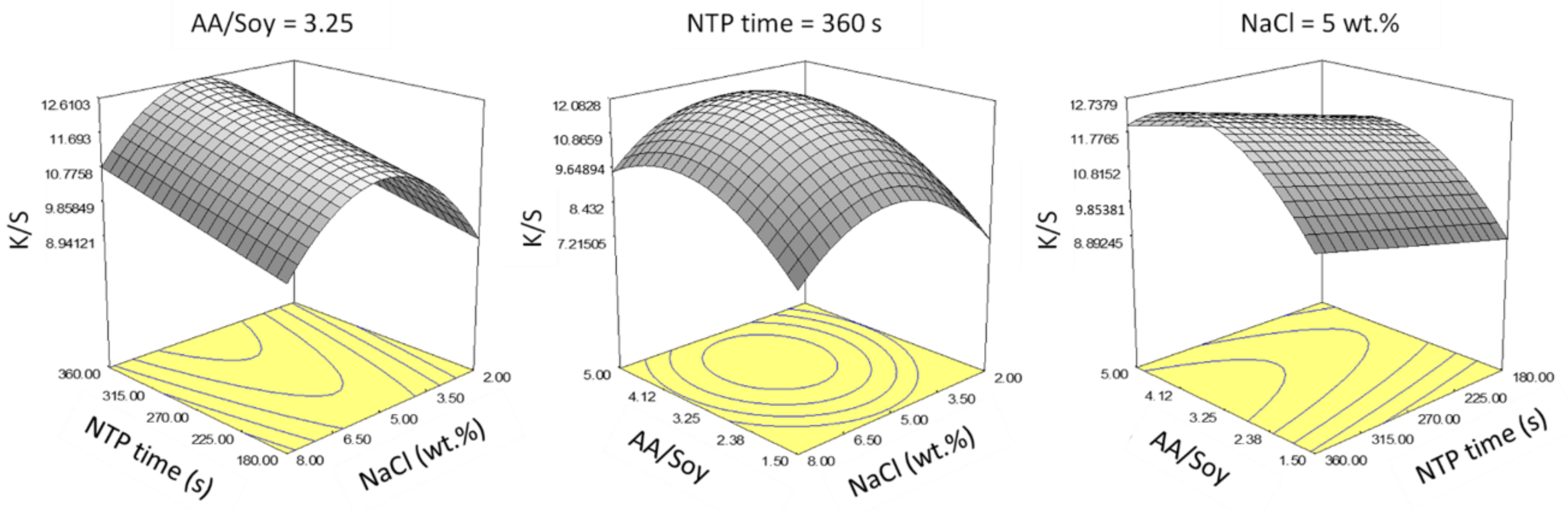
p < 0.05) after performing stepwise regression, including the three main factor effects and the quadratic terms of only AA/Soy and NaCl concentration. Representative response surfaces are presented in
Figure 7.
Within the selected ranges for the main factors, increasing NTP treatment time linearly increased color strength, so the longest NTP time = 360 s gave the best results. However, there existed an optimal point for AA/Soy and NaCl concentration. Based on the above plots, we determined an optimal condition as NTP time = 360 s, NaCl = 5% wt.%, and AA/Soy = 3.25.
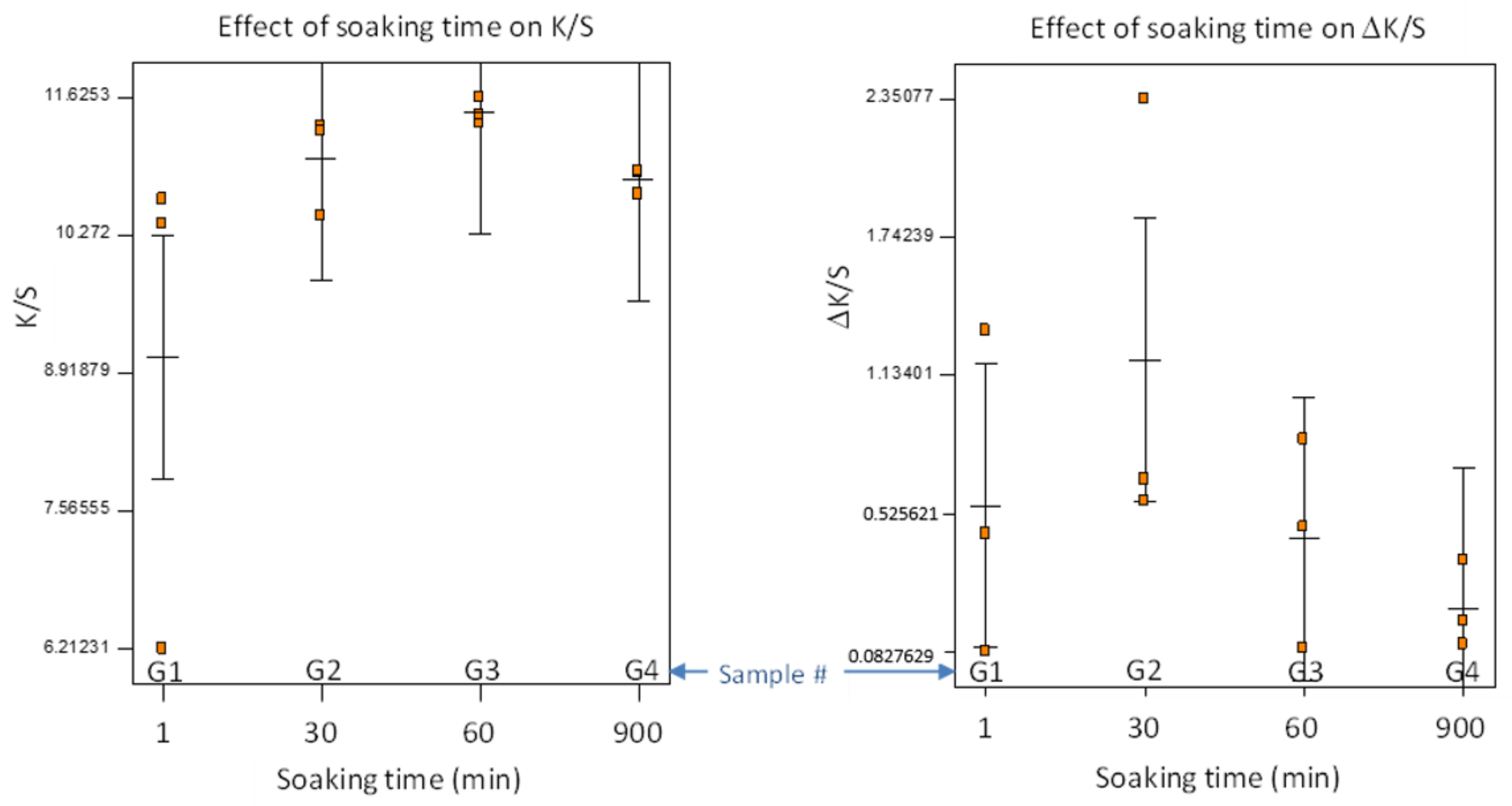
Experiment G was conducted at the determined optimal conditions, and we varied the pretreatment soaking time for practical applications, making it a one-factor factorial design with three replicates. Furthermore, all the samples in Experiment G were washed a second time with detergent by following the same protocol described in
Section 2.3, and the change of K/S values (denoted as ΔK/S) was used to evaluate the colorfastness against wash. The results are presented in
Figure 8, showing each data point, the means, and the least significant difference (LSD) lines.
ANOVA for the one-factor model did not detect any significant difference among the four pretreatment soaking times (F-test, p > 0.2), because of a large variation of K/S when the soaking time was short. There was an outlier with low K/S = 6.2, occurring with 1 min of soaking time. Generally, the soaking time appeared to affect the consistency of color strength. The ΔK/S indicated slight reduction in color strength after second detergent wash. Similarly, shorter soaking time resulted in larger variation of ΔK/S. It is recommended that a soaking time ≥60 min be used, or we need to develop a better method to evenly distribute AA/Soy into the fabrics in a shorter time (e.g., continuous soaking and pressing with rollers).
To provide a visual demonstration comparing the dyeing strengths of different treatments, images of selected samples acquired using a flatbed scanner are presented in the
Supplementary File (Figure S1).
3.2. FTIR Analysis
To understand chemical changes during the pretreatment and dyeing processes, ATR–FTIR spectroscopy was performed at different stages of the processes. As a foundation for interpreting the FTIR spectra,
Table S1 in the Supplementary File summarizes major FTIR bands of AESO, soybean oil, acrylic acid, and para-aramids.
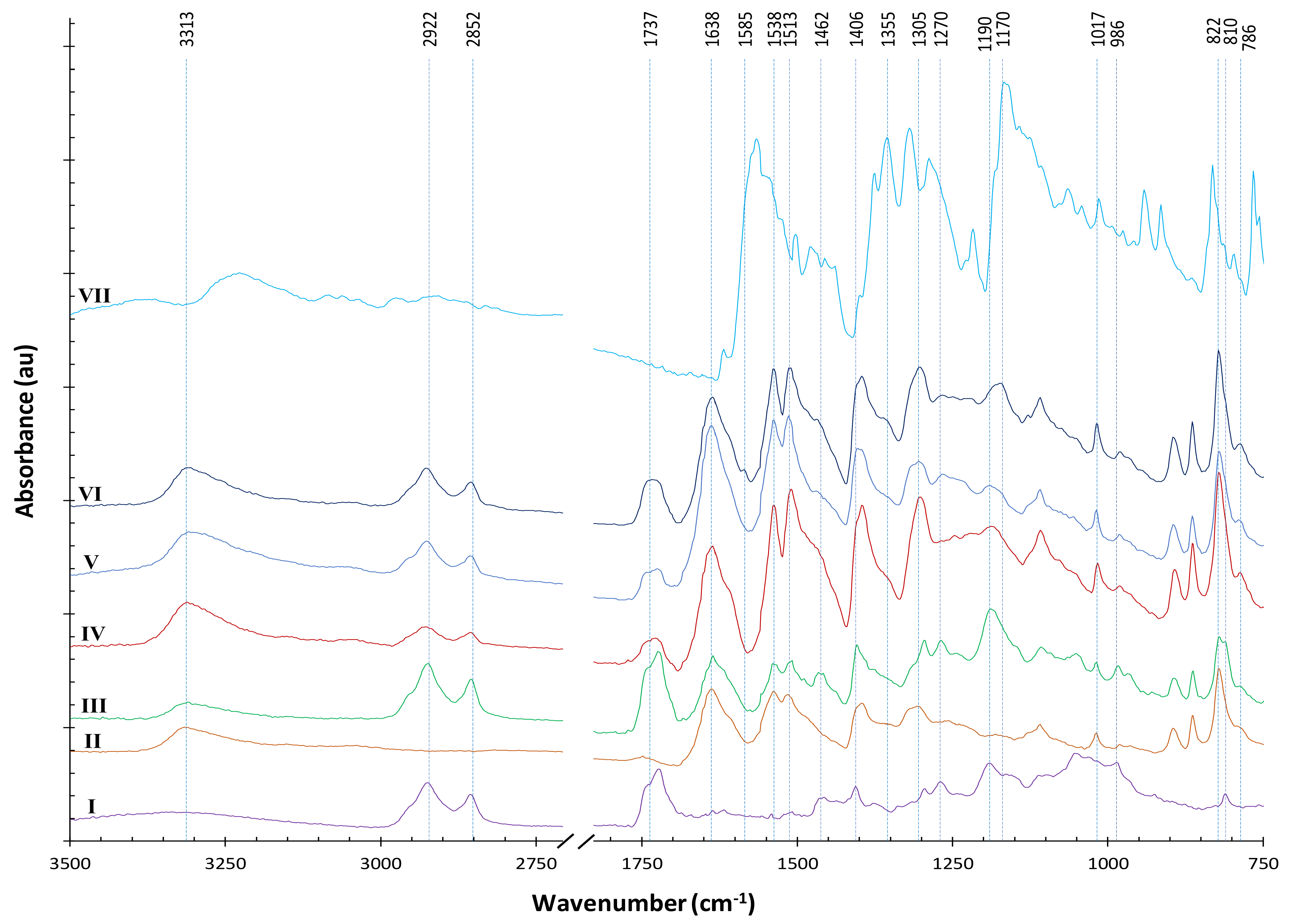
Figure 9 presents ATR–FTIR spectra of materials at different stages of our processes using AESO for pretreatment. The AESO signal is dominated by the major peaks of soybean oil backbone (line I). A strong peak for ester carbonyl groups should appear at 1742 cm
−1; however, due to the formation of acrylate esters, a peak should appear at nearby 1723 cm
−1 [
25]. Therefore, the peak with a deformed shape around 1737 cm
−1 should be the overlapping of 1742 and 1723 cm
−1. Compared with the dominant signal from soybean oil backbone, characteristics of AESO/acrylate can be found as small peaks. There are residual epoxides in the AESO that were not fully acrylated as revealed by the oxirane peaks at 1270 and 822 cm
−1. Although major acrylate peaks (such as 1638 and 1619 cm
−1) overlap with a strong signal from para-aramids, two distinguishable acrylate peaks at 1406 and 810 cm
−1 can be tracked along the way of our processes to indicate the consumption of the acrylates.
For para-aramids, the bands shown in
Table S1 and correspondingly in
Figure 9 (line II) can be considered as an internal standard [
26]. The FTIR spectrum of AESO-soaked para-aramids apparently show the addition of signals from both AESO and para-aramids (line III). After subsequent NTP treatment, changes can be seen (line IV): the acrylate peaks at 1406 and 810 cm
−1 disappeared or significantly decreased. Although this observation was somewhat obscured by a nearby peak of para-aramids, the shapes of the two peaks more closely resemble those of the para-aramid peaks nearby, indicating the consumption of the acrylate functional groups. A sobservation can be found around 1737 cm
−1, showing the consumption of 1723 cm
−1 acrylate esters. The oxirane peak at 1270 cm
−1 also disappeared (line IV). However, the signal of vinyl functional group at 986 cm
−1 remained, albeit decreased, indicating that the AESO was not fully polymerized. The 1190 cm
−1 peak of C–O stretching in the esters of soybean oil backbone also remained, revealing that the NTP treatment did not fully break the ester bonds.
Figure 9 also shows the FTIR spectrum of a dyed sample which was pretreated with AESO without subsequent NTP treatment (line V, sample A3, K/S = 6.14), and another dyed sample pretreated with AESO with subsequent NTP treatment (line VI, sample C9, K/S = 8.46). Other than differences at a few minor peaks, lines V and VI are very similar. The –C=O carbonyl stretching and C–O stretching in the esters at 1737 cm
−1 and 1190 cm
−1, respectively remained strong in lines V and VI, indicating that the NTP treatment and the dyeing process did not break much of the ester bonds, which remained after dyeing and detergent wash. However, the 1190 cm
−1 peak in line VI shifted to the right, probably due to the influence of the dye peak at 1170 cm
−1, because the sample of line VI was dyed to a higher K/S value. The three spectra (lines IV, V, and VI) all have a new peak emerging at 786 cm
−1, which is in the typical region of 1,3-disubstituted or 1,2,3-trisubstituted C–H bending, meaning a possible new attachment of AESO to the para-aromatic rings. Interestingly, this new peak at 786 cm
−1 could be generated by the NTP treatment or the thermal dyeing process, because the sample of line V was not treated with NTP after AESO soaking. In line VI, the weak absorption band at 1585 cm
−1 was assigned to aromatic rings conjugated with α-carbonyl group, indicating that some AESO covalently bonded to para-aramid rings after NTP treatment [
27].
Additionally, lines IV, V, and VI obviously have stronger FTIR signal because the AESO pretreatment, NTP treatment, and dyeing process changed the reflective index of the treated samples, enabling deeper ATR–FTIR penetration.
The FTIR spectrum of the Victoria Blue R dye is also shown in
Figure 9 (line VII). Because of low uptake of the dye by the fabrics and that most of the dye peaks were obscured by the characteristic peaks of the para-aramids and AESO, no significant dye signal was detected in the dyed samples, except for that at 1355 and 1170 cm
−1 that rendered a small shoulder in the spectra of dyed samples (lines V and VI), especially clear for the sample of line VI, which was treated with NTP and consequently dyed to a higher color strength.
Because the strong signal of para-aramids obscured many meaningful peaks that reveal chemical changes, we used ATR–FTIR to monitor reactions between soybean oil and acrylic acid on KBr plates (instead of on para-aramids) in contrast to those of AESO. It is important to note that the reagents acrylic acid and AESO contain 200 or 4000 ppm of monomethyl ether of hydroquinone (MEHQ), respectively, as an inhibitor of self-polymerization.
Figure 10 shows the results; line I is the spectrum of soybean oil; line II is that of acrylic acid; and line III is that of the mixture of acrylic acid and soybean oil in a molar ratio of 4:1, apparently showing the signal addition of soybean oil and acrylic acid.
After a 6 min NTP treatment of the mixture of soybean oil and acrylic acid, significant changes could be observed (line IV). A small but obvious broad peak appeared around 3650 cm
−1, which is assigned to O–H stretching usually from alcohols or phenols. Because the NTP generated in ambient air has ozone, singlet oxygen atoms, and hydrogen peroxide as highly reactive oxidants, the unstable C=C double bonds in soybean oil could be first attacked to form epoxidized soybean oil, which could be followed by ensuing reaction with acrylic acid catalyzed by UV and/or radical species in NTP to form AESO. However, a competitive reaction could also lead to the production of polyols [
28], which is evident in line IV, and the polyols could react with acrylic acid to form acrylate polymers [
29,
30].
After NTP treatment (line IV), the 3009 cm
−1 peak (=C–H in soybean oil) disappeared and some new peaks appeared between 3009 and 2922 cm
−1. The peak at 2980 cm
−1 can be assigned to C–H (sp
3 stretching), indicating hydrogen abstraction from the –CH
3 or –CH
2 of the soybean oil. However, if the FTIR spectrum was acquired on para-aramids pretreated with AA/Soy followed by NTP treatment, (see line A in
Figure S2 of Supplementary File), this peak at 2980 cm
−1 was not observed. Instead, a small peak appeared at 786 cm
−1, which is the typical region of 1,3-disubstituted or 1,2,3-trisubstituted C–H bending and similar to the case in
Figure 9 for samples pretreated with AESO. Therefore, we reasoned that the AESO or AA/Soy network was “anchored” to para-aramids at the C–H point.
The 1695 cm−1 peak (C=O stretching) in acrylic acid was significantly consumed with the application of NTP, and a new peak at 1556 cm−1 indicated nitro groups caused by the reactive nitrogen and oxygen species (RONS) in NTP; a small peak at the same wavelength can also be observed in NTP-treated AESO (line VII).
Interestingly, by simply adding NaCl to the mixture of soybean oil and acrylic acid, similar changes can be observed around 3650 and 2980 cm
−1 (line V), indicating that NaCl triggered reactions between the soybean oil and acrylic acid at room temperature, which explains the confounding effect of NaCl we observed in Experiment E, in that the reactions with or without NaCl might lead to different kinetics and pathways. With NTP application on the mixture of soybean oil and acrylic acid (line III vs. VI), the 1637 and 1617 cm
−1 of C=C double bonds in the acrylic acid were consumed, indicating that the NTP treatment did induce radical polymerization through the C=C double bonds. A similar observation could be found in NTP-treated AESO (line VI vs. VII). However, for the NTP-treated mixture of soybean oil and acrylic acid, the 984 cm
−1 peak (=CH
2) was almost completely consumed after a 6 min NTP treatment (line IV), whereas the 986 cm
−1 peak of vinyl functional groups in AESO was still visible (line VII), indicating that a 2.5 min NTP treatment did not totally consume the vinyl groups, and longer NTP time could be applied on the AESO. The FTIR spectra of dyed samples pretreated with AESO or AA/Soy were very similar, except for some small peaks (see line B vs. C in
Figure S2 of Supplementary File).
The NTP-induced chemical reactions in AESO and AA/Soy are complex, and the precise details of reaction pathways on the surface of para-aramids deserve further study. Nonetheless, based on the FTIR results, we can propose the following mechanism of improved dyeing with the pretreatment of AESO or AA/Soy. Because of the high crystallinity and inertness of para-aramids, few dye molecules can be directly adsorbed on the para-aramid surface. With the pretreatment of AESO or AA/Soy and subsequent NTP treatment, a cross-linked network (yet flexible due to the preserved long acyl chains) is formed, “anchoring” to the para-aramids with covalent bonds, and also providing functional groups that bind the dye molecules. Although the pretreatment with AESO vs. AA/Soy generated differences in the polymerized network on para-aramids, comparable dyeing effects were achieved, as evident in
Section 3.1.
3.3. Supporting Evidence: SEM, Extraction Test, and Tensile Strength
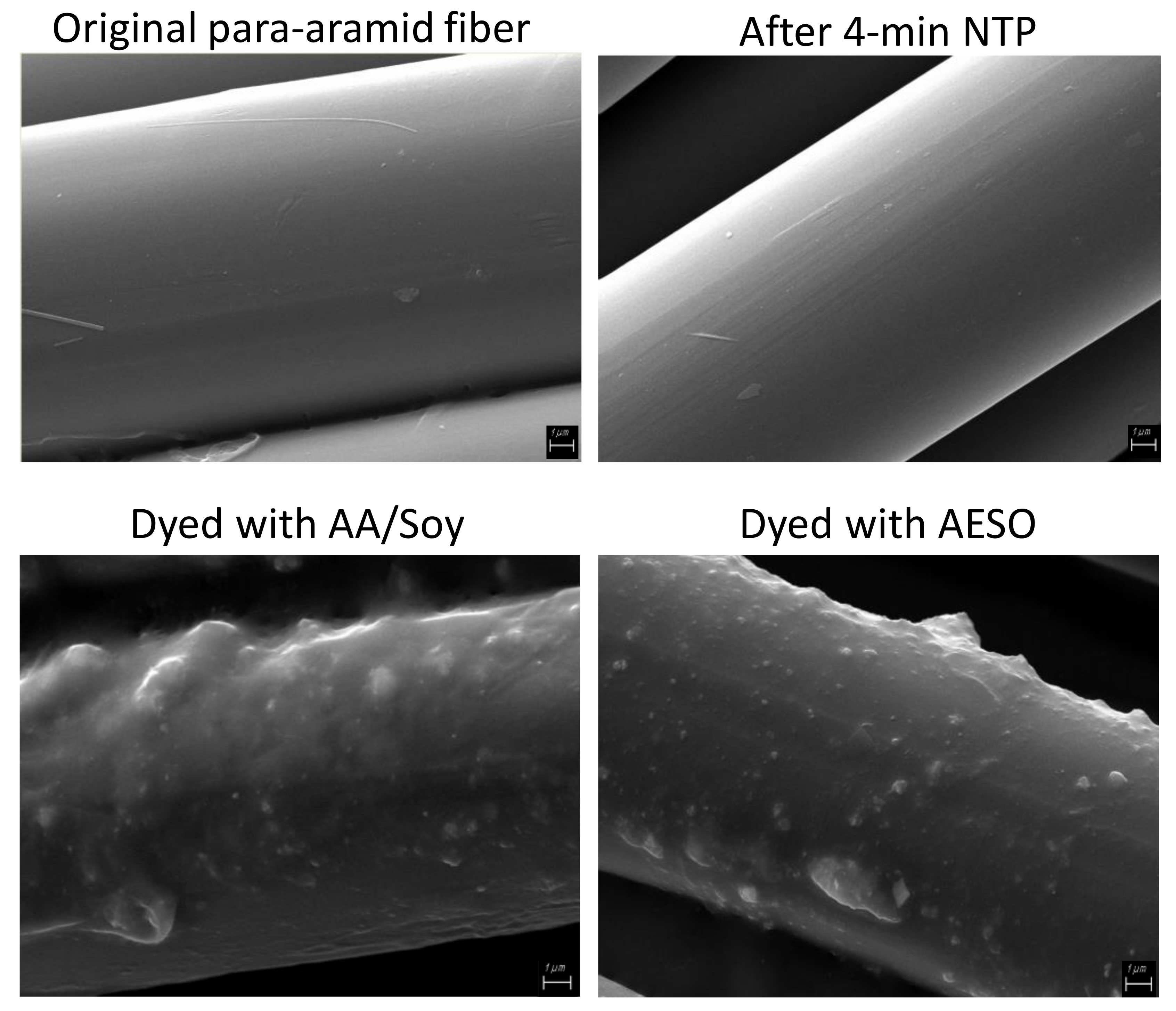
Scanning electron micrographs (SEM) reveal the smooth surface of original para-aramid fibers, and a 4 min NTP treatment did not show any visible changes on the surface (
Figure 11), explaining why the NTP treatment alone would not significantly improve dyeing. The samples dyed with AESO or AA/Soy pretreatment have a resin-like coating on the para-aramid fibers, in which dye particles are embedded. It is notable that the coating survived two washes with detergent, indicating a polymerized network. Agglomeration of dye particles can also be observed, especially in the AA/Soy pretreated sample, indicating that our pretreatment procedure needs further improvement in order to evenly distribute the AESO or AA/Soy and deliver the NTP treatment.
After a 15 h extraction with three solvents of different polarity, the color strength of all the tested samples decreased. Because the K/S values were measured at different wavelengths for the blue cationic dye vs. the red disperse dye, we report here the percentage decrease in K/S value after extraction for comparison. For the samples dyed with AA/Soy pretreatment followed by NTP treatment, the K/S value decreased by 20%, 64%, and 63% after the extraction using hexane, ethanol, or 2:1 (v/v) chloroform:methanol, respectively. For the sample dyed with Disperse Red 1 acrylate, the K/S value decreased by 6%, 54%, and 84% after the extraction using hexane, ethanol, or 2:1 (v/v) chloroform:methanol, respectively. This indicates that not all the AA/Soy were polymerized (and the unpolymerized soybean oil could be easily extracted by hexane), or not all the AA/Soy were covalently bonded to the fabric. However, there was evidence that almost all the dye molecules were covalently bonded to the NTP-induced polymerized network. When the solvents were evaporated from the extractable matters and the dry lipid-dye matrices were re-dissolved in hexane, then water was added to study the partition of the dye between the hexane and water, no blue color was observed in the aqueous layer, indicating that this water-soluble dye was chemically linked to the soybean oil network.
In addition, significant color strength remained after extraction with the three solvents of different polarities, indicating a covalently bonded network formed on the surface of para-aramid fabrics, with one exception of the 84% reduction in K/S value for the sample dyed with Disperse Red 1 acrylate and extracted with chloroform:methanol, which indicates the lack of bonding between the polymerized dye and the para-aramids; the extracted sample exhibited a yellow color close to that of the undyed fabric. In this respect, the method developed in this study performed better in binding with the inert para-aramids. Furthermore, the samples dyed with Disperse Red 1 acrylate became more rigid because of the short chain of the starting monomers, while the samples dyed with AA/Soy remained flexible due to the preserved long chains of soybean oil.
It is worthwhile to mention that in dyeing textiles, dyes are usually fixed on textiles through physical entrapment or the relatively weak hydrogen bonds and van der Waals force of attraction, or stronger ionic bonds in the case of acid dye or basic dye; few dye molecules binded to textiles via covalent bonds, except for reactive dyes with cotton, wool, or polyamides such as nylon [
31,
32].
Tensile test results show that our developed method of dyeing para-aramids did not reduce the strength of para-aramid fibers measured as breakpoint peak load.